Plant Microbiomes Alleviate Abiotic Stress-Associated Damage in Crops and Enhance Climate-Resilient Agriculture
Abstract
1. Introduction
2. Abiotic Stress in Plants: Impacts and Resilience
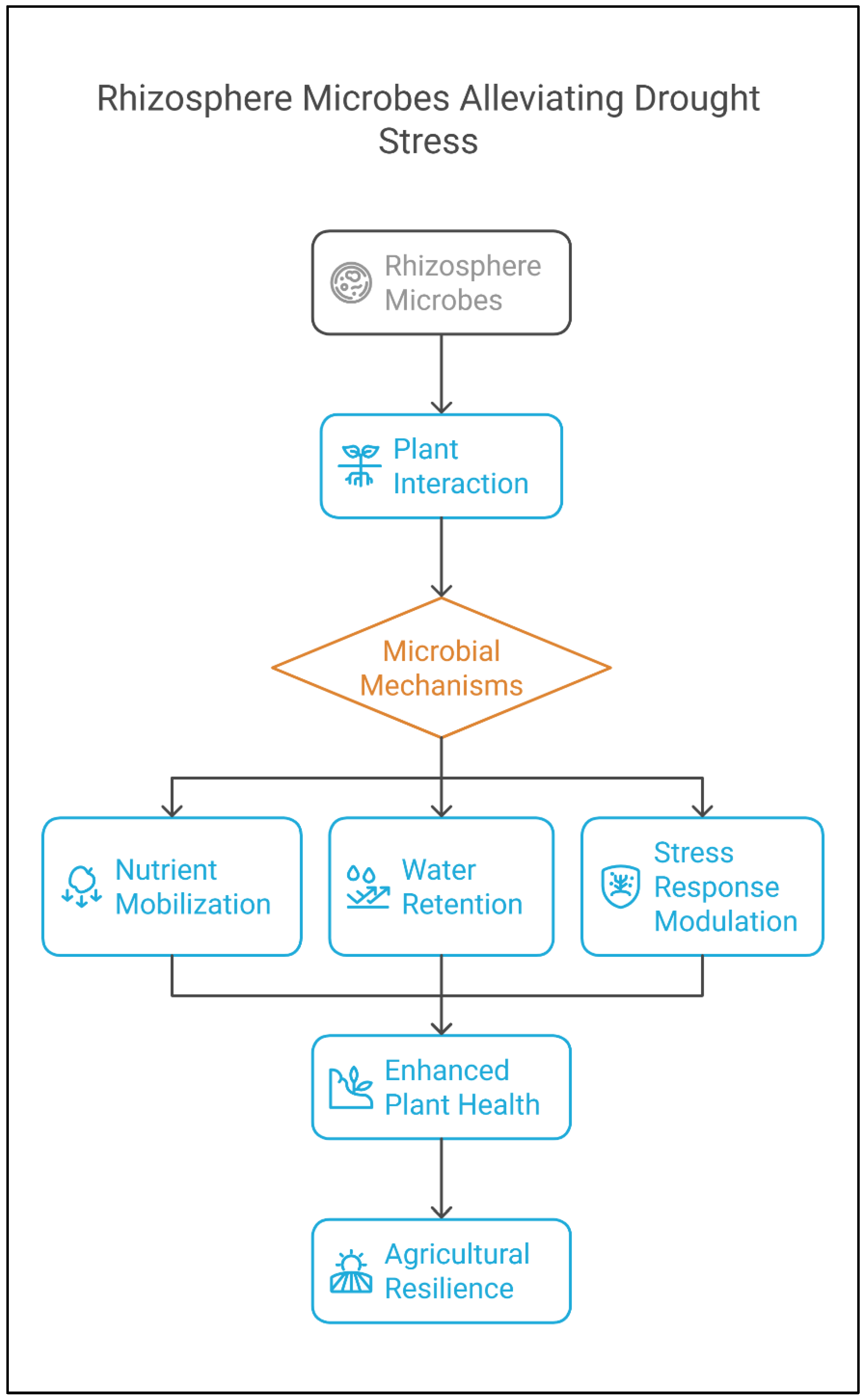
2.1. Drought Stress
2.2. Salinity Stress
2.3. Temperature Extremes (Heat and Cold Stress)
2.4. Nutrient Deficiencies
3. Plant Microbiomes and Stress Mitigation
3.1. Composition and Specific Microbial Groups in Plant Microbiomes
3.2. Mechanisms of Stress Alleviation
3.2.1. Enhanced Nutrient Uptake
3.2.2. Regulation of Plant Hormonal Pathways
3.2.3. Production of Stress-Relieving Metabolites
3.2.4. Induction of Systemic Tolerance and Improved Root Architecture
3.2.5. Heavy Metal Detoxification
4. Impacts of Climate Change on Plant–Microbe Interactions
5. Advances in Microbiome Engineering for Stress Resilience
5.1. Emerging Tools and Technologies
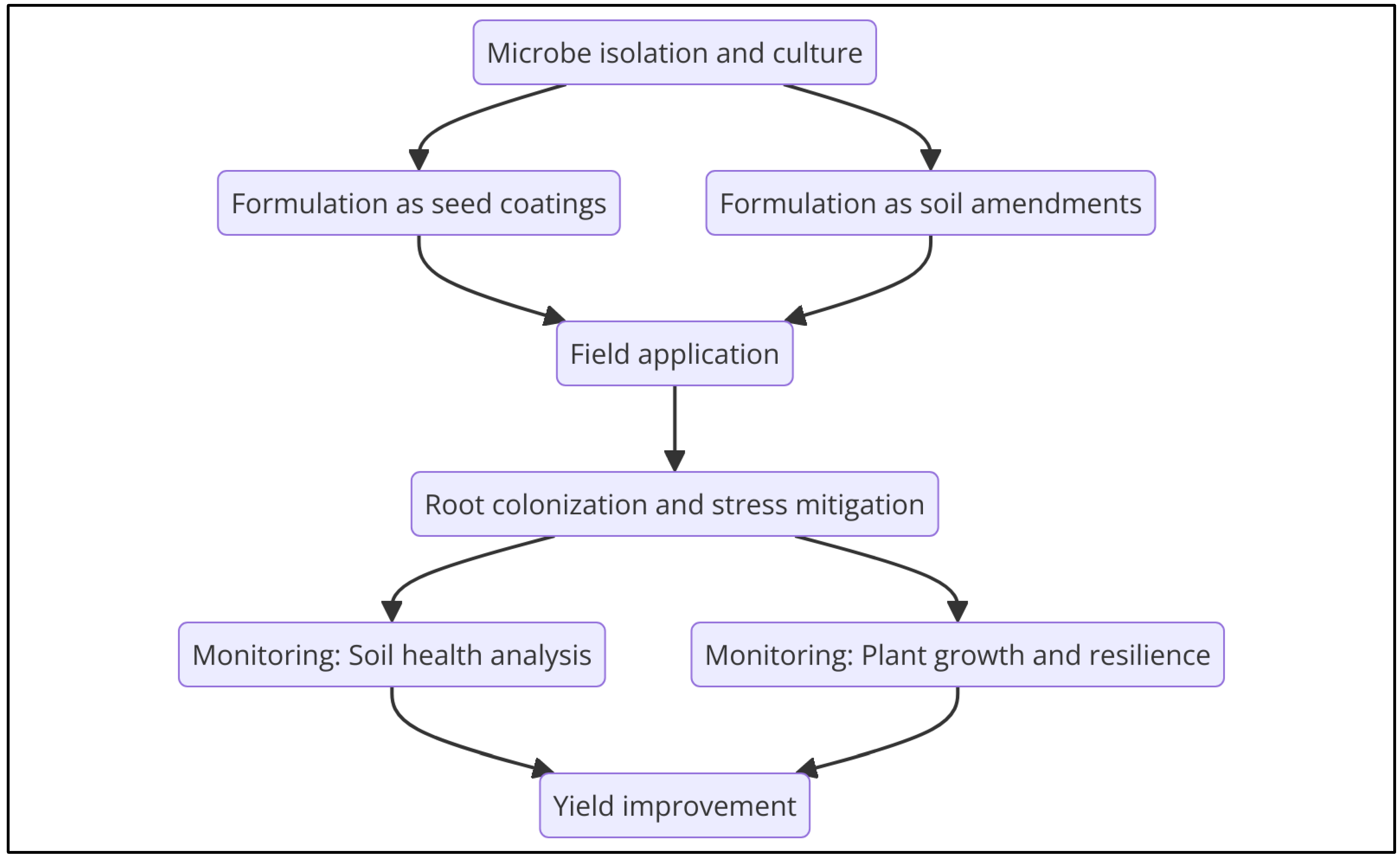
5.2. Bioinoculants and Biostimulants for Field Applications
5.3. CRISPR-Cas Tools for Microbial Enhancement
5.4. Crosstalk in Microbes and Climate Change Mitigation
6. Opportunities and Challenges in Utilizing Plant Microbiomes
Translating Lab-Scale Findings into Field Applications
7. Final Remarks and Future Perspectives
7.1. Reiterating the Importance of Plant Microbiomes in Building Climate-Resilient Systems
7.2. Call to Action for Interdisciplinary Research and Collaboration
7.3. Role of Plant Microbiomes in Sustainable Agriculture and Ecosystem Restoration
7.4. Integrating Microbiome Research with Climate-Smart Agricultural Practices
7.5. Policy and Funding Priorities for Microbiome-Based Solutions
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Albahri, G.; Alyamani, A.A.; Badran, A.; Hijazi, A.; Nasser, M.; Maresca, M.; Baydoun, E. Enhancing essential grains yield for sustainable food security and bio-safe agriculture through latest innovative approaches. Agronomy 2023, 13, 1709. [Google Scholar] [CrossRef]
- Lam, D. The Next 2 Billion: Can the World Support 10 Billion People? Popul. Dev. Rev. 2025, 51, 63–102. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, Y.; Sun, B.; Gao, Z.; Qin, P.; Li, Y.; Yue, W.; Cui, H. Combating land degradation through human efforts: Ongoing challenges for sustainable development of global drylands. J. Environ. Manag. 2024, 354, 120254. [Google Scholar] [CrossRef]
- Hossain, M.E.; Shahrukh, S.; Hossain, S.A. Chemical fertilizers and pesticides: Impacts on soil degradation, groundwater, and human health in Bangladesh. In Environmental Degradation: Challenges and Strategies for Mitigation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 63–92. [Google Scholar]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef]
- Nicaise, V.; Roux, M.; Zipfel, C. Recent advances in PAMP-triggered immunity against bacteria: Pattern recognition receptors watch over and raise the alarm. Plant Physiol. 2009, 150, 1638–1647. [Google Scholar] [CrossRef]
- Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and strategies of plant microbiome interactions to mitigate abiotic stresses. Agronomy 2022, 12, 2069. [Google Scholar] [CrossRef]
- Favela, A.; O Bohn, M.; D Kent, A. Maize germplasm chronosequence shows crop breeding history impacts recruitment of the rhizosphere microbiome. ISME J. 2021, 15, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Verma, H.; Kumar, D.; Kumar, V.; Kumari, M.; Singh, S.K.; Sharma, V.K.; Droby, S.; Santoyo, G.; White, J.F.; Kumar, A. The potential application of endophytes in management of stress from drought and salinity in crop plants. Microorganisms 2021, 9, 1729. [Google Scholar] [CrossRef]
- Dhungana, I.; Kantar, M.B.; Nguyen, N.H. Root exudate composition from different plant species influences the growth of rhizosphere bacteria. Rhizosphere 2023, 25, 100645. [Google Scholar] [CrossRef]
- Liu, D.; Xu, L.; Wang, H.; Xing, W.; Song, B.; Wang, Q. Root Exudates Promoted Microbial Diversity in the Sugar Beet Rhizosphere for Organic Nitrogen Mineralization. Agriculture 2024, 14, 1094. [Google Scholar] [CrossRef]
- Thepbandit, W.; Athinuwat, D. Rhizosphere microorganisms supply availability of soil nutrients and induce plant defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef]
- Prisa, D.; Fresco, R. Inoculants for Plant Drought Stress Tolerance: Mechanisms and Applications. Preprints 2023. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Santoyo, G.; Glick, B.R. Recent advances in the bacterial phytohormone modulation of plant growth. Plants 2023, 12, 606. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Beattie, G.A. Plant-Microbe Interactions in Adaptation of Agricultural Crops to Abiotic Stress Conditions. In Probiotics Plant Health; Springer: Singapore, 2017; pp. 163–200. [Google Scholar]
- Bennett, E.M.; Baird, J.; Baulch, H.; Chaplin-Kramer, R.; Fraser, E.; Loring, P.; Morrison, P.; Parrott, L.; Sherren, K.; Winkler, K.J. Ecosystem Services and the Resilience of Agricultural Landscapes. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 64, pp. 1–43. [Google Scholar]
- Khan, M.; Khan, T.; Tabassum, B.; Hashim, M. Microbiome-Driven Soil Fertility: Understanding Symbiotic Relationships. In Progress in Soil Microbiome Research; Springer: Berlin/Heidelberg, Germany, 2024; pp. 77–115. [Google Scholar]
- Joshi, N.; Joshi, K. Exploring Plant-Microbial Interactions for Improved Crop Health and Growth. MSW Manag. J. 2023, 33, 292–304. [Google Scholar]
- Iqbal, B.; Li, G.; Alabbosh, K.F.; Hussain, H.; Khan, I.; Tariq, M.; Javed, Q.; Naeem, M.; Ahmad, N. Advancing environmental sustainability through microbial reprogramming in growth improvement, stress alleviation, and phytoremediation. Plant Stress 2023, 10, 100283. [Google Scholar] [CrossRef]
- Calanca, P.P. Effects of abiotic stress in crop production. In Quantification of Climate Variability, Adaptation and Mitigation for Agricultural Sustainability; Springer International Publishing: Cham, Switzerland, 2017; pp. 165–180. [Google Scholar]
- Kumar, L.; Chhogyel, N.; Gopalakrishnan, T.; Hasan, M.K.; Jayasinghe, S.L.; Kariyawasam, C.S.; Kogo, B.K.; Ratnayake, S. Climate change and future of agri-food production. In Future Foods; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–79. [Google Scholar]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Chaturvedi, V.; Gupta, S. Climate change and abiotic stress-induced oxidative burst in rice. In Advances in Rice Research for Abiotic Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2019; pp. 505–535. [Google Scholar]
- Compant, S.; Cassan, F.; Kostić, T.; Johnson, L.; Brader, G.; Trognitz, F.; Sessitsch, A. Harnessing the plant microbiome for sustainable crop production. Nat. Rev. Microbiol. 2025, 23, 9–23. [Google Scholar] [CrossRef]
- Alzate Zuluaga, M.Y.; Fattorini, R.; Cesco, S.; Pii, Y. Plant-microbe interactions in the rhizosphere for smarter and more sustainable crop fertilization: The case of PGPR-based biofertilizers. Front. Microbiol. 2024, 15, 1440978. [Google Scholar] [CrossRef]
- Ali, S.; Akhtar, M.S.; Siraj, M.; Zaman, W. Molecular Communication of Microbial Plant Biostimulants in the Rhizosphere Under Abiotic Stress Conditions. Int. J. Mol. Sci. 2024, 25, 12424. [Google Scholar] [CrossRef]
- Zhang, W. Strategic Engineering of Synthetic Microbial Communities (SynComs) for Optimizing Plant Health and Yield in Agriculture. Mol. Microbiol. Res. 2024, 14, 3. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial biostimulants as response to modern agriculture needs: Composition, role and application of these innovative products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Fagunwa, O.E.; Olanbiwoninu, A.A. Accelerating the sustainable development goals through microbiology: Some efforts and opportunities. Access Microbiol. 2020, 2, e000112. [Google Scholar]
- Lal, R.; Bouma, J.; Brevik, E.; Dawson, L.; Field, D.J.; Glaser, B.; Hatano, R.; Hartemink, A.E.; Kosaki, T.; Lascelles, B.; et al. Soils and sustainable development goals of the United Nations: An International Union of Soil Sciences perspective. Geoderma Reg. 2021, 25, e00398. [Google Scholar] [CrossRef]
- Suman, A.; Govindasamy, V.; Ramakrishnan, B.; Aswini, K.; SaiPrasad, J.; Sharma, P.; Pathak, D.; Annapurna, K. Microbial community and function-based synthetic bioinoculants: A perspective for sustainable agriculture. Front. Microbiol. 2022, 12, 805498. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Sánchez-Bermúdez, M.; Del Pozo, J.C.; Pernas, M. Effects of combined abiotic stresses related to climate change on root growth in crops. Front. Plant Sci. 2022, 13, 918537. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of abiotic stress on crops. Sustain. Crop Prod. 2020, 3, 5–16. [Google Scholar]
- Rao, N.K.S.; Shivashankara, K.S.; Laxman, R.H. Abiotic Stress Physiology of Horticultural Crops; Springer: Berlin/Heidelberg, Germany, 2016; Volume 311. [Google Scholar]
- Hasanuzzaman, M.; Fujita, M.; Oku, H.; Nahar, K.; Hawrylak-Nowak, B. Plant Nutrients and Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Cavatte, P.C.; Martins, S.C.V.; Morais, L.E.; Silva, P.E.M.; DaMatta, F.M. The Physiology of Abiotic Stresses. In Plant Breeding for Abiotic Stress Tolerance; Fritsche-Neto, R., Borém, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 21–51. [Google Scholar]
- Koyro, H.-W.; Ahmad, P.; Geissler, N. Abiotic Stress Responses in Plants: An Overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- De Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, P.; Robinson, N. Stress Physiology: Abiotic Stresses. In Sugarcane: Physiology, Biochemistry, and Functional Biology; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 411–434. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Grant, O.M. Understanding and exploiting the impact of drought stress on plant physiology. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Springer: Berlin/Heidelberg, Germany, 2012; pp. 89–104. [Google Scholar]
- Impa, S.M.; Nadaradjan, S.; Jagadish, S.V.K. Drought stress induced reactive oxygen species and anti-oxidants in plants. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Springer: Berlin/Heidelberg, Germany, 2012; pp. 131–147. [Google Scholar]
- Vidal, C.; González, F.; Santander, C.; Pérez, R.; Gallardo, V.; Santos, C.; Aponte, H.; Ruiz, A.; Cornejo, P. Management of rhizosphere microbiota and plant production under drought stress: A Comprehensive Review. Plants 2022, 11, 2437. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Jabeen, M.; Ahmad, I. Pseudomonas azotoformans FAP5, a novel biofilm-forming PGPR strain, alleviates drought stress in wheat plant. Int. J. Environ. Sci. Technol. 2021, 18, 3855–3870. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Xing, Y.; Dao, J.; Zhao, D.; Li, Y.; Li, W.; Wang, Z. A meta-analysis on morphological, physiological and biochemical responses of plants with PGPR inoculation under drought stress. Plant Cell Environ. 2023, 46, 199–214. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.-J. Halotolerant rhizobacterial strains mitigate the adverse effects of NaCl stress in soybean seedlings. BioMed Res. Int. 2019, 2019, 9530963. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Keisham, M.; Mukherjee, S.; Bhatla, S.C. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.S. Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef] [PubMed]
- Soualiou, S.; Duan, F.; Li, X.; Zhou, W. Crop production under cold stress: An understanding of plant responses, acclimation processes, and management strategies. Plant Physiol. Biochem. 2022, 190, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Jahed, K.R.; Saini, A.K.; Sherif, S.M. Coping with the cold: Unveiling cryoprotectants, molecular signaling pathways, and strategies for cold stress resilience. Front. Plant Sci. 2023, 14, 1246093. [Google Scholar] [CrossRef]
- Ashraf, A.; Bano, A.; Ali, S.A. Characterisation of plant growth-promoting rhizobacteria from rhizosphere soil of heat-stressed and unstressed wheat and their use as bio-inoculant. Plant Biol. 2019, 21, 762–769. [Google Scholar] [CrossRef]
- Zhang, J.; Song, K.; Jin, F.; Jia, F.; Liang, J.; Wang, F.; Zhang, J. A novel strategy of artificially regulating plant rhizosphere microbial community to promote plant tolerance to cold stress. Sci. Total Environ. 2024, 949, 175184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Z.; Wang, L.; Yue, Y.; Wang, L.; Yang, X. The effects of plant growth-promoting rhizobacteria on plants under temperature stress: A meta-analysis. Rhizosphere 2023, 28, 100788. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, B.; Chakraborty, U. Plant growth promoting rhizobacteria protect wheat plants against temperature stress through antioxidant signalling and reducing chloroplast and membrane injury. J. Plant Growth Regul. 2018, 37, 1396–1412. [Google Scholar] [CrossRef]
- Thieringer, H.A.; Jones, P.G.; Inouye, M. Cold shock and adaptation. Bioessays 1998, 20, 49–57. [Google Scholar] [CrossRef]
- Thiagarajan, T.R.; Ames, R.N.; Ahmad, M.H. Response of cowpea (Vigna unguiculata) to inoculation with co-selected vesicular–arbuscular mycorrhizal fungi and Rhizobium strains in field trials. Can. J. Microbiol. 1992, 38, 573–576. [Google Scholar] [CrossRef]
- Maiti, A.; Erimban, S.; Daschakraborty, S. Extreme makeover: The incredible cell membrane adaptations of extremophiles to harsh environments. Chem. Commun. 2024, 60, 10280–10294. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Adl, S.M. Plant growth-promoting rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. In Phyto-Microbiome Stress Regulation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 147–203. [Google Scholar]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ahmed, W.; Yang, J.; Yao, X.; Zhang, J.; Wei, L.; Ji, G. Seed coat treatment by plant-growth-promoting rhizobacteria Lysobacter antibioticus 13–6 enhances maize yield and changes rhizosphere bacterial communities. Biol. Fertil. Soils 2023, 59, 317–331. [Google Scholar] [CrossRef]
- Aizaz, M.; Lubna; Jan, R.; Asaf, S.; Bilal, S.; Kim, K.-M.; AL-Harrasi, A. Regulatory dynamics of plant hormones and transcription factors under salt stress. Biology 2024, 13, 673. [Google Scholar] [CrossRef]
- Li, J.; Cao, X.; Jia, X.; Liu, L.; Cao, H.; Qin, W.; Li, M. Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front. Plant Sci. 2021, 12, 710093. [Google Scholar]
- Farhan, M.; Sathish, M.; Kiran, R.; Mushtaq, A.; Baazeem, A.; Hasnain, A.; Hakim, F.; Hasan Naqvi, S.A.; Mubeen, M.; Iftikhar, Y. Plant Nitrogen Metabolism: Balancing Resilience to Nutritional Stress and Abiotic Challenges. Phyton 2024, 93, 581–609. [Google Scholar] [CrossRef]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Zahid Nabi, S.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Dimkpa, C.; Adzawla, W.; Pandey, R.; Atakora, W.K.; Kouame, A.K.; Jemo, M.; Bindraban, P.S. Fertilizers for food and nutrition security in sub-Saharan Africa: An overview of soil health implications. Front. Soil Sci. 2023, 3, 1123931. [Google Scholar] [CrossRef]
- Rashmi, I.; Roy, T.; Kartika, K.; Pal, R.; Coumar, V.; Kala, S.; Shinoji, K. Organic and inorganic fertilizer contaminants in agriculture: Impact on soil and water resources. In Contaminants in Agriculture: Sources, Impacts and Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–41. [Google Scholar]
- Fontaine, S.; Abbadie, L.; Aubert, M.; Barot, S.; Bloor, J.M.; Derrien, D.; Duchene, O.; Gross, N.; Henneron, L.; Le Roux, X.; et al. Plant–soil synchrony in nutrient cycles: Learning from ecosystems to design sustainable agrosystems. Glob. Change Biol. 2024, 30, e17034. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mazahar, S.; Chapadgaonkar, S.S.; Giri, P.; Shourie, A. Phyto-microbiome to mitigate abiotic stress in crop plants. Front. Microbiol. 2023, 14, 1210890. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.M.; Florentine, S.; Mahmood, A.; Wasaya, A.; Javed, T.; Sattar, A.; Sarwar, N.; Kalaji, H.M.; Ahmad, H.B.; Worbel, J.; et al. Interactive effect of elevated CO2 and drought on physiological traits of Datura stramonium. Front. Plant Sci. 2022, 13, 929378. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef]
- Jayaprakashvel, M.; Chitra, C.; Mathivanan, N. Metabolites of plant growth-promoting rhizobacteria for the management of soilborne pathogenic fungi in crops. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 293–315. [Google Scholar]
- Ortiz, N.; Armada, E.; Duque, E.; Roldán, A.; Azcón, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015, 174, 87–96. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Santoyo, G.; Carmen, O.-M.M.d.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The Rules of Engagement in the Legume-Rhizobial Symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Remus-Emsermann, M.N.P.; Schlechter, R.O. Phyllosphere microbiology: At the interface between microbial individuals and the plant host. New Phytol. 2018, 218, 1327–1333. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Park, S.; Mir, R.A.; Mushtaq, M.; Bhat, B.; Mahmoudi, H.; Bae, H. Deciphering the plant microbiome to improve drought tolerance: Mechanisms and perspectives. Environ. Exp. Bot. 2022, 201, 104933. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Saharan, B.S.; Brar, B.; Duhan, J.S.; Kumar, R.; Marwaha, S.; Rajput, V.D.; Minkina, T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life 2022, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells 2021, 10, 1551. [Google Scholar] [CrossRef]
- Bhagat, N.; Raghav, M.; Dubey, S.; Bedi, N. Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. J. Microbiol. Biotechnol. 2021, 31, 1045. [Google Scholar] [CrossRef]
- Dimkpa, C.; Weinand, T.; Asch, F. Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ. 2009, 32, 1682–1694. [Google Scholar] [CrossRef]
- Selvakumar, G.; Panneerselvam, P.; Ganeshamurthy, A.N. Bacterial Mediated Alleviation of Abiotic Stress in Crops. In Bacteria in Agrobiology: Stress Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 205–224. [Google Scholar]
- Pattnaik, S.; Mohapatra, B.; Gupta, A. Plant growth-promoting microbe mediated uptake of essential nutrients (Fe, P, K) for crop stress management: Microbe–soil–plant continuum. Front. Agron. 2021, 3, 689972. [Google Scholar] [CrossRef]
- Imran, A.; Hakim, S.; Tariq, M.; Nawaz, M.S.; Laraib, I.; Gulzar, U.; Hanif, M.K.; Siddique, M.J.; Hayat, M.; Fraz, A.; et al. Diazotrophs for Lowering Nitrogen Pollution Crises: Looking Deep Into the Roots. Front. Microbiol. 2021, 12, 637815. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Andrews, M.E. Specificity in Legume-Rhizobia Symbioses. Int. J. Mol. Sci. 2017, 18, 705. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.N.; Islam, N.F.; Sarma, B.; Nath, B.C.; Al-Ani, L.K.T.; Lesueur, D. Frankia-actinorhizal symbiosis: A non-chemical biological assemblage for enhanced plant growth, nodulation and reclamation of degraded soils. Symbiosis 2024, 92, 1–26. [Google Scholar] [CrossRef]
- Vassileva, M.; Mendes, G.d.O.; Deriu, M.A.; Benedetto, G.d.; Flor-Peregrin, E.; Mocali, S.; Martos, V.; Vassilev, N. Fungi, P-solubilization, and plant nutrition. Microorganisms 2022, 10, 1716. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Chauhan, P.K.; Upadhyay, S.K.; Singh, R.K.; Dwivedi, P.; Wang, J.; Jain, D.; Jiang, M. Mechanistic insights and potential use of siderophores producing microbes in rhizosphere for mitigation of stress in plants grown in degraded land. Front. Microbiol. 2022, 13, 898979. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. How Do Plant Growth-Promoting Bacteria Use Plant Hormones to Regulate Stress Reactions? Plants 2024, 13, 2371. [Google Scholar] [CrossRef]
- Kang, J.; Peng, Y.; Xu, W. Crop root responses to drought stress: Molecular mechanisms, nutrient regulations, and interactions with microorganisms in the rhizosphere. Int. J. Mol. Sci. 2022, 23, 9310. [Google Scholar] [CrossRef]
- Quintas-Nunes, F.; Brandão, P.R.; Barreto Crespo, M.T.; Glick, B.R.; Nascimento, F.X. Plant growth promotion, phytohormone production and genomics of the rhizosphere-associated microalga, Micractinium rhizosphaerae sp. nov. Plants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; González-Andrés, F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biotechnol. 2021, 105, 8629–8645. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, A.P.; Chakraborty, R. Understanding the potential of root microbiome influencing salt-tolerance in plants and mechanisms involved at the transcriptional and translational level. Physiol. Plant. 2021, 173, 1657–1681. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Zhao, P.; Li, P.; Wu, S.; Zhou, M.; Zhi, R.; Gao, H. Volatile organic compounds (VOCs) from Bacillus subtilis CF-3 reduce anthracnose and elicit active defense responses in harvested litchi fruits. AMB Express 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Harish; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Castañeda, T.; Lynch, J.P.; Six, J.; Hartmann, M. Improving soil resource uptake by plants through capitalizing on synergies between root architecture and anatomy and root-associated microorganisms. Front. Plant Sci. 2022, 13, 827369. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a key player in sustainable agriculture and human health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Phurailatpam, L.; Dalal, V.K.; Singh, N.; Mishra, S. Heavy metal stress alleviation through omics analysis of soil and plant microbiome. Front. Sustain. Food Syst. 2022, 5, 817932. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Khan, M.S.; Rajput, V.D.; Umar, S.; Minkina, T.; Lee, J. Maize associated bacterial microbiome linked mitigation of heavy metal stress: A multidimensional detoxification approach. Environ. Exp. Bot. 2022, 200, 104911. [Google Scholar] [CrossRef]
- Tsyganov, V.E.; Tsyganova, A.V.; Gorshkov, A.P.; Seliverstova, E.V.; Kim, V.E.; Chizhevskaya, E.P.; Belimov, A.A.; Serova, T.A.; Ivanova, K.A.; Kulaeva, O.A.; et al. Efficacy of a Plant-Microbe System: Pisum sativum (L.) Cadmium-Tolerant Mutant and Rhizobium leguminosarum Strains, Expressing Pea Metallothionein Genes PsMT1 and PsMT2, for Cadmium Phytoremediation. Front. Microbiol. 2020, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Jurado, S.; Fuentes-Romero, F.; Ruiz-Sainz, J.-E.; Janczarek, M.; Vinardell, J.-M. Rhizobial Exopolysaccharides: Genetic Regulation of Their Synthesis and Relevance in Symbiosis with Legumes. Int. J. Mol. Sci. 2021, 22, 6233. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, Z.; Chen, Y.; Tang, J.; Zeng, L.; Peng, C.; Chen, L.; Wang, J. Unlocking soil revival: The role of sulfate-reducing bacteria in mitigating heavy metal contamination. Environ. Geochem. Health 2024, 46, 417. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V.; Anshumali. A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 170. [Google Scholar] [CrossRef]
- Juwarkar, A.A.; Yadav, S.K. Bioaccumulation and biotransformation of heavy metals. In Bioremediation Technology: Recent Advances; Springer: Berlin/Heidelberg, Germany, 2010; pp. 266–284. [Google Scholar]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of Heavy Metal Stress in Plants and Remediation of Soil by Rhizosphere Microorganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, S.; Prajapati, S.C.; Kumar, S.; Priyanka, K.; Saxena, R. Bioremediation of Arsenic metal from water and soil by Bacillus species—A review. J. Integr. Sci. Technol. 2025, 13, 1038. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sah, D.; Chakraborty, M.; Rai, J.P.N. Mechanism and application of bacterial exopolysaccharides: An advanced approach for sustainable heavy metal abolition from soil. Carbohydr. Res. 2024, 544, 109247. [Google Scholar] [CrossRef]
- Pal, A.; Bhattacharjee, S.; Saha, J.; Sarkar, M.; Mandal, P. Bacterial survival strategies and responses under heavy metal stress: A comprehensive overview. Crit. Rev. Microbiol. 2022, 48, 327–355. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; He, T.; Saleem, M. Signaling and Detoxification Strategies in Plant-Microbes Symbiosis under Heavy Metal Stress: A Mechanistic Understanding. Microorganisms 2023, 11, 69. [Google Scholar] [CrossRef]
- Gomes, A.F.R.; Almeida, M.C.; Sousa, E.; Resende, D.I.S.P. Siderophores and metallophores: Metal complexation weapons to fight environmental pollution. Sci. Total Environ. 2024, 932, 173044. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Singh, D. Role of Genetically Modified Microorganisms in Heavy Metal Bioremediation. In Advances in Environmental Biotechnology; Kumar, R., Sharma, A.K., Ahluwalia, S.S., Eds.; Springer: Singapore, 2017; pp. 197–214. [Google Scholar]
- Yuan, X.; Li, S.; Chen, J.; Yu, H.; Yang, T.; Wang, C.; Huang, S.; Chen, H.; Ao, X. Impacts of global climate change on agricultural production: A comprehensive review. Agronomy 2024, 14, 1360. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Iglesius, A.; Yang, X.-B.; Epstein, P.R.; Chivian, E. Climate change and extreme weather events-Implications for food production, plant diseases, and pests. Global Change & Human Health 2001, 2, 90–104. [Google Scholar]
- Trivedi, P.; Batista, B.D.; Bazany, K.E.; Singh, B.K. Plant–microbiome interactions under a changing world: Responses, consequences and perspectives. New Phytol. 2022, 234, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Jurburg, S.D.; Nunes, I.; Brejnrod, A.; Jacquiod, S.; Priemé, A.; Sørensen, S.J.; Van Elsas, J.D.; Salles, J.F. Legacy effects on the recovery of soil bacterial communities from extreme temperature perturbation. Front. Microbiol. 2017, 8, 1832. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Flooding and prolonged drought have differential legacy impacts on soil nitrogen cycling, microbial communities and plant productivity. Plant Soil 2018, 431, 371–387. [Google Scholar] [CrossRef]
- Aslam, M.M.; IDRIS, A.L.; Zhang, Q.; Xu, W.; KARANJA, J.K.; Wei, Y. Rhizosphere microbiomes can regulate plant drought tolerance. Pedosphere 2022, 32, 61–74. [Google Scholar] [CrossRef]
- Hanaka, A.; Ozimek, E.; Reszczyńska, E.; Jaroszuk-Ściseł, J.; Stolarz, M. Plant tolerance to drought stress in the presence of supporting bacteria and fungi: An efficient strategy in horticulture. Horticulturae 2021, 7, 390. [Google Scholar] [CrossRef]
- Jorquera, H.; Barraza, F.; Heyer, J.; Valdivia, G.; Schiappacasse, L.N.; Montoya, L.D. Indoor PM2.5 in an urban zone with heavy wood smoke pollution: The case of Temuco, Chile. Environ. Pollut. 2018, 236, 477–487. [Google Scholar] [CrossRef]
- Goulden, M.L.; McMillan, A.; Winston, G.; Rocha, A.; Manies, K.; Harden, J.W.; Bond-Lamberty, B. Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Glob. Change Biol. 2011, 17, 855–871. [Google Scholar] [CrossRef]
- Zeng, Q.; Hu, H.W.; Ge, A.H.; Xiong, C.; Zhai, C.C.; Duan, G.L.; Han, L.L.; Huang, S.Y.; Zhang, L.M. Plant–microbiome interactions and their impacts on plant adaptation to climate change. J. Integr. Plant Biol. 2025, 67, 826–844. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Yadav, A.N.; Kumar, V.; Singh, D.P.; Saxena, A.K. Beneficial plant-microbes interactions: Biodiversity of microbes from diverse extreme environments and its impact for crop improvement. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Volume 2: Microbial Interactions and Agro-Ecological Impacts; Springer: Berlin/Heidelberg, Germany, 2017; pp. 543–580. [Google Scholar]
- Misu, I.J.; Kayess, M.O.; Siddiqui, M.N.; Gupta, D.R.; Islam, M.N.; Islam, T. Microbiome Engineering for Sustainable Rice Production: Strategies for Biofertilization, Stress Tolerance, and Climate Resilience. Microorganisms 2025, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Arif, I.; Batool, M.; Schenk, P.M. Plant microbiome engineering: Expected benefits for improved crop growth and resilience. Trends Biotechnol. 2020, 38, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.H.; Baki, M.Z.I.; Ahmed, B.; Mostofa, M.G. Current, faltering, and future strategies for advancing microbiome-assisted sustainable agriculture and environmental resilience. New Crops 2024, 1, 100013. [Google Scholar] [CrossRef]
- Ahmed, V.; Verma, M.K.; Gupta, S.; Mandhan, V.; Chauhan, N.S. Metagenomic Profiling of Soil Microbes to Mine Salt Stress Tolerance Genes. Front. Microbiol. 2018, 9, 159. [Google Scholar] [CrossRef]
- Hempel, C.A.; Buchner, D.; Mack, L.; Brasseur, M.V.; Tulpan, D.; Leese, F.; Steinke, D. Predicting environmental stressor levels with machine learning: A comparison between amplicon sequencing, metagenomics, and total RNA sequencing based on taxonomically assigned data. Front. Microbiol. 2023, 14, 1217750. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C. Microbial modulation of hormone signaling, proteomic dynamics, and metabolomics in plant drought adaptation. Food Energy Secur. 2024, 13, 513. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Tani, E.; Papasotiropoulos, V.; Letsiou, S.; Gerakari, M.; Abraham, E.; Bebeli, P.J. Enhancing Abiotic Stress Resilience in Mediterranean Woody Perennial Fruit Crops: Genetic, Epigenetic, and Microbial Molecular Perspectives in the Face of Climate Change. Int. J. Mol. Sci. 2025, 26, 3160. [Google Scholar] [CrossRef]
- Tariq, A.; Guo, S.; Farhat, F.; Shen, X. Engineering Synthetic Microbial Communities: Diversity and Applications in Soil for Plant Resilience. Agronomy 2025, 15, 513. [Google Scholar] [CrossRef]
- Wang, Y.; Zafar, N.; Ali, Q.; Manghwar, H.; Wang, G.; Yu, L.; Ding, X.; Ding, F.; Hong, N.; Wang, G. CRISPR/Cas genome editing technologies for plant improvement against biotic and abiotic stresses: Advances, limitations, and future perspectives. Cells 2022, 11, 3928. [Google Scholar] [CrossRef]
- Nadarajah, K.K. Building Resilience: Engineering the Plant Microbiome for Biotic Stress Management. In Plant Microbiome and Biological Control: Emerging Trends and Applications; Mathur, P., Roy, S., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 59–84. [Google Scholar]
- Zhao, L.; Walkowiak, S.; Fernando, W.G.D. Artificial intelligence: A promising tool in exploring the phytomicrobiome in managing disease and promoting plant health. Plants 2023, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Abdi, G.; Patil, N.; Tendulkar, R.; Dhariwal, R.; Mishra, P.; Tariq, M.; Tarighat, M.A.; Jain, M.; Mudgal, G. Engineering Genomic Landscapes: Synthetic Biology Approaches in Genomic Rearrangement. In Advances in Genomics: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 227–264. [Google Scholar]
- De Haro, L.P. Biosecurity in the Age of Synthetic Biology; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Mikiciuk, G.; Miller, T.; Kisiel, A.; Cembrowska-Lech, D.; Mikiciuk, M.; Łobodzińska, A.; Bokszczanin, K. Harnessing Beneficial Microbes for Drought Tolerance: A Review of Ecological and Agricultural Innovations. Agriculture 2024, 14, 2228. [Google Scholar] [CrossRef]
- Schmitz, L.; Yan, Z.; Schneijderberg, M.; de Roij, M.; Pijnenburg, R.; Zheng, Q.; Franken, C.; Dechesne, A.; Trindade, L.M.; van Velzen, R.; et al. Synthetic bacterial community derived from a desert rhizosphere confers salt stress resilience to tomato in the presence of a soil microbiome. ISME J. 2022, 16, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- McCarty, N.S.; Ledesma-Amaro, R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019, 37, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.K.; Rahayu, F.; Alkahtani, A.M.; Alreshidi, M.A.; Yadav, K.K.; Fauziah, L.; Murianingrum, M.; Akhtar, N.; Mufidah, E.; Rahayu, D.M. Metagenomic profiling of rhizosphere microbiota: Unraveling the plant-soil dynamics. Physiol. Mol. Plant Pathol. 2024, 133, 102381. [Google Scholar] [CrossRef]
- Priya, P.; Aneesh, B.; Harikrishnan, K. Genomics as a potential tool to unravel the rhizosphere microbiome interactions on plant health. J. Microbiol. Methods 2021, 185, 106215. [Google Scholar] [CrossRef]
- Xu, J. Invited review: Microbial ecology in the age of genomics and metagenomics: Concepts, tools, and recent advances. Mol. Ecol. 2006, 15, 1713–1731. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Suh, D.H.; Lee, C.H.; Yoo, S.M.; Lee, S.Y.; Yoon, S.H. Heat-responsive and time-resolved transcriptome and metabolome analyses of Escherichia coli uncover thermo-tolerant mechanisms. Sci. Rep. 2020, 10, 17715. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhou, R.; Wang, X.; Dossa, K.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; Zhang, X. Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol. 2019, 19, 66. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.; Reddy, M.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Concha, C.; Doerner, P. The impact of the rhizobia–legume symbiosis on host root system architecture. J. Exp. Bot. 2020, 71, 3902–3921. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.J.; Bhople, B.S.; Kumar, A.; Erneste, H.; Emmanuel, B.; Singh, Y.N. Contribution of arbuscular mycorrhizal fungi (AM fungi) and rhizobium inoculation on crop growth and chemical properties of rhizospheric soils in high plants. IOSR-JAVS 2014, 7, 45–55. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial Consortia: Promising Probiotics as Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Akensous, F.-Z.; Anli, M.; Meddich, A. Biostimulants as innovative tools to boost date palm (Phoenix dactylifera L.) performance under drought, salinity, and heavy metal (Oid) s’ stresses: A concise review. Sustainability 2022, 14, 15984. [Google Scholar] [CrossRef]
- Aamir, M.; Rai, K.K.; Zehra, A.; Dubey, M.K.; Kumar, S.; Shukla, V.; Upadhyay, R.S. Microbial bioformulation-based plant biostimulants: A plausible approach toward next generation of sustainable agriculture. In Microbial Endophytes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–225. [Google Scholar]
- Abavisani, M.; Faraji, N.; Faraji, S.; Ebadpour, N.; Kesharwani, P.; Sahebkar, A. A comprehensive review on utilizing CRISPR/Cas system for microbiome modification. Biochem. Eng. J. 2024, 211, 109443. [Google Scholar] [CrossRef]
- Thankappan, S.; Binodh, A.K.; Kumar, P.R.; Kurien, S.; Narayanasamy, S.; Prabina, J.B.; Uthandi, S. Genome Editing of Plant Growth-Promoting Microbes (PGPM) Towards Developing Smart Bio-Formulations for Sustainable Agriculture: Current Trends and Perspectives. In Genome Editing in Bacteria (Part 2); Bentham Science: Sharjah, United Arab Emirates, 2024; pp. 106–149. [Google Scholar]
- Sabri, S.; Mustofa, M.K.; Fouad, M.; Mukherjee, S.; Fakruddin, M.; Shishir, M.A. Comprehensive Analysis of CRISPR-Cas Systems in Microbial and Their Multifaceted Applications. Microb. Bioact. 2024, 7, 1–11. [Google Scholar]
- Sudheer, S.; Bai, R.G.; Usmani, Z.; Sharma, M. Insights on engineered microbes in sustainable agriculture: Biotechnological developments and future prospects. Curr. Genom. 2020, 21, 321–333. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Anand, U.; Kumar, R.; Ghorai, M.; Aftab, T.; Jha, N.K.; Rajapaksha, A.U.; Bundschuh, J.; Bontempi, E.; Dey, A. Phytoremediation and sequestration of soil metals using the CRISPR/Cas9 technology to modify plants: A review. Environ. Chem. Lett. 2023, 21, 429–445. [Google Scholar] [CrossRef]
- Sahoo, S.; Routray, S.P.; Lenka, S.; Bhuyan, R.; Mohanty, J.N. CRISPR/Cas-Mediated functional gene editing for improvement in bioremediation: An emerging strategy. In Omics Insights in Environmental Bioremediation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 635–664. [Google Scholar]
- Ahmad, A.; Mustafa, G.; Rana, A.; Zia, A.R. Improvements in bioremediation agents and their modified strains in mediating environmental pollution. Curr. Microbiol. 2023, 80, 208. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Kong, X.; Zheng, S.; Bai, N.; Li, L.; Khan, M.H.U.; Fiaz, S.; Zhang, Z. Exploring plant-microbe interactions in adapting to abiotic stress under climate change: A review. Front. Plant Sci. 2024, 15, 1482739. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, H.-S. Interplay between Plants and Microbial Communities: Insights from Holobionts and Environmental Interactions. Phyton 2024, 93, 2519. [Google Scholar] [CrossRef]
- Chatterjee, D.; Saha, S. Response of soil properties and soil microbial communities to the projected climate change. In Advances in Crop Environment Interaction; Springer: Berlin/Heidelberg, Germany, 2018; pp. 87–136. [Google Scholar]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.; Parra-Saldívar, R. Soil carbon sequestration–An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y. The long-term relationship between microbial metabolism and greenhouse gases. Trends Microbiol. 2020, 28, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Gurung, R.; Camargo, A.F.; Fongaro, G.; Treichel, H.; Mainali, B.; Angove, M.J.; Ngo, H.H.; Guo, W.; Puadel, S.R. Nitrous oxide emission in altered nitrogen cycle and implications for climate change. Environ. Pollut. 2022, 314, 120272. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.E.; Franzosa, E.A.; Everett, C.; Li, C.; Hu, F.B.; Wirth, D.F.; Song, M.; Chan, A.T. A framework for microbiome science in public health. Nat. Med. 2021, 27, 766–774. [Google Scholar] [CrossRef]
- Zaura, E.; Pappalardo, V.Y.; Buijs, M.J.; Volgenant, C.M.; Brandt, B.W. Optimizing the quality of clinical studies on oral microbiome: A practical guide for planning, performing, and reporting. Periodontology 2000 2021, 85, 210–236. [Google Scholar] [CrossRef]
- Afridi, M.S.; Javed, M.A.; Ali, S.; De Medeiros, F.H.V.; Ali, B.; Salam, A.; Sumaira; Marc, R.A.; Alkhalifah, D.H.M.; Selim, S. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 899464. [Google Scholar] [CrossRef]
- Clagnan, E.; Costanzo, M.; Visca, A.; Di Gregorio, L.; Tabacchioni, S.; Colantoni, E.; Sevi, F.; Sbarra, F.; Bindo, A.; Nolfi, L. Culturomics-and metagenomics-based insights into the soil microbiome preservation and application for sustainable agriculture. Front. Microbiol. 2024, 15, 1473666. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.; Wahab, A.; Waheed, A.; Mohamed, H.I.; Hakeem, K.R.; Li, L.; Li, W.-J. Harnessing bacterial endophytes for environmental resilience and agricultural sustainability. J. Environ. Manag. 2024, 368, 122201. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, A.; Malusà, E.; Costa, C.; Pallottino, F.; Mocali, S.; Pinzari, F.; Canfora, L. Current methods, common practices, and perspectives in tracking and monitoring bioinoculants in soil. Front. Microbiol. 2021, 12, 698491. [Google Scholar] [CrossRef] [PubMed]
- Pabar, S.A.; Kotroczó, Z.; Takács, T.; Biró, B. Evaluating the Efficacy of Selected Plant Growth-Promoting Microorganisms in Optimizing Plant Growth and Soil Health in Diverse Soil Types. Agriculture 2024, 14, 1586. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar]
- French, E.; Kaplan, I.; Iyer-Pascuzzi, A.; Nakatsu, C.H.; Enders, L. Emerging strategies for precision microbiome management in diverse agroecosystems. Nat. Plants 2021, 7, 256–267. [Google Scholar] [CrossRef]
- Khan, A.; Singh, A.V.; Gautam, S.S.; Agarwal, A.; Punetha, A.; Upadhayay, V.K.; Kukreti, B.; Bundela, V.; Jugran, A.K.; Goel, R. Microbial bioformulation: A microbial assisted biostimulating fertilization technique for sustainable agriculture. Front. Plant Sci. 2023, 14, 1270039. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Xiong, C.; Egidi, E.; Singh, B.K. Formulation challenges associated with microbial biofertilizers in sustainable agriculture and paths forward. J. Sustain. Agric. Environ. 2024, 3, e70006. [Google Scholar] [CrossRef]
- Maitra, S.; Brestic, M.; Bhadra, P.; Shankar, T.; Praharaj, S.; Palai, J.B.; Shah, M.M.R.; Barek, V.; Ondrisik, P.; Skalický, M. Bioinoculants—Natural biological resources for sustainable plant production. Microorganisms 2021, 10, 51. [Google Scholar] [CrossRef]
- Meena, R.P.; Jha, A. Conservation agriculture for climate change resilience: A microbiological perspective. In Microbes for Climate Resilient Agriculture; Wiley Online Books: Hoboken, NJ, USA, 2018; pp. 165–190. [Google Scholar]
- Siyanbola, K.F.; Ejiohuo, O.; Ade-adekunle, O.A.; Adekunle, F.O.; Onyeaka, H.; Furr, C.-L.L.; Hodges, F.E.; Carvalho, P.; Oladipo, E.K. Bacteriophages: Sustainable and effective solution for climate-resilient agriculture. Sustain. Microbiol. 2024, 1, qvae025. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Innocent, M.O.; Livinus, M.U.; Elelu, S.-A.; Ibrahim, G.O.; Lateefat, S.O.; Saheed, S.K.; Muhammad, A.S. Future Research of Soil Microbiomes and Green Technology Innovation for a Better Tomorrow. In Soil Microbiome in Green Technology Sustainability; Springer: Berlin/Heidelberg, Germany, 2024; pp. 569–585. [Google Scholar]
- Microbiome Support. Deliverable 3.4 Strategic Research and Innovation Agenda for Future Microbiome Activities and Applications; Microbiome Support: Vienna, Austria, 2020. [Google Scholar]
- Singh, V.; Rastogi, M. Future and Challenges of Microbiome Engineering. In Microbiome Engineering; CRC Press: Boca Raton, FL, USA, 2024; pp. 263–280. [Google Scholar]
- Meisner, A.; Kostic, T.; Vernooij, M.; Sessitsch, A.; Bunthof, C.J. The Global Microbiome Research Landscape: Mapping of Research, Infrastructures, Policies and Institutions in 2021; MicrobiomeSupport Consortium: Wageningen, The Netherlands, 2022. [Google Scholar]
- Kamilova, F.; Okon, Y.; de Weert, S.; Hora, K. Commercialization of microbes: Manufacturing, inoculation, best practice for objective field testing, and registration. In Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2014; pp. 319–327. [Google Scholar]
- Jagadesh, M.; Dash, M.; Kumari, A.; Singh, S.K.; Verma, K.K.; Kumar, P.; Bhatt, R.; Sharma, S.K. Revealing the hidden world of soil microbes: Metagenomic insights into plant, bacteria, and fungi interactions for sustainable agriculture and ecosystem restoration. Microbiol. Res. 2024, 285, 127764. [Google Scholar] [CrossRef] [PubMed]
- Igiehon, N.O.; Babalola, O.O. Rhizosphere microbiome modulators: Contributions of nitrogen fixing bacteria towards sustainable agriculture. Int. J. Environ. Res. Public Health 2018, 15, 574. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Kalhoro, M.T.; Faqir, Y.; Ma, J.; Osei, M.D.; Khaliq, G. Climate-resilient microbial biotechnology: A perspective on sustainable agriculture. Sustainability 2022, 14, 5574. [Google Scholar] [CrossRef]
- Bouri, M.; Taieb, K.H.; Bolouri, P.; Rhouma, A.; Şahin, F. Microbial Biostimulants for Improving Crop Nutrition, Health, and Productivity in Climate-Smart Agriculture. In Plant Holobiome Engineering for Climate-Smart Agriculture; Springer: Berlin/Heidelberg, Germany, 2024; pp. 235–255. [Google Scholar]
- Purohit, H.J.; Pandit, P.; Pal, R.; Warke, R.; Warke, G.M. Soil microbiome: An intrinsic driver for climate smart agriculture. J. Agric. Food Res. 2024, 18, 101433. [Google Scholar] [CrossRef]
- Benmrid, B.; Ghoulam, C.; Zeroual, Y.; Kouisni, L.; Bargaz, A. Bioinoculants as a means of increasing crop tolerance to drought and phosphorus deficiency in legume-cereal intercropping systems. Commun. Biol. 2023, 6, 1016. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Khan, I.M.; Shah, T.I.; Bangroo, S.A.; Kirmani, N.A.; Nazir, S.; Malik, A.R.; Aezum, A.M.; Mir, Y.H.; Hilal, A. Soil microbiome: A treasure trove for soil health sustainability under changing climate. Land 2022, 11, 1887. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Liu, S.; Xie, Z.; Chang, H.; Wu, T. Phytohormones-mediated strategies for mitigation of heavy metals toxicity in plants focused on sustainable production. Plant Cell Rep. 2024, 43, 99. [Google Scholar] [CrossRef]
- Mishra, H. Nanobiostimulants and Precision Agriculture: A Data-Driven Approach to Farming and Market Dynamics. In Nanobiostimulants: Emerging Strategies for Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2024; pp. 365–398. [Google Scholar]
- Yadav, A.; Yadav, K. Challenges and Opportunities in Biofertilizer Commercialization. SVOA Microbiol. 2024, 5, 1–14. [Google Scholar] [CrossRef]



| Abiotic Stress | Physiological Impact | References |
|---|---|---|
| Drought | Reduced water availability, stomatal closure, decreased photosynthesis, oxidative stress, and leaf wilting. | [38] |
| Salinity | Osmotic stress, ionic imbalance (Na+ and Cl− toxicity), reduced water uptake, impaired photosynthesis. | [39] |
| Extreme Heat | Protein denaturation, membrane fluidity disruption, increased transpiration, oxidative damage. | [40] |
| Extreme Cold | Membrane rigidification, reduced enzymatic activities, decreased photosynthesis. | [41] |
| Nutrient Deficiency | Limited chlorophyll production, impaired metabolic pathways, reduced growth and yield. | [42] |
| Waterlogging | Oxygen deprivation in roots, reduced nutrient uptake, increased ethylene production. | [43] |
| Microbial Mechanism | Description | References |
|---|---|---|
| Enhancing Nutrient Uptake | Microbes like mycorrhizal fungi and rhizobacteria improve nutrient availability (e.g., phosphorus and nitrogen) and facilitate uptake. | [8] |
| Phytohormone Modulation | Microbial production of auxins, gibberellins, and cytokinins regulates plant growth, while ACC deaminase-producing bacteria reduce ethylene stress. | [92] |
| Osmoprotectant Production | Bacteria produce osmolytes (proline, trehalose) to help plants maintain water balance under drought and salinity stress. | [93] |
| Inducing Systemic Tolerance (IST) | Rhizobacteria trigger plant defense responses, improving resistance to drought, salinity, and heat stress. | [94] |
| Exopolysaccharide (EPS) Production | Microbial EPS helps in water retention around plant roots, preventing desiccation under drought conditions. | [95] |
| Antioxidant Enzyme Activation | Microbes enhance the activity of superoxide dismutase (SOD) and catalase (CAT), reducing oxidative stress. | [96] |
| Heavy Metal Detoxification | Certain microbes sequester toxic heavy metals through biosorption, enhancing plant survival in contaminated soils. | [97] |
| Microbial Strategy | Description | Examples | References |
|---|---|---|---|
| Biosorption | Bacteria and fungi absorb and immobilize heavy metals through their cell walls, reducing metal toxicity in plants. | Pseudomonas putida efficiently removes cadmium (Cd) from contaminated soils. | [124] |
| Bioaccumulation | Microbes internalize heavy metals, preventing uptake by plants. | Bacillus subtilis accumulates arsenic (As), reducing its availability in rice fields. | [125] |
| Biotransformation | Enzymatic conversion of toxic metals into less harmful forms (e.g., reduction of Cr6+ to Cr3+). | Pseudomonas aeruginosa converts toxic Cr6+ to Cr3+, reducing its toxicity. | [126] |
| Exopolysaccharide (EPS) Production | Microbial EPS binds heavy metals, preventing their transport into plant tissues. | Azotobacter chroococcum produces EPS that binds Pb, Zn, and Cd, reducing plant uptake. | [127] |
| Metal Precipitation | Sulfate-reducing bacteria precipitate heavy metals as insoluble sulfides, limiting bioavailability. | Desulfovibrio desulfuricans reduces U6+ to insoluble U4+ in uranium-contaminated soils. | [128] |
| Rhizoremediation | Rhizobacteria enhance metal uptake and promote plant growth. | Rhizobium leguminosarum assists pea plants in lead (Pb) tolerance and uptake reduction. | [129] |
| Chelation & Siderophore Production | Microbial siderophores chelate heavy metals, reducing toxicity and promoting sequestration. | Pseudomonas fluorescens produces siderophores that bind iron (Fe) and lead (Pb). | [130] |
| Genetically Engineered Microbes | Engineered microbes enhance metal tolerance and detoxification in contaminated soils. | Escherichia coli engineered to express metallothioneins for cadmium (Cd) detoxification. | [131] |
| Tool/Technology | Description | Potential Application | References |
|---|---|---|---|
| Metagenomics | High-throughput sequencing to profile entire microbial communities in plant-associated environments. | Identifies beneficial microbes that enhance drought and salt stress tolerance. | [146] |
| Transcriptomics | RNA sequencing to analyze microbial gene expression under different stress conditions. | Determines microbial responses to environmental stressors, guiding microbiome engineering. | [147] |
| Metabolomics | Analyzes plant-microbe metabolite interactions to understand stress adaptation. | Identifies microbial metabolites that promote stress tolerance in plants. | [148] |
| Proteomics | Large-scale study of microbial and plant protein expression under stress conditions. | Identifies functional proteins and stress-responsive pathways involved in microbiome-mediated plant resilience. | [149] |
| Synthetic Microbial Communities (SynComs) | Assembles beneficial microbial consortia to improve plant resilience. | Enhances crop productivity by introducing beneficial microbiomes in degraded soils. | [150] |
| CRISPR-based Microbiome Editing | Uses CRISPR-Cas systems to engineer beneficial microbial strains for stress resistance. | Enhances microbial traits that help plants tolerate extreme environmental conditions. | [151] |
| Artificial Microbial Consortia | Designs microbial communities with specific plant-growth-promoting traits. | Introduces engineered microbes that increase plant stress tolerance. | [152] |
| Bioinformatics & Machine Learning | Uses computational models to predict plant-microbiome interactions. | Optimizes microbiome engineering strategies for targeted plant benefits. | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, F.; Ali, S.; Siraj, M.; Akhtar, M.S.; Zaman, W. Plant Microbiomes Alleviate Abiotic Stress-Associated Damage in Crops and Enhance Climate-Resilient Agriculture. Plants 2025, 14, 1890. https://doi.org/10.3390/plants14121890
Ullah F, Ali S, Siraj M, Akhtar MS, Zaman W. Plant Microbiomes Alleviate Abiotic Stress-Associated Damage in Crops and Enhance Climate-Resilient Agriculture. Plants. 2025; 14(12):1890. https://doi.org/10.3390/plants14121890
Chicago/Turabian StyleUllah, Fazal, Sajid Ali, Muhammad Siraj, Muhammad Saeed Akhtar, and Wajid Zaman. 2025. "Plant Microbiomes Alleviate Abiotic Stress-Associated Damage in Crops and Enhance Climate-Resilient Agriculture" Plants 14, no. 12: 1890. https://doi.org/10.3390/plants14121890
APA StyleUllah, F., Ali, S., Siraj, M., Akhtar, M. S., & Zaman, W. (2025). Plant Microbiomes Alleviate Abiotic Stress-Associated Damage in Crops and Enhance Climate-Resilient Agriculture. Plants, 14(12), 1890. https://doi.org/10.3390/plants14121890









