Effects of Different Management Practices on Ramet System Dynamics in Moso Bamboo (Phyllostachys edulis) Forests, China
Abstract
1. Introduction
2. Results
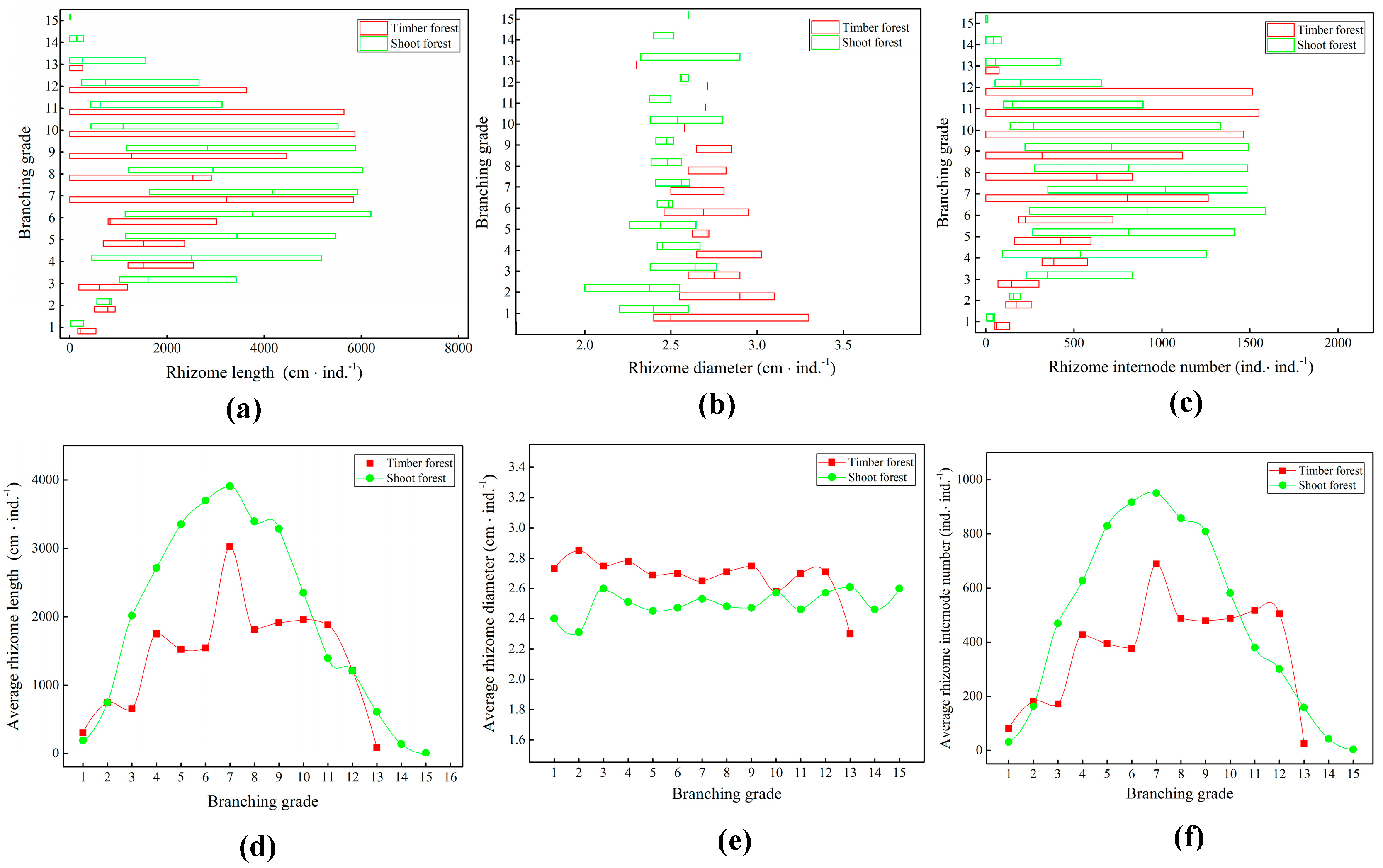
2.1. Growth of Underground Rhizomes of Bamboo Ramet Systems
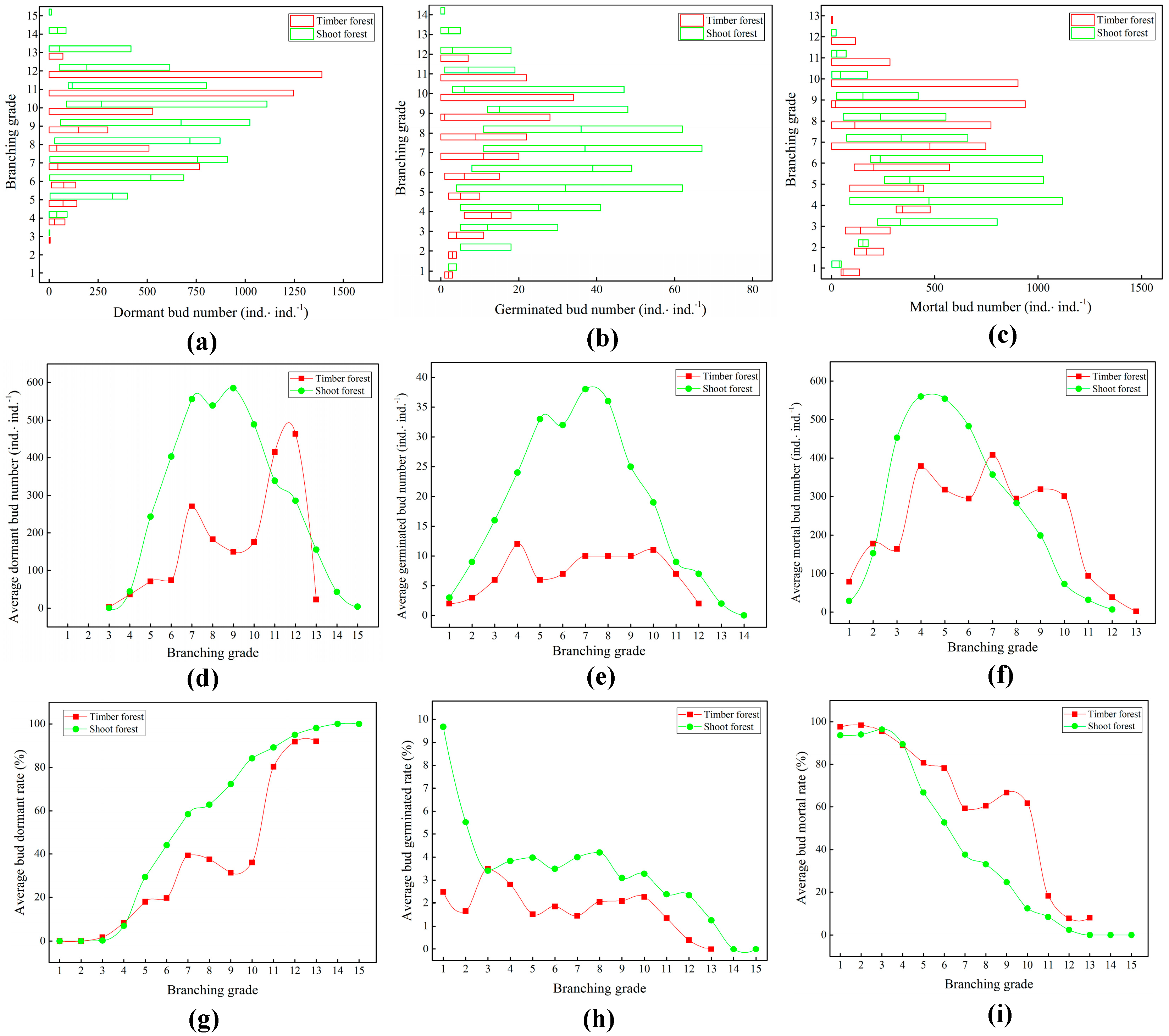
2.2. Structure of Underground Bud Banks of Bamboo Ramet Systems
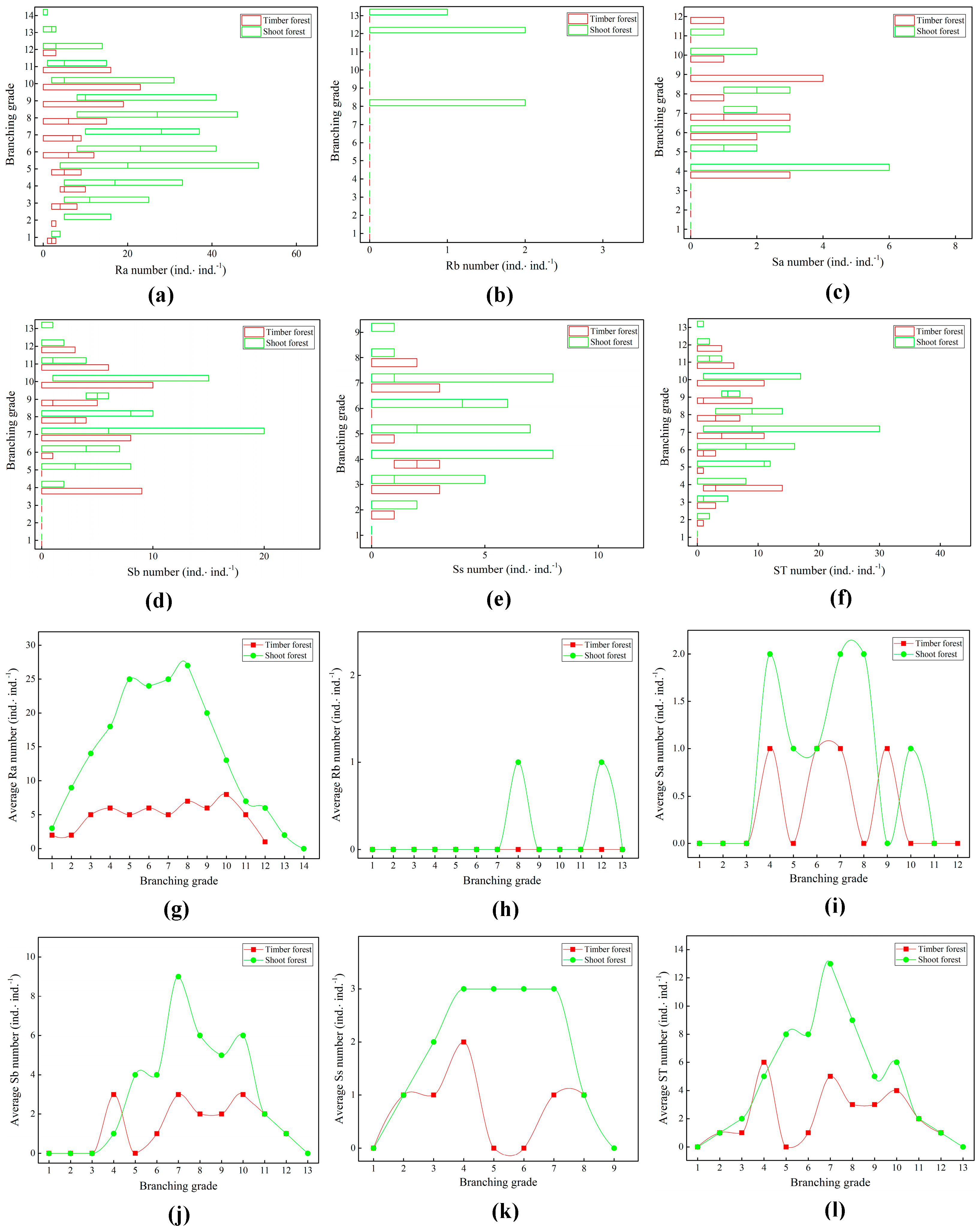
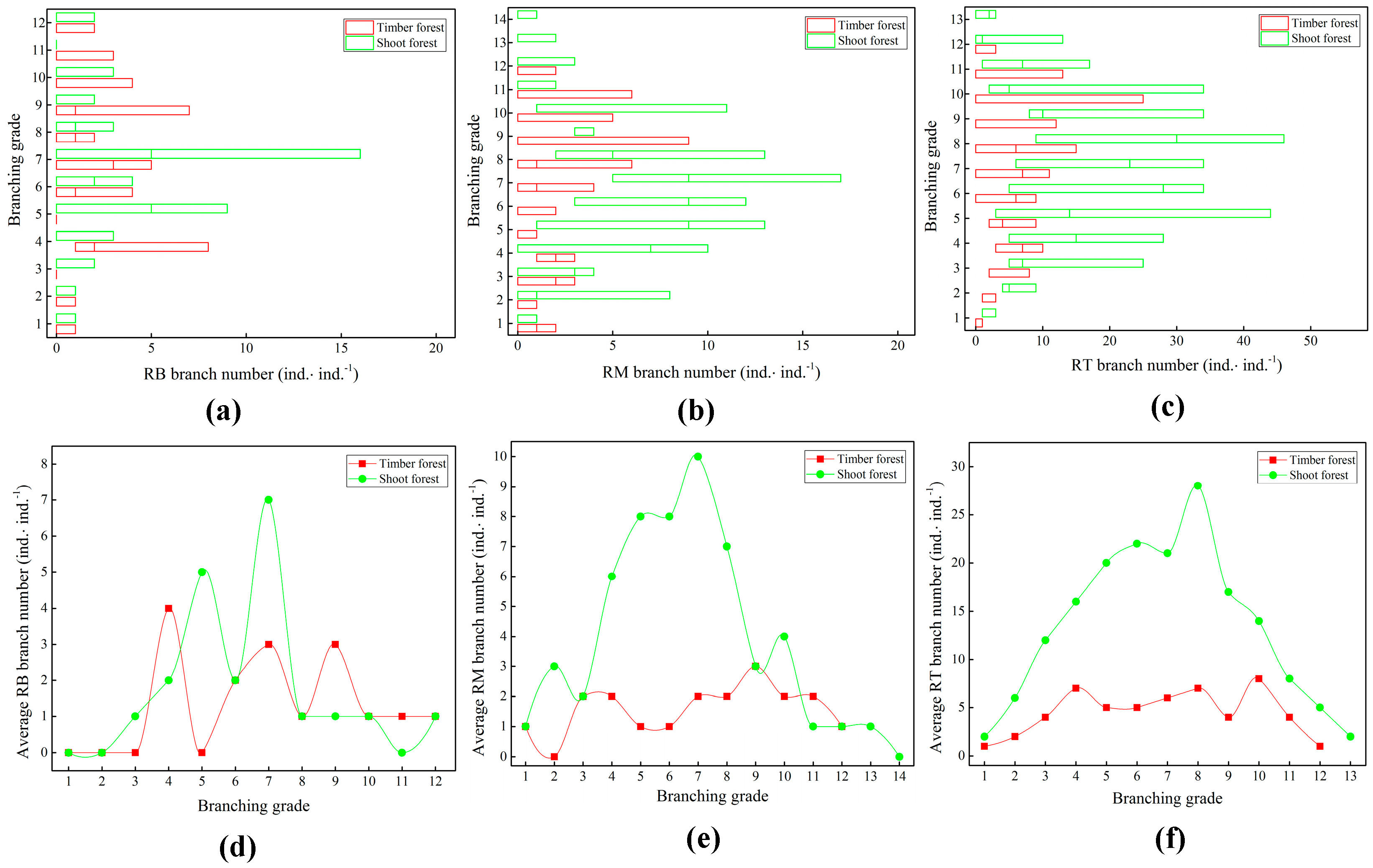
2.3. Branch Growth of Bamboo Ramet Systems
2.4. Branch Distribution Patterns in Bamboo Ramet Systems
3. Discussion
3.1. Influence of Different Management Methods on Underground Rhizome Morphology of Ramet Systems
3.2. Dynamic Relationship Between Bud Bank Structure and Ramet System Renewal
3.3. Responses of Branch Growth and Distribution of Ramet Systems to Artificial Management
4. Materials and Methods
4.1. Experimental Site
4.2. Investigation and Analysis
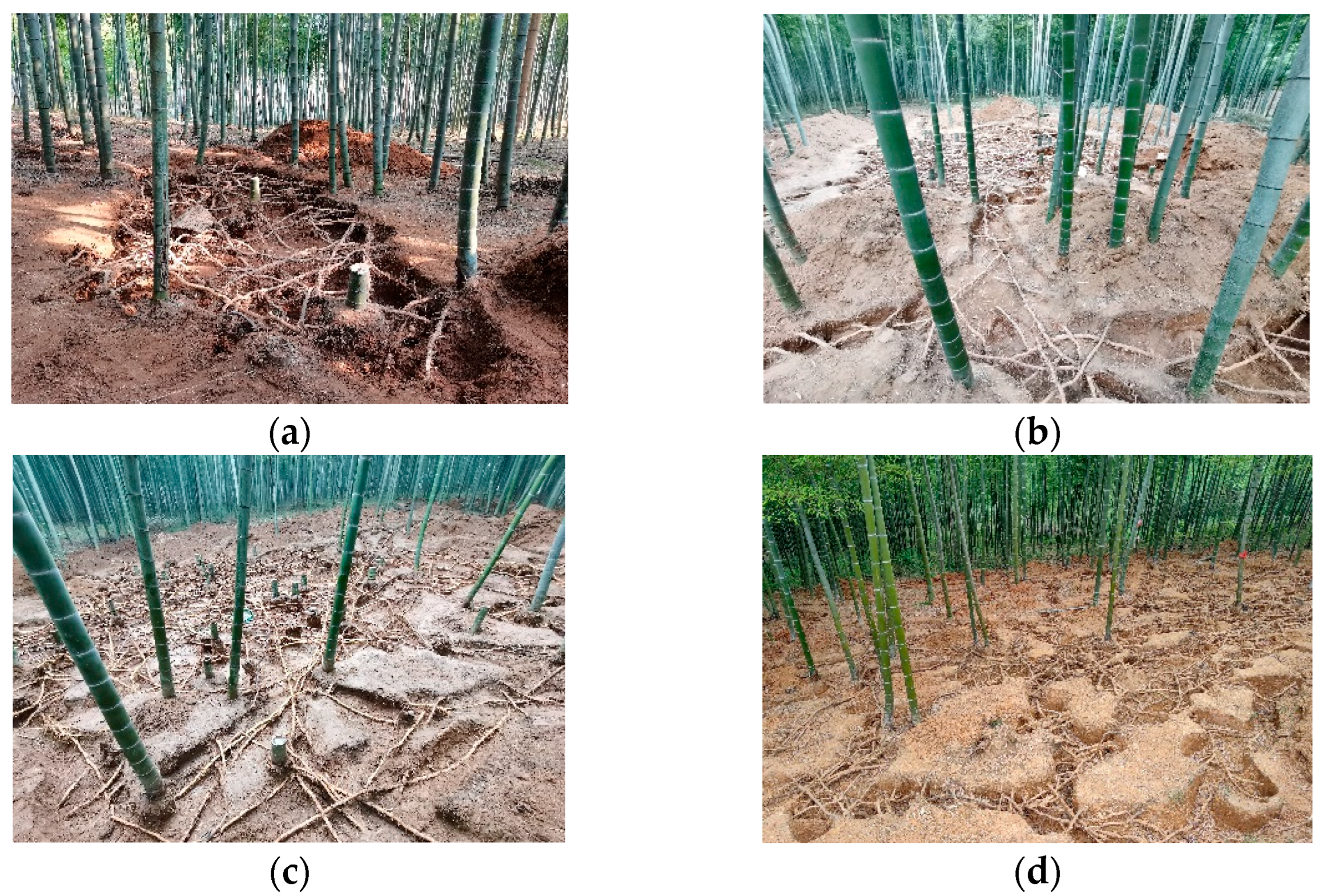
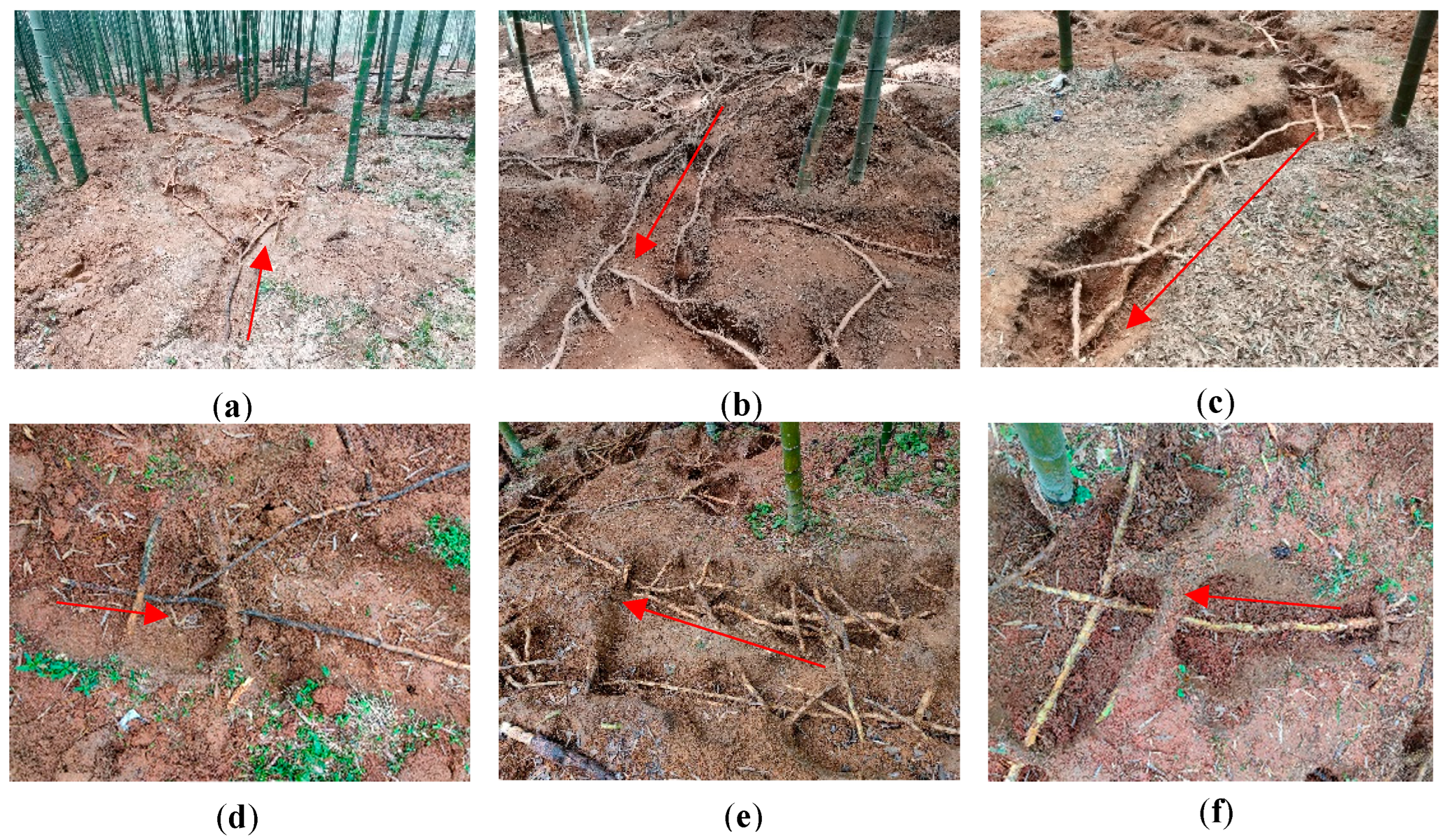
4.2.1. Excavating Ramet Systems by Opening Exposed Rhizome Pits
4.2.2. Investigation of Branch Growth of Bamboo Ramet Systems
4.2.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roiloa, S.R.; Xue, W.; Dong, B.C.; Yu, F.H. Ecological implications of plant clonality. Flora 2023, 309, 152420. [Google Scholar] [CrossRef]
- Franklin, S.; Alpert, P.; Salguero-Gómez, R.; Janovský, Z.; Herben, T.; Klimešová, J.; Douhovnikoff, V. Next-gen plant clonal ecology. Perspect. Plant Ecol. 2021, 49, 125601. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Baoyin, T.; Zhang, L.; Yuan, T. The Effects of different grazing periods on the functional traits of Leymus chinensis (Trin.) Tzvelev in a typical Inner Mongolia steppe. Agronomy 2024, 14, 2370. [Google Scholar] [CrossRef]
- Zhang, Y.; Nan, Z.; Christensen, M.J.; Xin, X.; Zhang, N. Effects of rust on plant growth and stoichiometry of Leymus chinensis under different grazing intensities in Hulunber grassland. Agriculture 2022, 12, 961. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, C.; Zhang, X.; Sun, K. Impacts of sand burial and wind erosion on regeneration and growth of a desert clonal shrub. Front. Plant Sci. 2018, 9, 1696. [Google Scholar] [CrossRef]
- Martin, F.M.; Dommanget, F.; Lavallée, F.; Evette, A. Clonal growth strategies of Reynoutria japonica in response to light, shade, and mowing, and perspectives for management. NeoBiota 2020, 56, 89–110. [Google Scholar] [CrossRef]
- Guo, Y.H.; Yuan, C.; Tang, L.; Peng, J.M.; Zhang, K.L.; Li, G.; Ma, X.J. Responses of clonal growth and photosynthesis in Amomum villosum to different light environments. Photosynthetica 2016, 54, 396–404. [Google Scholar] [CrossRef]
- Xing, Y.P.; Wei, G.W.; Luo, F.L.; Li, C.Y.; Dong, B.C.; Ji, J.S.; Yu, F.H. Effects of salinity and clonal integration on the amphibious plant Paspalum paspaloides: Growth, photosynthesis and tissue ion regulation. J. Plant Ecol. 2019, 12, 45–55. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Wu, M.; Liu, X.; Wang, X.; Yang, J.; Zhou, D.; Zhang, X. Clonal integration promotes the growth of Phragmites australis populations in saline wetlands of the Yellow River Delta. Front. Plant Sci. 2023, 14, 1162923. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Q.; Wu, J.; Yang, L.; Fan, L.; Zhang, L.; Qin, M.; Chen, S. Clonal integration alters metabolic non-structural carbohydrate processes of a dwarf bamboo under negatively correlated light and soil water conditions. Agr. Water Manag. 2024, 306, 109152. [Google Scholar] [CrossRef]
- Zou, Z.; Li, Y.; Song, H. Influence of the size of clonal fragment on the nitrogen turnover processes in a bamboo ecosystem. Front. Plant Sci. 2023, 14, 1308072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cai, C. Effects of physiological integration on nitrogen use efficiency of moso bamboo in homogeneous and heterogeneous environments. Front. Plant Sci. 2023, 14, 1203881. [Google Scholar] [CrossRef]
- Gao, G.; Wen, X.; Wu, Z.; Zhong, H.; Zhang, X. Deciphering the ramet system of a bamboo plant in response to intensive management. Forests 2022, 13, 1968. [Google Scholar] [CrossRef]
- Gao, G.; Wen, X.; Wu, Z.; Zhong, H.; Pan, Y.; Zhang, X. Growth characteristics of ramet system in Phyllostachys praecox forest under mulch management. Plants 2024, 13, 1761. [Google Scholar] [CrossRef]
- Gu, R.; Wei, S.; Li, J.; Zheng, S.; Li, Z.; Liu, G.; Fan, S. Predicting the impacts of climate change on the geographic distribution of moso bamboo in China based on biomod2 model. Eur. J. For. Res. 2024, 143, 1499–1512. [Google Scholar] [CrossRef]
- Paudyal, K.; Yanxia, L.; Long, T.T.; Adhikari, S.; Lama, S.; Bhatta, K.P. Ecosystem Services from Bamboo Forests: Key Findings, Lessons Learnt and Call for Actions from Global Synthesis; INBAR: Beijing, China, 2022. [Google Scholar]
- Fei, B. Economic value and research significance of Moso bamboo. In The Moso Bamboo Genome; Gao, J., Ed.; Springer International Publishing Press: Cham, Switzerland, 2021; pp. 1–12. [Google Scholar]
- Kim, J.H.; Choi, H.; Kim, J.G. Phenotypic and genetic variations of endangered Lysimachia maritima at global distribution limit. Glob. Ecol. Conserv. 2024, 51, e02910. [Google Scholar] [CrossRef]
- Pruchniewicz, D.; Żołnierz, L. Spatial aggregation and biometric variability of the grass Calamagrostis epigejos (L.) roth during different expansion stages in mesic mountain meadows. Int. J. Env. Res. Pub. He. 2022, 19, 7903. [Google Scholar] [CrossRef]
- Harris, T.; Kučerová, A.; Bitomský, M.; Bartušková, A.; Lubbe, F.C.; Klimešová, J. Capital and income breeders among herbs: How relative biomass allocation into a storage organ relates to clonal traits, phenology and environmental gradients. New Phytol. 2025, 245, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Adomako, M.O.; Xue, W.; Du, D.L.; Yu, F.H. Soil biota and soil substrates influence responses of the rhizomatous clonal grass Leymus chinensis to nutrient heterogeneity. Plant Soil 2021, 465, 19–29. [Google Scholar] [CrossRef]
- Klimešová, J.; Mudrák, O.; Martínková, J.; Lisner, A.; Lepš, J.; Filartiga, A.L.; Ottaviani, G. Are belowground clonal traits good predictors of ecosystem functioning in temperate grasslands? Funct. Ecol. 2021, 35, 787–795. [Google Scholar] [CrossRef]
- An, Y.; Gao, Y.; Tong, S.; Liu, B. Morphological and physiological traits related to the response and adaption of Bolboschoenus planiculmis seedlings grown under salt-alkaline stress conditions. Front. Plant Sci. 2021, 12, 567782. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Meckstroth, S.; Garai, J. The Amelioration of grazing through physiological integration by a clonal dune plant. Plants 2023, 12, 724. [Google Scholar] [CrossRef]
- Yang, C.; Shi, X.; Nan, J.; Huang, Q.; Shen, X.; Li, J. Morphological responses of the submerged macrophyte Vallisneria natans along an underwater light gradient: A mesocosm experiment reveals the importance of the Secchi depth to water depth ratio. Sci. Total Environ. 2022, 808, 152199. [Google Scholar] [CrossRef]
- Xu, T.; Abdullah, I.; Akram, N.A.; Wang, Y.; Zhang, L.; Feng, X.; Wang, J.; Wang, L. Defoliation facilitates Leymus chinensis clones spatial expansion into saline-alkali soils under different population densities. Flora 2022, 296, 152154. [Google Scholar] [CrossRef]
- Reijers, V.C.; Hoeks, S.; Van Belzen, J.; Siteur, K.; De Rond, A.J.A.; Van De Ven, C.N.; Lammers, C.; Van De Koppel, J.; Van Der Heide, T. Sediment availability provokes a shift from Brownian to Lévy-like clonal expansion in a dune building grass. Ecol. Lett. 2021, 24, 258–268. [Google Scholar] [CrossRef]
- Wu, J.; Hou, X.; Xu, L.; Zhou, Q.; Wang, Y.; Guo, Z.; Adomako, M.O.; Ma, Q. Belowground bud banks and land use change: Roles of vegetation and soil properties in mediating the composition of bud banks in different ecosystems. Front. Plant Sci. 2024, 14, 1330664. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Ye, D.; Gao, J.J.; Luo, F.L.; Zhu, Y.J.; Yu, F.H. Responses of belowground bud bank density of geophytes to environmental perturbations: A meta-analysis. J. Plant Ecol. 2024, 17, rtae029. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Ma, Q.; Liu, Z. Roles of aboveground vegetation, soil properties, and disturbance in determining belowground bud bank in sand dune ecosystems. Environ. Exp. Bot. 2020, 178, 104155. [Google Scholar] [CrossRef]
- Gao, G.; Wu, Z.; Xing, W.; Zhang, X.; Zhong, H.; Pan, Y. Bud population characteristics of Phyllostachys praecox ‘prevernalis’ under different mulching cultivation periods. Bangl. J. Bot. 2018, 47, 969–974. [Google Scholar] [CrossRef]
- Li, Y.; Bao, G.; Zhang, P.; Feng, X.; Ma, J.; Lu, H.; Shi, H.; Wei, X.; Tang, B.; Liu, K. Changes in bud bank and their correlation with plant community composition in degraded alpine meadows. Front. Plant Sci. 2023, 14, 1259340. [Google Scholar] [CrossRef]
- Yu, D.; Zheng, X.; Mu, C.; Wang, J. Irrigation and nitrogen application promote population density through altered bud bank size and components in Leymus chinensis. Agronomy 2022, 12, 1436. [Google Scholar] [CrossRef]
- Vanderlei, R.S.; Barros, M.F.; Dexter, K.G.; Tabarelli, M.; Santos, M.G. Human disturbances reduce tree abundance and stimulate woody plant resprouting and clonal growth in a tropical dry forest. Forest Ecol. Manag. 2024, 555, 121694. [Google Scholar] [CrossRef]
- Yang, P.; Huang, L.; He, S.; Zeng, X.; Chen, Y.; Wang, H. Adaptive strategies employed by clonal plants in heterogeneous patches. Forests 2023, 14, 1648. [Google Scholar] [CrossRef]
- Gao, G.; Zhong, H.; Wu, Z.; Li, N.; Zhong, Z.; Pan, Y.; Wu, L. Effects of key growth conditions on endogenous hormone content in tillering stem bases, germination of lateral buds, and biomass allocation in Indocalamus decorus. J. For. Res. 2018, 29, 1547–1555. [Google Scholar] [CrossRef]
- Zuo, K.; Fan, L.; Guo, Z.; Zhang, J.; Duan, Y.; Zhang, L.; Chen, S.; Lin, H.; Hu, R. Aboveground biomass component plasticity and allocation variations of bamboo (Pleioblastus amarus) of different regions. Forests 2024, 15, 43. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Li, P.; Hancock, J.T.; Hu, X. Research progress and application of plant branching. Phyton-Int. J. Exp. Bot. 2023, 92, 679–689. [Google Scholar] [CrossRef]
- Li, L.; Zhu, H.; Wu, T.; Wei, L.; Li, N. New landscape-perspective exploration of Moso bamboo forests under on/off-year phenomena and human activities. Front. For. Glob. Change 2023, 6, 1204329. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Chen, J.; Sun, L.; Hou, Y.; Wang, Y.; Wang, J.; Gan, J.; Barmukh, R.; Li, S.; et al. Dynamics of rhizosphere microbial structure and function associated with the biennial bearing of moso bamboo. J. Environ. Manag. 2024, 351, 119977. [Google Scholar] [CrossRef]
- Li, B.; Xu, L.; Chen, W.; Pan, Y.; He, T.; Chen, L.; Rong, J.; Zheng, Y. Variation in nitrogen utilization and nutrient composition across various organs under different strip logging management models in Moso bamboo (Phyllostachys edulis) forest. Plants 2024, 13, 1448. [Google Scholar] [CrossRef]

| Bamboo Forest Type | Bamboo Forest Structure | Bamboo Forest Basic Soil Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Diameter at Breast Height (cm) | Standing Density (Plants/ha) | pH Value | Total Nitrogen (g/kg−1) | Total Phosphorus (g⋅kg−1) | Hydrolyzable Nitrogen (mg⋅kg−1) | Available Potassium (mg⋅kg−1) | Organic Matter (g⋅kg−1) | Available Phosphorus (mg⋅kg−1) | |
| Timber forest | 12.10 ± 1.47 | 3611 ± 278 | 4.65 ± 0.09 | 1.29 ± 0.18 | 0.33 ± 0.02 | 108.00 ± 13.45 | 33.50 ± 8.43 | 25.70 ± 3.80 | 2.02 ± 0.21 |
| Shoot forest | 11.08 ± 1.12 | 3014 ± 285 | 4.83 ± 0.05 | 1.28 ± 0.17 | 0.23 ± 0.01 | 129.67 ± 24.79 | 67.97 ± 27.33 | 24.67 ± 4.19 | 3.31 ± 1.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, G.; Wen, X.; Qian, J.; Huang, Y.; Wu, Z.; Zhong, H.; Pan, Y.; Zhang, X. Effects of Different Management Practices on Ramet System Dynamics in Moso Bamboo (Phyllostachys edulis) Forests, China. Plants 2025, 14, 1835. https://doi.org/10.3390/plants14121835
Gao G, Wen X, Qian J, Huang Y, Wu Z, Zhong H, Pan Y, Zhang X. Effects of Different Management Practices on Ramet System Dynamics in Moso Bamboo (Phyllostachys edulis) Forests, China. Plants. 2025; 14(12):1835. https://doi.org/10.3390/plants14121835
Chicago/Turabian StyleGao, Guibin, Xing Wen, Jinfang Qian, Yiji Huang, Zhizhuang Wu, Hao Zhong, Yanhong Pan, and Xiaoping Zhang. 2025. "Effects of Different Management Practices on Ramet System Dynamics in Moso Bamboo (Phyllostachys edulis) Forests, China" Plants 14, no. 12: 1835. https://doi.org/10.3390/plants14121835
APA StyleGao, G., Wen, X., Qian, J., Huang, Y., Wu, Z., Zhong, H., Pan, Y., & Zhang, X. (2025). Effects of Different Management Practices on Ramet System Dynamics in Moso Bamboo (Phyllostachys edulis) Forests, China. Plants, 14(12), 1835. https://doi.org/10.3390/plants14121835






