Beyond Peat: Wood Fiber and Two Novel Organic Byproducts as Growing Media—A Systematic Review
Abstract
1. Introduction
2. Organic Byproducts as Growing Media
3. Methodology
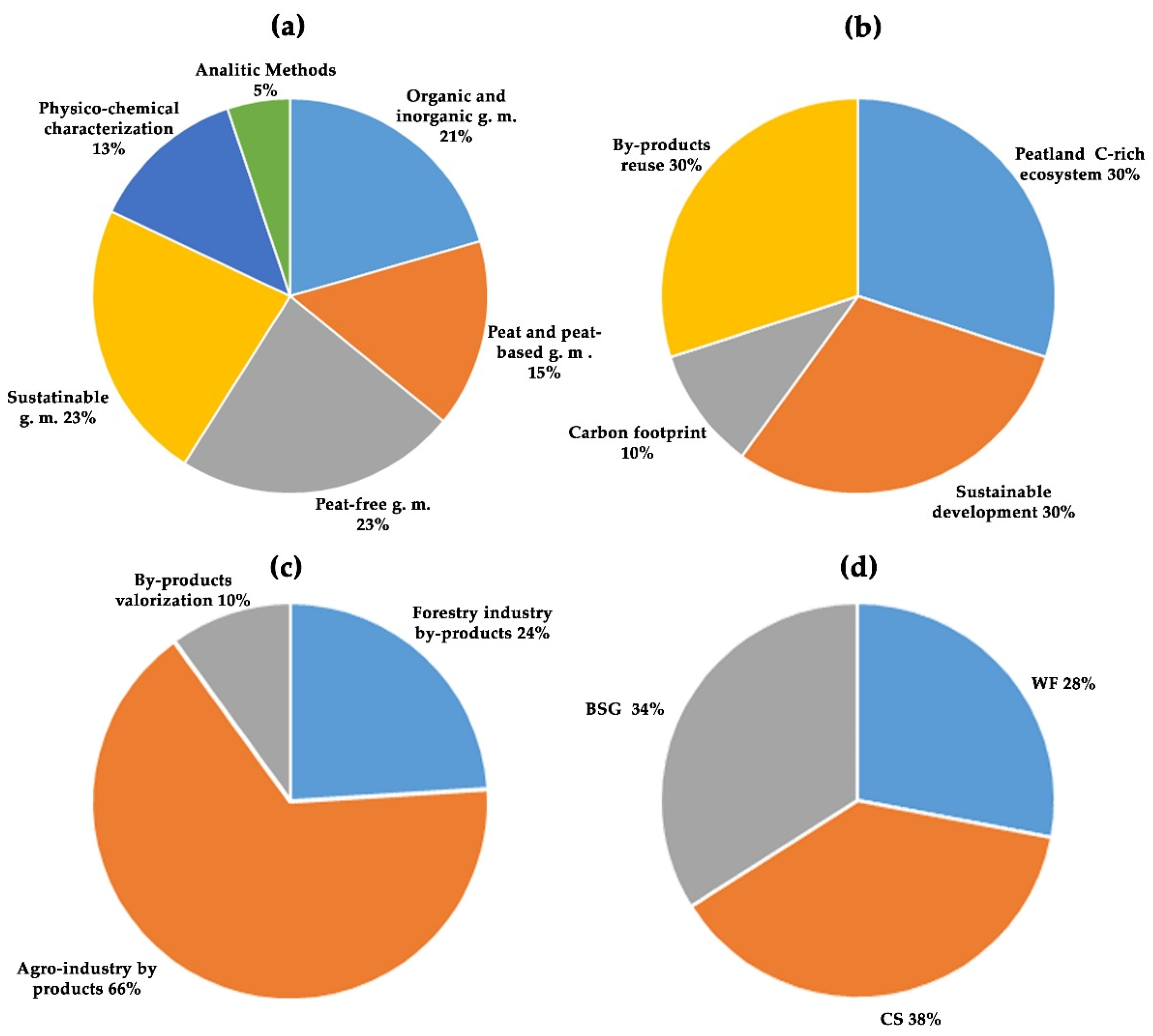
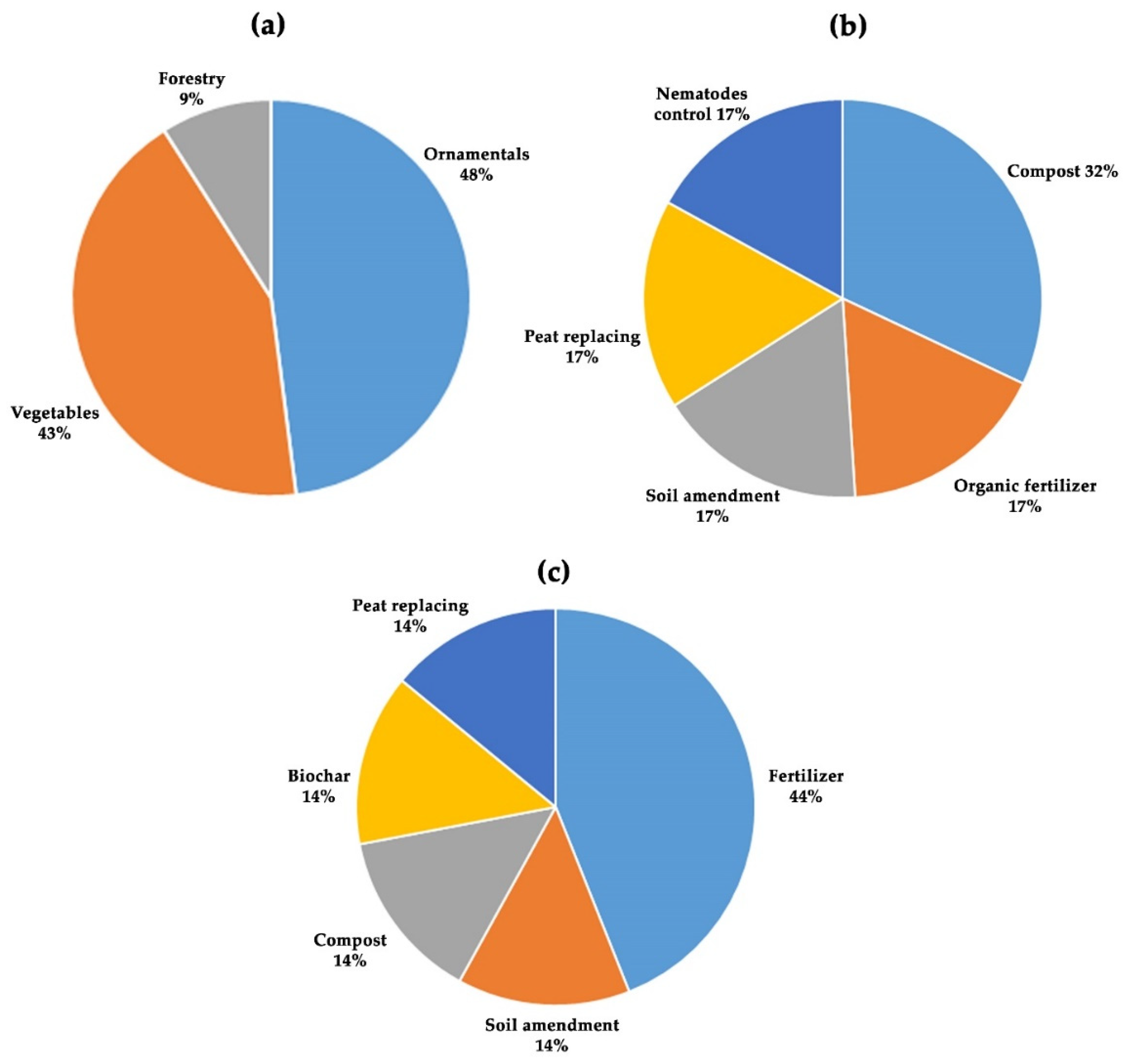
3.1. Formulation of the Problem
3.2. Keyword Identification
4. Wood Fiber
4.1. The Production Process
4.2. Agricultural and Non-Agricultural Uses
4.3. Physical, Hydrological, and Chemical Characterizations
4.3.1. Physical and Hydrological Characterizations
4.3.2. Chemical Characterization
4.3.3. Practical Applications
4.4. Crop’s Performance
4.4.1. Wood Fiber Peat Replacement up to 50%
4.4.2. Wood Fiber Peat-Replacement Beyond 50%
4.4.3. Wood Fiber and Fertilization
5. Coffee Silverskin
5.1. The Production Process
5.2. Agricultural and Non-Agricultural Uses
5.3. Physical, Hydrological, and Chemical Characterization
5.3.1. Physical and Hydrological Characterization
5.3.2. Chemical Characterization
5.3.3. Phytotoxicity
5.3.4. Practical Applications
5.4. Crop’s Performance
5.4.1. Coffee Silverskin as Organic Fertilizer
5.4.2. Coffee Silverskin as Co-Composting Matrix
5.4.3. Coffee Silverskin as Peat-Based Growing Medium
6. Brewer’s Spent Grain
6.1. The Production Process
6.2. Agricultural and Non-Agricultural Uses
6.3. Physical, Hydrological, and Chemical Characterization
6.3.1. Physical and Hydrological Characterization
6.3.2. Chemical Characterization
6.3.3. Practical Applications
6.4. Crop’s Performance
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| WF | Wood fiber |
| CS | Coffee silverskin |
| BSG | Brewer’s spent grain |
| R.R | Recommended range |
| G.M. | Growing Media |
References
- Gruda, N.S. Soilless culture systems and growing media in horticulture: An overview. In Advances in Horticultural Soilless Culture; Gruda, N., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The Contribution of Ornamental Plants to Urban Ecosystem Services. Earth 2022, 3, 1258–1274. [Google Scholar] [CrossRef]
- Baumgarten, A. Analytical methods for growing media–challenges and perspectives. Acta Hortic. 2008, 779, 97–104. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Kaudal, B.B.; Chen, D.; Madhavan, D.B.; Downie, A.; Weatherley, A. An Examination of Physical and Chemical Properties of Urban Biochar for Use as Growing Media Substrate. Biomass Bioenergy 2016, 84, 49–58. [Google Scholar] [CrossRef]
- Gruda, N.S. Advances in Soilless Culture and Growing Media in Today’s Horticulture—An Editorial. Agronomy 2022, 12, 2773. [Google Scholar] [CrossRef]
- Gruda, N.S.; Hirschler, O.; Stuart, J. Peat reduction in horticulture—An overview of Europe. Acta Hortic. 2024, 1391, 545–560. [Google Scholar] [CrossRef]
- Bar-Tal, A.; Saha, U.K.; Raviv, M.; Tuller, M. Inorganic and synthetic organic components of soilless culture and potting mixtures. In Soilless Culture, 2nd ed.; Raviv, M., Lieth, J.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 259–301. [Google Scholar] [CrossRef]
- Jozinović, A.; Šubarić, D.; Ačkar, Đ.; Babić, J.; Orkić, V.; Guberac, S.; Miličević, B. Food Industry By-Products as Raw Materials in the Production of Value-Added Corn Snack Products. Foods 2021, 10, 946. [Google Scholar] [CrossRef]
- Kitir, N.; Yildirim, E.; Şahin, Ü.; Turan, M.; Ekinci, M.; Ors, S.; Ünlü, H. Peat use in horticulture. In Peat Use in Horticulture; IntechOpen: London, UK, 2018; pp. 75–90. [Google Scholar] [CrossRef]
- Di Lonardo, S.; Cacini, S.; Becucci, L.; Lenzi, A.; Orsenigo, S.; Zubani, L.; Rossi, G.; Zaccheo, P.; Massa, D. Testing new peat-free substrate mixtures for the cultivation of perennial herbaceous species: A case study on Leucanthemum vulgare Lam. Sci. Hortic. 2021, 289, 110472. [Google Scholar] [CrossRef]
- Dargie, G.C.; Lewis, S.L.; Lawson, I.T.; Mitchard, E.T.; Page, S.E.; Bocko, Y.E.; Ifo, S.A. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 2017, 542, 86–90. [Google Scholar] [CrossRef]
- Harenda, K.M.; Lamentowicz, M.; Samson, M.; Chojnicki, B.H. The role of peatlands and their carbon storage function in the context of climate change. In Interdisciplinary Approaches for Sustainable Development Goals; Zielinski, T., Sagan, I., Surosz, W., Eds.; Springer: Cham, Switzerland, 2018; pp. 169–187. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef] [PubMed]
- González-Orozco, M.M.; Prieto-Ruíz, J.Á.; Aldrete, A.; Hernández-Díaz, J.C.; Chávez-Simental, J.A.; Rodríguez-Laguna, R. Nursery Production of Pinus engelmannii Carr. with Substrates Based on Fresh Sawdust. Forests 2018, 9, 678. [Google Scholar] [CrossRef]
- Durand, S.; Jackson, B.E.; Fonteno, W.C.; Michel, J.-C. Quantitative Description and Classification of Growing Media Particle Morphology through Dynamic Image Analysis. Agriculture 2023, 13, 396. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, V.; Martínez-Corta, J.; Martínez-Herrero, M.D.; Fornes, F. Physical and Chemical Properties of Sedge Peat-Based Media and Their Relation to Plant Growth. Acta Hortic. 1989, 238, 45–56. [Google Scholar] [CrossRef]
- Jackson, B.E.; Wright, R.D.; Gruda, N. Container medium pH in a pine tree substrate amended with peat moss and dolomitic limestone affects plant growth. HortScience 2009, 44, 1983–1987. [Google Scholar] [CrossRef]
- Gruda, N.; Rau, B.; Wright, R.D. Laboratory bioassay and greenhouse evaluation of a pine tree substrate used as a container substrate. Eur. J. Hortic. Sci. 2009, 74, 73–78. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid-State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Kour, R.; Singh, S.; Sharma, H.B.; Naik, T.S.K.; Shehata, N.; Ali, W.; Kapoor, D.; Dhanjal, D.S.; Singh, J.; Khan, A.H.; et al. Persistence and Remote Sensing of Agri-Food Wastes in the Environment: Current State and Perspectives. Chemosphere 2023, 317, 137822. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From Waste to Sustainable Industry: How Can Agro-Industrial Wastes Help in the Development of New Products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Asdrubali, F.; Evangelisti, L.; Guattari, C.; Roncone, M.; Milone, D. Experimental analysis of the thermal performance of wood fiber insulating panels. Sustainability 2023, 15, 1963. [Google Scholar] [CrossRef]
- Hirschler, O.; Osterburg, B. Peat extraction, trade and use in Europe: A material flow analysis. Mires Peat 2022, 28, 24–27. [Google Scholar] [CrossRef]
- Blok, C.; Eveleens, B.; van Winkel, A. Growing Media for Food and Quality of Life in the Period 2020–2050. Acta Hortic. 2021, 1305, 341–356. [Google Scholar] [CrossRef]
- Maher, M.; Prasad, M.; Raviv, M. Organic soilless media components. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2008; pp. 459–504. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Wood fiber substrates as a peat alternative for vegetable production. Eur. J. Wood Wood Prod. 2006, 64, 347–350. [Google Scholar] [CrossRef]
- Zaccheo, P.; Cattivello, C.; Longo, C.; Crippa, L.; Notaristefano, P.; Orfeo, D. A Comparative Study on Some Locally Available Organic Materials for Their Potential Utilization in Sustainable Growing Media Blends. Acta Hortic. 2024, 1389, 123–130. [Google Scholar] [CrossRef]
- Laiche, A.J., Jr.; Nash, V.E. Evaluation of Pine Bark, Pine Bark with Wood, and Pine Tree Chips as Components of a Container Plant Growing Media. J. Environ. Hortic. 1986, 4, 22–25. [Google Scholar] [CrossRef]
- Benoit, F.; Ceustermans, N. Growing Cucumber on Ecologically Sound Substrates. Hydroponics Transpl. Prod. 1994, 396, 55–66. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. The influence of organic substrates on growth and physiological parameters of vegetable seedlings. Acta Hortic. 1997, 450, 487–494. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Influence of wood fiber substrates and application rates on the growth of tomato transplants. Adv. Hortic. Sci. 1999, 13, 20–24. [Google Scholar]
- Wu, L.; Li, R.; Liu, J.; Cui, W.; Qi, Z.; Zhou, W. Nitrogen Immobilization by Wood Fiber Substrates Strongly Affects the Photosynthetic Performance of Lettuce. Plants 2025, 14, 1518. [Google Scholar] [CrossRef]
- Gruda, N.; Tucher, S.v.; Schnitzler, W.H. N-Immobilisierung in Holzfasersubstraten bei der Anzucht von Tomatenjungpflanzen (Lycopersicon lycopersicum (L.) Karst. ex Farw.). J. Appl. Bot. 2000, 74, 32–37. [Google Scholar] [CrossRef]
- Gumy, N. Toresa and other wood-fibre products: Advantages and drawbacks when used in growing media. In Proceedings of the International Peat Symposium Peat in Horticulture: Peat and Its Alternatives in Growing Media, Amsterdam, The Netherlands, 30 October 2001; pp. 39–44. [Google Scholar]
- Bobo, J.G.; Jackson, B.E. North American and European Conifer Species Evaluated for Use as Wood Components in Growing Media: A Mini Review. Acta Hortic. 2024, 1389, 131–138. [Google Scholar] [CrossRef]
- Bunt, B.R. Media and Mixes for Container-Grown Plants: A Manual on the Preparation and Use of Growing Media for Pot Plants; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Paquet, G.; Nguyen, T.T.A.; Brégard, A.; Barrada, A.; Poirier, G.; Dorais, M. The agronomic potential of a new vertical growing farming system using biofertilizers and peat-based wood fibre growing medium: Lettuce as a case study. Acta Hortic. 2022, 1355, 455–462. [Google Scholar] [CrossRef]
- Čepulienė, R.; Butkevičienė, L.M.; Steponavičienė, V. Nutrient Use Efficiency and Cucumber Productivity as a Function of the Nitrogen Fertilization Rate and the Wood Fiber Content in Growing Media. Plants 2024, 13, 2911. [Google Scholar] [CrossRef]
- Woznicki, T.; Jackson, B.E.; Sønsteby, A.; Kusnierek, K. Wood Fiber from Norway Spruce—A Stand-Alone Growing Medium for Hydroponic Strawberry Production. Horticulturae 2023, 9, 815. [Google Scholar] [CrossRef]
- Robert, F.S.; Thériault, L.; Allaire, M.; Charland, M. Characterization of selected growing media for greenhouse-grown strawberries. Acta Hortic. 2024, 1389, 91–98. [Google Scholar] [CrossRef]
- Haraldsen, T.K.; Aurdal, S.M.; Woznicki, T.L. Peat-free and peat-reduced growing media for greenhouse petunia (Petunia × hybrida Vilm.) production. Acta Hortic. 2024, 1389, 41–50. [Google Scholar] [CrossRef]
- Reineke, T.; Muhammed, H.H.A.; Anlauf, R.; Daum, D. Impact of thermo-hydrolytically treated wood fibers as a substrate component on the growth of petunias. Acta Hortic. 2024, 1389, 105–112. [Google Scholar] [CrossRef]
- Di Lonardo, S.; Sodini, M.; Massa, D.; Nesi, B.; Orsenigo, S.; Zubani, L.; Cacini, S. The effect of different peat-free growing media and fertilization levels on the plant nutrition of Leucanthemum vulgare (Lam.) and Dianthus barbatus (L.). J. Plant Nutr. 2025, 48, 114–129. [Google Scholar] [CrossRef]
- Mariotti, B.; Oliet, J.A.; Andivia, E.; Tsakaldimi, M.; Villar-Salvador, P.; Ivetić, V.; Montagnoli, A.; Janković, I.K.; Bilir, N.; Bohlenius, H.; et al. A Global Review on Innovative, Sustainable, and Effective Materials Composing Growing Media for Forest Seedling Production. Curr. For. Rep. 2023, 9, 413–428. [Google Scholar] [CrossRef]
- Heiskanen, J.; Ruhanen, H.; Himanen, K.; Kivimäenpää, M.; Silvan, N. Growth of Nordic container forest tree seedlings in some peatless and peat-reduced growing media. New For. 2024, 55, 1499–1517. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Chen, Y.; Weadock, N.; Wan, J.; Vaaland, O.; Han, X.; Li, T.; Hu, L. Tin Anode for Sodium-Ion Batteries Using Natural Wood Fiber as a Mechanical Buffer and Electrolyte Reservoir. Nano Lett. 2013, 13, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrate for production of vegetable transplants: I. Physical properties of wood fiber substrates. Sci. Hortic. 2004, 100, 309–322. [Google Scholar] [CrossRef]
- Gruda, N.; Schnitzler, W.H. Suitability of wood fiber substrates for production of vegetable transplants II. The effect of wood fiber substrates and their volume weights on the growth of tomato transplants. Sci. Hortic. 2004, 100, 333–340. [Google Scholar] [CrossRef]
- Domeño, I.; Irigoyen, I.; Muro, J. New Wood Fibre Substrates Characterization and Evaluation in Hydroponic Tomato Culture. Eur. J. Hortic. Sci. 2010, 75, 89–94. [Google Scholar] [CrossRef]
- Zawadzińska, A.; Salachna, P.; Nowak, J.S.; Kowalczyk, W. Response of Interspecific Geraniums to Waste Wood Fiber Substrates and Additional Fertilization. Agriculture 2021, 11, 119. [Google Scholar] [CrossRef]
- De Boodt, M.; Verdonck, O. The physical properties of the substrates in horticulture. Acta Hortic. 1972, 26, 37–44. [Google Scholar] [CrossRef]
- Michel, J.C. Wettability of organic growing media used in horticulture: A review. Vadose Zone J. 2015, 14, vzj2014-09. [Google Scholar] [CrossRef]
- Fain, G.B.; Gilliam, C.H.; Sibley, J.L.; Boyer, C.R. WholeTree substrates derived from three species of pine in production of annual vinca. HortTechnology 2008, 18, 13–17. [Google Scholar] [CrossRef]
- Sdao, A.E.; Mondelli, D.; Piscitelli, L.; Scaltrito, E.; Leoni, B.; Cristiano, G.; De Lucia, B. Morpho-Physiological Response of Bedding Plants Quality to Unconventional Agro-Industrial Organic Matrices to Peat Replacing: Preliminary Outcomes. Acta Hortic. 2025, 1417, 125–132. [Google Scholar] [CrossRef]
- Theurl, M.C.; Hörtenhuber, S.J.; Lindenthal, T.; Palme, W. Unheated Soil-Grown Winter Vegetables in Austria: Greenhouse Gas Emissions and Socio-Economic Factors of Diffusion Potential. J. Clean. Prod. 2017, 151, 134–144. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production—A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Hirschler, O.; Osterburg, B.; Weimar, H.; Glasenapp, S.; Ohmes, M.F. Peat Replacement in Horticultural Growing Media: Availability of Bio-Based Alternative Materials; Thünen Working Paper No. 190; Thünen Institute: Braunschweig, Germany, 2022. [Google Scholar] [CrossRef]
- Beretta, D.; Ripamonti, M. Evaluation of Wood Fiber as Component of Substrates for Container Nursery Crops. Acta Hortic. 2021, 1305, 77–82. [Google Scholar] [CrossRef]
- Laun, N.; Weinheimer, S.; Lutz, F.; Emmel, M.; Gruda, N. Peat reduced substrates for vegetable seedlings. Acta Hortic. 2021, 1321, 23–30. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Sønsteby, A.; Aurdal, S.M.; Kusnierek, K.; Haraldsen, T.K. Optimizing Peat and Wood Fiber Blends: Impacts of Liming and Fertilization on Growth of Petunia (Petunia x hybrida Vilm.) and Basil (Ocimum basilicum L.). Horticulturae 2024, 10, 895. [Google Scholar] [CrossRef]
- Woznicki, T.; Kusnierek, K.; Roos, U.M.; Andersen, S.; Zimmer, K.; Sønsteby, A. Exploration of Alternative Growing Media in Strawberry Production with Focus on Wood Fiber from Norway Spruce. Acta Hortic. 2021, 1305, 15–22. [Google Scholar] [CrossRef]
- Aurdal, S.M.; Woznicki, T.L.; Haraldsen, T.K.; Kusnierek, K.; Sønsteby, A.; Remberg, S.F. Wood fiber-based growing media for strawberry cultivation: Effects of incorporation of peat and compost. Horticulturae 2022, 9, 36. [Google Scholar] [CrossRef]
- Woznicki, T.; Kusnierek, K.; Vandecasteele, B.; Sønsteby, A. Reuse of Coir, Peat, and Wood Fiber in Strawberry Production. Front. Plant Sci. 2024, 14, 1307240. [Google Scholar] [CrossRef]
- Harris, C.N.; Dickson, R.W.; Fisher, P.R.; Jackson, B.E.; Poleatewich, A.M. Evaluating Peat Substrates Amended with Pine Wood Fiber for Nitrogen Immobilization and Effects on Plant Performance with Container-grown Petunia. HortTechnology 2020, 30, 107–116. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Teipel, J.; Scharinger, A.; Kuballa, T.; Walch, S.G.; Grosch, F.; Bunzel, M.; Okaru, A.O.; Schwarz, S. Fully automated identification of coffee species and simultaneous quantification of furfuryl alcohol using NMR spectroscopy. J. AOAC Int. 2020, 103, 306–314. [Google Scholar] [CrossRef]
- Coffee Statistics: Consumption, Preferences, & Spending. Available online: https://www.driveresearch.com/market-research-company-blog/coffee-survey (accessed on 16 May 2025).
- Coffee Production 2023/2024. Available online: https://www.fas.usda.gov/data/production/commodity/0711100 (accessed on 18 May 2025).
- Coffee Report and Outlook December 2023—International Coffee Organization. Available online: https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf (accessed on 16 May 2025).
- Haro, N.; Meza-Mori, G.; Lopez, J.L.Z.; Rascón, J.; Pariente, E.; Condori-Apfata, J.A.; Granda-Santos, M.; Inga, B.M.F.; Oliva-Cuz, M.; Lopez, R.Y.R.; et al. Influence of agroforestry systems on Coffea arabica L. yield and quality at different altitudes in Amazonas, Peru. J. Agric. Food Res. 2025, 19, 101574. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Xotlanihua-Flores, D.; Bacchetta, L.; Diretto, G.; Maccioni, O.; Frusciante, S.; Rojas-Abarca, L.M.; Sánchez-Rodríguez, E. Bioactive Compounds and Valorization of Coffee By-Products from the Origin: A Circular Economy Model from Local Practices in Zongolica, Mexico. Plants 2024, 13, 2741. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, R.; Wirkowska-Wojdyła, M.; Piasecka, I.; Górska, A. Application of Response Surface Methodology to Optimize the Extraction Process of Bioactive Compounds Obtained from Coffee Silverskin. Appl. Sci. 2023, 13, 5388. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; de Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Martuscelli, M.; Esposito, L.; Di Mattia, C.D.; Ricci, A.; Mastrocola, D. Characterization of coffee silver skin as potential food-safe ingredient. Foods 2021, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Ansanelli, G.; Fiorentino, G.; Chifari, R.; Meisterl, K.; Leccisi, E.; Zucaro, A. Sustainability assessment of coffee silverskin waste management in the metropolitan city of Naples (Italy): A life cycle perspective. Sustainability 2023, 15, 16281. [Google Scholar] [CrossRef]
- del Pozo, C.; Rego, F.; Yang, Y.; Puy, N.; Bartrolí, J.; Fàbregas, E.; Bridgwater, A.V. Converting coffee silverskin to value-added products by a slow pyrolysis-based biorefinery process. Fuel Process. Technol. 2021, 214, 106708. [Google Scholar] [CrossRef]
- Pongsiriyakul, K.; Wongsurakul, P.; Kiatkittipong, W.; Premashthira, A.; Kuldilok, K.; Najdanovic-Visak, V.; Adhikari, S.; Cognet, P.; Kida, T.; Assabumrungrat, S. Upcycling coffee waste: Key industrial activities for advancing circular economy and overcoming commercialization challenges. Processes 2024, 12, 2851. [Google Scholar] [CrossRef]
- Pyrzynska, K. Useful Extracts from Coffee By-Products: A Brief Review. Separations 2024, 11, 334. [Google Scholar] [CrossRef]
- Farm to Fork Strategy: The Role of Nutrients. Available online: https://www.fertilizerseurope.com/farm-to-fork-strategy-the-role-of-nutrients/ (accessed on 12 May 2025).
- Salbitani, G.; Chianese, M.R.; Bossa, R.; Bencivenga, T.; Carraturo, F.; Nappo, A.; Guida, M.; Loreto, F.; Carfagna, S. Cultivation of barley seedlings in a coffee silverskin-enriched soil: Effects in plants and in soil. Plant Soil 2024, 498, 199–211. [Google Scholar] [CrossRef]
- Carnier, R.; Berton, R.S.; Coscione, A.R.; Pires, A.M.M.; Corbo, J.Z. Coffee Silverskin and Expired Coffee Powder Used as Organic Fertilizers. Coffee Sci. 2019, 14, 24–32. [Google Scholar] [CrossRef]
- Picca, G.; Plaza, C.; Madejón, E.; Panettieri, M. Composting of coffee silverskin with carbon rich materials leads to high quality soil amendments. Waste Biomass Valor. 2024, 14, 297–307. [Google Scholar] [CrossRef]
- González-Moreno, M.A.; García Gracianteparaluceta, B.; Marcelino Sádaba, S.; Zaratiegui Urdin, J.; Robles Domínguez, E.; Pérez Ezcurdia, M.A.; Seco Meneses, A. Feasibility of vermicomposting of spent coffee grounds and silverskin from coffee industries: A laboratory study. Agronomy 2020, 10, 1125. [Google Scholar] [CrossRef]
- Lorbeer, L.; Schwarz, S.; Franke, H.; Lachenmeier, D.W. Toxicological Assessment of Roasted Coffee Silver Skin (Testa of Coffea sp.) as Novel Food Ingredient. Molecules 2022, 27, 6839. [Google Scholar] [CrossRef]
- Franca, A.S.; Basílio, E.P.; Resende, L.M.; Fante, C.A.; Oliveira, L.S. Coffee Silverskin as a Potential Ingredient for Functional Foods: Recent Advances and a Case Study with Chocolate Cake. Foods 2024, 13, 3935. [Google Scholar] [CrossRef] [PubMed]
- Lestari, W.; Hasballah, K.; Listiawan, M.Y.; Sofia, S. Coffee by-products as the source of antioxidants: A systematic review. F1000Research 2022, 11, 220. [Google Scholar] [CrossRef]
- Ramos, F.J.H.T.V.; Marques, M.D.F.V.; de Oliveira Aguiar, V.; Gondim, F.F.; dos Santos Gomes, L.; de Oliveira Gomes, P.H. Geopolymer composites reinforced with silverskin fibers from the coffee industry waste. J. Mater. Res. Technol. 2024, 31, 3287–3300. [Google Scholar] [CrossRef]
- Garcia, C.V.; Kim, Y.T. Spent coffee grounds and coffee silverskin as potential materials for packaging: A review. J. Polym. Environ. 2021, 29, 2372–2384. [Google Scholar] [CrossRef]
- Dos Santos, É.M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.P.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Grigolon, G.; Nowak, K.; Poigny, S.; Hubert, J.; Kotland, A.; Waldschütz, L.; Wandrey, F. From coffee waste to active ingredient for cosmetic applications. Int. J. Mol. Sci. 2023, 24, 8516. [Google Scholar] [CrossRef]
- Ruschioni, S.; Duca, D.; Tulli, F.; Zarantoniello, M.; Cardinaletti, G.; Corsi, L.; Olivotto, I.; Basili, D.; Naspetti, S.; Truzzi, C.; et al. Evaluation of Growth Performance and Environmental Impact of Hermetia illucens Larvae Reared on Coffee Silverskins Enriched with Schizochytrium limacinum or Isochrysis galbana Microalgae. Animals 2024, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Prandi, B.; Ferri, M.; Monari, S.; Zurlini, C.; Cigognini, I.; Verstringe, S.; Schaller, D.; Walter, M.; Navarini, L.; Tassoni, A.; et al. Extraction and chemical characterization of functional phenols and proteins from coffee (Coffea arabica) by-products. Biomolecules 2021, 11, 1571. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT-Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The wastes of coffee bean processing for utilization in food: A review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioproc. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Sdao, A.E.; Cristiano, G.; De Lucia, B. Department of Soil, Plant and Food Sciences. University of Bari Aldo Moro: Bari, Italy, 2025; Unpublished work. [Google Scholar]
- Thligene, N.; Mezzapesa, G.N.; Mondelli, D.; Trani, A.; Veronico, P.; Melillo, M.T.; Dumontet, S.; Miano, T.; Sasanelli, N. Effect of Coffee Silver Skin and Brewers’ Spent Grain in the Control of Root-Knot Nematodes. Helminthologia 2019, 56, 30–41. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Ziemah, J.; Ullrich, M.S.; Kuhnert, N. Antibacterial Activity Potential of Industrial Food Production Waste Extracts Against Pathogenic Bacteria: Comparative Analysis and Characterization. Foods 2024, 13, 1902. [Google Scholar] [CrossRef]
- Al-Charchafchi, F.; Al-Quadan, F. Effect of chlorogenic acid on germination and seedling growth, and on the enzymes activity extracted from Artemisia herba alba ASSO. Part I: Germination and seedling growth. Dirasat Pure Sci. 2006, 33, 168–175. [Google Scholar]
- Serna-Jiménez, J.A.; Siles, J.A.; de los Ángeles Martín, M.; Chica, A.F. A Review on the Applications of Coffee Waste Derived from Primary Processing: Strategies for Revalorization. Processes 2022, 10, 2436. [Google Scholar] [CrossRef]
- European Beer Trends—2024 Edition. Available online: https://brewersofeurope.eu/european-beer-trends/ (accessed on 11 May 2025).
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and nutrient value proposition of brewers spent grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Duan, Y.; Zhang, H.; Ma, H. A Mini-Review on Brewer’s Spent Grain Protein: Isolation, Physicochemical Properties, Application of Protein, and Functional Properties of Hydrolysates. J. Food Sci. 2019, 84, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A.; Szulc, J.; Błaszak, B. Brewers’ spent grain—Simply waste or potential ingredient of functional food? Zywnosc Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2023, 30, 5–23. [Google Scholar] [CrossRef]
- Bamforth, C.W. Progress in brewing science and beer production. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 161–176. [Google Scholar] [CrossRef]
- Amoriello, T.; Ciccoritti, R. Sustainability: Recovery and Reuse of Brewing-Derived By-Products. Sustainability 2021, 13, 2355. [Google Scholar] [CrossRef]
- Pasquet, P.-L.; Villain-Gambier, M.; Trébouet, D. By-Product Valorization as a Means for the Brewing Industry to Move toward a Circular Bioeconomy. Sustainability 2024, 16, 3472. [Google Scholar] [CrossRef]
- Yoo, J.H.; Luyima, D.; Lee, J.H.; Park, S.Y.; Yang, J.W.; An, J.Y.; Yun, Y.U.; Oh, T.K. Effects of Brewer’s Spent Grain Biochar on the Growth and Quality of Leaf Lettuce (Lactuca sativa L. var. crispa.). Appl. Biol. Chem. 2021, 64, 10. [Google Scholar] [CrossRef]
- Cacace, C.; Cocozza, C.; Traversa, A.; Coda, R.; Rizzello, C.G.; Pontonio, E.; De Mastro, F.; Brunetti, G.; Verni, M. Potential of Native and Bioprocessed Brewers’ Spent Grains as Organic Soil Amendments. Front. Sustain. Food Syst. 2022, 6, 1010890. [Google Scholar] [CrossRef]
- Ebido, N.E.; Nnadi, A.L.; Adeoluwa, O.O.; Ndubuaku, U.M.; Obalum, S.E.; Ugwuoju, C.L.; Ajoagu, G.M.; Baiyeri, K.P. Influence of brewery waste and animal manure-based compost on the growth of green amaranth in sandy tropical soils. Org. Farming 2024, 10, 69–79. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Arapoglou, D.; Chorianopoulos, N.; Markou, G.; Haroutounian, S.A. Conversion of brewers’ spent grain into proteinaceous animal feed using solid state fermentation. Environ. Sci. Pollut. Res. 2022, 29, 29562–29569. [Google Scholar] [CrossRef]
- Paiva, C.F.; Almeida, T.D.S.F.D.; Arelhano, G.E.; Alvarado, A.V.R.; De Menezes, M.B.; Argandoña, E.J.S.; De Alencar Gomes, I.L.; Moya, A.M.T.M.; Loubet Filho, P.S.; Dos Santos, E.F. Potential of brewer’s spent grain as a nutritional ingredient in bakery products. Plant Food Hum. Nutr. 2025, 80, 12. [Google Scholar] [CrossRef]
- Da Silva, A.M.M.; Lima, M.A.; Koksel, F.; Sato, A.C.K. Incorporation of brewer’s spent grain into plant-based meat analogues: Benefits to physical and nutritional quality. Int. J. Food Sci. Technol. 2024, 59, 3870–3882. [Google Scholar] [CrossRef]
- Naibaho, J.; Setiawan, R.D.; Korzeniowska, M. Biological properties of bioactive compounds from brewers’ spent grain: Current trends, challenges, and perspectives. Curr. Opin. Food Sci. 2024, 62, 101268. [Google Scholar] [CrossRef]
- Naibaho, J.; Butula, N.; Jonuzi, E.; Korzeniowska, M.; Laaksonen, O.; Föste, M.; Kütt, M.L.; Yang, B. Potential of brewers’ spent grain in yogurt fermentation and evaluation of its impact in rheological behaviour, consistency, microstructural properties and acidity profile during the refrigerated storage. Food Hydrocoll. 2022, 125, 107412. [Google Scholar] [CrossRef]
- Qazanfarzadeh, Z.; Masek, A.; Chakraborty, S.; Kumaravel, V. Development of brewer’s spent grain-derived bio nanocomposites through a multiproduct biorefinery approach for food packaging. Ind. Crop Prod. 2024, 220, 119226. [Google Scholar] [CrossRef]
- Panić, S.; Đurišić-Mladenović, N.; Petronijević, M.; Stijepović, I.; Milanović, M.; Kozma, G.; Kukovecz, Á. Valorization of waste biomass towards biochar production—Characterization and perspectives for sustainable applications in Serbia. Environ. Technol. Innov. 2025, 37, 104043. [Google Scholar] [CrossRef]
- Belardi, I.; De Francesco, G.; Alfeo, V.; Bravi, E.; Sileoni, V.; Marconi, O.; Marrocchi, A. Advances in the valorization of brewing by-products. Food Chem. 2025, 465, 141882. [Google Scholar] [CrossRef]
- Birk, L.; Dos Santos, B.P.; Ossanes, D.S.; de Souza Schwarz, P.; Bachmann, S.A.L.; Sebben, V.C.; Eller, S.; de Oliveira, T.F. Brewer’s Spent Grain as a Potential Sorbent for Toxicology Methods: Application to Antidepressant Analysis in Urine. J. Pharm. Biomed. Anal. 2025, 254, 116564. [Google Scholar] [CrossRef]
- Estevão-Rodrigues, T.; Fernandes, H.; Moutinho, S.; Ferreira, M.; Castro, C.; Belo, I.; Salgado, J.M.; Oliva-Teles, A.; Peres, H. Effect of Solid-Fermented Brewer’s Spent Grain on Growth, Metabolism, and Oxidative Status of European Seabass (Dicentrarchus labrax). Fishes 2025, 10, 49. [Google Scholar] [CrossRef]
- Ferri, I.; Dametti, M.R.; Frazzini, S.; Dell’Anno, M.; Rossi, L. Valorization of Carob and Brewer’s Spent Grain as Growth-Substrate Supplements in Tenebrio molitor Rearing. Animals 2025, 15, 1697. [Google Scholar] [CrossRef]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-products in the malting and brewing industries—Re-usage possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M. The Variability of Physico-Chemical Properties of Brewery Spent Grain from 8 Different Breweries. Heliyon 2021, 7, e06583. [Google Scholar] [CrossRef] [PubMed]
- Meneses, N.G.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Colpini, L.M.S. All-Around Characterization of Brewers’ Spent Grain. Eur. Food Res. Technol. 2021, 247, 3013–3021. [Google Scholar] [CrossRef]
- Belardi, I.; Marrocchi, A.; Alfeo, V.; Sileoni, V.; De Francesco, G.; Paolantoni, M.; Marconi, O. Sequential Extraction and Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy Monitoring in the Biorefining of Brewer’s Spent Grain. Molecules 2023, 28, 7992. [Google Scholar] [CrossRef]
- Mussatto, S.I. Biotechnological potential of brewing industry by-products. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Springer: Dordrecht, The Netherlands, 2009; pp. 313–326. [Google Scholar]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Potential applications of brewery spent grain: Critical an overview. J. Environ. Chem. Eng. 2022, 10, 106951. [Google Scholar] [CrossRef]
- Mainali, K.; Yadav, M.P.; Sharma, B.K.; Sarker, M.I.; Ngo, H.; Hotchkiss, A.; Simon, S. Isolation and Characterization of the Physiochemical Properties of Brewer’s Spent Grain. Agriculture 2024, 15, 47. [Google Scholar] [CrossRef]
- García, D.C.; Villalba, I.; Savino, N.; Nazareno, M.A. Nutritional and Functional Characterization of Different Types of Brewer’s Spent Grain Flours. Food Biosci. 2025, 64, 105890. [Google Scholar] [CrossRef]
- Kebede, Y. Analysis of the physico-chemical characteristics of brewers spent grain (BSG). J. Nat. Sci. Res. 2020, 11, 9–12. [Google Scholar] [CrossRef]
- Abbas, A.; Siddiq, Z.; Hayyat, M.U.; Zhang, Y.J.; Ghaffar, R.; Gatasheh, M.K. Na+ and K+ compartmentalization in Spinacea oleracea and their effects on growth, water relations, endogenous melatonin, and non-structural carbohydrates. Sci. Hortic. 2024, 323, 112467. [Google Scholar] [CrossRef]
- González-Díaz, R.L.; Saldarriaga-Hernandez, S.; Ramírez-Aguirre, M.D.; Guerrero-Higareda, S.; García-Cayuela, T.; Garcia-Amezquita, L.E.; Carrillo-Nieves, D. Valorization of brewer’s spent grains through Ganoderma lucidum cultivation: Functional food ingredients and bread prototypes. LWT 2024, 214, 117131. [Google Scholar] [CrossRef]
- Parchami, M.; Agnihotri, S.; Taherzadeh, M.J. Aqueous ethanol organosolv process for the valorization of brewer’s spent grain (BSG). Bioresour. Technol. 2022, 362, 127764. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Roberto, I.C. Chemical Characterization and Liberation of Pentose Sugars from Brewer’s Spent Grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Jin, Z.; Lan, Y.; Ohm, J.B.; Gillespie, J.; Schwarz, P.; Chen, B. Physicochemical composition, fermentable sugars, free amino acids, phenolics, and minerals in brewers’ spent grains obtained from craft brewing operations. J. Cereal Sci. 2022, 104, 103413. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Cruz-Narváez, Y.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. In silico bioactivity analysis of peptide fractions derived from brewer’s spent grain hydrolysates. Int. J. Food Sci. Technol. 2024, 59, 2804–2815. [Google Scholar] [CrossRef]
- Assandri, D.; Pampuro, N.; Zara, G.; Cavallo, E.; Budroni, M. Suitability of Composting Process for the Disposal and Valorization of Brewer’s Spent Grain. Agriculture 2021, 11, 2. [Google Scholar] [CrossRef]
- Mbagwu, J.S.C.; Ekwealor, G.C. Agronomic potential of brewers’ spent grains. Biol. Wastes 1990, 34, 335–347. [Google Scholar] [CrossRef]
- Tumbure, A.; Pulver, C.; Black, L.; Walsh, L.; Prasad, M.; Leahy, J.J.; Corbett, E.; Gaffney, M.T. Bio-Resource Availability in Ireland: A Practical Review of Potential Replacement Materials for Use in Horticultural Growth Media. Horticulturae 2025, 11, 378. [Google Scholar] [CrossRef]

| Parameters | Unit of Measure | Value | References |
|---|---|---|---|
| B.D. | g cm−3 | 0.07 | [52] |
| P.D. | g cm−3 | 1.56 | [52] |
| M. | % | 10.3–41.0 | [41,53] |
| T.P.S. | % | >85 | [54] |
| E.A.W. | vol. % | 13.8 | [52] |
| Parameters | Unit of Measure | Value | References |
|---|---|---|---|
| pH | 4.1–6.0 | [30,53] | |
| E.C. | mS cm−1 | 4.0–28 | [30,53,57] |
| C.E.C. | meq g−1 | 0.22 | [53] |
| T.O.M. | % | 95–98 | [30,53] |
| O.C. | % | 64–67 | [30] |
| T.N. | % | 0.20–0.45 | [30,41,53,57] |
| C/N | 168–456 | [30,41,52] | |
| P | mg kg−1 | 57 | [41] |
| K | mg kg−1 | 472 | [41] |
| Ca | mg kg−1 | 1574 | [53] |
| Mg | mg kg−1 | 346 | [53] |
| Fe | mg kg−1 | 46.5 | [53] |
| Lignin | g kg−1 d.w. | 360 | [28] |
| Cellulose | g kg−1 d.w. | 180 | [52] |
| Hemicellulose | mg kg−1 d.w. | 489 | [52] |
| Parameters | Unit of Measure | Value | References |
|---|---|---|---|
| F.S. | mm | 2–5 | [94] |
| B.D. | g cm−3 | 0.18 | [94] |
| P.D. | g cm−3 | 0.71 | [94] |
| M. | % | 7.1–7.3 | [94,95,96] |
| W.H.C. | g H2O g−1 dry sample | 5.11 | [97] |
| W.R. | % | 91.4 | [97] |
| Parameters | Unit of Measure | Value | References |
|---|---|---|---|
| pH | 5.3–5.6 | [78,98,99] | |
| E.C. | dS m−1 | 1.8–4.9 | [95,96,97] |
| Ash | g 100 g−1 | 5–7 | [95,96,97,98] |
| T.O.M. | g 100 g−1 | 43.3–93.4 | [83,85,98] |
| T.N. | g 100 g−1 | 0.14–5.1 | [57,83,85] |
| C/N | 11–19.1 | [83,85,97] | |
| P | g 100 g−1 | 0.76 | [57] |
| K | g kg−1 | 7.6 | [57] |
| Lignin | g 100 g−1 | 28.6–30.2 | [96,100] |
| Cellulose | g 100 g−1 | 23.7 | [96] |
| Hemicellulose | g 100 g−1 | 62.1 | [96] |
| Protein | g 100 g−1 | 16.2–18.7 | [96,100] |
| T.P. | mg kg−1 | 140 | [101] |
| Parameters | Unit of Measure | Value | References |
|---|---|---|---|
| B.D. | g cm−3 | 0.129–0.159 | [126] |
| M. | % | 78–84 | [112,127,128,129] |
| W.R. | % | 207 | [57] |
| Parameters | Unit of Measurement | Value | References |
|---|---|---|---|
| pH | 4.5–6.0 | [98,113,128] | |
| E.C. | dS m−1 | 3480 | [57] |
| Ash | % | 1.9–8.5 | [128,129,132,133] |
| T.O.M. | g 100 g−1 | 93.8 | [57] |
| O.C. | % | 47.3–53.0 | [132,133,134] |
| T.N. | % | 3.8–5.1 | [132,133,134] |
| C/N | 10.8–13.9 | [132,133,134] | |
| P | mg kg−1 | 6000 | [127] |
| Ca | mg kg−1 | 3600 | [127] |
| Mg | mg kg−1 | 1900 | [127] |
| S | mg kg−1 | 2900 | [127] |
| Na | mg kg−1 | 137 | [105,127] |
| Lignin | g 100 g−1 | 15.4–28.0 | [137,138] |
| Cellulose | % | 16.8–26.0 | [131] |
| Hemicellulose | % | 19.2–41.9 | [131] |
| Protein | g 100 g−1 d.w. | 15–25 | [136] |
| Amino acids | mg kg−1 | 2571.7 | [117,139] |
| Fiber | g 100 g−1 d.w. | 50–70 | [136] |
| Fat | g 100 g−1 d.w. | 5–10 | [136] |
| T.P. | mg GAE g−1 d.m. | 7.41 | [129] |
| Material | Origin | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Sphagnum peat | Surface of the rewetted peat soils | Good T.P.S., air content, and W.H.C. The acidic pH value can easily be adjusted. | Contain herbal or grass seeds, poorly re-wettable; investment costs are still high. | [8] |
| Wood fiber | Byproducts from the wood industry (sawdust, chips, bark). | Good drainability, re-wetting. Low B.D. is used to optimize the physical properties of the blend. It reduces B.D., increases airspace, and improves rewettability. | May cause nitrogen immobilization (due to degradation by microorganisms). High C/N. Degradation by microorganisms leads to deterioration of physical properties. Relatively low N content. It can impair W.R. and nitrogen availability if used in excessive proportions. High proportions (≥75%) can lead to chlorosis and reduced growth unless pH and nutrient supply are managed. | [4,5,8,12,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] |
| Coffee silverskin | Byproduct of coffee roasting. | Rich in nutrients (N, P, K, etc.). High W.R. capacity, high O.M., acidic pH. Antioxidant properties. Compostable to eliminate phytotoxicity. | Phytotoxicity (polyphenols, chlorogenic acid) inhibits germination. Low C/N. Compositional variability. Low nitrogen mineralization. | [57,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102] |
| Brewer’s spent grain | Byproduct of brewing (mashing). | Rich in fiber, protein, and bioactives (amino acids). Biochar, soil conditioner, compost, and peat substitute (low doses) are used in agriculture. Good W.R. Sub-acid pH. | High M. and sugar content (rapid deterioration: 7–10 days); needs refrigeration/drying. High Na levels (negative impact on plants). At high doses, it reduces growth and flowering due to high EC and P deficiency. | [57,98,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sdao, A.E.; Gruda, N.S.; De Lucia, B. Beyond Peat: Wood Fiber and Two Novel Organic Byproducts as Growing Media—A Systematic Review. Plants 2025, 14, 1945. https://doi.org/10.3390/plants14131945
Sdao AE, Gruda NS, De Lucia B. Beyond Peat: Wood Fiber and Two Novel Organic Byproducts as Growing Media—A Systematic Review. Plants. 2025; 14(13):1945. https://doi.org/10.3390/plants14131945
Chicago/Turabian StyleSdao, Anna Elisa, Nazim S. Gruda, and Barbara De Lucia. 2025. "Beyond Peat: Wood Fiber and Two Novel Organic Byproducts as Growing Media—A Systematic Review" Plants 14, no. 13: 1945. https://doi.org/10.3390/plants14131945
APA StyleSdao, A. E., Gruda, N. S., & De Lucia, B. (2025). Beyond Peat: Wood Fiber and Two Novel Organic Byproducts as Growing Media—A Systematic Review. Plants, 14(13), 1945. https://doi.org/10.3390/plants14131945








