Growth and Biomass Distribution Responses of Populus tomentosa to Long-Term Water–Nitrogen Coupling in the North China Plain
Abstract
1. Introduction
2. Results
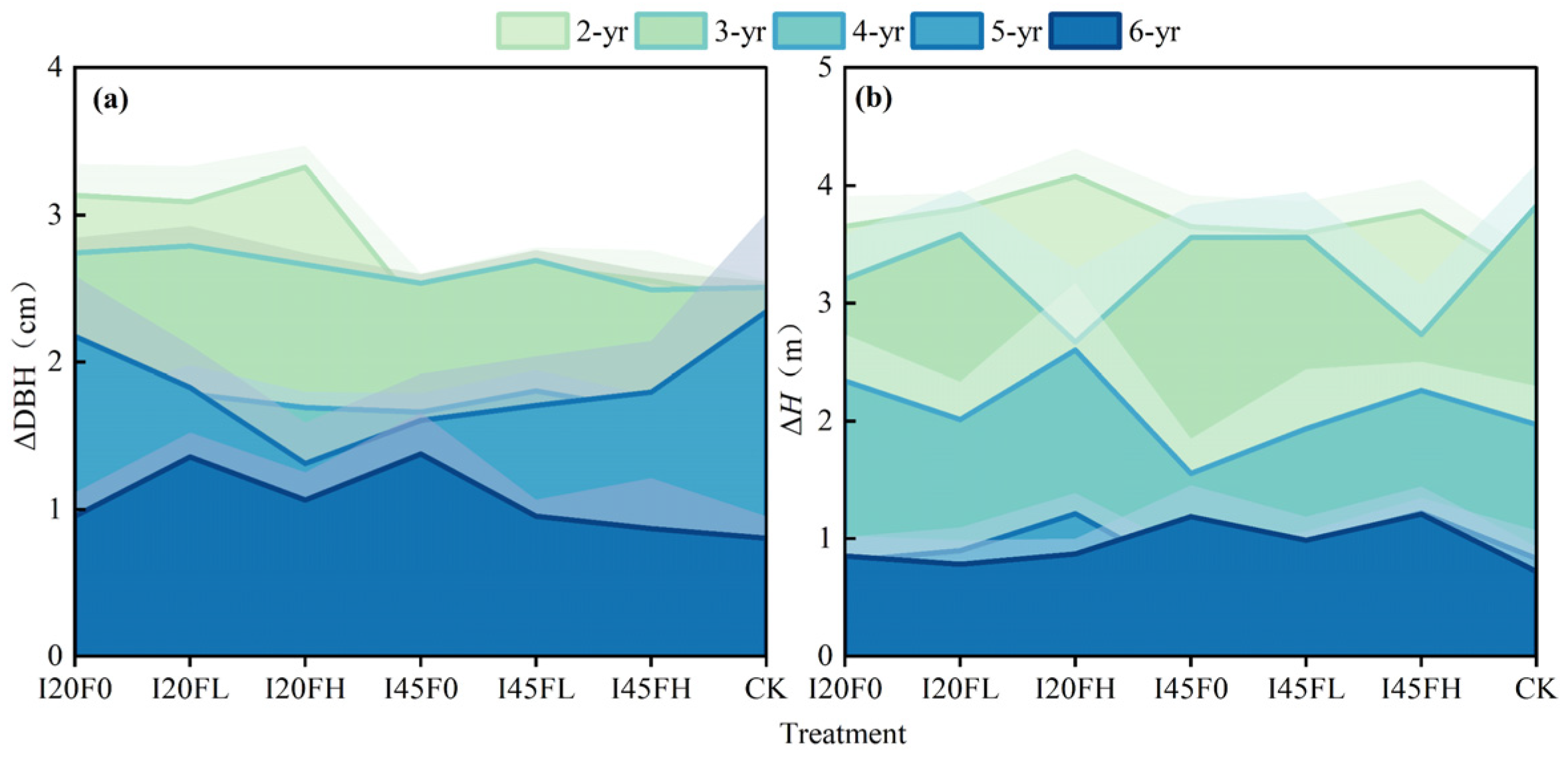
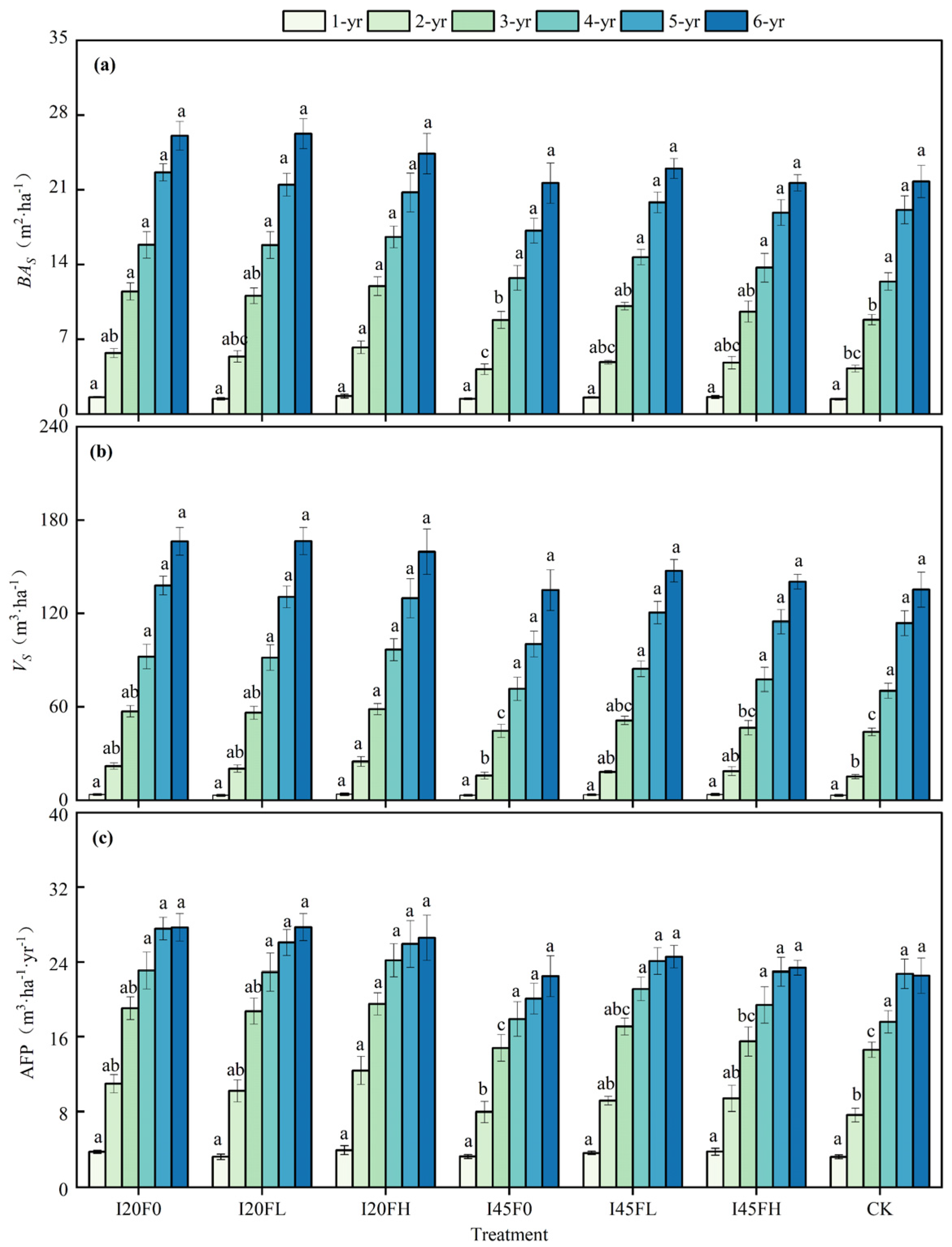
2.1. Long-Term Effects of Different Water–Nitrogen Coupling Treatments on Tree Growth
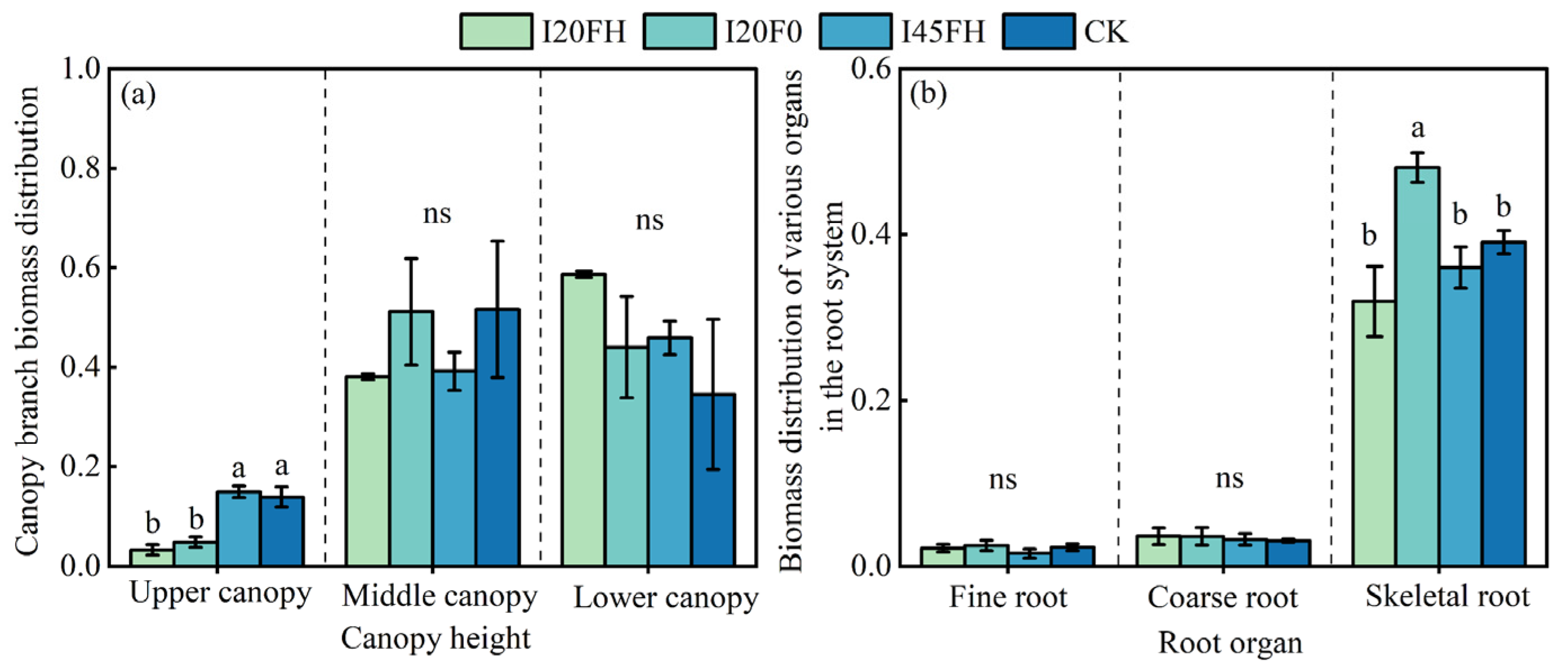
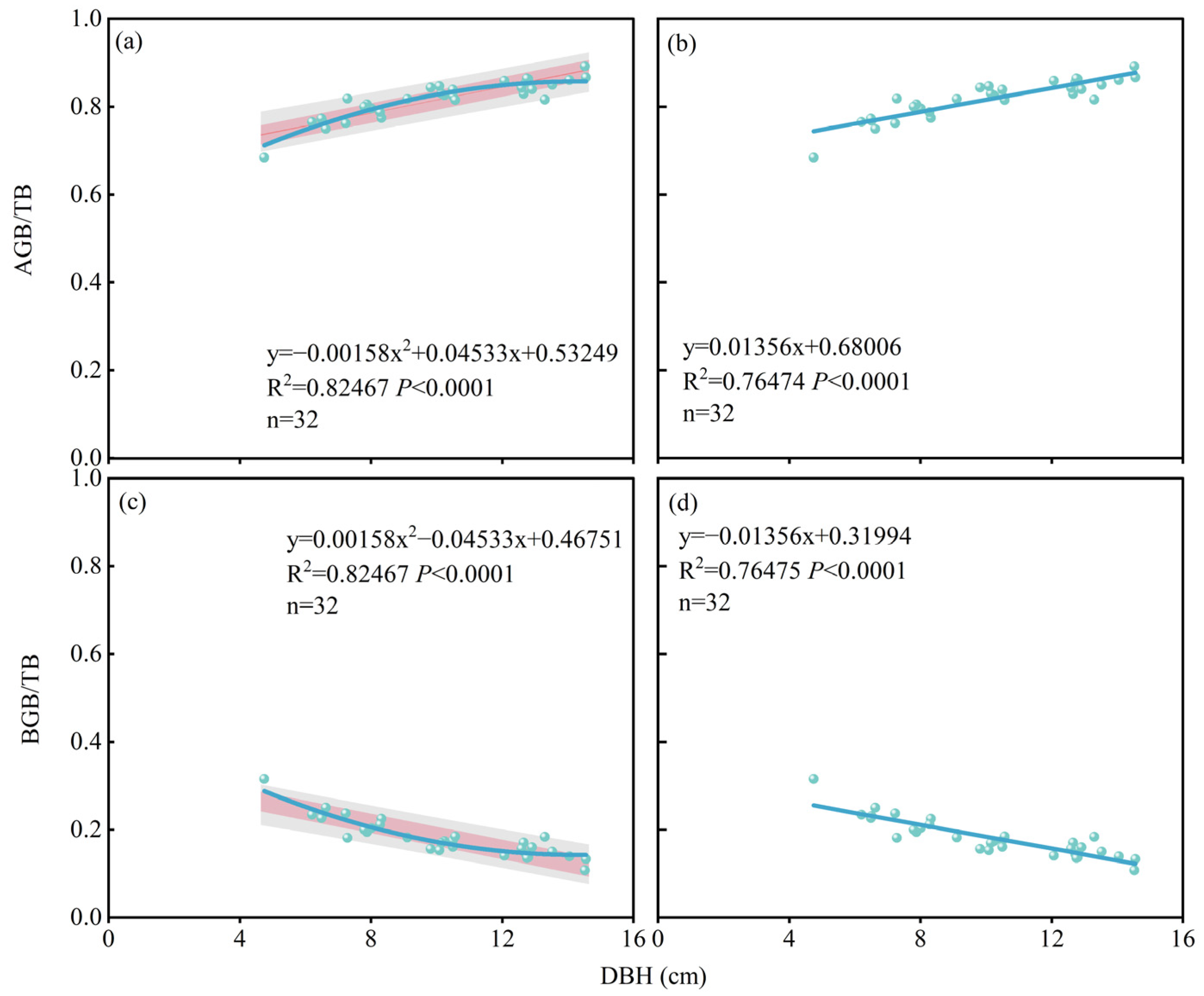
2.2. Long-Term Effects of Different Water–Nitrogen Coupling Treatments on Biomass Distribution Pattern
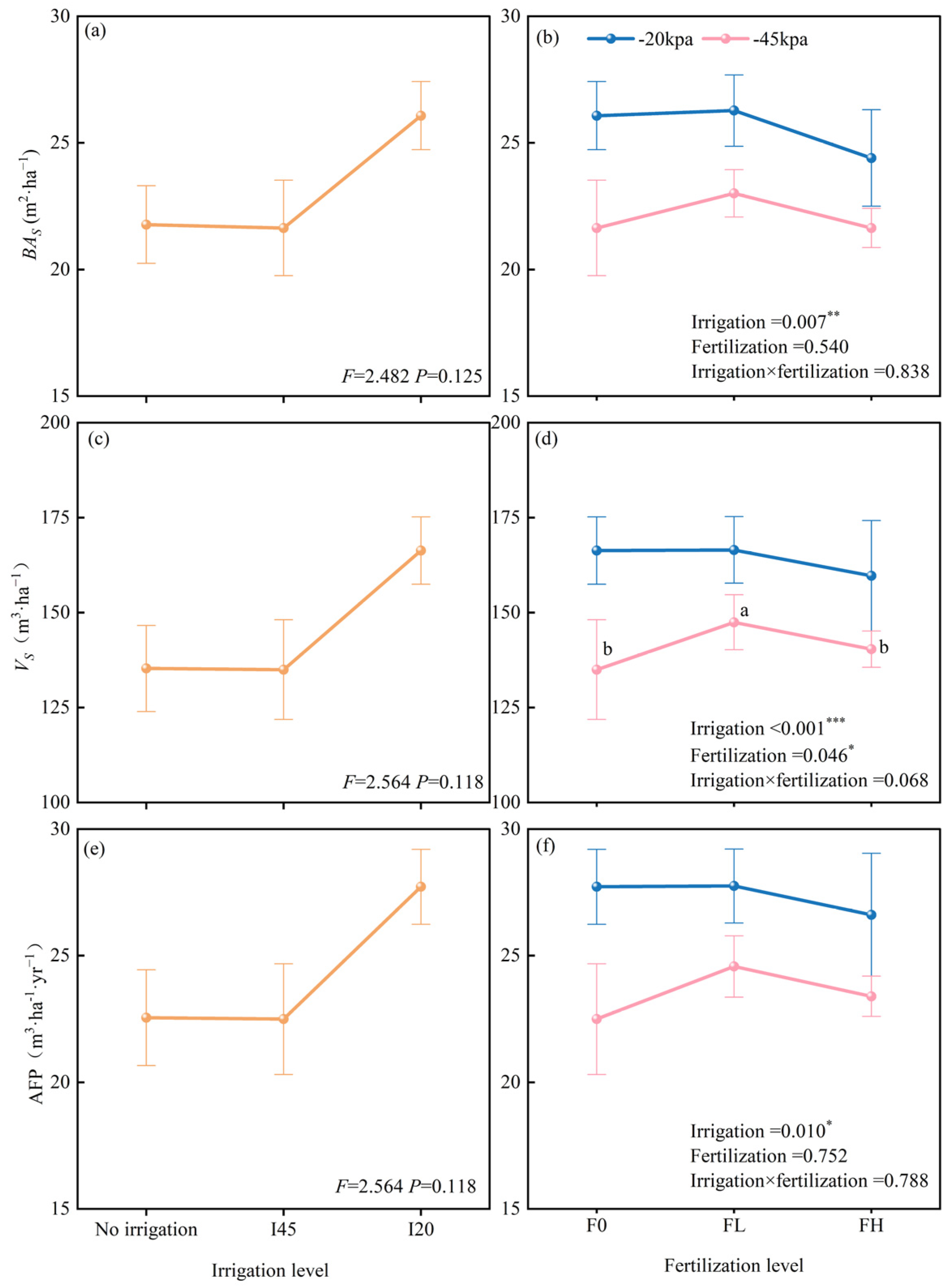
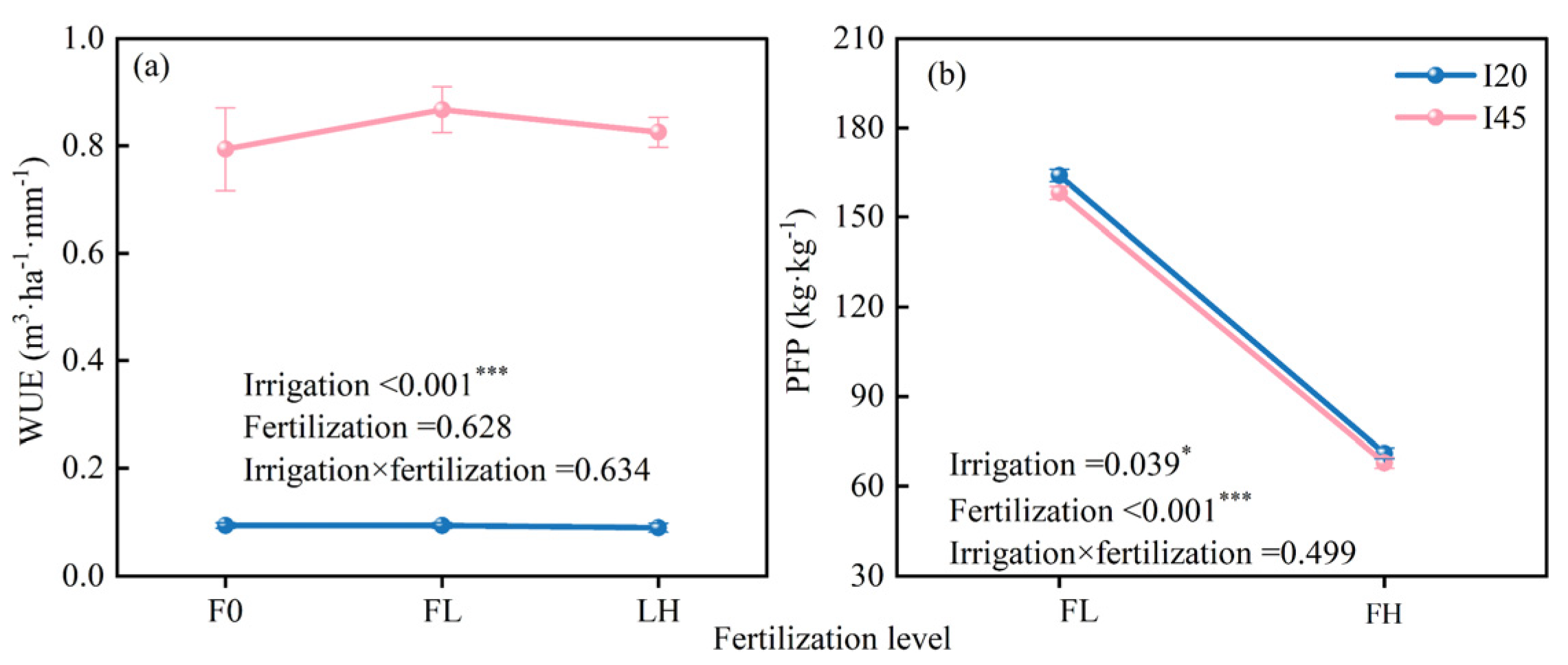
2.3. Long-Term Effects of Different Water–Nitrogen Coupling Treatments on WUE and PFP
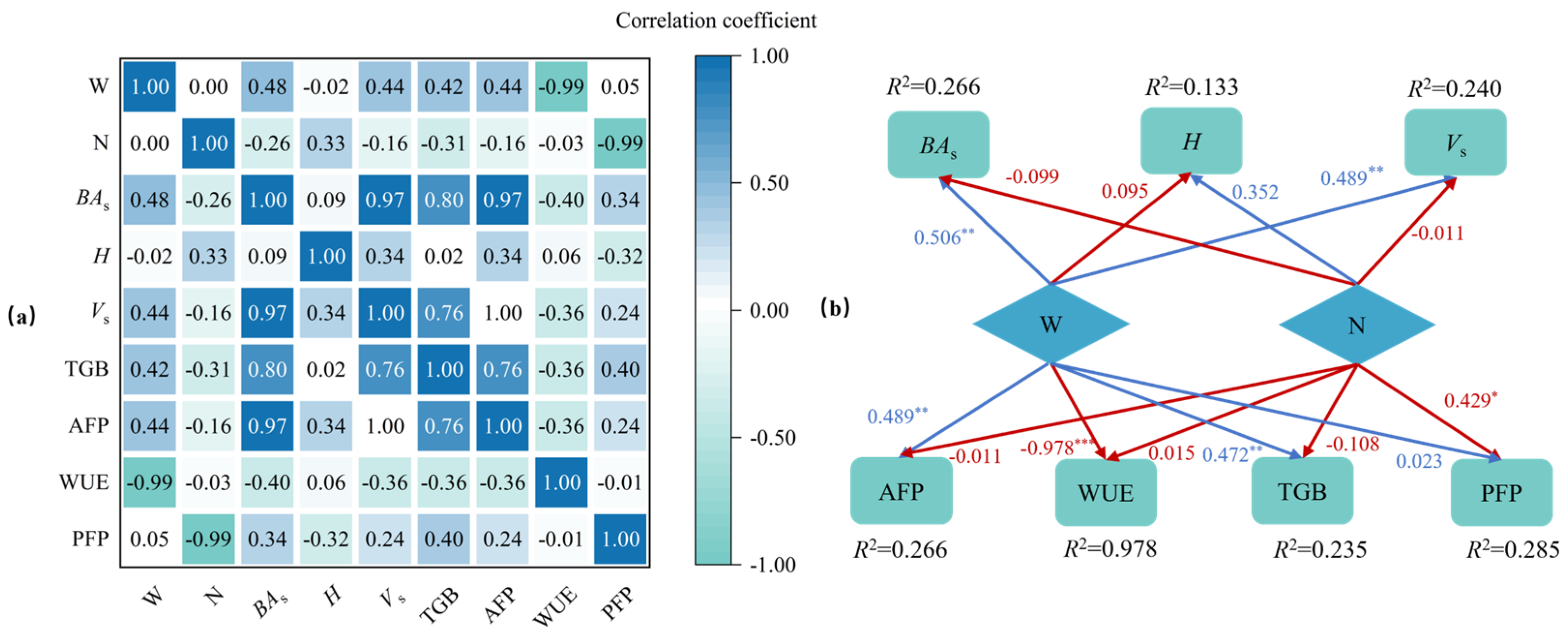
2.4. Correlation and Path Analysis
3. Discussion
3.1. Long-Term Effects of Water–Nitrogen Coupling on Tree Growth
3.2. Long-Term Effects of Water–Nitrogen Coupling on Biomass Allocation
3.3. Effects of Water–Nitrogen Coupling on WUE and PFP at the End of the Rotation Period
4. Materials and Methods
4.1. General Description of the Experimental Site
4.2. Experimental Design
4.3. Growth Measurement and Biomass Acquisition
4.3.1. Acquisition of Forest Growth Data
4.3.2. Acquisition and Calculation of Forest Biomass Data
4.3.3. Irrigation Water Use Efficiency and Nitrogen Fertilizer Partial Productivity
4.4. Statistics and Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warman, R.D. Global Wood Production from Natural Forests Has Peaked. Biodivers. Conserv. 2014, 23, 1063–1078. [Google Scholar] [CrossRef]
- Forrester, D.I. Growth Responses to Thinning, Pruning and Fertiliser Application in Eucalyptus Plantations: A Review of Their Production Ecology and Interactions. For. Ecol. Manag. 2013, 310, 336–347. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Qu, G.; Xi, B.; Jia, L. Cultivar and Tree Size, but Not Climate, Are Principal Factors Affecting Stem Quality of Populus Tomentosa Plantations in the North China Plain. For. Ecol. Manag. 2024, 573, 122348. [Google Scholar] [CrossRef]
- Gu, X.-B.; Li, Y.-N.; Du, Y.-D. Effects of Ridge-Furrow Film Mulching and Nitrogen Fertilization on Growth, Seed Yield and Water Productivity of Winter Oilseed Rape (Brassica napus L.) in Northwestern China. Agric. Water Manag. 2018, 200, 60–70. [Google Scholar] [CrossRef]
- Ahmed, A.K.M.; Jiang, L.; Wang, F.; Chen, S.; Zhou, X.; Pei, X.; Zhao, X.; Qu, G. Variation Analysis of Growth Traits of Four Poplar Clones under Different Water and Fertilizer Management. J. For. Res. 2020, 31, 45–55. [Google Scholar] [CrossRef]
- Fu, Y.; Ding, G.; Quan, W.; Zhao, X.; Tao, Q. Coupling Optimization of Water-Fertilizer for Coordinated Development of the Environment and Growth of Pinus Massoniana Seedlings. Agric. Water Manag. 2024, 300, 108895. [Google Scholar] [CrossRef]
- Coyle, D.R.; Coleman, M.D. Forest Production Responses to Irrigation and Fertilization Are Not Explained by Shifts in Allocation. For. Ecol. Manag. 2005, 208, 137–152. [Google Scholar] [CrossRef]
- He, Y.; Xi, B.; Li, G.; Wang, Y.; Jia, L.; Zhao, D. Influence of Drip Irrigation, Nitrogen Fertigation, and Precipitation on Soil Water and Nitrogen Distribution, Tree Seasonal Growth and Nitrogen Uptake in Young Triploid Poplar (Populus Tomentosa) Plantations. Agric. Water Manag. 2021, 243, 106460. [Google Scholar] [CrossRef]
- He, Y.; Xi, B.; Bloomberg, M.; Jia, L.; Zhao, D. Effects of Drip Irrigation and Nitrogen Fertigation on Stand Growth and Biomass Allocation in Young Triploid Populus Tomentosa Plantations. For. Ecol. Manag. 2020, 461, 117937. [Google Scholar] [CrossRef]
- Van Den Driessche, R.; Thomas, B.R.; Kamelchuk, D.P. Effects of N, NP, and NPKS Fertilizers Applied to Four-Year Old Hybrid Poplar Plantations. New For. 2008, 35, 221–233. [Google Scholar] [CrossRef]
- Rappe-George, M.O.; Gärdenäs, A.I.; Kleja, D.B. The Impact of Four Decades of Annual Nitrogen Addition on Dissolved Organic Matter in a Boreal Forest Soil. Biogeosci. Discuss. 2012, 9, 12433. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, D.; Ma, S.; Zhou, X.; Xu, L.; Zhu, J.; Jing, X.; Zheng, C.; Shen, H.; Zhou, Z.; et al. The Response of Tree Growth to Nitrogen and Phosphorus Additions in a Tropical Montane Rainforest. Sci. Total Environ. 2018, 618, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Fisk, M.C.; Vadeboncoeur, M.A.; Garrison-Johnston, M.; Yanai, R.D.; Fahey, T.J. Phosphorus Limitation of Aboveground Production in Northern Hardwood Forests. Ecology 2018, 99, 438–449. [Google Scholar] [CrossRef] [PubMed]
- DeForest, J.L.; Snell, R.S. Tree Growth Response to Shifting Soil Nutrient Economy Depends on Mycorrhizal Associations. New Phytol. 2020, 225, 2557–2566. [Google Scholar] [CrossRef]
- Nasto, M.K.; Winter, K.; Turner, B.L.; Cleveland, C.C. Nutrient Acquisition Strategies Augment Growth in Tropical N2 -fixing Trees in Nutrient-poor Soil and under Elevated CO2. Ecology 2019, 100, e02646. [Google Scholar] [CrossRef]
- Liu, W.; Duan, H.; Shen, F.; Liao, Y.; Li, Q.; Wu, J. Effects of Long-term Nitrogen Addition on Water Use by Cunninghamia Lanceolate in a Subtropical Plantation. Ecosphere 2022, 13, e4033. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.-S.; Bai, J.; Khan, A.; Zhao, L.; Wang, W.; Zhu, S.-G.; Liu, S.-T.; Jin, J.-M.; Nyanchera, G.D.; et al. Reduced Fertilization Boosts Soil Quality and Economic Benefits in Semiarid Apple Orchard: A Two-Year Appraisal of Fertigation Strategy. Agric. Water Manag. 2024, 295, 108766. [Google Scholar] [CrossRef]
- Zhang, R.; Nie, L.; Huang, M.; Yang, H.; Shi, C.; Wei, Y.; Song, L.; Zhu, J.; Bo, H.; Wang, J.; et al. Effects of Irrigation and Nitrogen Application on Soil Nutrients in Triploid Populus Tomentosa Stands. Forests 2022, 13, 1046. [Google Scholar] [CrossRef]
- Roa-Fuentes, L.L.; Campo, J.; Parra-Tabla, V. Plant Biomass Allocation across a Precipitation Gradient: An Approach to Seasonally Dry Tropical Forest at Yucatán, Mexico. Ecosystems 2012, 15, 1234–1244. [Google Scholar] [CrossRef]
- Wu, J.; Hong, J.; Wang, X.; Sun, J.; Lu, X.; Fan, J.; Cai, Y. Biomass Partitioning and Its Relationship with the Environmental Factors at the Alpine Steppe in Northern Tibet. PLoS ONE 2013, 8, e81986. [Google Scholar] [CrossRef]
- Bebre, I.; Marques, I.; Annighöfer, P. Biomass Allocation and Leaf Morphology of Saplings Grown under Various Conditions of Light Availability and Competition Types. Plants 2022, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Kara, F. Effects of Light Transmittance on Growth and Biomass of Understory Seedlings in Mixed Pine-Beech Forests. Eur. J. For. Res. 2022, 141, 1189–1200. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant Root-Shoot Biomass Allocation over Diverse Biomes: A Global Synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Zhang, H.; Song, T.; Wang, K.; Yang, H.; Yue, Y.; Zeng, Z.; Peng, W.; Zeng, F. Influences of Stand Characteristics and Environmental Factors on Forest Biomass and Root–Shoot Allocation in Southwest China. Ecol. Eng. 2016, 91, 7–15. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How Does Biomass Distribution Change with Size and Differ among Species? An Analysis for 1200 Plant Species from Five Continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef]
- Baret, M.; Pepin, S.; Ward, C.; Pothier, D. Long-Term Changes in Belowground and Aboveground Resource Allocation of Boreal Forest Stands. For. Ecol. Manag. 2015, 350, 62–69. [Google Scholar] [CrossRef]
- Byambadorj, S.-O.; Nyam-Osor, B.; Park, B.B.; Avirmed, T.; Scippa, G.S.; Chiatante, D.; Montagnoli, A.; Dimitrova, A. Afforestation of Mongolian Steppe: Patterns of Biomass Partitioning in Populus Sibirica and Ulmus Pumila Trees in Response to Management Supporting Measures. Plant Biosyst.-Int. J. Deal. All. Asp. Plant Biol. 2022, 156, 969–981. [Google Scholar] [CrossRef]
- Fang, S.; Xu, X.; Lu, S.; Tang, L. Growth Dynamics and Biomass Production in Short-Rotation Poplar Plantations: 6-Year Results for Three Clones at Four Spacings. Biomass Bioenergy 1999, 17, 415–425. [Google Scholar] [CrossRef]
- Lie, Z.; Xue, L.; Jacobs, D.F. Allocation of Forest Biomass across Broad Precipitation Gradients in China’s Forests. Sci. Rep. 2018, 8, 10536. [Google Scholar] [CrossRef]
- Wang, Y.; Xi, B.; Bloomberg, M.; Moltchanova, E.; Li, G.; Jia, L. Response of Diameter Growth, Biomass Allocation and N Uptake to N Fertigation in a Triploid Populus Tomentosa Plantation in the North China Plain: Ontogenetic Shift Does Not Exclude Plasticity. Eur. J. For. Res. 2015, 134, 889–898. [Google Scholar] [CrossRef]
- Wang, Q.-W.; Daumal, M.; Nagano, S.; Yoshida, N.; Morinaga, S.-I.; Hikosaka, K. Plasticity of Functional Traits and Optimality of Biomass Allocation in Elevational Ecotypes of Arabidopsis Halleri Grown at Different Soil Nutrient Availabilities. J. Plant Res. 2019, 132, 237–249. [Google Scholar] [CrossRef]
- Oliveira, N.; Rodríguez-Soalleiro, R.; Pérez-Cruzado, C.; Cañellas, I.; Sixto, H.; Ceulemans, R. Above- and below-Ground Carbon Accumulation and Biomass Allocation in Poplar Short Rotation Plantations under Mediterranean Conditions. For. Ecol. Manag. 2018, 428, 57–65. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature Drives Global Patterns in Forest Biomass Distribution in Leaves, Stems, and Roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef] [PubMed]
- Xi, B.; Clothier, B.; Coleman, M.; Duan, J.; Hu, W.; Li, D.; Di, N.; Liu, Y.; Fu, J.; Li, J.; et al. Irrigation Management in Poplar (Populus Spp.) Plantations: A Review. For. Ecol. Manag. 2021, 494, 119330. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Feng, Z.; Liu, Y.; Xu, Y.; Uscola, M. Uptake of Nitrogen Forms by Diploid and Triploid White Poplar Depends on Seasonal Carbon Use Strategy and Elevated Summer Ozone. J. Exp. Bot. 2021, 72, 7180–7190. [Google Scholar] [CrossRef]
- Coleman, M. Spatial and Temporal Patterns of Root Distribution in Developing Stands of Four Woody Crop Species Grown with Drip Irrigation and Fertilization. Plant Soil. 2007, 299, 195–213. [Google Scholar] [CrossRef]
- Yan, X.-L.; Dai, T.-F.; Jia, L.-M. Evaluation of the Cumulative Effect of Drip Irrigation and Fertigation on Productivity in a Poplar Plantation. Ann. For. Sci. 2018, 75, 5. [Google Scholar] [CrossRef]
- Campoe, O.C.; Stape, J.L.; Albaugh, T.J.; Lee Allen, H.; Fox, T.R.; Rubilar, R.; Binkley, D. Fertilization and Irrigation Effects on Tree Level Aboveground Net Primary Production, Light Interception and Light Use Efficiency in a Loblolly Pine Plantation. For. Ecol. Manag. 2013, 288, 43–48. [Google Scholar] [CrossRef]
- Zou, S.; Li, D.; Di, N.; Liu, J.; Li, L.; Liu, Y.; Xi, B.; Coleman, M. Stand Development Modifies Effects of Soil Water Availability on Poplar Fine-Root Traits: Evidence from a Six-Year Experiment. Plant Soil. 2022, 480, 165–184. [Google Scholar] [CrossRef]
- Tan, J.; Yu, W.; Liu, Y.; Guo, Y.; Liu, N.; Fu, H.; Di, N.; Duan, J.; Li, X.; Xi, B. Response of Fine-Root Traits of Populus Tomentosa to Drought in Shallow and Deep Soil. Forests 2023, 14, 951. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, W.; Currell, M.; Yang, Y.; Zhao, H.; Lv, M. Relationship between Land-Use and Sources and Fate of Nitrate in Groundwater in a Typical Recharge Area of the North China Plain. Sci. Total Environ. 2017, 609, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.C.; Bouillet, J.-P.; Regina Pivello, V.; Aló, L.L.; Costa, V.E.; Van Den Meersche, K.; Guerrini, I.A.; Laclau, J.-P. Roots Take up Labeled Nitrogen from a Depth of 9 m in a Wooded Savanna in Brazil. Soil. Biol. Biochem. 2021, 160, 108282. [Google Scholar] [CrossRef]
- Henriksson, N.; Lim, H.; Marshall, J.; Franklin, O.; McMurtrie, R.E.; Lutter, R.; Magh, R.; Lundmark, T.; Näsholm, T. Tree Water Uptake Enhances Nitrogen Acquisition in a Fertilized Boreal Forest—But Not under Nitrogen-poor Conditions. New Phytol. 2021, 232, 113–122. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Warren, C.R.; Baker, T.G. Effect of Thinning, Pruning and Nitrogen Fertiliser Application on Transpiration, Photosynthesis and Water-Use Efficiency in a Young Eucalyptus Nitens Plantation. For. Ecol. Manag. 2012, 266, 286–300. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Baker, T.G. Effect of Thinning, Pruning and Nitrogen Fertiliser Application on Light Interception and Light-Use Efficiency in a Young Eucalyptus Nitens Plantation. For. Ecol. Manag. 2013, 288, 21–30. [Google Scholar] [CrossRef]
- Brinkhoff, R.; Mendham, D.; Hunt, M.; Unwin, G.; Hovenden, M. Nitrogen Fertiliser Only Increases Leaf Area in the Lower Crown of Mid-Rotation Eucalyptus Nitens Plantations. For. Ecol. Manag. 2022, 508, 120048. [Google Scholar] [CrossRef]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Zhou, G.; Zou, X.; Bai, E.; Scanlon, T.M.; Hou, E.; et al. Plant Acclimation to Long-Term High Nitrogen Deposition in an N-Rich Tropical Forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef]
- Mao, Q.; Lu, X.; Zhou, K.; Chen, H.; Zhu, X.; Mori, T.; Mo, J. Effects of Long-Term Nitrogen and Phosphorus Additions on Soil Acidification in an N-Rich Tropical Forest. Geoderma 2017, 285, 57–63. [Google Scholar] [CrossRef]
- Lu, X.; Mo, J.; Gilliam, F.S.; Zhou, G.; Fang, Y. Effects of Experimental Nitrogen Additions on Plant Diversity in an Old-growth Tropical Forest. Glob. Change Biol. 2010, 16, 2688–2700. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global Allocation Rules for Patterns of Biomass Partitioning in Seed Plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef]
- Yue, K.; Fornara, D.A.; Li, W.; Ni, X.; Peng, Y.; Liao, S.; Tan, S.; Wang, D.; Wu, F.; Yang, Y. Nitrogen Addition Affects Plant Biomass Allocation but Not Allometric Relationships among Different Organs across the Globe. J. Plant Ecol. 2021, 14, 361–371. [Google Scholar] [CrossRef]
- Niklas, K.J. Modelling Below- and Above-Ground Biomass for Non-Woody and Woody Plants. Ann. Bot. 2005, 95, 315–321. [Google Scholar] [CrossRef]
- Mccarthy, M.C.; Enquist, B.J. Consistency between an Allometric Approach and Optimal Partitioning Theory in Global Patterns of Plant Biomass Allocation. Funct. Ecol. 2007, 21, 713–720. [Google Scholar] [CrossRef]
- Zhou, L.; Hong, Y.; Li, C.; Lu, C.; He, Y.; Shao, J.; Sun, X.; Wang, C.; Liu, R.; Liu, H.; et al. Responses of Biomass Allocation to Multi-Factor Global Change: A Global Synthesis. Agric. Ecosyst. Environ. 2020, 304, 107115. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Wang, X.; Ullah, H.; Liu, Y.; Duan, J. Tree Crown Affects Biomass Allocation and Its Response to Site Conditions and the Density of Platycladus Orientalis Linnaeus Plantation. Forests 2023, 14, 2433. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Ji, C.; Han, W. Above- and Belowground Biomass Allocation in Tibetan Grasslands. J. Veg. Sci. 2009, 20, 177–184. [Google Scholar] [CrossRef]
- He, Y.; Li, G.; Xi, B.; Zhao, H.; Jia, L. Fine Root Plasticity of Young Populus Tomentosa Plantations under Drip Irrigation and Nitrogen Fertigation in the North China Plain. Agric. Water Manag. 2022, 261, 107341. [Google Scholar] [CrossRef]
- Jia, S.; Meng, F.; Li, X. Soil Nutrients and Season Drive Fine Root Traits, Soil Microbial Community and Their Interaction in a Pinus Koraiensis Seed Orchard. For. Ecol. Manag. 2025, 580, 122503. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, X.; Jia, L.; Xi, B. Effects of Key Forest Management Practices and Climatic Factors on the Growth of Populus Tomentosa Plantations in the North China Plain. For. Ecol. Manag. 2022, 521, 120444. [Google Scholar] [CrossRef]
- Kröner, K.; Larysch, E.; Schindler, Z.; Obladen, N.; Frey, J.; Stangler, D.F.; Seifert, T. Influence of Crown Morphology and Branch Architecture on Tree Radial Growth of Drought-Affected Fagus sylvatica L. For. Ecosyst. 2024, 11, 100237. [Google Scholar] [CrossRef]
- Samariks, V.; Brizga, D.; Rūba, J.; Seipulis, A.; Jansons, Ā. Root-Plate Characteristics of Common Aspen in Hemiboreal Forests of Latvia: A Case Study. Forests 2020, 12, 32. [Google Scholar] [CrossRef]
- Hassan, S.; Liguori, G.; Inglese, P.; Louhaichi, M.; Sortino, G. The Effect of Soil Volume Availability on Opuntia Ficus-Indica Canopy and Root Growth. Agronomy 2020, 10, 635. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Zhou, L.; Liu, H.; Ma, Y.; Ding, Y.; et al. The Impacts of Climate Change on Water Resources and Agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef]
- Haddeland, I.; Heinke, J.; Biemans, H.; Eisner, S.; Flörke, M.; Hanasaki, N.; Konzmann, M.; Ludwig, F.; Masaki, Y.; Schewe, J.; et al. Global Water Resources Affected by Human Interventions and Climate Change. Proc. Natl. Acad. Sci. USA 2014, 111, 3251–3256. [Google Scholar] [CrossRef]
- Xi, B.; Di, N.; Wang, Y.; Duan, J.; Jia, L. Modeling Stand Water Use Response to Soil Water Availability and Groundwater Level for a Mature Populus Tomentosa Plantation Located on the North China Plain. For. Ecol. Manag. 2017, 391, 63–74. [Google Scholar] [CrossRef]
- Gu, B.; Ge, Y.; Chang, S.X.; Luo, W.; Chang, J. Nitrate in Groundwater of China: Sources and Driving Forces. Glob. Environ. Change 2013, 23, 1112–1121. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Baker, T.G. Interactive Effects of Simultaneously Applied Thinning, Pruning and Fertiliser Application Treatments on Growth, Biomass Production and Crown Architecture in a Young Eucalyptus Nitens Plantation. For. Ecol. Manag. 2012, 267, 104–116. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Fernández, J.-E.; Coleman, M.; Hu, W.; Di, N.; Zou, S.; Liu, Y.; Xi, B.; Clothier, B. Variations in Water-Balance Components and Carbon Stocks in Poplar Plantations with Differing Water Inputs over a Whole Rotation: Implications for Sustainable Forest Management under Climate Change. Agric. For. Meteorol. 2022, 320, 108958. [Google Scholar] [CrossRef]
- Xi, B.; Bloomberg, M.; Watt, M.S.; Wang, Y.; Jia, L. Modeling Growth Response to Soil Water Availability Simulated by HYDRUS for a Mature Triploid Populus Tomentosa Plantation Located on the North China Plain. Agric. Water Manag. 2016, 176, 243–254. [Google Scholar] [CrossRef]
- Chen, Z. Compilation of Poplar Two-dimensional Standing Wood Volume table. For. Res. 1989, 01, 78–83. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Yan, X.; Jia, L.; Chen, N.; Liu, J.; Zhao, P.; Zhou, L.; Cao, Q. Effect of Nitrogen, Phosphorus and Potassium Fertilization Management on Soil Properties and Leaf Traits and Yield of Sapindus Mukorossi. Front. Plant Sci. 2024, 15, 1300683. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Guo, H.; Zhao, H.; Jiao, J.; Deyholos, M.K.; Yan, X.; Liu, Y.; Liang, Z. Optimal Fertilizer Application for Panax Notoginseng and Effect of Soil Water on Root Rot Disease and Saponin Contents. J. Ginseng. Res. 2016, 40, 38–46. [Google Scholar] [CrossRef] [PubMed]

| Indices | Source of Variation | Stand Ages (Years Old) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |||||||
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| ΔDBH (cm) | IF vs. CK | 3.886 | 0.006 | 1.654 | 0.17 | 0.459 | 0.832 | 0.759 | 0.608 | 1.133 | 0.369 |
| I | 15.88 | 0.001 | 0.387 | 0.061 | 0.009 | 0.926 | 0.063 | 0.804 | 0.106 | 0.748 | |
| F | 0.494 | 0.616 | 1.378 | 0.271 | 0.653 | 0.53 | 0.527 | 0.597 | 0.501 | 0.612 | |
| I × F | 0.463 | 0.635 | 0.147 | 0.864 | 0.021 | 0.979 | 1.278 | 0.297 | 1.848 | 0.179 | |
| ΔH (m) | IF vs. CK | 1.247 | 0.313 | 1.177 | 0.347 | 0.727 | 0.632 | 1.230 | 0.321 | 0.865 | 0.533 |
| I | 0.694 | 0.413 | 0.138 | 0.714 | 1.468 | 0.238 | 0.021 | 0.886 | 3.035 | 0.094 | |
| F | 0.746 | 0.485 | 2.32 | 0.12 | 0.898 | 0.421 | 3.711 | 0.039 | 0.343 | 0.713 | |
| I × F | 0.183 | 0.834 | 0.107 | 0.899 | 0.387 | 0.683 | 0.176 | 0.840 | 0.069 | 0.934 | |
| BAS (m2·ha−1) | IF vs. CK | 2.631 | 0.038 | 2.893 | 0.025 | 2.191 | 0.074 | 2.197 | 0.073 | 1.996 | 0.100 |
| I | 8.628 | 0.007 | 9.877 | 0.004 | 6.445 | 0.018 | 9.227 | 0.006 | 8.814 | 0.007 | |
| F | 0.714 | 0.500 | 0.355 | 0.705 | 0.426 | 0.658 | 0.281 | 0.757 | 0.632 | 0.540 | |
| I × F | 0.633 | 0.539 | 0.678 | 0.517 | 0.449 | 0.644 | 1.548 | 0.233 | 0.178 | 0.838 | |
| VS (m3·ha−1) | IF vs. CK | 2.302 | 0.062 | 2.840 | 0.027 | 2.272 | 0.065 | 2.325 | 0.060 | 1.841 | 0.127 |
| I | 6.229 | 0.020 | 9.662 | 0.005 | 6.878 | 0.015 | 9.207 | 0.006 | 7.894 | 0.010 | |
| F | 0.893 | 0.423 | 0.288 | 0.753 | 0.405 | 0.617 | 0.287 | 0.753 | 0.288 | 0.752 | |
| I × F | 0.472 | 0.629 | 0.608 | 0.553 | 0.516 | 0.603 | 1.522 | 0.239 | 0.241 | 0.788 | |
| AFP (m3·ha−1·yr−1) | IF vs. CK | 2.302 | 0.062 | 2.840 | 0.027 | 2.272 | 0.065 | 2.325 | 0.060 | 1.841 | 0.127 |
| I | 6.229 | 0.020 | 9.662 | 0.005 | 6.878 | 0.015 | 9.207 | 0.006 | 7.894 | 0.010 | |
| F | 0.893 | 0.423 | 0.288 | 0.753 | 0.405 | 0.617 | 0.287 | 0.753 | 0.288 | 0.752 | |
| I × F | 0.472 | 0.629 | 0.608 | 0.553 | 0.516 | 0.603 | 1.522 | 0.239 | 0.241 | 0.799 | |
| Treatment | Irrigation When SWP at 20 cm Depth (kPa) | Irrigation Amount (mm·ha−1·year−1) | Fertigation Amount (kg ha−1 year−1N) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | 2017 | 2018 | 2019 | 2020 | 2021 | ||
| I20F0 | −20 | 347.40 | 455.73 | 291.67 | 309.90 | 364.57 | 0 | 0 | 0 | 0 | 0 |
| I20FL | −20 | 347.40 | 455.73 | 291.67 | 309.90 | 364.57 | 80 | 120 | 120 | 120 | 120 |
| I20FH | −20 | 347.40 | 455.73 | 291.67 | 309.90 | 364.57 | 220 | 260 | 260 | 260 | 260 |
| I45F0 | −45 | 16.67 | 0.00 | 25.52 | 51.10 | 76.78 | 0 | 0 | 0 | 0 | 0 |
| I45FL | −45 | 16.67 | 0.00 | 25.52 | 51.10 | 76.78 | 80 | 120 | 120 | 120 | 120 |
| I45FH | −45 | 16.67 | 0.00 | 25.52 | 51.10 | 76.78 | 220 | 260 | 260 | 260 | 260 |
| Control | Non-irrigation | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, J.; He, Y.; Zhu, W.; Jia, L.; Xi, B. Growth and Biomass Distribution Responses of Populus tomentosa to Long-Term Water–Nitrogen Coupling in the North China Plain. Plants 2025, 14, 1833. https://doi.org/10.3390/plants14121833
Wang Y, Liu J, He Y, Zhu W, Jia L, Xi B. Growth and Biomass Distribution Responses of Populus tomentosa to Long-Term Water–Nitrogen Coupling in the North China Plain. Plants. 2025; 14(12):1833. https://doi.org/10.3390/plants14121833
Chicago/Turabian StyleWang, Yafei, Juntao Liu, Yuelin He, Wei Zhu, Liming Jia, and Benye Xi. 2025. "Growth and Biomass Distribution Responses of Populus tomentosa to Long-Term Water–Nitrogen Coupling in the North China Plain" Plants 14, no. 12: 1833. https://doi.org/10.3390/plants14121833
APA StyleWang, Y., Liu, J., He, Y., Zhu, W., Jia, L., & Xi, B. (2025). Growth and Biomass Distribution Responses of Populus tomentosa to Long-Term Water–Nitrogen Coupling in the North China Plain. Plants, 14(12), 1833. https://doi.org/10.3390/plants14121833








