Managing Arsenic Pollution from Soil–Plant Systems: Insights into the Role of Biochar
Abstract
1. Introduction
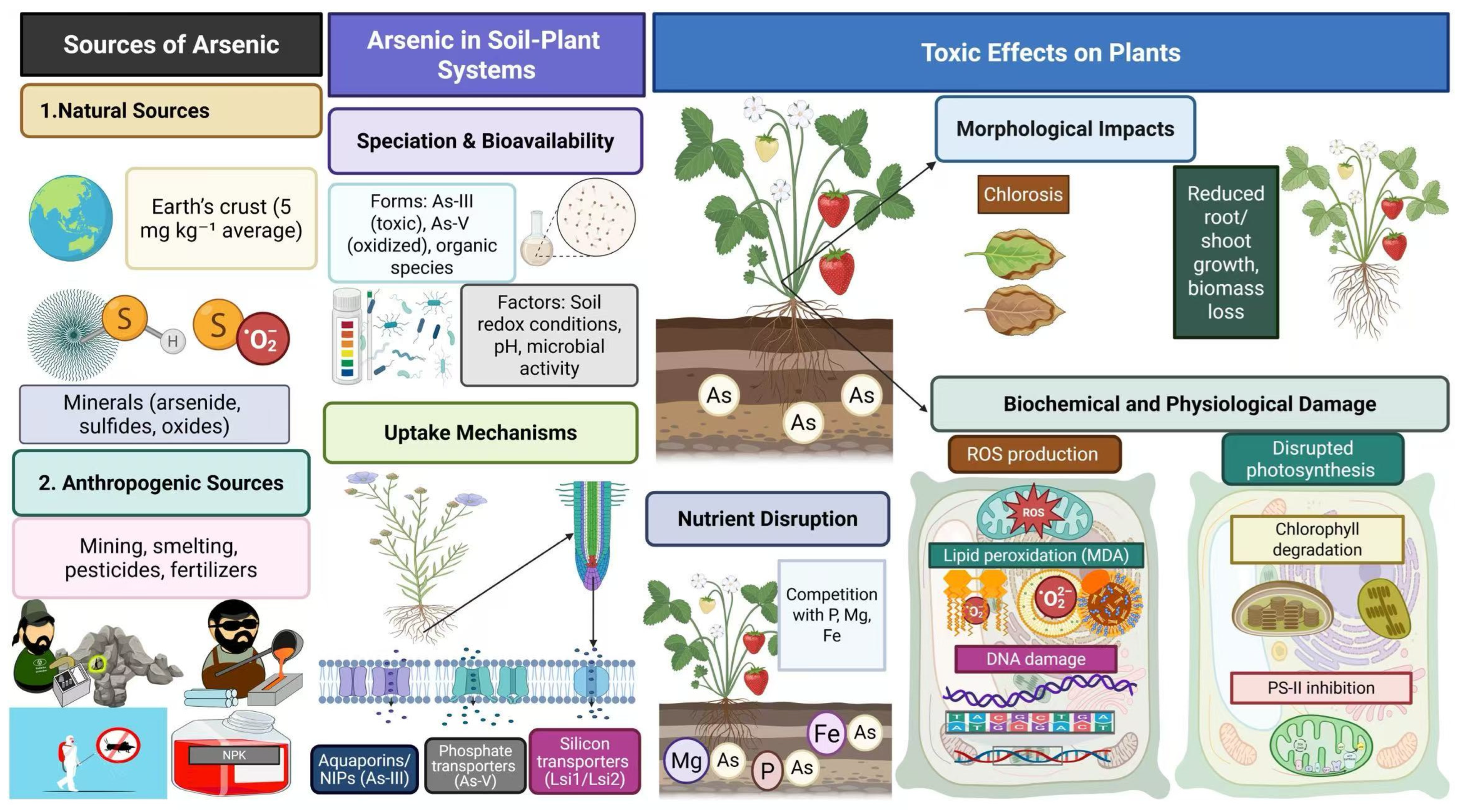
2. Arsenic, a Toxic Environmental Contaminant
3. Arsenic Occurrence and Distribution in the Environment
4. Arsenic Speciation and Bioavailability
5. Mechanisms of Arsenic Uptake and Transport Ion in Plants
6. Toxic Impacts of Arsenic on Plants
| Plant Species | As Concentrations | Growth Media | Major Effects | References |
|---|---|---|---|---|
| Maize | 50 mg kg−1 | Pot | Arsenic toxicity decreased RWC, chlorophyll and carotenoid synthesis, stomatal size, and density and increased As accumulation in maize shoots. | [71] |
| Maize | 3.2 mg L−1 | Pot | Arsenic decreases leaves, plant height, stem girth, pedunle length, chlorophyll and carotenoid synthesis, APX, and SOD activity and increased lipid peroxidation, phytochelatins production, and soil As availability. | [72] |
| Maize | 120 mg kg−1 | Pot | Arsenic toxicity decreased the plant morphological performance, phosphorous accumulation, chlorophyll synthesis, transpiration rate, stomatal conductance, and water use efficiency and increased MDA and ROS production and As accumulation. | [73] |
| Date Palm | 1 mM | Pot | Arsenic stress decreased root and shoot growth and biomass production, and chlorophyll synthesis and increased oxidative damages, lipid peroxidation, MDA, and O2•− production. | [74] |
| Mustard | 2 mM | Pot | Arsenic stress decreased plant fresh weight (35–47%), root length (38%), shoot length (39%), and chlorophyll synthesis (9–16%) and increased thiobarbituric acid reactive substances (53–125%), H2O2 production, and nonprotein thiols. | [5] |
| Mustard | 75 mg kg−1 | Pot study | Arsenic toxicity decreased root (25%) and shoot (27%) dry weight, plant height (39%) and leaf area (23%), chlorophyll-a (12%), chlorophyll-b (15%), carotenoid (6%), SOD (65%), POD (23%), APX (28%), GR (32%), and GST (46%) and increased ROS and MDA production. As also increased non-protein thiols, cysteine and phytochelatins, and accumulation and translocation of As. | [75] |
| Wheat | 2 mM | Petri dish | Arsenic toxicity reduced seed germination, seedling growth, chlorophyll synthesis, and antioxidant activities (APX, POD, SOD, and CAT) and increased the production of TBARS, lipid peroxidation, and H2O2. | [76] |
| Wheat | 100 μM | Pot | Arsenic stress increased EL, antioxidant activities, MDA and H2O2 production, and accumulation of osmolytes. Further, As also decreased RWC, photosynthetic efficiency, chlorophyll synthesis, stomatal conductance, and transpiration rate. | [77] |
| Wheat | 60 mg kg−1 | Pot | Arsenic toxicity inhibited the plant growth, productivity, photosynthetic pigments, oxidative damages, and As accumulation in roots and shoots and increased APX, SOD, and POD activities. | [78] |
| Wheat | 70 μM | Pot | Arsenic toxicity declined plant height, tillers, spike length, crop growth rate, stomatal conductance, and soil N, P, and K availability, and increased EL and As accumulation in wheat tissues. | [79] |
| Wheat | 2.02 mg kg−1 | Pot | Arsenic toxicity decreased plant height, plant biomass, spike length, grain weight, chlorophyll synthesis, and SPAD contents and increased MDA, EL, and H2O2 production, and As accumulation in roots, shoots, and grains. | [80] |
| Rice | 70 µM | Pot | Arsenic stress decreased shoot (53%) and root length (64%) and their biomass (51–67%), photosynthetic rate (49%), stomatal conductance (2%), CO2 concentration (51%), MDA (33%) and transpiration rate (38%), tissue nitrogen (12%), potassium (16%), and zinc (18%) concentration and increased SOD (28%), POD (49%), and CAT (46%) activities. | [81] |
| Rice | 2 mg L−1 | Hydroponic | Arsenic decreased root and shoot growth and biomass, chlorophyll synthesis (27.3%), SOD activity (34.46%), increased EL (8.8–15.4%), and increased root and shoot As concentration. | [82] |
| Rice | 10 μmol L−1 | Hydroponic | Arsenic toxicity decreased root and shoot elongation, biomass production, root surface area, grain weight, and grain yield and increased As accumulation in plant tissues. | [83] |
| Rice | 150 μM | Hydroponic | Arsenic toxicity declined root length (21%), shoot length (11%), fresh biomass (35%), dry biomass (36%), chlorophyll synthesis (55%), and anthocyanins (25%) and increased Mg concentration (61%), AAO activity (36%), and proline synthesis (97%). | [84] |
| Rice | 1 mM | Pot | Arsenic toxicity decreased plant dry biomass (35%), RWC (27%), and chlorophyll synthesis (44%) and increased As accumulation, proline synthesis (177%), MDA (27%), H2O2 (89%) production, and increased antixidant activities. | [85] |
| Spinach | 100 mg kg−1 | Pot | The plant growth, chlorophyll synthesis, chlorophyll fluorescence, free amino acid synthesis, and tissue zinc and manganese synthesis were significantly decreased under As stress. | [86] |
| Tomato | 3.2 mg L−1 | Peat moss | Arsenic decreased shoot and root dry biomass by 8.53% and 11.57%, Ca concentration in leaves (43.7%) and fruits (38.31%), and increased As accumulation, H2O2 production, and flavonoids contents. | [87] |
| Barley | 150 μm | Hydroponic | Arsenic treatment decreased shoot length (33.4%), root length (27.9%), shoot (36.3%) and root (25.6%) fresh biomass, chlorophyll synthesis, and fluorescence and increased MDA and ROS production, As accretion in roots and shoots, and decreased Ca uptake. Further, As toxicity also increased the expression of As transport genes. | [88] |
| Bamboo | 250 μM | Tissue culture chamber | Arsenic accumulation, translocation factor, bioaccumulation factor, soluble sugars, and membrane stability were decreased under As stress. Further, As increased ROS production and antioxidant activities. | [89] |
| Lentil | 100 mg kg−1 | Pot | Arsenic toxicity decreased soil phosphorous, potassium, nitrogen and sulfur availability, root and shoot length, and biomass production and increased As uptake and accumulation in roots and shoots of lentil. | [90] |
7. Mechanisms Mediated by Biochar to Mitigate Arsenic Toxicity
7.1. Direct Mechanisms Mediated by Biochar to Mitigate as Toxicity
7.1.1. Biochar Improves Water Uptake and Maintains Membrane Stability to Counter Arsenic Toxicity
7.1.2. Biochar Improves Synthesis of Potential Osmolytes and Increases Antioxidant Activity to Alleviate Arsenic Toxicity
7.1.3. Biochar Ensures Better Photosynthetic Efficiency and Gene Expression Under Arsenic Toxicity
7.2. Indirect Mechanisms Mediated by Biochar to Mitigate as Toxicity
7.2.1. Biochar Modulates Soil pH and Improves Nutrient Availability to Counter Arsenic Toxicity
7.2.2. Biochar Causes Arsenic Immobilization and Decreases Its Uptake to Counter Arsenic Toxicity
7.2.3. Biochar Improves Soil Microbial Activities and Biological Properties Under Arsenic Stress
7.2.4. Biochar Ensures Sustainable and Safe Crop Production in Arsenic-Polluted Soils
8. Integrative Application of Biochar and Other Amendments to Alleviate Arsenic Toxicity
9. Practical Application, Challenges, and Perspectives of Biochar Application to Remediate Arsenic-Polluted Soils
10. Conclusions and Future Prospective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angon, P.B.; Islam, M.S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The journey of arsenic from soil to grain in rice. Front. Plant Sci. 2017, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.H.; Chandrasekharam, D.; Dhanachandra, W.; Ram, K. Relationship of arsenic accumulation with irrigation practices and crop type in agriculture soils of Bengal Delta, India. Appl. Water Sci. 2019, 9, 119. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Ali, A.A.; Ahmad, J.; Baig, M.A.; Ahmad, A.; AAl-Huqail, A.; Qureshi, M.I. Impact of ferrous sulfate on thylakoidal multiprotein complexes, metabolism and defence of Brassica juncea L. under Arsenic Stress. Plants 2022, 11, 1559. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, M.; García-Montalvo, E.A.; Arellano-Mendoza, M.G.; Sánchez-Peña, L.D.C.; Soria Jasso, L.E.; Izquierdo-Vega, J.A.; Valenzuela, O.L.; Hernández-Zavala, A. Arsenic exposure and non-carcinogenic health effects. Hum. Exp. Toxicol. 2021, 40, S826–S850. [Google Scholar] [CrossRef]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic Pollution Sources. In Reviews of Environmental Contamination Volume 197: International Perspectives on Arsenic Pollution and Remediation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 17–60. [Google Scholar]
- Reis, V.; Duarte, A.C. Occurrence, distribution, and significance of arsenic speciation. Compr. Anal. Chem. 2019, 85, 1–14. [Google Scholar]
- Bhattacharya, S.; Gupta, K.; Debnath, S.; Ghosh, U.C.; Chattopadhyay, D.; Mukhopadhyay, A. Arsenic bioaccumulation in rice and edible plants and subsequent transmission through food chain in Bengal basin: A review of the perspectives for environmental health. Toxicol. Environ. Chem. 2012, 94, 429–441. [Google Scholar] [CrossRef]
- Gahlowt, P.; Singh, S.; Gupta, R.; Zheng, B.; Tripathi, D.K.; Singh, V.P. Arsenite in plant biology: How plants tackle it? Plant Physiol. Biochem. 2025, 219, 109332. [Google Scholar] [CrossRef]
- de Freitas-Silva, L.; de Araújo, T.O.; da Silva, L.C.; de Oliveira, J.A.; de Araujo, J.M. Arsenic accumulation in Brassicaceae seedlings and its effects on growth and plant anatomy. Ecotoxicol. Environ. Safe 2016, 124, 1–9. [Google Scholar] [CrossRef]
- Vezza, M.E.; Llanes, A.; Travaglia, C.; Agostini, E.; Talano, M.A. Arsenic stress effects on root water absorption in soybean plants: Physiological and morphological aspects. Plant Physiol. Biochem. 2018, 123, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Vogelsang, L.; Seidel, T.; Schmidt, R.; Weber, M.; Reichelt, M.; Dietz, K.J. Interference between arsenic-induced toxicity and hypoxia. Plant Cell Environ. 2019, 42, 574–590. [Google Scholar] [CrossRef]
- Kalita, J.; Pradhan, A.K.; Shandilya, Z.M.; Tanti, B. Arsenic stress responses and tolerance in rice: Physiological, cellular and molecular approaches. Rice Sci. 2018, 25, 235–249. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C. The role of reactive oxygen species in arsenic toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hamza, A.; Xie, Z.; Hussain, S.; Brestic, M.; Tahir, M.A.; Ulhassan, Z.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Arsenic transport and interaction with plant metabolism: Clues for improving agricultural productivity and food safety. Environ. Pollut. 2021, 290, 117987. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K.; Sablok, G.; Deshpande, T.U.; Suprasanna, P. Transcriptomics profiling of Indian mustard (Brassica juncea) under arsenate stress identifies key candidate genes and regulatory pathways. Front. Plant Sci. 2015, 6, 646. [Google Scholar] [CrossRef]
- Das, S.; Biswas, A.K. Comparative study of silicon and selenium to modulate chloroplast pigments levels, Hill activity, photosynthetic parameters and carbohydrate metabolism under arsenic stress in rice seedlings. Environ. Sci. Pollut. Res. 2022, 29, 19508–19529. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; El-Serehy, H.A.; Moustakas, M.; Ahmad, P. Magnesium oxide nanoparticles (MgO-NPs) alleviate arsenic toxicity in soybean by modulating photosynthetic function, nutrient uptake and antioxidant potential. Metals 2022, 12, 2030. [Google Scholar] [CrossRef]
- Sun, H.; Shi, Y.; Zhao, P.; Long, G.; Li, C.; Wang, J.; Qiu, D.; Lu, C.; Ding, Y.; Liu, L.; et al. Effects of polyethylene and biodegradable micro-plastics on photosynthesis, antioxidant defense systems, and arsenic accumulation in maize (Zea mays L.) seedlings grown in arsenic-contaminated soils. Sci. Total Environ. 2023, 868, 161557. [Google Scholar] [CrossRef]
- Dixit, G.; Singh, A.P.; Kumar, A.; Mishra, S.; Dwivedi, S.; Kumar, S.; Trivedi, P.K.; Pandey, V.; Tripathi, R.D. Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol. Biochem. 2016, 99, 86–96. [Google Scholar] [CrossRef]
- Vezza, M.E.; Luna, D.F.; Agostini, E.; Talano, M.A. Glutathione, a key compound for as accumulation and tolerance in soybean plants treated with AsV and AsIII. Environ. Exp. Bot. 2019, 162, 272–282. [Google Scholar] [CrossRef]
- Farooq, M.A.; Hannan, F.; Zou, H.X.; Zhou, W.; Zhao, D.S.; Abbas, T.; Ahmad, R.; Ayyaz, A.; Yan, X. Comparative transcriptome and physiological analyses reveal involvement of photosynthesis, phytohormone signaling, and cysteine-methionine metabolism in arsenic toxicity tolerance in Brassica napus. J. Hazard. Mater. 2025, 494, 138521. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Rhaman, M.S.; Parvin, K.; Bardhan, K.; Marques, D.N.; García-Caparrós, P.; Hasanuzzaman, M. Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses 2022, 2, 179–209. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Abd Manan, F. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Senn, A.C.; Hug, S.J.; Kaegi, R.; Hering, J.G.; Voegelin, A. Arsenate co-precipitation with Fe(II) oxidation products and retention or release during precipitate aging. Water Res. 2018, 131, 334–345. [Google Scholar] [CrossRef]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd, Y.M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Zheng, X.; Zou, M.; Zhang, B.; Lai, W.; Zeng, X.; Chen, S.; Wang, M.; Yi, X.; Tao, X.; Lu, G. Remediation of Cd-, Pb-, Cu-, and Zn-contaminated soil using cow bone meal and oyster shell meal. Ecotoxicol. Environ. Safe 2022, 229, 113073. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Bai, L.; Han, C.; Sun, X. Application of biochar-based materials for remediation of arsenic contaminated soil and water: Preparation, modification, and mechanisms. J. Environ. Chem. Eng. 2022, 10, 108292. [Google Scholar] [CrossRef]
- Zama, E.F.; Li, G.; Tang, Y.T.; Reid, B.J.; Ngwabie, N.M.; Sun, G.X. The removal of arsenic from solution through biochar-enhanced precipitation of calcium-arsenic derivatives. Environ. Pollut. 2022, 292, 118241. [Google Scholar] [CrossRef]
- Qian, W.; Liang, J.Y.; Zhang, W.X.; Huang, S.T.; Diao, Z.H. A porous biochar supported nanoscale zero-valent iron material highly efficient for the simultaneous remediation of cadmium and lead contaminated soil. J. Environ. Sci. 2022, 113, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Noerpel, M.R.; Scheckel, K.G.; Ippolito, J.A. Wheat straw biochar reduces environmental cadmium bioavailability. Environ. Int. 2019, 126, 69–75. [Google Scholar] [CrossRef]
- Abdin, Y.; Usman, A.; Ok, Y.S.; Tsang, Y.F.; Al-Wabel, M. Competitive sorption and availability of coexisting heavy metals in mining-contaminated soil: Contrasting effects of mesquite and fishbone biochars. Environ. Res. 2020, 181, 108846. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y.; Huang, L.; Li, Y.; Huang, Q.; Xu, G.; Müller, K.; Wang, H.; Ok, Y.S.; Liu, Z. The ratio of H/C is a useful parameter to predict adsorption of the herbicide metolachlor to biochars. Environ. Res. 2020, 184, 109324. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.; Ru, J.; Zhao, J.; Xiao, S. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci. Total Environ. 2018, 637, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Upadhyay, M.K.; Giri, B.; Karwadiya, J.; Bose, S.; Jaiswal, M.K. Iron oxide doped rice biochar reduces soil-plant arsenic stress, improves nutrient values: An amendment towards sustainable development goals. Chemosphere 2023, 312, 137117. [Google Scholar] [CrossRef]
- Song, P.; Xu, H.; Sun, S.; Xiong, W.; Yang, Z. Remediation of arsenic-spiked soil by biochar-loaded nanoscale zero-valent iron: Performance, mechanism, and microbial response. J. Clean. Prod. 2022, 380, 134985. [Google Scholar] [CrossRef]
- Xiao, B.; Jia, J.; Wang, W.; Zhang, B.; Ming, H.; Ma, S.; Kang, Y.; Zhao, M. A review on magnetic biochar for the removal of heavy metals from contaminated soils: Preparation, application, and microbial response. J. Hazard. Mater. 2023, 10, 100254. [Google Scholar] [CrossRef]
- Sevak, P.; Pushkar, B. Arsenic pollution cycle, toxicity and sustainable remediation technologies: A comprehensive review and bibliometric analysis. J. Environ. Manag. 2024, 349, 119504. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Mikutta, C.; Mandaliev, P.N.; Mahler, N.; Kotsev, T.; Kretzschmar, R. Bioaccessibility of arsenic in mining-impacted circumneutral river floodplain soils. Environ. Sci. Technol. 2014, 48, 13468–13477. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Saha, D.; Saha, R.; Ghosh, T.; Saha, B. A review on sources, toxicity and remediation technologies for removing arsenic from drinking water. Res. Chem. Intermed. 2014, 40, 447–485. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Nawaz, R.; Hussain, F.; Raza, M.; Ali, S.; Rizwan, M.; Oh, S.E.; Ahmad, S. Human health implications, risk assessment and remediation of As- contaminated water: A critical review. Sci. Total Environ. 2017, 601, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.Y.; Chen, T.B.; Xie, H.; Liu, Y.R. Soil as contamination and its risk assessment in areas near the industrial districts of Chenzhou City, Southern China. Environ. Int. 2005, 31, 791–798. [Google Scholar] [CrossRef]
- De las Torres, A.I.G.; Gir´ aldez, I.; Martínez, F.; Palencia, P.; Corns, W.T.; S’anchez-Rodas, D. Arsenic accumulation and speciation in strawberry plants exposed to inorganic arsenic enriched irrigation. Food Chem. 2020, 315, 126215. [Google Scholar] [CrossRef]
- Gao, M.; Su, Y.; Gao, J.; Zhong, X.; Li, H.; Wang, H.; Lü, C.; He, J. Arsenic speciation transformation in soils with high geological background: New insights from the governing role of Fe. Chemosphere 2022, 302, 134860. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The chemistry and behaviour of antimony in the soil environment with comparisons to arsenic: A critical review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef]
- Das, S.; Chou, M.L.; Jean, J.S.; Liu, C.C.; Yang, H.J. Water management impacts on arsenic behavior and rhizosphere bacterial communities and activities in a rice agro-ecosystem. Sci. Total Environ. 2016, 542, 642–652. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.H.; Cao, Y.; Zhu, Y.G.; Rathinasabapathi, B.; Ma, L.Q. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front. Plant Sci. 2017, 8, 268. [Google Scholar] [CrossRef]
- Zeng, K.; Liu, L.; Zheng, N.; Yu, Y.; Xu, S.; Yao, H. Iron at the helm: Steering arsenic speciation through redox processes in soils. Environ. Res. 2025, 274, 121327. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.S.; Sidhu, G.P.S.; Kumar, V. Root exudates ameliorate cadmium tolerance in plants: A review. Environ. Chem. Lett. 2020, 18, 1243–1275. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, H.; Sun, D.; Xu, G.; Rathinasabapathi, B.; Chen, Y.; Ma, L.Q. Heterologous expression of Pteris vittata phosphate transporter PvPht1; 3 enhances arsenic translocation to and accumulation in tobacco shoots. Environ. Sci. Technol. 2019, 53, 10636–10644. [Google Scholar] [CrossRef]
- Sun, D.; Feng, H.; Li, X.; Ai, H.; Sun, S.; Chen, Y.; Xu, G.; Rathinasabapathi, B.; Cao, Y.; Ma, L.Q. Expression of new Pteris vittata phosphate transporter PvPht1; 4 reduces arsenic translocation from the roots to shoots in tobacco plants. Environ. Sci. Technol. 2019, 54, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Tanaka, M.; Mitani, N.; Ma, J.F.; Maeshima, M.; Fujiwara, T. NIP1; 1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J. Biol. Chem. 2009, 284, 2114–2120. [Google Scholar] [CrossRef]
- Sun, S.K.; Chen, Y.; Che, J.; Konishi, N.; Tang, Z.; Miller, A.J.; Ma, J.F.; Zhao, F.J. Decreasing arsenic accumulation in rice by overexpressing Os NIP 1; 1 and Os NIP 3; 3 through disrupting arsenite radial transport in roots. New Phytol. 2018, 219, 641–653. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, S.K.; Tang, Z.; Liu, G.; Moore, K.L.; Maathuis, F.J.; Miller, A.J.; McGrath, S.P.; Zhao, F.J. The Nodulin 26-like intrinsic membrane protein OsNIP3; 2 is involved in arsenite uptake by lateral roots in rice. J. Exp. Bot. 2017, 68, 3007–3016. [Google Scholar] [CrossRef]
- Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Ma, J.F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11401–11406. [Google Scholar] [CrossRef]
- Sinha, D.; Datta, S.; Mishra, R.; Agarwal, P.; Kumari, T.; Adeyemi, S.B.; Kumar Maurya, A.; Ganguly, S.; Atique, U.; Seal, S.; et al. Negative impacts of arsenic on plants and mitigation strategies. Plants 2023, 12, 1815. [Google Scholar] [CrossRef]
- Chandrakar, V.; Pandey, N.; Keshavkant, S. Plant responses to arsenic toxicity: Morphology and physiology. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Springer: Singapore, 2018; pp. 27–48. [Google Scholar]
- Wang, N.; Xue, X.M.; Juhasz, A.L.; Chang, Z.Z.; Li, H.B. Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic. Environ. Pollut. 2017, 220, 514–522. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Malik, A.; Naheed, K.; Hussain, S.; Yasin, N.A.; Javad, S.; Siddiqui, M.H.; Ali, H.M.; Ali, A. Potassium silicate and zinc oxide nanoparticles modulate antioxidant system, membranous H+-ATPase and Nitric oxide content in faba bean (Vicia faba) seedlings exposed to arsenic toxicity. Funct. Plant Biol. 2022, 50, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Thapar, K.R.; Ingo, H.D.; Ahmad, A. Nitric oxide and spermidine alleviate arsenic-incited oxidative damage In. Funct. Plant Biol. 2021, 50, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Husain, T.; Kushwaha, B.K.; Suhel, M.; Fatima, A.; Mishra, V.; Singh, S.K.; Bhatt, J.A.; Rai, M.; Prasad, S.M. Regulation of ascorbate-glutathione cycle by exogenous nitric oxide and hydrogen peroxide in soybean roots under arsenate stress. J. Hazard. Mater. 2021, 409, 123686. [Google Scholar] [CrossRef]
- Alamri, S.; Kushwaha, B.K.; Singh, V.P.; Siddiqui, M.H.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M. Ascorbate and Glutathione independently alleviate arsenate toxicity in brinjal but both require endogenous nitric oxide. Physiol. Plant. 2021, 173, 276–286. [Google Scholar] [CrossRef]
- Alamri, S.; Siddiqui, M.H.; Kushwaha, B.K.; Singh, V.P.; Ali, H.M. Mitigation of arsenate toxicity by indole-3-acetic acid in brinjal roots: Plausible association with endogenous hydrogen peroxide. J. Hazard. Mater. 2021, 405, 124336. [Google Scholar] [CrossRef]
- Chandrakar, V.; Naithani, S.C.; Keshavkant, S. Arsenic-induced metabolic disturbances and their mitigation mechanisms in crop plants: A review. Biologia 2017, 71, 367–377. [Google Scholar] [CrossRef]
- Talukdar, D. Effect of Arsenic-Induced Toxicity on Morphological Traits of Trigonella foenum-Graecum L. and Lathyrus sativus L. during germination and early seedling growth. Curr. Res. J. Biol. Sci. 2011, 3, 116–123. [Google Scholar]
- Kaur, S.; Chowhan, N.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R. β-pinene alleviates arsenic (as)-induced oxidative stress by modulating enzymatic antioxidant activities in roots of Oryza sativa. Ecotoxicol. Environ. Safe 2022, 229, 113080. [Google Scholar] [CrossRef]
- Piršelová, B.; Galuščáková, Ľ.; Lengyelová, L.; Kubová, V.; Jandová, V.; Hegrová, J. Assessment of the hormetic effect of arsenic on growth and physiology of two cultivars of maize (Zea mays L.). Plants 2022, 11, 3433. [Google Scholar] [CrossRef]
- David, O.A.; Labulo, A.H.; Hassan, I.; Olawuni, I.; Oseghale, C.O.; Terna, A.D.; Ajayi, O.O.; Ayegbusi, S.A.; Owolabi, M.O. Complexation and immobilization of arsenic in maize using green synthesized silicon nanoparticles (SiNPs). Sci. Rep. 2024, 14, 6176. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Mohamed, A.K.; Salam, M.A. Self-decoration of N-doped graphene oxide 3-D hydrogel onto magnetic shrimp shell biochar for enhanced removal of hexavalent chromium. J. Hazard. Mater. 2021, 408, 124951. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Bilal, S.; Asaf, S.; Alamri, S.S.; Imran, M.; Khan, A.L.; Al-Rawahi, A.; Lee, I.J.; Al-Harrasi, A. Silicon-induced tolerance against arsenic toxicity by activating physiological, anatomical and biochemical regulation in Phoenix dactylifera (date palm). Plants 2022, 11, 2263. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, W.U.; Shah, A.A.; Yasin, N.A.; Naz, S.; Ali, A.; Tahir, A.; Batool, A.I. Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere 2021, 262, 128384. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nawaz, S.; Iqbal, K.; Rehman, S.; Ullah, R.; Nawaz, G.; Almeer, R.; Sayed, A.A.; Peluso, I. Plant-derived smoke solution alleviates cellular oxidative stress caused by arsenic and mercury by modulating the cellular antioxidative defense system in wheat. Plants 2022, 11, 1379. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Arvin, M.J.; Ashraf, M. Mitigation of arsenic toxicity in wheat by the exogenously applied salicylic acid, 24-epi-brassinolide and silicon. J. Soil. Sci. Plant Nutr. 2020, 20, 577–588. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hefnawy, S.F.; Elhamahmy, M.M.; Abdelrazik, E.M.; Sardarov, Y.B.; Ahmad, P.; Zivcak, M.; Brestic, M.; Allakhverdiev, S.I. 5-Aminolevulinic acid (ALA) reduces arsenic toxicity stress in wheat (Triticum aestivum L.). J. Plant Growth Regul. 2023, 42, 3303–3322. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Aijaz, N.; Hameed, A.; Buttar, N.A.; Rehman, S.; Riaz, M.W.; Ahmad, A.; Manzoor, M.A.; Asaduzzaman, M. Cultivating resilience in wheat: Mitigating arsenic toxicity with seaweed extract and Azospirillum brasilense. Front. Microbiol. 2024, 15, 1441719. [Google Scholar] [CrossRef]
- Manzoor, N.; Ali, L.; Al-Huqail, A.A.; Alghanem, S.M.S.; Al-Haithloul, H.A.S.; Abbas, T.; Chen, G.; Huan, L.; Liu, Y.; Wang, G. Comparative efficacy of silicon and iron oxide nanoparticles towards improving the plant growth and mitigating arsenic toxicity in wheat (Triticum aestivum L.). Ecotoxicol. Environ. Safe 2023, 264, 115382. [Google Scholar] [CrossRef]
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of cellular redox status and antioxidant defense system after synergistic application of zinc oxide nanoparticles and salicylic acid in rice (Oryza sativa) plant under arsenic stress. Plants 2021, 10, 2254. [Google Scholar] [CrossRef]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 150. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, Y.; Wang, X.; Cao, Q.; Fang, Z.; Chang, M.; Cai, Q.; Lou, L. Exogenous IAA alleviates arsenic toxicity to rice and reduces arsenic accumulation in rice grains. J. Plant Growth Regul. 2022, 41, 734–741. [Google Scholar] [CrossRef]
- Samanta, S.; Banerjee, A.; Roychoudhury, A. Arsenic toxicity is counteracted by exogenous application of melatonin to different extents in arsenic-susceptible and arsenic-tolerant rice cultivars. J. Plant Growth Regul. 2022, 41, 2210–2231. [Google Scholar] [CrossRef]
- Rahman, A.; Mostofa, M.G.; Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. BioMed Res. Int. 2015, 2015, 340812. [Google Scholar] [CrossRef] [PubMed]
- Zemanová, V.; Pavlíková, D.; Hnilička, F.; Pavlík, M. Arsenic toxicity-induced physiological and metabolic changes in the shoots of Pteris cretica and Spinacia oleracea. Plants 2021, 10, 2009. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effect of silicon nanoparticles on tomato plants exposed to two forms of inorganic arsenic. Agronomy 2022, 12, 2366. [Google Scholar] [CrossRef]
- Nazir, M.M.; Li, Q.; Noman, M.; Ulhassan, Z.; Ali, S.; Ahmed, T.; Zeng, F.; Zhang, G. Calcium oxide nanoparticles have the role of alleviating arsenic toxicity of barley. Front. Plant Sci. 2022, 13, 843795. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Hasanuzzaman, M.; Barker, J.; Liu, G.; Li, Y.; Mokhberdoran, F. Insight into the biochemical and physiological mechanisms of nanoparticles-induced arsenic tolerance in bamboo. Front. Plant Sci. 2023, 14, 1121886. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Hoque, M.A.; Ahammed, G.J.; Carpenter-Boggs, L. Arbuscular mycorrhizal fungi reduce arsenic uptake and improve plant growth in Lens culinaris. PLoS ONE 2019, 14, e0211441. [Google Scholar] [CrossRef]
- Sharma, I. Arsenic Induced Oxidative Stress in Plants. Biologia 2012, 67, 447–453. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular Mechanism of Arsenic-Induced Neurotoxicity Including Neuronal Dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef] [PubMed]
- Zargari, F.; Rahaman, M.S.; KazemPour, R.; Hajirostamlou, M. Arsenic, Oxidative Stress and Reproductive System. J. Xenobiotics 2022, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc Oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha, N. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Guzhova, I.V.; Margulis, B.A. Glyceraldehyde-3-phosphate dehydrogenase is a multifaceted therapeutic target. Pharmaceutics 2020, 12, 416. [Google Scholar] [CrossRef]
- Zaidi, S.; Hayat, S.; Pichtel, J. Arsenic-induced plant stress: Mitigation strategies and omics approaches to alleviate toxicity. Plant Physiol. Biochem. 2024, 213, 108811. [Google Scholar] [CrossRef]
- Guo, W.; Xing, Y.; Luo, X.; Li, F.; Ren, M.; Liang, Y. Reactive oxygen species: A crosslink between plant and human eukaryotic cell systems. Int. J. Mol. Sci. 2023, 24, 13052. [Google Scholar] [CrossRef]
- de Dios Alché, J. A Concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef]
- Yu, L.; Luo, Y.; Liao, B.; Xie, L.; Chen, L.; Xiao, S.; Li, J.; Hu, S.; Shu, W. Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol. 2012, 195, 97–112. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Villasuso, A.L.; Travaglia, C.; Racagni, G.E.; Reinoso, H.; Agostini, E. Arsenic stress induces changes in lipid signaling and evokes the stomata closure in soybean. Plant Physiol. Biochem. 2016, 103, 45–52. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Zhang, L. The core triacylglycerol toolbox in woody oil plants reveals targets for oil production bioengineering. Front. Plant Sci. 2023, 14, 1170723. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dubey, R.S. Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: Role of proline as enzyme protectant. J. Plant Physiol. 2006, 163, 927–936. [Google Scholar] [CrossRef]
- Ravindiran, G.; Rajamanickam, S.; Janardhan, G.; Hayder, G.; Alagumalai, A.; Mahian, O.; Lam, S.S.; Sonne, C. Production and modifications of biochar to engineered materials and its application for environmental sustainability: A review. Biochar 2024, 6, 62. [Google Scholar] [CrossRef]
- Yun, X.; Ma, Y.; Zheng, H.; Zhang, Y.; Cui, B.; Xing, B. Pb(II) adsorption by biochar from co-pyrolysis of corn stalks and alkali-fused fly ash. Biochar 2022, 4, 66. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Alharby, H.F.; Bamagoos, A.A.; Pirzadah, T.B. Biochar promotes arsenic (As) immobilization in contaminated soils and alleviates the As-toxicity in soybean (Glycine max (L.) Merr.). Chemosphere 2022, 292, 133407. [Google Scholar] [CrossRef]
- Liao, Y.; Ashraf, H.; Huang, S.; Ramzan, M.; Saba, R.; Baqir, M.; Salmen, S.H.; Alharbi, S.A.; Hareem, M. Unveiling the efficacy of Bacillus faecalis and composted biochar in alleviating arsenic toxicity in maize. BMC Plant Biol. 2024, 24, 660. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.K.; Noman, A.; Alhaithloul, H.A.S.; Adeel, M.; Rui, Y.K.; Shah, T.; Zhu, S.H.; Shang, J.Y. Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing Cd and As-induced oxidative stress in Cd and As co-contaminated paddy soil. Sci. Total Environ. 2020, 717, 137086. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Irfan, Q.M.; Rehman, R.U. Characterization of mercury-induced stress biomarkers in Fagopyrum tataricum plants. Int. J. Phytoremediat. 2018, 20, 225–236. [Google Scholar] [CrossRef]
- Kofroňová, M.; Hrdinová, A.; Mašková, P.; Tremlová, J.; Soudek, P.; Petrová, Š.; Pinkas, D.; Lipavská, H. Multi-component antioxidative system and robust carbohydrate status, the essence of plant arsenic tolerance. Antioxidants 2020, 9, 283. [Google Scholar] [CrossRef]
- Alwutayd, K.M.; Alghanem, S.M.S.; Alshehri, D.; Saleem, M.H.; Hussain, S.; Ali, B.; Abeed, A.H.A. Advancing arsenic toxicity mitigation in rice (Oryza sativa L.) with rice straw biochar and silicon: A study on morpho-physio-biochemical responses. J. Soil. Sci. Plant Nutr. 2024, 24, 2152–2166. [Google Scholar] [CrossRef]
- Islam, M.A.; Pang, J.H.; Meng, F.W.; Li, Y.W.; Ning, X.U.; Chao, Y.A.N.G.; Jun, L.I.U. Putrescine, spermidine, and spermine play distinct roles in rice salt tolerance. J. Integ. Agric. 2020, 19, 643–655. [Google Scholar] [CrossRef]
- Qiao, J.; Yu, H.; Wang, X.; Li, F.; Wang, Q.; Yuan, Y.; Liu, C. The applicability of biochar and zero-valent iron for the mitigation of arsenic and cadmium contamination in an alkaline paddy soil. Biochar 2019, 1, 203–212. [Google Scholar] [CrossRef]
- Cañas, R.A.; Yesbergenova-Cuny, Z.; Belanger, L.; Rouster, J.; Brulé, L.; Gilard, F.; Quilleré, I.; Sallaud, C.; Hirel, B. NADH-GOGAT overexpression does not improve maize (Zea mays L.) performance even when pyramiding with NAD-IDH, GDH and GS. Plants 2020, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Arikan, B.; Alp-Turgut, F.N.; Gulenturk, C. The regulatory effects of biochar on PSII photochemistry, antioxidant system and nitrogen assimilation in Lemna minor exposed to inorganic pollutants, arsenic and fluoride. J. Environ. Chem. Eng. 2023, 11, 110713. [Google Scholar] [CrossRef]
- Alam, M.Z.; McGee, R.; Hoque, M.A.; Ahammed, G.J.; Carpenter-Boggs, L. Effect of arbuscular mycorrhizal fungi, selenium and biochar on photosynthetic pigments and antioxidant enzyme activity under arsenic stress in mung bean (Vigna radiata). Front. Physiol. 2019, 10, 193. [Google Scholar] [CrossRef]
- Shabbir, A.; Saqib, M.; Murtaza, G.; Abbas, G.; Imran, M.; Rizwan, M.; Naeem, M.A.; Ali, S.; Javeed, H.M.R. Biochar mitigates arsenic-induced human health risks and phytotoxicity in quinoa under saline conditions by modulating ionic and oxidative stress responses. Environ. Pollut. 2021, 287, 117348. [Google Scholar] [CrossRef]
- Wen, E.; Yang, X.; Chen, H.; Shaheen, S.M.; Sarkar, B.; Xu, S.; Song, H.; Liang, Y.; Rinklebe, J.; Hou, D.; et al. Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phyto-availability of arsenic, cadmium and lead in a paddy soil. J. Hazard. Mater. 2020, 407, 124344. [Google Scholar] [CrossRef]
- Rahman, M.M.; Das, A.K.; Sultana, S.; Ghosh, P.K.; Islam, M.R.; Keya, S.S.; Ahmed, M.; Nihad, S.A.I.; Khan, M.A.R.; Lovell, M.C.; et al. Biochar potentially enhances maize tolerance to arsenic toxicity by improving physiological and biochemical responses to excessive arsenate. Biochar 2023, 5, 71. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Abourehab, M.A.; Ijaz, M.; Nawaz, M.; Ul-Allah, S.; Abbas, T.; Shah, A.N.; Imam, M.S.; Abdelsalam, N.R.; et al. Application of silicon and biochar alleviates the adversities of arsenic stress in maize by triggering the morpho-physiological and antioxidant defense mechanisms. Front. Environ. Sci. 2022, 10, 2086. [Google Scholar] [CrossRef]
- Alsamadany, H.; Alharby, H.F.; Al-Zahrani, H.S.; Alzahrani, Y.M.; Almaghamsi, A.A.; Abbas, G.; Farooq, M.A. Silicon-nanoparticles doped biochar is more effective than biochar for mitigation of arsenic and salinity stress in Quinoa: Insight to human health risk assessment. Front. Plant Sci. 2022, 13, 989504. [Google Scholar] [CrossRef]
- Sahin, O.; Taskın, M.B.; Kaya, E.C.; Taskın, H. Poultry manure biochar reduces arsenic induced oxidative stress and arsenic levels in rice plants. J. Agric. Fac. Uludag Univ. 2017, 31, 103–113. [Google Scholar]
- Ferdousi, N.; Imamul Huq, S.M. Arsenic mitigation approach in soil by some indigenous sources of biochar made at low pyrolysis temperature. Int. J. Plant Soil Sci. 2020, 32, 93–108. [Google Scholar] [CrossRef]
- Kowitwiwat, A.; Sampanpanish, P. Phytostabilization of arsenic and manganese in mine tailings using Pennisetum purpureum cv. Mott supplemented with cow manure and acacia wood-derived biochar. Heliyon 2020, 6, e04552. [Google Scholar] [CrossRef]
- Irshad, M.K.; Noman, A.; Wang, Y.; Yin, Y.; Chen, C.; Shang, J. Goethite modified biochar simultaneously mitigates the arsenic and cadmium accumulation in paddy rice (Oryza sativa) L. Environ. Res. 2022, 206, 112238. [Google Scholar] [CrossRef]
- Jin, W.; Wang, Z.; Sun, Y.; Wang, Y.; Bi, C.; Zhou, L.; Zheng, X. Impacts of biochar and silicate fertilizer on arsenic accumulation in rice (Oryza sativa L.). Ecotoxicol. Environ. Safe 2020, 189, 109928. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, H.; Yan, X.L. The Fe3O4-modified biochar reduces arsenic availability in soil and arsenic accumulation in indica rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2021, 28, 18050–18061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, X.; Gao, M.; Song, Z. Effect of Fe–Mn–Ce modified biochar composite on microbial diversity and properties of arsenic-contaminated paddy soils. Chemosphere 2020, 250, 126249. [Google Scholar] [CrossRef]
- Yu, Z.; Qiu, W.; Wang, F.; Lei, M.; Wang, D.; Song, Z. Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere 2017, 168, 341–349. [Google Scholar] [CrossRef]
- Manzoor, N.; Ali, L.; Ahmad, T.; Khan, M.Y.; Ali, H.M.; Liu, Y.; Wang, G. Biochar and nanoscale silicon synergistically alleviate arsenic toxicity and enhance productivity in chili peppers (Capsicum annuum L.). Chemosphere 2024, 368, 143682. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef]
- Huang, X.; Li, M.; Hou, Y.; Huang, P.; Wen, H.; Li, H.; Ma, C. Comparison of arsenic remediation effects between selenium-rich biochar and selenium-modified biochar. J. Environ. Chem. Eng. 2024, 12, 113488. [Google Scholar] [CrossRef]
- Rahimzadeh, S.; Kazem, G. The roles of nanoparticle-enriched biochars in improving soil enzyme activities and nutrient uptake by basil plants under arsenic toxicity. Inter. J. Phytorem. 2025, 27, 307–315. [Google Scholar] [CrossRef]
- Manikandan, R.; Sahi, S.V.; Venkatachalam, P. Impact assessment of mercury accumulation and biochemical and molecular response of Mentha arvensis: A potential hyperaccumulator plant. Sci. World J. 2015, 2015, 715217. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Hasanuzzaman, M. Unlocking the potential of co-application of steel slag and biochar in mitigation of arsenic-induced oxidative stress by modulating antioxidant and glyoxalase system in Abelmoschus esculentus L. Chemosphere 2024, 351, 141232. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative effects of biochar on rapeseed (Brassica napus L.) growth and heavy metal immobilization in soil irrigated with untreated wastewater. J. Plant Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Xu, M.; Gao, P.; Wu, J.; Ma, J.; Zhang, X.; Yang, G.; Long, L.; Chen, C.; Song, C.; Xiao, Y. Biochar promotes arsenic sequestration on iron plaques and cell walls in rice roots. Chemosphere 2022, 288, 132422. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi-Golezani, K.; Rahimzadeh, S. Biochar-based nanoparticles mitigated arsenic toxicity and improved physiological performance of basil via enhancing cation exchange capacity and ferric chelate reductase activity. Chemosphere 2024, 362, 142623. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Ran, C.; Zhang, Y.; Wang, X.; Lu, S.; Geng, Y.; Guo, L.; Shao, X. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 2022, 185, 112–122. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Islam, M.S.; Magid, A.S.I.A.; Chen, Y.; Weng, L.; Ma, J.; Arafat, M.Y.; Khan, Z.H.; Li, Y. Effect of calcium and iron-enriched biochar on arsenic and cadmium accumulation from soil to rice paddy tissues. Sci. Total Environ. 2021, 785, 147163. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Imtiaz, M.; Qaswar, M.; Ikram, M.; Bashir, S.; Rizwan, M.; Irshad, S.; Tu, S.; Li, W.; et al. Chitosan-modified biochar immobilised arsenic in root medium and enhanced the growth of zucchini (cv. Courgette d’Italie) seedlings. Crop. Past. Sci. 2021, 73, 170–180. [Google Scholar] [CrossRef]
- Khanam, R.; Tripathy, L.; Chidambaranathan, P. Impact of biochar and water regimes on arsenic transfer and uptake in rice: Insights into transporter behaviour and soil–plant dynamics. Expo. Health 2025, 17, 467–480. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, N.; Yi, P.; Chen, L.; Dai, H.; Zhang, J.; Li, W.; Li, R.; Liu, A.; Zhou, Z.; et al. Integrated physio-biochemistry and RNA-seq revealed the mechanism underlying biochar-mediated alleviation of compound heavy metals (Cd, Pb, As) toxicity in cotton. Ecotoxicol. Environ. Safe 2024, 284, 116974. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Li, C.; Chen, Y.; Zheng, L.; Ding, D.; Shan, S. Synergistic mechanism of iron manganese supported biochar for arsenic remediation and enzyme activity in contaminated soil. J. Environ. Manag. 2023, 347, 119127. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Roy, A. Sustainable soil management under drought stress through biochar application: Immobilizing arsenic, ameliorating soil quality, and augmenting plant growth. Environ. Res. 2024, 259, 119531. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.; Chow, K.L.; Chen, X.W.; Ng, C.W.; Wong, M.H. Effects of biochar on soil water retention curves of compacted clay during wetting and drying. Biochar 2022, 4, 4. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ghosh, T.K.; Kabir, A.H.; Abdelrahman, M.; Khan, M.A.R.; Mochida, K.; Tran, L.S.P. Potassium in plant physiological adaptation to abiotic stresses. Plant Physiol. Biochem. 2022, 186, 279–289. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, M.; Azim, U.; Vithanage, M.; Bhattacharya, T. Garbage to Gains: The role of biochar in sustainable soil quality improvement, arsenic remediation, and crop yield enhancement. Chemosphere 2023, 344, 140417. [Google Scholar] [CrossRef]
- Dias, Y.N.; da Silveira Pereira, W.V.; da Costa, M.V.; de Souza, E.S.; Ramos, S.J.; do Amarante, C.B.; Campos, W.E.O.; Fernandes, A.R. Biochar mitigates bioavailability and environmental risks of arsenic in gold mining tailings from the eastern Amazon. J. Environ. Manag. 2022, 311, 114840. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, Z.; Sun, Y.; Cao, X.; Hou, D.; Alessi, D.S.; Ok, Y.S.; Tsang, D.C. Unraveling iron speciation on Fe-biochar with distinct arsenic removal mechanisms and depth distributions of As and Fe. Chem. Eng. J. 2021, 425, 131489. [Google Scholar] [CrossRef]
- El-Naggar, A.; Shaheen, S.M.; Hseu, Z.Y.; Wang, S.L.; Ok, Y.S.; d Rinklebe, J. Release dynamics of As, Co, and Mo in a biochar treated soil under pre-definite redox conditions. Sci. Total Environ. 2019, 657, 686–695. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Tao, Q.I.; Chen, Y.; Zhao, J.; Li, B.; Li, Y.; Tao, S.; Li, M.; Li, Q.; Xu, Q.; Li, Y.; et al. Enhanced Cd removal from aqueous solution by biologically modified biochar derived from digestion residue of corn straw silage. Sci. Total Environ. 2019, 674, 213–222. [Google Scholar] [CrossRef]
- Wu, C.; Huang, L.; Xue, S.-G.; Huang, Y.-Y.; Hartley, W.; Cui, M.-q.; Wong, M.-H. Arsenic sorption by red mud-modified biochar produced from rice straw. Environ. Sci. Pollut. Res. 2017, 24, 18168–18178. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Wang, M.; Shohag, M.J.I.; Lu, L.; He, T.; Liao, C.; Zhang, Z.; Chen, J.; You, X.; Zhao, Y.; et al. Biochar performance for preventing cadmium and arsenic accumulation, and the health risks associated with mustard (Brassica juncea) grown in co-contaminated soils. Ecotoxicol. Environ. Safe 2023, 263, 115216. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Ju, L.; Ling, X.; Liu, C.; Wei, X.; Qiu, H.; Tang, Y.; Morel, J.L.; Qiu, R.; Li, C.; et al. Simultaneous attenuation of phytoaccumulation of Cd and As in soil treated with inorganic and organic amendments. Environ. Pollut. 2019, 250, 464–474. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Bundschuh, J.; Seneweera, S.; Marchuk, A.; Ok, Y.S. Iron modification to silicon-rich biochar and alternative water management to decrease arsenic accumulation in rice (Oryza sativa L.). Environ. Pollut. 2021, 286, 117661. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lamb, D.; Rahman, M.M.; Bahar, M.M.; Sanderson, P.; Abbasi, S.; Bari, A.F.; Naidu, R. Removal of arsenate from contaminated waters by novel zirconium and zirconium-iron modified biochar. J. Hazard. Mater. 2021, 409, 124488. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, S.; Liu, C.; Chen, T.; Tang, Y.; Ma, J.; Yin, K.; Luo, S. Efficient removal of arsenic from groundwater using iron oxide nanoneedle array-decorated biochar fibers with high Fe utilization and fast adsorption kinetics. Water Res. 2019, 167, 115107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Luo, J.; Song, W.; Chen, H.; Zhang, S. Influence of biochar on the partitioning of iron and arsenic from paddy soil contaminated by acid mine drainage. Sci. Rep. 2025, 15, 4852. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Verma, Y.; Lai, C.W.; Naushad, M.; Iqbal, J.; Kumar, A.; Dhiman, P. Biochar and biosorbents derived from biomass for arsenic remediation. Heliyon 2024, 10, e36288. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Kasuga, J.; Makino, T.; Arao, T. Evaluation of the effects of application of iron materials on the accumulation and speciation of arsenic in rice grain grown on uncontaminated soil with relatively high levels of arsenic. Environ. Exp. Bot. 2016, 125, 42–51. [Google Scholar] [CrossRef]
- Asadishad, B.; Chahal, S.; Akbari, A.; Cianciarelli, V.; Azodi, M.; Ghoshal, S.; Tufenkji, N. Amendment of agricultural soil with metal nanoparticles: Effects on soil enzyme activity and microbial community composition. Environ. Sci. Technol. 2018, 52, 1908–1918. [Google Scholar] [CrossRef]
- Bandara, T.; Franks, A.; Xu, J.; Bolan, N.; Wang, H.; Tang, C. Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit. Rev. Environ. Sci. Technol. 2020, 50, 903–978. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.; Gao, J.; Wang, F.; Hou, Y.; Cai, Q.; Liu, Q. Biochar assisted bioremediation of soils with combined contamination of petroleum hydrocarbons and heavy metals: A review. Appl. Soil Ecol. 2024, 204, 105720. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Bian, R.; Cheng, K.; Zhang, X.; Zheng, J.; Joseph, S.; Crowley, D.; Pan, G.; Li, L. Effects of biochar on availability and plant uptake of heavy metals–A meta-analysis. J. Environ. Manag. 2018, 222, 76–85. [Google Scholar] [CrossRef]
- Zou, Q.; An, W.; Wu, C.; Li, W.; Fu, A.; Xiao, R.; Chen, H.; Xue, S. Red mud-modified biochar reduces soil arsenic availability and changes bacterial composition. Environ. Chem. Lett. 2018, 16, 615–622. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Chen, G.; Zhou, J.; Chen, Z.; Li, Z.; Zhu, J.; Feng, T.; Chen, Y. Response of soil microbial communities to additions of straw biochar, iron oxide, and iron oxide–modified straw biochar in an arsenic-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 23761–23768. [Google Scholar] [CrossRef]
- Tang, X.; Zou, L.; Su, S.; Lu, Y.; Zhai, W.; Manzoor, M.; Liao, Y.; Nie, J.; Shi, J.; Ma, L.Q.; et al. Long-term manure application changes bacterial communities in rice rhizosphere and arsenic speciation in rice grains. Environ. Sci. Technol. 2021, 55, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Yuan, Y.; Zhang, X.; Wang, L.; Tao, Y.; Jiang, Z.; Yu, H.; Dong, M.; Zhang, Y. Stabilization of lead and cadmium in soil by sulfur-iron functionalized biochar: Performance, mechanisms and microbial community evolution. J. Hazard. Mater. 2022, 425, 127876. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dai, Z.; Ge, C.; Yu, H.; Bolan, N.; Tsang, D.C.; Song, H.; Hou, D.; Shaheen, S.M.; Wang, H.; et al. Multiple-functionalized biochar affects rice yield and quality via regulating arsenic and lead redistribution and bacterial community structure in soils under different hydrological conditions. J. Hazard. Mater. 2023, 443, 130308. [Google Scholar] [CrossRef]
- Chen, S.; Qi, G.; Ma, G.; Zhao, X. Biochar amendment controlled bacterial wilt through changing soil chemical properties and microbial community. Microbiol. Res. 2020, 231, 126373. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.; Li, B.; Huang, W.; Qin, J.; Li, H.; Chen, G. Hydrous zirconium oxide modified biochar for in situ remediation of arsenic contaminated agricultural soil. J. Environ. Chem. Eng. 2022, 10, 108360. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Zhang, L.; Zheng, Y.; Liu, X.; Zhang, Y. The critical role of biochar to mitigate the adverse impacts of drought and salinity stress in plants. Front. Plant Sci. 2023, 14, 1163451. [Google Scholar] [CrossRef]
- Guo, G.; Chen, S.; Zhang, D.; Wang, J.; Lei, M.; Ju, T.; Wei, H. Influence of biochar on the arsenic phytoextraction potential of Pteris vittata in soils from an abandoned arsenic mining site. Chemosphere 2024, 352, 141389. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phyto-availability, translocation, and phytoremediation—A review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M.; Yu, K. Release of As, Ba, Cd, Cu, Pb, and Sr under pre-definite redox conditions in different rice paddy soils originating from the USA and Asia. Geoderma 2016, 270, 21–32. [Google Scholar] [CrossRef]
- Lin, L.; Gao, M.; Qiu, W.; Wang, D.; Huang, Q.; Song, Z. Reduced arsenic accumulation in indica rice (Oryza sativa L.) cultivar with ferromanganese oxide impregnated biochar composites amendments. Environ. Pollut. 2017, 231, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Hossain, A.; Sharif, M.O.; Das, M.; Sarker, T. Optimizing biochar, vermicompost, and duckweed amendments to mitigate arsenic uptake and accumulation in rice (Oryza sativa L.) cultivated on arsenic-contaminated soil. BMC Plant Biol. 2024, 24, 545. [Google Scholar] [CrossRef]
- Shukla, K.; Khanam, R.; Kumar, B.J.; Srivastava, S. Zinc oxide nanoparticles in combination with biochar alleviates arsenic accumulation in field grown rice crop. Rhizosphere 2023, 27, 100764. [Google Scholar] [CrossRef]
- Mensah, A.K.; Marschner, B.; Shaheen, S.M.; Rinklebe, J. Biochar, compost, iron oxide, manure, and inorganic fertilizer affect bioavailability of arsenic and improve soil quality of an abandoned arsenic-contaminated gold mine spoil. Ecotoxicol. Environ. Safe 2022, 234, 113358. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Yu, Z.; Chen, J.; Zhang, J.; Zhu, J.; Yang, W.; Yang, R.; Wu, P.; Wang, S. Synergistic effect between biochar and nitrate fertilizer facilitated arsenic immobilization in an anaerobic contaminated paddy soil. Sci. Total Environ. 2024, 955, 177007. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, B.; Liu, G.; Cai, Z.; Zhang, C. Potential toxic compounds in biochar: Knowledge gaps between biochar research and safety. In Biochar from Biomass and Waste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 349–384. [Google Scholar]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Visioli, G.; Conti, F.D.; Menta, C.; Bandiera, M.; Malcevschi, A.; Jones, D.L.; Vamerali, T. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environ. Monit. Assess. 2016, 188, 166. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Wang, L.; Liu, X.; Tang, C.; Xu, J. A novel calcium-based magnetic biochar reduces the accumulation of As in grains of rice (Oryza sativa L.) in As-contaminated paddy soils. J. Hazard. Mater. 2020, 394, 122507. [Google Scholar] [CrossRef]
- Chen, T.; Wei, Y.; Yang, W.; Liu, C. Highly efficient As (III) removal in water using millimeter-sized porous granular MgO-biochar with high adsorption capacity. J. Hazard. Mater. 2021, 416, 125822. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Kim, B.; Jang, J.; Lee, D.S. Buckwheat hull-derived biochar immobilized in alginate beads for the adsorptive removal of cobalt from aqueous solutions. J. Hazard. Mater. 2022, 436, 129245. [Google Scholar] [CrossRef] [PubMed]
- Nkoh, J.N.; Ajibade, F.O.; Atakpa, E.O.; Abdulaha-Al Baquy, M.; Mia, S.; Odii, E.C.; Xu, R. Reduction of heavy metal uptake from polluted soils and associated health risks through biochar amendment: A critical synthesis. J. Hazard. Mater. Adv. 2022, 6, 100086. [Google Scholar] [CrossRef]


| Plant Species | As Stress | Rate of BC | Effects on Plant | References |
|---|---|---|---|---|
| Maize | 10 mg kg−1 | 5% | Biochar application decreased oxidative stress and MDA production by increasing SOD (46.55%), CAT (82.82%), and GST (153.83%) activity, flavonoid synthesis (75.37%), soluble sugars, amino acids, and nutrient availability and decreasing As uptake and accumulation. | [120] |
| Maize | 600 mg kg−1 | 0.5% | Co-applying BC with bacteria enhanced plant height (99%), shoot (96%) and root dry biomass (91%), chlorophyll synthesis (94%), and N, P, and K concentration in plant tissues. | [108] |
| Maize | 12 mg kg−1 | 50 g kg−1 | Biochar increased plant height (2.91%), leaf area (24.41%), cob length (5.29%), grains/cob (9.73%), grain weight (11.24%), grain yield (9.91%), chlorophyll synthesis (11.35%), TSP (26.76%), FAA (26.50%), SS (46.95%), SOD (20.44%), POD (16.91%), CAT (12.78%), and APX (20%) activities and decreased MDA (39%) and H2O2 (28.05%) production and As concentration in shoots (31.03%) and grains (70.58%). | [121] |
| Quinoa | 20 mg kg−1 | 1% | Biochar addition enhanced APX, CAT, and SOD activities, grain and biomass yield, chlorophyll synthesis, tissue, N, P, and K contents and reduced the As uptake, transport, and accumulation. | [122] |
| Quinoa | 20 mg L−1 | 2% | Biochar increased the root and shoot lengths by 2.6%% and 2.4%, their dry weights by 2.9% and 0%, and grain yield by 30%. Further, BC also enhanced the RWC by 28%, stomatal conductance by 156%, chlorophyll contents by 2.8%, shoot and root K by 18% and 115%, and membrane stability by 136%. Additionally, BC also decreased As accretion in shoots (75%), roots (32%), and grains (95%) and increased SOD (33%), POD (31%), and CAT (34%) activities | [118] |
| Rice | 60 mg kg−1 | 20 g kg−1 | Biochar application decreased H2O2 production and enhanced the APX and CAT activities and N, P, K, and S concentration in plant tissues and decreased As accumulation. | [123] |
| Water Spinach | 1 mg L−1 | 20 t ha−1 | Biochar addition reduced As accumulation and improved plant growth and As adsorption. | [124] |
| Napier grass | 68 mg kg−1 | 5% | BC application reduced As uptake and accumulation by causing stabilization and immobilization of As. Further, BC also improved the plant relative growth rates, biomass production, and chlorophyll synthesis. | [125] |
| Rice | 100 µM | 5% | Biochar application decreased ROS production and membrane damage and increased organic acids, proline synthesis, antioxidants activities, plant growth, biomass production, gas exchange characteristics, and decreased the As accumulation in plant tissues. | [112] |
| Rice | 231 mg kg−1 | 3% | Biochar addition enhanced root, shoot, husk, and grain weight and decreased As accumulation in roots, husks, and grains. Biochar application also increased synthesis of glutamate, histidine, arginine, aspartate, serine, glycine, and proline and increased the abundance of Acidobacteria, Proteobacteria, Choloroflexi, Actinobacteria, and Firmicutes. | [126] |
| Rice | 105 mg kg−1 | 5% | Biochar decreased As in roots, straw, and grain and increased As dilution and biomass production. | [127] |
| Rice | 120 mg kg−1 | 3% | Biochar supply increased the As in soil solution and decreased As in amorphous Fe/Al oxide fraction. Biochar also increased the abundance of Fe-reducing bacteria, including Clostridum (27.3%), Bacillus (2.39%), and Caloramator (4.46%), and As-reducing (19%) genes. | [62] |
| Rice | 1.6% | Biochar supplementation increased cation exchange capacity and reduced the As concentration in rice lower than 0.2 mg kg−1. Biochar supply also decreased As concentration in iron plaque, rice stems, leaves, husks, and roots. | [128] | |
| Rice | 138 mg kg−1 | 2% | Biochar application enhanced soil pH and CEC and reduced the bioavailable forms of As. Further, BC also converted the specifically bound forms of As into hydrous oxide bound and crystalline hydrous oxide forms and increased soil urease, catalase, phosphate, and peroxidase activities and abundance of Proteobacteria, Acidobacteria, and Gemmatimonadetes. | [129] |
| Rice | 73 mg kg−1 | 2% | Biochar supply improved root growth and aboveground biomass and decreased the As accumulation in rice plant parts, which was linked with oxidation of As by Mn oxides. Biochar also enhanced amino acid synthesis and Mn concentration by 36%. | [130] |
| Chilli | 7.5 mg kg−1 | 10 g kg−1 | Biochar enhanced shoot (34.24%) and root length (50.47%) and their biomass (43.55 and 52.07%), chlorophyll synthesis, SOD (18.12%), CAT (15.78%), soluble sugars (37%), and protein (27.20%) and decreased soil As (52.42%) availability. | [131] |
| Tomato | 3003 mg kg−1 | 30% | Biochar application reduced As accumulation in soil, water, roots, shoots, and fruits and increased water and soil pH, Fe availability, and plant fresh and dry biomass production. | [132] |
| Pak choi | 1000 mg L−1 | 3% | Biochar application increases aboveground biomass, chlorophyll synthesis, RWC, APX, CAT, and POD activities and decreased MDA production and As accumulation in roots and stem. | [133] |
| Basil | 100 mg kg−1 | 5% | Biochar enhanced soil organic matter, microbial biomass carbon, soil respiration, and soil enzyme activities (urease, alkaline phosphatase, and dehydrogenase) and decreased As availability. | [134] |
| Okra | 10 mg kg−1 | 2 g kg−1 | Biochar application decreased As accumulation in root and shoots of okra, increased antioxidant activities and performance of glyoxalase enzyme, and decreased methylglyoxal production. Further, BC also decreased oxidative damages and increased the synthesis of thiol and phytochelatins. | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Q.; Du, Z.; Huang, X.; Hassan, M.U.; Altihani, F.A. Managing Arsenic Pollution from Soil–Plant Systems: Insights into the Role of Biochar. Plants 2025, 14, 1553. https://doi.org/10.3390/plants14101553
Su Q, Du Z, Huang X, Hassan MU, Altihani FA. Managing Arsenic Pollution from Soil–Plant Systems: Insights into the Role of Biochar. Plants. 2025; 14(10):1553. https://doi.org/10.3390/plants14101553
Chicago/Turabian StyleSu, Qitao, Zhixuan Du, Xinyi Huang, Muhammad Umair Hassan, and Faizah Amer Altihani. 2025. "Managing Arsenic Pollution from Soil–Plant Systems: Insights into the Role of Biochar" Plants 14, no. 10: 1553. https://doi.org/10.3390/plants14101553
APA StyleSu, Q., Du, Z., Huang, X., Hassan, M. U., & Altihani, F. A. (2025). Managing Arsenic Pollution from Soil–Plant Systems: Insights into the Role of Biochar. Plants, 14(10), 1553. https://doi.org/10.3390/plants14101553







