Structure-Based Function of Humic Acid in Abiotic Stress Alleviation in Plants: A Review
Abstract
1. Introduction
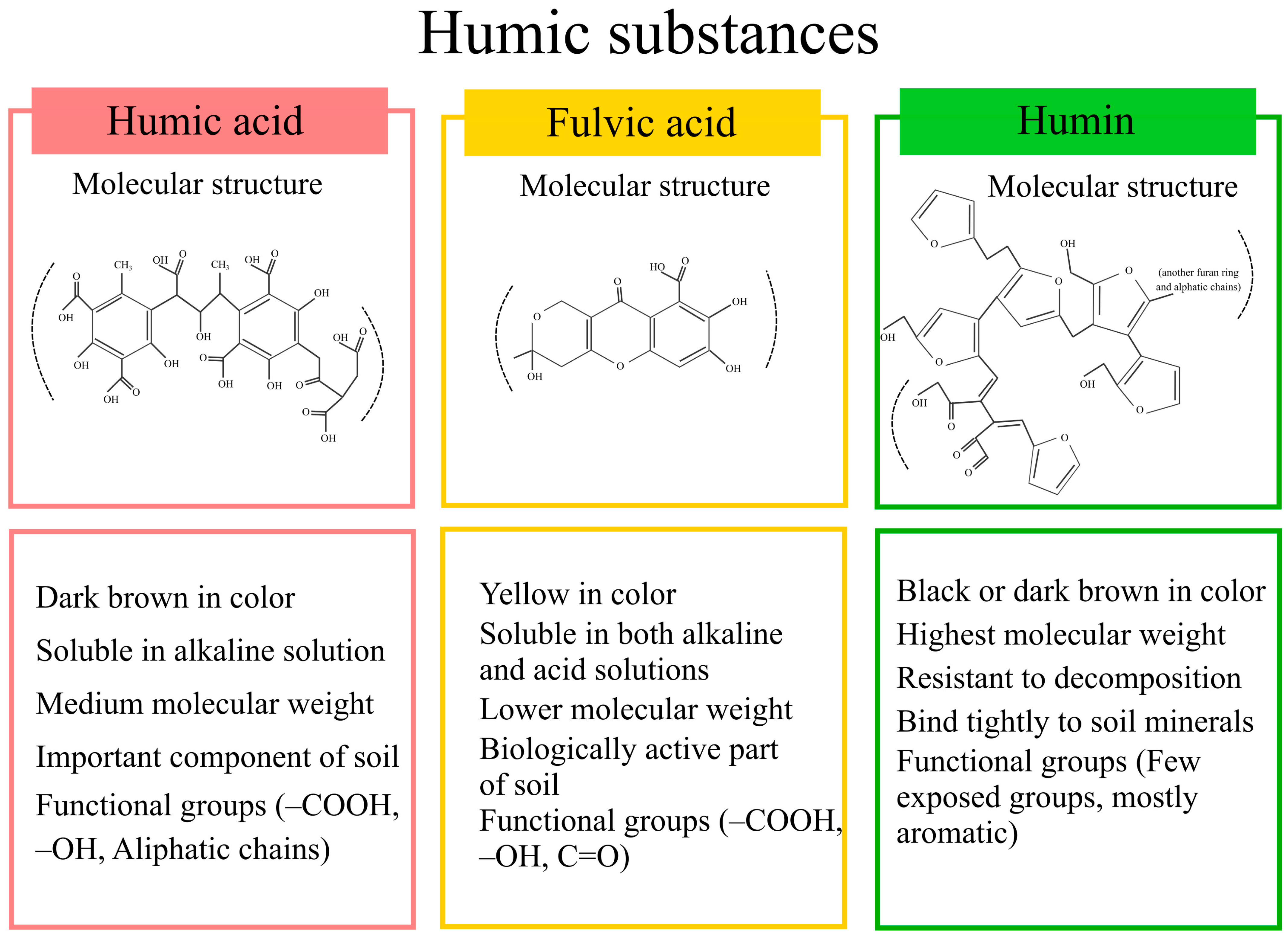
2. Humic Acid Molecular Structure and Functional Groups
3. Structure–Function Relationships of Humic Acid
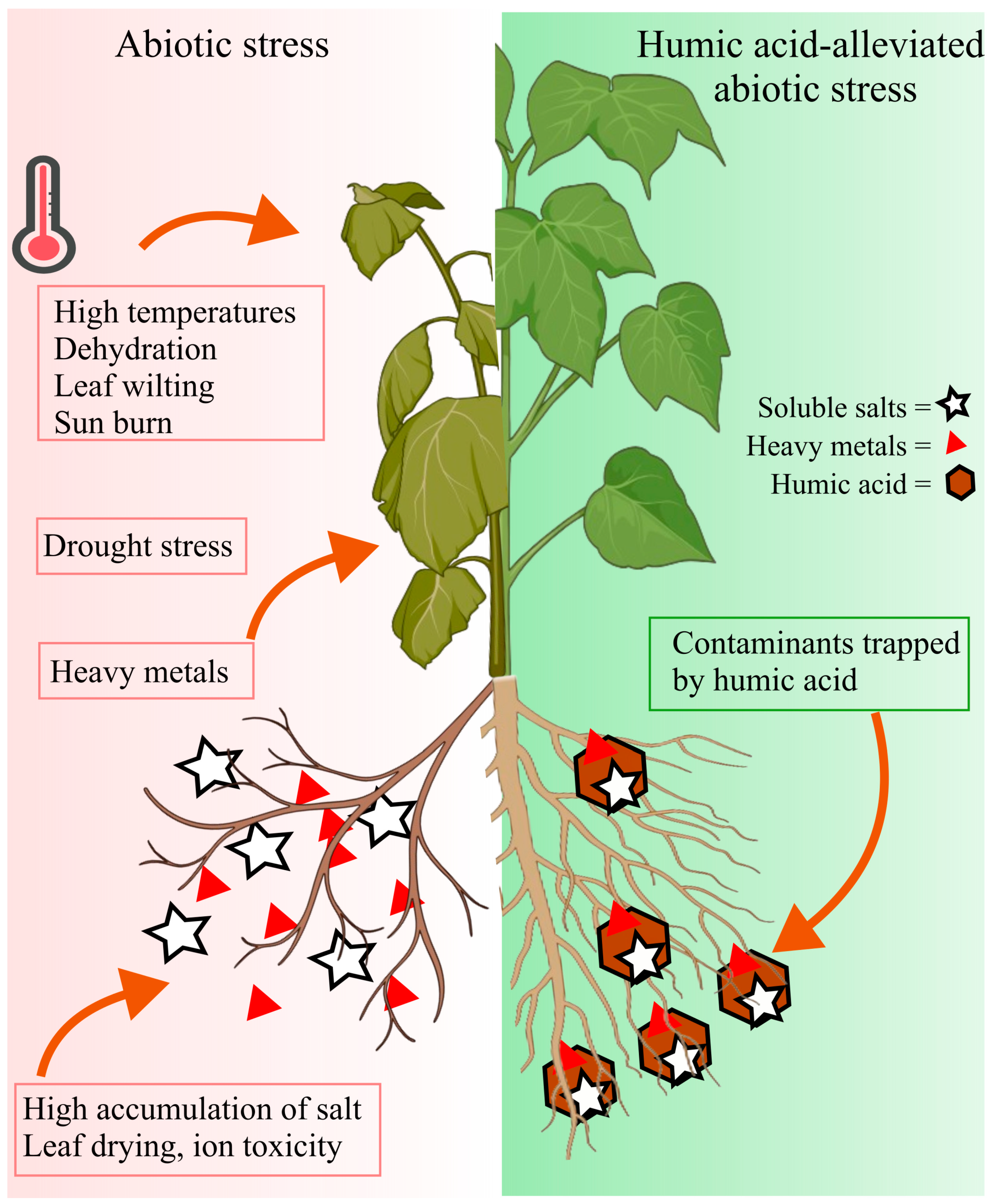
4. Humic Acid-Mediated Stress Alleviation in Plants
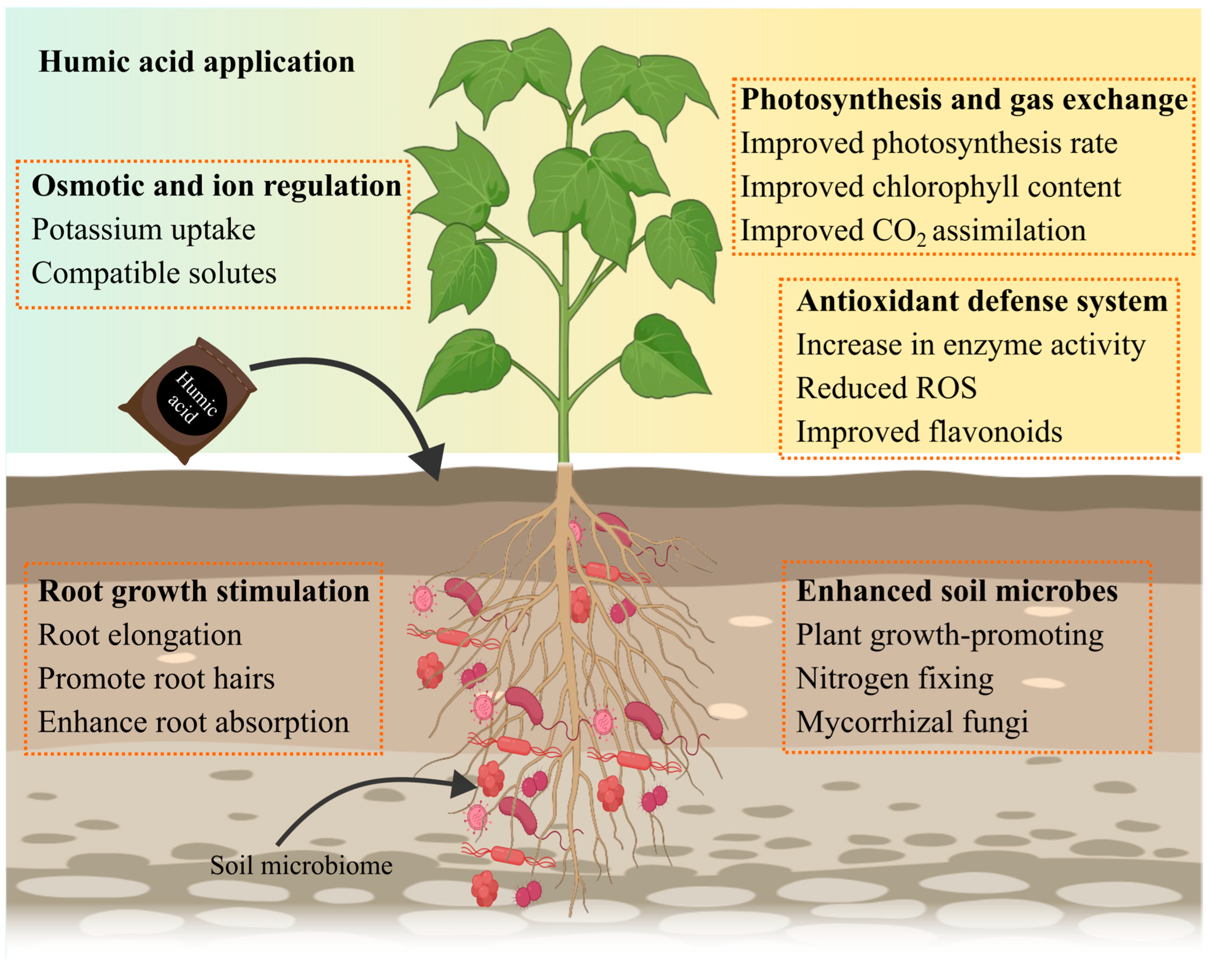
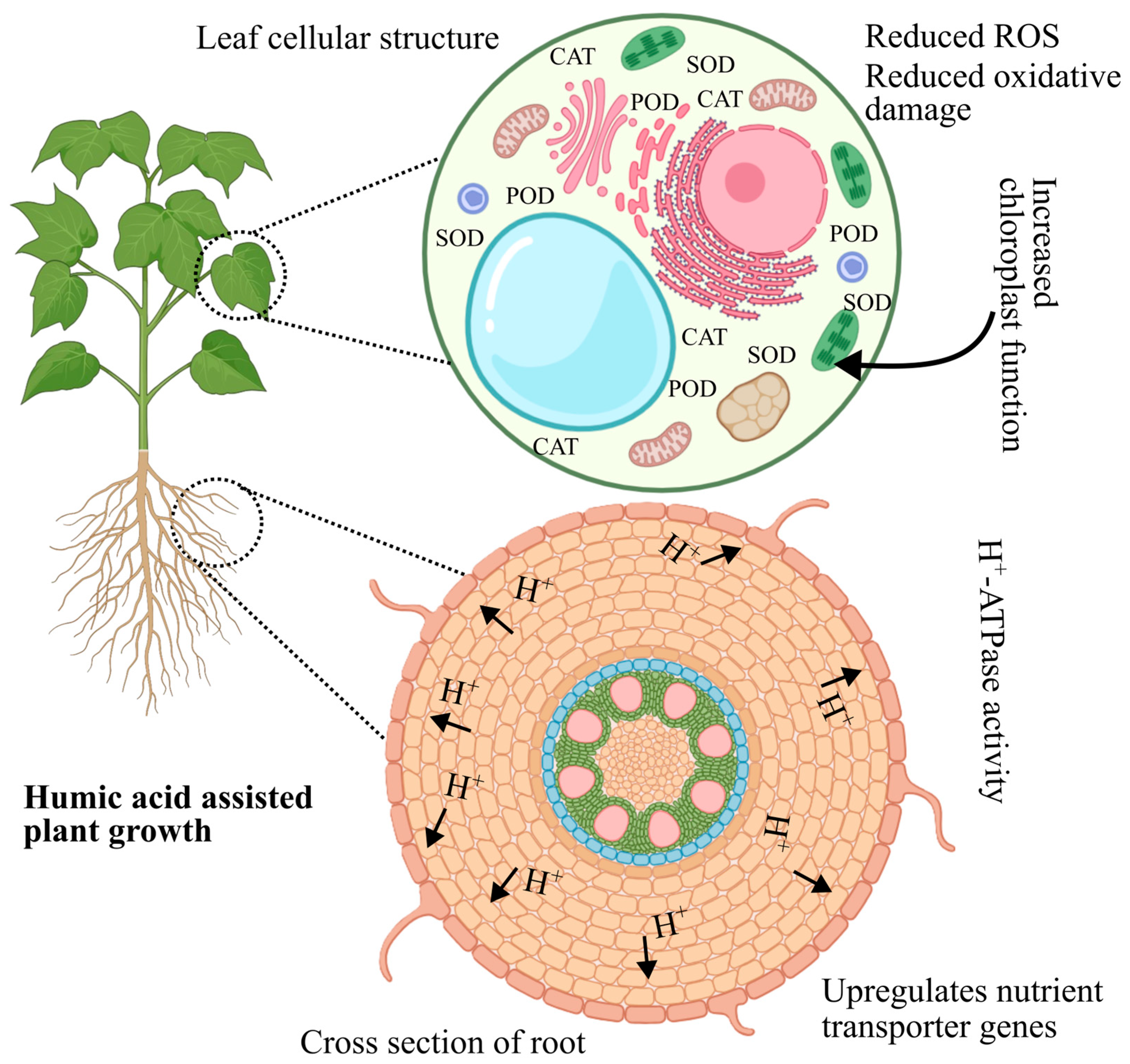
5. Mechanism of Humic Acid-Mediated Stress Alleviation
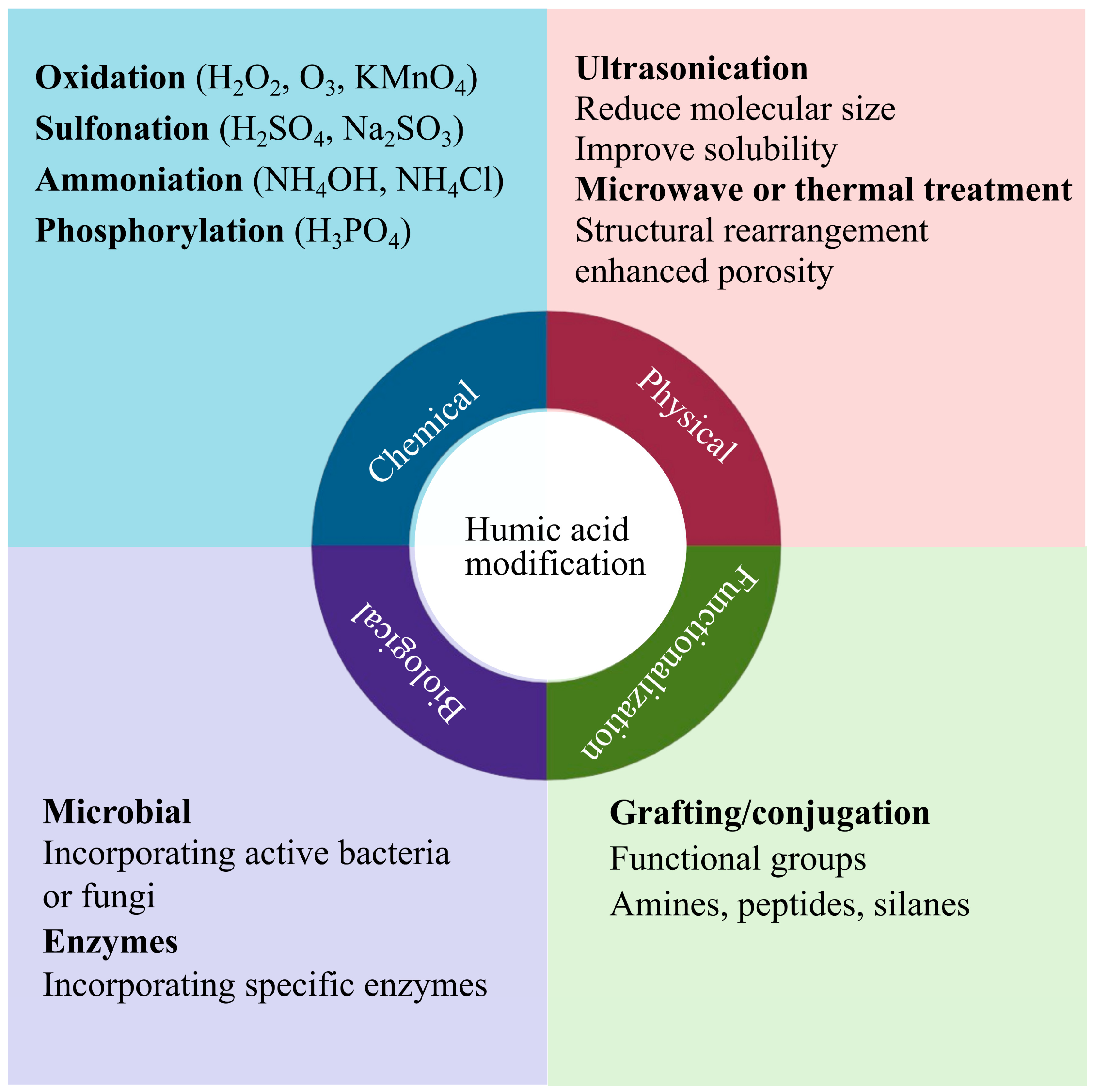
6. Humic Acid Modification for Stress Alleviation
| Functional Group | Associated Chemical or Biological Modifications | Plant Benefit | Ref |
| –COOH (Carboxyl Acid) | Promotes interaction with plant growth-promoting rhizobacteria (PGPR), and arbuscular mycorrhizal fungi (AMF) | Enhances antioxidant production, improves growth and abiotic stress tolerance | [117] |
| –OH (Hydroxyl Group) | Antioxidant-modulated pathways | Enhances soil moisture retention and scavenging of ROS | [118] |
| Aromatic Rings | Modified by sulfonation or phenolic enrichment | Increases hydrophilicity, contributes to structural stability and stress resilience | [119,120] |
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Orlov, D.S. Humic Substances of Soils and General Theory of Humification; CRC Press: Boca Raton, FL, USA, 2020; 266p. [Google Scholar]
- Hayes, M.H.; Swift, R.S. Vindication of humic substances as a key component of organic matter in soil and water. Adv. Agron. 2020, 163, 1–37. [Google Scholar] [CrossRef]
- Wu, A.; You, C.; Yin, R.; Xu, Z.; Zhang, L.; Liu, Y.; Li, H.; Wang, L.; Xu, L.; Xu, H. Forest gaps slow the humification process of fir (Abies faxoniana Rehder & E.H. Wilson) twig litter during eight years of decomposition in an alpine forest. Forests 2023, 14, 868. [Google Scholar] [CrossRef]
- Wiesler, F.; Hund-Rinke, K.; Gäth, S.; George, E.; Greef, J.; Hoelzle, L.; Holz, D.; Hülsbergen, P.; Pfeil, D.; Severin, D.; et al. Anwendung von organischen Düngern und organischen Reststoffen in der Landwirtschaft. Berichte über Landwirtsch.-Z. Für Agrarpolit. Und Landwirtsch. 2016, 94, 6–31. [Google Scholar] [CrossRef]
- Gerke, J. Concepts and misconceptions of humic substances as the stable part of soil organic matter: A review. Agronomy 2018, 8, 76. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humates—Facts and fantasies on their value as commercial soil amendments. Crops Soils 1979, 31, 14–16. [Google Scholar]
- Gerke, J. Carbon accumulation in arable soils: Mechanisms and the effect of cultivation practices and organic fertilizers. Agronomy 2021, 11, 1079. [Google Scholar] [CrossRef]
- Weber, J.; Jerzykiewicz, M.; Ukalska-Jaruga, A.; Ćwieląg-Piasecka, I.; Jamroz, E.; Kocowicz, A.; Debicka, M.; Bekier, J.; Mielnik, L.; Bejger, R. Properties of humin isolated from Polish arable soils: The most recalcitrant fraction of soil organic matter that prevent soil degradation. Land Degrad. Dev. 2024, 35, 2425–2436. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Billingham, K.L. Humic products—Potential or presumption for agriculture. do humic products have a place in australian grazing enterprises? In Proceedings of the 22nd International Grassland Congress; Taro, P.T., Ed.; NSW Department of Primary Industries: Sydney, Australia, 2013; pp. 1485–1488. [Google Scholar]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant biostimulants: A categorical review, their implications for row crop production, and relation to soil health indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Urrutia, O.; Fuentes, M.; Olaetxea, M.; Garnica, M.; Baigorri, R.; Movila, M.; De Hita, D.; Garcia-Mina, J. The effect of soil organic matter on plant mineral nutrition. In Achieving Sustainable Crop Nutrition; Burleigh Dodds Science Publishing: Cambridge, UK, 2020; pp. 291–306. [Google Scholar]
- Fuentes, M.; Baigorri, R.; González-Gaitano, G.; García-Mina, J.M. New methodology to assess the quantity and quality of humic substances in organic materials and commercial products for agriculture. J. Soils Sediments 2018, 18, 1389–1399. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.S.; Ali, S.; et al. Humic substances: Determining potential molecular regulatory processes in plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Nardi, S.; Ertani, A.; Francioso, O. Soil–root cross-talking: The role of humic substances. J. Plant Nutr. Soil Sci. 2017, 180, 5–13. [Google Scholar] [CrossRef]
- Bezuglova, O.; Klimenko, A. Application of humic substances in agricultural industry. Agronomy 2022, 12, 584. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Laskosky, J.; Mante, A.; Zvomuya, F.; Amarakoon, I.; Leskiw, L. A bioassay of long-term stockpiled salvaged soil amended with biochar, peat, and humalite. Agrosyst. Geosci. Environ. 2020, 3, e20068. [Google Scholar] [CrossRef]
- Canellas, L.P.; Canellas, N.O.; da, S. Irineu, L.E.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020, 7, 12. [Google Scholar] [CrossRef]
- Bybordi, A.; Ebrahimian, E. Growth, yield and quality components of canola fertilized with urea and zeolite. Commun. Soil Sci. Plant Anal. 2013, 44, 2896–2915. [Google Scholar] [CrossRef]
- El-Bassiouny, H.; Bakry, B.A.; El-Monem, A.; Allah, M. Physiological role of humic acid and nicotinamide on improving plant growth, yield, and mineral nutrient of wheat (Triticum durum) grown under newly reclaimed sandy soil. Agric. Sci. 2014, 5, 687–700. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R.; Zimmerman, A. Impacts of 1.5-year field aging on biochar, humic acid, and water treatment residual amended soil. Soil Sci. 2014, 179, 333–339. [Google Scholar] [CrossRef]
- Gollenbeek, L.; van der Weide, R. Prospects for Humic Acid Products from Digestate in The Netherlands: Quickscan; Wageningen Plant Research: Wageningen, The Netherlands, 2020. [Google Scholar]
- Rose, M.; Patti, A.; Little, K.; Brown, A.; Jackson, W.; Cavagnaro, T. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- PubChem. Humic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Humic-acid (accessed on 31 May 2025).
- Yang, F.; Fu, Q.; Antonietti, M. Anthropogenic, carbon-reinforced soil as a living engineered material. Chem. Rev. 2023, 123, 2420–2435. [Google Scholar] [CrossRef] [PubMed]
- Khuda, F.; Anjum, M.; Khan, S.; Khan, H.; Sahibzada, M.U.K.; Khusro, A.; Jan, A.; Ullah, N.; Shah, Y.; Abbas, M. Antimicrobial, anti-inflammatory and antioxidant activities of natural organic matter extracted from cretaceous shales in district Nowshera-Pakistan. Arab. J. Chem. 2022, 15, 103633. [Google Scholar] [CrossRef]
- Yang, F.; Lan, Y.; Li, R.; Fu, Q.; Cheng, K.; Liu, Z.; Antonietti, M. Anthropogenic soil as an environmental material, as exemplified with improved growth of rice seedlings. Carbon Res. 2024, 3, 46. [Google Scholar] [CrossRef]
- Zheng, A.; Huang, Z.; Wei, G.; Zhao, K.; Jiang, L.; Zhao, Z.; Tian, Y.; Li, H. Controlling deoxygenation pathways in catalytic fast pyrolysis of biomass and its components by using metal-oxide nanocomposites. iScience 2020, 23, 101511. [Google Scholar] [CrossRef]
- Bosch-Serra, À.D.; Jiménez-de-Santiago, D.E.; González-Pérez, J.A.; Almendros, G. Pig slurry fertilization changes the pyrolytic signature of humic substances in calcareous soil. Agronomy 2025, 15, 725. [Google Scholar] [CrossRef]
- Tiwari, J.; Ramanathan, A.L.; Bauddh, K.; Korstad, J. Humic substances: Structure, function and benefits for agroecosystems—A review. Pedosphere 2023, 33, 237–249. [Google Scholar] [CrossRef]
- Zykova, M.V.; Bratishko, K.A.; Buyko, E.E.; Azarkina, L.A.; Ivanov, V.V.; Mihalyov, D.A.; Trofimova, E.S.; Danilets, M.G.; Ligacheva, A.A.; Konstantinov, A.I.; et al. Coal-derived humic substances: Insight into chemical structure parameters and biomedical properties. Molecules 2024, 29, 1530. [Google Scholar] [CrossRef]
- Jin, Y.; Yuan, Y.; Liu, Z.; Gai, S.; Cheng, K.; Yang, F. Effect of humic substances on nitrogen cycling in soil-plant ecosystems: Advances, issues, and future perspectives. J. Environ. Manag. 2024, 351, 119738. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, C.; Jin, Y.; Cheng, K.; Yang, F. Contribution of exogenous humic substances to phosphorus availability in soil-plant ecosystem: A review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1085–1102. [Google Scholar] [CrossRef]
- Ore, O.; Adeola, A.; Fapohunda, O.; Adedipe, D.; Bayode, A.; Adebiyi, M. Humic substances derived from unconventional resources: Extraction, properties, environmental impacts, and prospects. Environ. Sci. Pollut. Res. 2023, 30, 59106–59127. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, M.; Salzano, M.; Savy, D.; Di Meo, V.; Valentini, M.; Cozzolino, V.; Piccolo, A. Antibacterial and antioxidant properties of humic substances from composted agricultural biomasses. Chem. Biol. Technol. Agric. 2022, 9, 1. [Google Scholar] [CrossRef]
- Maffia, A.; Oliva, M.; Marra, F.; Mallamaci, C.; Nardi, S.; Muscolo, A. Humic substances: Bridging ecology and agriculture for a greener future. Agronomy 2025, 15, 410. [Google Scholar] [CrossRef]
- Lau, S.E.; Lim, L.W.T.; Hamdan, M.F.; Chan, C.; Saidi, N.B.; Ong-Abdullah, J.; Tan, B.C. Enhancing plant resilience to abiotic stress: The power of biostimulants. Phyton-Int. J. Exp. Bot. 2025, 94, 1–31. [Google Scholar] [CrossRef]
- Boutahiri, S.; Benrkia, R.; Tembeni, B.; Idowu, O.E.; Olatunji, O.J. Effect of biostimulants on the chemical profile of food crops under normal and abiotic stress conditions. Curr. Plant Biol. 2024, 40, 100410. [Google Scholar] [CrossRef]
- Amerian, M.; Palangi, A.; Gohari, G.; Ntatsi, G. Humic acid and grafting as sustainable agronomic practices for increased growth and secondary metabolism in cucumber subjected to salt stress. Sci. Rep. 2024, 14, 15883. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Advances in humeomics: Enhanced structural identification of humic molecules after size fractionation of a soil humic acid. Anal. Chim. Acta 2012, 720, 77–90. [Google Scholar] [CrossRef]
- Olk, D.; Dinnes, D.; Scoresby, J.; Callaway, C.; Darlington, J. Humic products in agriculture: Potential benefits and research challenges—A review. J. Soils Sediments 2018, 18, 2881–2891. [Google Scholar] [CrossRef]
- Yildiztekin, M.; Tuna, A.L.; Kaya, C. Physiological effects of the brown seaweed (Ascophyllum nodosum) and humic substances on plant growth, enzyme activities of certain pepper plants grown under salt stress. Acta Biol. Hung. 2018, 69, 325–335. [Google Scholar] [CrossRef]
- Dinçsoy, M.; Sönmez, F. The effect of potassium and humic acid applications on yield and nutrient contents of wheat (Triticum aestivum L. var. Delfii) with same soil properties. J. Plant Nutr. 2019, 42, 2757–2772. [Google Scholar] [CrossRef]
- Mirza, M.; Agarwal, S.; Rahman, M.; Rauf, A.; Ahmad, N.; Alam, M.A.; Iqbal, Z. Role of humic acid on oral drug delivery of an antiepileptic drug. Drug Dev. Ind. Pharm. 2011, 37, 310–319. [Google Scholar] [CrossRef]
- van Tol de Castro, T.A.; Berbara, R.L.L.; Tavares, O.C.H.; Mello, D.; Pereira, E.G.; Souza, C.; Espinosa, L.M.; García, A.C. Humic acids induce a eustress state via photosynthesis and nitrogen metabolism leading to a root growth improvement in rice plants. Plant Physiol. Biochem. 2021, 162, 171–184. [Google Scholar] [CrossRef]
- García, A.C.; de Souza, L.G.; Pereira, M.G.; Castro, R.N.; García-Mina, J.M.; Zonta, E.; Lisboa, F.J.; Berbara, R.L. Structure-property-function relationship in humic substances to explain the biological activity in plants. Sci. Rep. 2016, 6, 20798. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar] [CrossRef]
- Garcia-Mina, J.; Mora, V.; Olaetxea, M.; Baigorri, R.; Fuentes, M.; Garnica, M.; San Francisco, S.; Erro, J.; Urrutia, O.; Casanova, E. Main mechanisms involved in the effects of humic substances on soil-plant systems. Agrocienc. Urug. 2012, 16, 188–190. [Google Scholar] [CrossRef]
- Hayes, M.H.; Swift, R.S. An appreciation of the contribution of Frank Stevenson to the advancement of studies of soil organic matter and humic substances. J. Soils Sediments 2018, 18, 1212–1231. [Google Scholar] [CrossRef]
- Xue, S.; Xiao, Y.; Wang, G.; Fan, J.; Wan, K.; He, Q.; Gao, M.; Miao, Z. Adsorption of heavy metals in water by modifying Fe3O4 nanoparticles with oxidized humic acid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126333. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, H.; Xu, C.; Ba, Y.; Geng, Z.; She, D. Effective adsorption of ammonium nitrogen by sulfonic-humic acid char and assessment of its recovery for application as nitrogen fertilizer. Sci. Total Environ. 2023, 867, 161591. [Google Scholar] [CrossRef]
- Sarlaki, E.; Kianmehr, M.H.; Ghorbani, M.; Kermani, A.M.; Asefpour Vakilian, K.; Angelidaki, I.; Wang, Y.; Gupta, V.K.; Pan, J.; Tabatabaei, M.; et al. Highly humified nitrogen-functionalized lignite activated by urea pretreatment and ozone plasma oxidation. Chem. Eng. J. 2023, 456, 140978. [Google Scholar] [CrossRef]
- Al-Akbari, R.; Manasrah, A.D.; Nassar, N.N. Production of humic and fulvic acid analogs through the ultrasonication of low-rank lignite coals. React. Chem. Eng. 2024, 9, 566–582. [Google Scholar] [CrossRef]
- Mirzaei Varoei, M.; Oustan, S.; Reyhanitabar, A.; Najafi, N. Preparation, characterization and nitrogen availability of nitrohumic acid as a slow-release nitrogen fertilizer. Arch. Agron. Soil Sci. 2023, 69, 3345–3361. [Google Scholar] [CrossRef]
- Thorn, K.A.; Cox, L.G. Nitrosation and nitration of fulvic acid, peat and coal with nitric acid. PLoS ONE 2016, 11, e0154981. [Google Scholar] [CrossRef] [PubMed]
- Doskočil, L.; Grasset, L.; Válková, D.; Pekař, M. Hydrogen peroxide oxidation of humic acids and lignite. Fuel 2014, 134, 406–413. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Ma, G.; Zhang, K.; Qu, Z.; E, G.; Wang, C.; Zhang, P.; Liu, Z. Humic acid extracted from danty via catalytic oxidation using H2O2/birnessite: Characteristics and agricultural beneficial effects. ACS Omega 2022, 7, 47192–47201. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Zhang, Y.; Hu, X.; Li, Q.; Su, Y.; Zhao, W. Exploration of the H2O2 oxidation process and characteristic evaluation of humic acids from two typical lignites. ACS Omega 2021, 6, 24051–24061. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, L.; Zhao, B.; Li, Y.; Lin, Z. Structural characteristics of humic acids derived from Chinese weathered coal under different oxidizing conditions. PLoS ONE 2019, 14, e0217469. [Google Scholar] [CrossRef]
- Sarlaki, E.; Kianmehr, M.H.; Marzban, N.; Shafizadeh, A.; Sheikh Ahmad Tajuddin, S.A.F.; Hu, S.; Tabatabaei, M.; Aghbashlo, M. Advances and challenges in humic acid production technologies from natural carbonaceous material wastes. Chem. Eng. J. 2024, 498, 155521. [Google Scholar] [CrossRef]
- Sarlaki, E.; Kianmehr, M.H.; Kermani, A.M.; Ghorbani, M.; Aghbashlo, M. Activation of nitro-humic substances from lignite using solid-phase nitro-humification process assisted by nitrogen enrichment and ozone oxidation. Iran. J. Biosyst. Eng. 2022, 53, 289–309. [Google Scholar] [CrossRef]
- Canellas, L.P.; da Silva, R.M.; Busato, J.G.; Olivares, F.L. Humic substances and plant abiotic stress adaptation. Chem. Biol. Technol. Agric. 2024, 11, 66. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Sonmez, O. Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Cereal Res. Commun. 2018, 46, 67–78. [Google Scholar] [CrossRef]
- Kutlu, I.; Gulmezoglu, N. Suitable humic acid application methods to maintain physiological and enzymatic properties of bean plants under salt stress. Gesunde Pflanz. 2023, 75, 1075–1086. [Google Scholar] [CrossRef]
- Joshi, S.; Nath, J.; Singh, A.K.; Pareek, A.; Joshi, R. Ion transporters and their regulatory signal transduction mechanisms for salinity tolerance in plants. Physiol. Plant. 2022, 174, e13702. [Google Scholar] [CrossRef]
- Olías, R.; Eljakaoui, Z.; Pardo, J.M.; Belver, A. The Na(+)/H(+) exchanger SOS1 controls extrusion and distribution of Na(+) in tomato plants under salinity conditions. Plant Signal. Behav. 2009, 4, 973–976. [Google Scholar] [CrossRef]
- Xie, Q.; Zhou, Y.; Jiang, X. Structure, function, and regulation of the plasma membrane Na+/H+ antiporter salt overly sensitive 1 in plants. Front. Plant Sci. 2022, 13, 866265. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, R.; Liu, S.; Li, R.; Zhou, Y.; Chen, Y.; Hou, H.; Dai, Q. Transcriptome-wide analysis revealed the potential of the high-affinity potassium transporter (HKT) gene family in rice salinity tolerance via ion homeostasis. Bioengineering 2022, 9, 410. [Google Scholar] [CrossRef]
- Ali, A.; Raddatz, N.; Pardo, J.M.; Yun, D.J. HKT sodium and potassium transporters in Arabidopsis thaliana and related halophyte species. Physiol. Plant. 2021, 171, 546–558. [Google Scholar] [CrossRef]
- Alsudays, I.M.; Alshammary, F.H.; Alabdallah, N.M.; Alatawi, A.; Alotaibi, M.M.; Alwutayd, K.M.; Alharbi, M.M.; Alghanem, S.M.S.; Alzuaibr, F.M.; Gharib, H.S.; et al. Applications of humic and fulvic acid under saline soil conditions to improve growth and yield in barley. BMC Plant Biol. 2024, 24, 191. [Google Scholar] [CrossRef]
- Malik, Z.; Malik, N.; Noor, I.; Kamran, M.; Ali, M.; Sabir, F.; Elansary, H.; Zin Elabadin, T.; Mahmoud, E.; Sh, F. Combined effect of rice-straw biochar and humic acid on growth, antioxidative capacity, and ion uptake in maize (Zea mays L.) grown under saline soil conditions. J. Plant Growth Regul. 2022, 42, 3211–3228. [Google Scholar] [CrossRef]
- Abu-Ria, M.E.; Elghareeb, E.M.; Shukry, W.M.; Abo-Hamed, S.A.; Ibraheem, F. Mitigation of drought stress in maize and sorghum by humic acid: Differential growth and physiological responses. BMC Plant Biol. 2024, 24, 514. [Google Scholar] [CrossRef] [PubMed]
- Bijanzadeh, E.; Naderi, R.; Egan, T.P. Exogenous application of humic acid and salicylic acid to alleviate seedling drought stress in two corn (Zea mays L.) hybrids. J. Plant Nutr. 2019, 42, 1483–1495. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Shen, J.; Zhang, L.; Wei, P.; Liu, A.; Song, H. The alleviating effect on the growth, chlorophyll synthesis, and biochemical defense system in sunflowers under cadmium stress achieved through foliar application of humic acid. BMC Plant Biol. 2024, 24, 792. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ağar, G.; Dursun, A.; Kul, R.; Alim, Z.; Argin, S. Humic + fulvic acid mitigated Cd adverse effects on plant growth, physiology and biochemical properties of garden cress. Sci. Rep. 2021, 11, 8040. [Google Scholar] [CrossRef]
- Wen, J.; Tang, X.; Wang, M.; Mu, L.; Hao, W.; Weng, J.; Gao, Z.; Hu, X. Regulation and mechanism of pyrite and humic acid on the toxicity of arsenate in lettuce. Sci. Total Environ. 2024, 912, 168980. [Google Scholar] [CrossRef]
- Yang, F.; Yuan, Y.; Liu, Q.; Zhang, X.; Gai, S.; Jin, Y.; Cheng, K. Artificial humic acid promotes growth of maize seedling under alkali conditions. Environ. Pollut. 2023, 327, 121588. [Google Scholar] [CrossRef]
- Rekaby, S.; Al-Huqail, A.; Gebreel, M.; Alotaibi, S.; Ghoneim, A. Compost and humic acid mitigate the salinity stress on quinoa (Chenopodium quinoa Willd L.) and improve some sandy soil properties. J. Soil Sci. Plant Nutr. 2023, 23, 2651–2661. [Google Scholar] [CrossRef]
- Abu-Ria, M.; Shukry, W.; Abo-Hamed, S.; Albaqami, M.; Almuqadam, L.; Ibraheem, F. Humic acid modulates ionic homeostasis, osmolytes content, and antioxidant defense to improve salt tolerance in rice. Plants 2023, 12, 1834. [Google Scholar] [CrossRef]
- Huang, R. The effect of humic acid on the desalinization of coastal clayey saline soil. Water Supply 2022, 22, 7242–7255. [Google Scholar] [CrossRef]
- Silva, E.; Matias, S.; Souza, J.; Rodrigues, E.; Ribeiro, É.; Freitas, J.; Cunha, J. Analise do crescimento de mudas de mamoeiro Hawai (Carica Papaya) produzidas sob diferentes proporções de esterco bovino e solo. Contrib. Cienc. Soc. 2025, 18, e15355. [Google Scholar] [CrossRef]
- Dias, T.; Leal, M.; Nascimento, E.; Veras, M.; da Silva, T.; Lopes, A. Morphological and physiological changes in papaya seedlings irrigated with saline water and application of humic substances. Comun. Sci. 2020, 11, e3290. [Google Scholar] [CrossRef]
- Chen, Q.; Qu, Z.; Ma, G.; Wang, W.; Dai, J.; Zhang, M.; Wei, Z.; Liu, Z. Humic acid modulates growth, photosynthesis, hormone and osmolytes system of maize under drought conditions. Agric. Water Manag. 2022, 263, 107447. [Google Scholar] [CrossRef]
- Matuszak-Slamani, R.; Bejger, R.; Włodarczyk, M.; Kulpa, D.; Sienkiewicz, M.; Gołębiowska, D.; Skórska, E.; Ukalska-Jaruga, A. Effect of humic acids on soybean seedling growth under polyethylene-glycol-6000-induced drought stress. Agronomy 2022, 12, 1109. [Google Scholar] [CrossRef]
- Qin, K.; Leskovar, D.I. Humic substances improve vegetable seedling quality and post-transplant yield performance under stress conditions. Agriculture 2020, 10, 254. [Google Scholar] [CrossRef]
- Turan, M.; Yildirim, E.; Ekinci, M.; Argin, S. Effect of biostimulants on yield and quality of cherry tomatoes grown in fertile and stressed soils. HortScience 2021, 56, 414–423. [Google Scholar] [CrossRef]
- Cha, J.Y.; Kang, S.H.; Ali, I.; Lee, S.C.; Ji, M.G.; Jeong, S.Y.; Shin, G.I.; Kim, M.G.; Jeon, J.R.; Kim, W.Y. Humic acid enhances heat stress tolerance via transcriptional activation of heat-shock proteins in Arabidopsis. Sci. Rep. 2020, 10, 15042. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Hussein, O.S.; Abdelkader, A.F.; Farag, I.A.; Hassan, Y.E.; Ibrahim, M. Metabolic activities and molecular investigations of the ameliorative impact of some growth biostimulators on chilling-stressed coriander (Coriandrum sativum L.) plant. BMC Plant Biol. 2021, 21, 361. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T.; Zhang, Q.; Zhu, Q.H.; Huang, D.Y.; Zhu, H.H.; Xu, C.; Su, S.M.; Zeng, X.B. Influence of straw-derived humic acid-like substance on the availability of Cd/As in paddy soil and their accumulation in rice grain. Chemosphere 2022, 300, 134368. [Google Scholar] [CrossRef]
- Dogan, M.; Bolat, I.; Karakas, S.; Dikilitas, M.; Gutiérrez-Gamboa, G.; Kaya, O. Remediation of cadmium stress in strawberry plants using humic acid and silicon applications. Life 2022, 12, 1962. [Google Scholar] [CrossRef]
- Boysan Canal, S.; Bozkurt, M.; Yilmaz, H. The effect of humic acid on rapeseed (Brassica napus L.) plant growth, heavy metal uptake, phytoremediation parameters (BCF, TF and TI), and antioxidant activity in heavy metal polluted soil. Yüzüncü Yıl Üniv. Tarım Bilim. Derg. 2022, 32, 237–248. [Google Scholar] [CrossRef]
- Duan, D.; Tong, J.; Xu, Q.; Dai, L.; Ye, J.; Wu, H.; Xu, C.; Shi, J. Regulation mechanisms of humic acid on Pb stress in tea plant (Camellia sinensis L.). Environ. Pollut. 2020, 267, 115546. [Google Scholar] [CrossRef] [PubMed]
- Ran, S.; He, T.; Zhou, X.; Yin, D. Effects of fulvic acid and humic acid from different sources on Hg methylation in soil and accumulation in rice. J. Environ. Sci. 2022, 119, 93–105. [Google Scholar] [CrossRef] [PubMed]
- García, A.C.; Santos, L.A.; Izquierdo, F.G.; Sperandio, M.V.L.; Castro, R.N.; Berbara, R.L.L. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol. Eng. 2012, 47, 203–208. [Google Scholar] [CrossRef]
- García, A.C.; Olaetxea, M.; Santos, L.A.; Mora, V.; Baigorri, R.; Fuentes, M.; Zamarreño, A.M.; Berbara, R.L.L.; Garcia-Mina, J.M. Involvement of hormone-and ROS-signaling pathways in the beneficial action of humic substances on plants growing under normal and stressing conditions. Biomed. Res. Int. 2016, 2016, 3747501. [Google Scholar] [CrossRef]
- Souza, A.C.; Olivares, F.L.; Peres, L.E.P.; Piccolo, A.; Canellas, L.P. Plant hormone crosstalk mediated by humic acids. Chem. Biol. Technol. Agric. 2022, 9, 29. [Google Scholar] [CrossRef]
- Vioratti Telles de Moura, O.; Luiz Louro Berbara, R.; França de Oliveira Torchia, D.; Fernanda Oliveira Da Silva, H.; Augusto van Tol de Castro, T.; Carlos Huertas Tavares, O.; Fernandes Rodrigues, N.; Zonta, E.; Azevedo Santos, L.; Calderín García, A. Humic foliar application as sustainable technology for improving the growth, yield, and abiotic stress protection of agricultural crops. A review. J. Saudi Soc. Agric. Sci. 2023, 22, 493–513. [Google Scholar] [CrossRef]
- Shen, J.; Guo, M.J.; Wang, Y.G.; Yuan, X.; Wen, Y.Y.; Song, X.E.; Dong, S.Q.; Guo, P.Y. Humic acid improves the physiological and photosynthetic characteristics of millet seedlings under drought stress. Plant Signal. Behav. 2020, 15, 1774212. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Zhang, Y.; Wang, M.; Wang, P.; Liu, D. Process condition optimization and structural feature analysis of humic acid extraction from weathered lignite. ACS Omega 2024, 9, 38409–38422. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, N.; Li, J.; Wang, Y.; Liu, Y.; Cao, M.; Yan, Q. Characterization of humic acids from original coal and its oxidization production. Sci. Rep. 2021, 11, 15381. [Google Scholar] [CrossRef]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Kah, M. Nanopesticides and nanofertilizers: Emerging contaminants or opportunities for risk mitigation? Front. Chem. 2015, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Nardi, S.; Concheri, G. Structural characteristics of humic substances as related to nitrate uptake and growth regulation in plant systems. Soil Biol. Biochem. 1992, 24, 373–380. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Gessa, C.; Ferrarese, L.; Trainotti, L.; Casadoro, G. A low molecular weight humic fraction on nitrate uptake and protein synthesis in maize seedlings. Soil Biol. Biochem. 2000, 32, 415–419. [Google Scholar] [CrossRef]
- Hamad, M.; Tantawy, M. Effect of different humic acids sources on the plant growth, calcium and iron utilization by sorghum. Egypt. J. Soil Sci. 2018, 58, 291–307. [Google Scholar] [CrossRef]
- Kluczek-Turpeinen, B.; Steffen, K.T.; Tuomela, M.; Hatakka, A.; Hofrichter, M. Modification of humic acids by the compost-dwelling deuteromycete Paecilomyces inflatus. Appl. Microbiol. Biotechnol. 2005, 66, 443–449. [Google Scholar] [CrossRef]
- Boguta, P.; Skic, K.; Sokołowska, Z.; Frąc, M.; Sas-Paszt, L. Chemical transformation of humic acid molecules under the influence of mineral, fungal and bacterial fertilization in the context of the agricultural use of degraded soils. Molecules 2021, 26, 4921. [Google Scholar] [CrossRef]
- Cha, J.Y.; Kim, T.W.; Choi, J.H.; Jang, K.S.; Khaleda, L.; Kim, W.Y.; Jeon, J.R. Fungal laccase-catalyzed oxidation of naturally occurring phenols for enhanced germination and salt tolerance of Arabidopsis thaliana: A green route for synthesizing humic-like fertilizers. J. Agric. Food Chem. 2017, 65, 1167–1177. [Google Scholar] [CrossRef]
- Tang, Y.; Hou, S.; Yang, Y.; Cheng, D.; Gao, B.; Wan, Y.; Li, Y.C.; Yao, Y.; Zhang, S.; Xie, J. Activation of humic acid in lignite using molybdate-phosphorus hierarchical hollow nanosphere catalyst oxidation: Molecular characterization and rice seed germination-promoting performances. J. Agric. Food Chem. 2020, 68, 13620–13631. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Cheng, D.; Gao, B.; Wan, Y.; Li, Y.C.; Yao, Y.; Xie, J.; Liu, L. Multifunctional slow-release fertilizer prepared from lignite activated by a 3d-molybdate-sulfur hierarchical hollow nanosphere catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10533–10543. [Google Scholar] [CrossRef]
- Dinu, M. Comparison of complexing ability of fulvic and humic acids in the aquatic environment with iron and zinc ions. Water Res. 2010, 37, 65–69. [Google Scholar] [CrossRef]
- Motojima, H.; Yamada, P.; Irie, M.; Ozaki, M.; Shigemori, H.; Isoda, H. Amelioration effect of humic acid extracted from solubilized excess sludge on saline-alkali soil. J. Mater. Cycles Waste Manag. 2012, 14, 169–180. [Google Scholar] [CrossRef]
- Pinos, N.Q.; Louro Berbara, R.L.; Elias, S.S.; van Tol de Castro, T.A.; García, A.C. Combination of humic substances and arbuscular mycorrhizal fungi affecting corn plant growth. J. Environ. Qual. 2019, 48, 1594–1604. [Google Scholar] [CrossRef]
- Zandonadi, D.B.; Monda, H.; Gralian, J.; James, A.; Lamar, R.T.; Santos, M.P. Humic acids as drivers of plant growth: Regulating root development and photobiology through redox modulation. Chem. Biol. Technol. Agric. 2025, 12, 71. [Google Scholar] [CrossRef]
- Muscolo, A.; Pizzeghello, D.; Francioso, O.; Sanchez Cortes, S.; Nardi, S. Effectiveness of humic substances and phenolic compounds in regulating plant-biological functionality. Agronomy 2020, 10, 1553. [Google Scholar] [CrossRef]
- Senesi, N.; Loffredo, E. Metal ion complexation by soil humic substances. In Chemical Processes in Soils; Huang, P.M., Senesi, N., Bollag, J.M., Eds.; Soil Science Society of America: Madison, WI, USA, 2005; Volume 8, pp. 563–617. [Google Scholar]
| Stress Type | Crop | Environment | HA Form & Dose | Application Details (Frequency, Duration, Volume/Area) | Key Findings | Ref |
| Salinity | Zea mays L. (maize) | Greenhouse | HA, 50 mg L−1 | Single application; duration not reported | Increased seed germination and seedling growth under salinity | [81] |
| Chenopodium quinoa L. (quinoa) | Field | HA, 1% (v/v) | Weekly application; 60 days; approx. 1 L/m2 | Increased plant height, fresh weight, and dry matter | [82] | |
| Oryza sativa L. (rice) | Greenhouse | HA, 100 mg L−1 | Applied at transplant; duration 30 days | Enhanced antioxidant enzyme activity and root growth | [83] | |
| Triticum aestivum L. (wheat) | Greenhouse | HA, 200 mg kg−1 soil | Single soil application; 45 days | Increased yield and productivity | [84] | |
| Carica papaya L. (papaya) | Greenhouse | HA, 3.5 mL L−1 | Biweekly foliar spray; 8 weeks | Alleviated salt stress, promoted growth, and improved photosynthesis | [85] | |
| Carica papaya L. (papaya) | Greenhouse | HS, 20 g kg−1 | Soil mixed before planting; 60 days | Increased photosynthesis, CO2 assimilation., WUE, and chlorophyll in saline conditions | [86] | |
| Drought | Zea mays L. (maize) | Greenhouse | HA, 45 kg ha−1 | Soil application at sowing; 90 days | Improved nutrient availability, WUE, Rubisco activity, sugars, and osmolyte content | [87] |
| Glycine max L. (soybean) | Greenhouse | HA, 5 mg dm−3 | Applied at sowing; 45 days | Increased antioxidant enzyme activity, biomass, and root length | [88] | |
| Capsicum annuum L. (pepper) | Field | HA, 4.5 L ha−1 | Weekly irrigation with HA; 75 days | Enhanced shoot biomass and growth under drought | [89] | |
| Extreme Temp. | Solanum lycopersicum L. (tomato) | Laboratory | HA, 500 mg L−1 | Foliar spray before heat exposure | Improved growth, fluorescence, antioxidant activity, and heat-responsive gene expression | [90] |
| Arabidopsis thaliana | Laboratory | Commercial HA, 860 mg L−1 | Single spray before heat stress | Enhanced heat stress tolerance via HSP gene expression | [91] | |
| Coriandrum sativum L. (coriander) | Greenhouse | HA, 50 mg L−1 | Applied during irrigation; 30 days | Promoted growth, enhanced antioxidants, and secondary metabolites | [92] | |
| Heavy Metals | Triticum aestivum L. (wheat) | Field | HA, 40 mg kg−1 | Soil amendment before planting; 60 days | Increased biomass, reduced oxidative stress under Cd stress | [93] |
| Fragaria × ananassa Duch. (strawberry) | Greenhouse | HA, 5 mM | Foliar application; 45 days | Reduced Cd toxicity via improved membrane stability and increased proline | [94] | |
| Brassica napus L. (rapeseed) | Greenhouse | HA, 2000 mg kg−1 | Soil amendment; 60 days | Increased growth, reduced metal accumulation and oxidative stress | [95] | |
| Lepidium sativum L. (garden cress) | Greenhouse | HS, 7000 mg L−1 | Hydroponic treatment; 20 days | Increased biomass and root diameter; reduced Cd uptake by up to 95% | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabi, F.; Sarfaraz, A.; Kama, R.; Kanwal, R.; Li, H. Structure-Based Function of Humic Acid in Abiotic Stress Alleviation in Plants: A Review. Plants 2025, 14, 1916. https://doi.org/10.3390/plants14131916
Nabi F, Sarfaraz A, Kama R, Kanwal R, Li H. Structure-Based Function of Humic Acid in Abiotic Stress Alleviation in Plants: A Review. Plants. 2025; 14(13):1916. https://doi.org/10.3390/plants14131916
Chicago/Turabian StyleNabi, Farhan, Ahmed Sarfaraz, Rakhwe Kama, Razia Kanwal, and Huashou Li. 2025. "Structure-Based Function of Humic Acid in Abiotic Stress Alleviation in Plants: A Review" Plants 14, no. 13: 1916. https://doi.org/10.3390/plants14131916
APA StyleNabi, F., Sarfaraz, A., Kama, R., Kanwal, R., & Li, H. (2025). Structure-Based Function of Humic Acid in Abiotic Stress Alleviation in Plants: A Review. Plants, 14(13), 1916. https://doi.org/10.3390/plants14131916








