Optimized Carbon–Nitrogen Fertilization Boosts Fragrant Rice (Oryza sativa L.) Yield and Quality via Enhanced Photosynthesis, Antioxidant Defense, and Osmoregulation
Abstract
1. Introduction
2. Materials and Methods
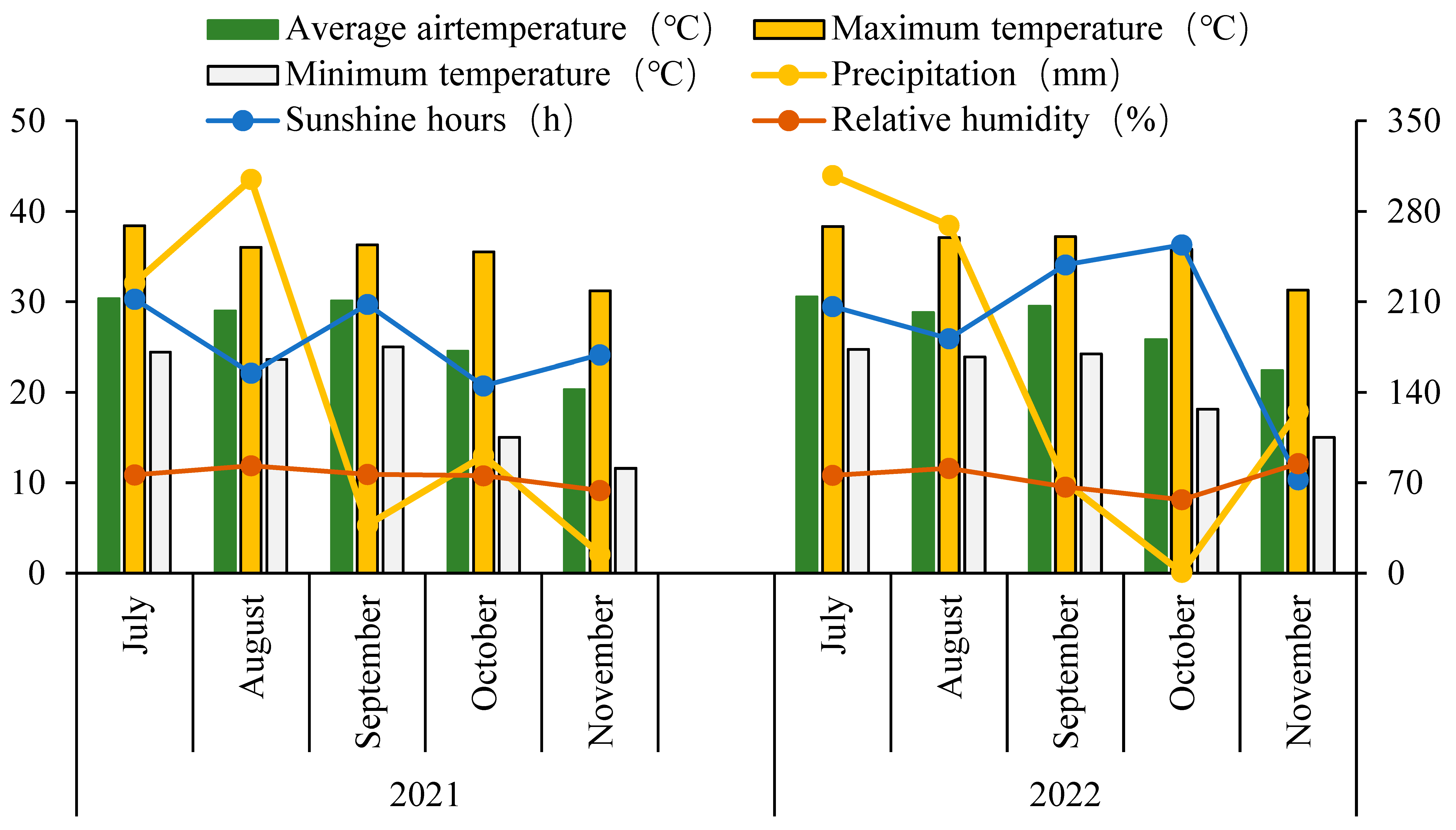
2.1. Experimental Description
2.2. Experimental Design
2.3. Sampling and Measurements
2.3.1. Measurement of Yield and Yield Components
2.3.2. Measurement of Rice Quality
2.3.3. Measurement of Dry Weight and Leaf Area Index
2.3.4. Measurement of Photosynthesis Rate and Total Chlorophyll Content
2.3.5. Measurement of Non-Structural Carbohydrate Content
2.3.6. Measurement of Antioxidant Defense, and Osmoregulation Parameters
2.4. Statistical Analysis
3. Results
3.1. Grain Yield, Total Dry Weight, and Yield Components
3.2. Grain Quality
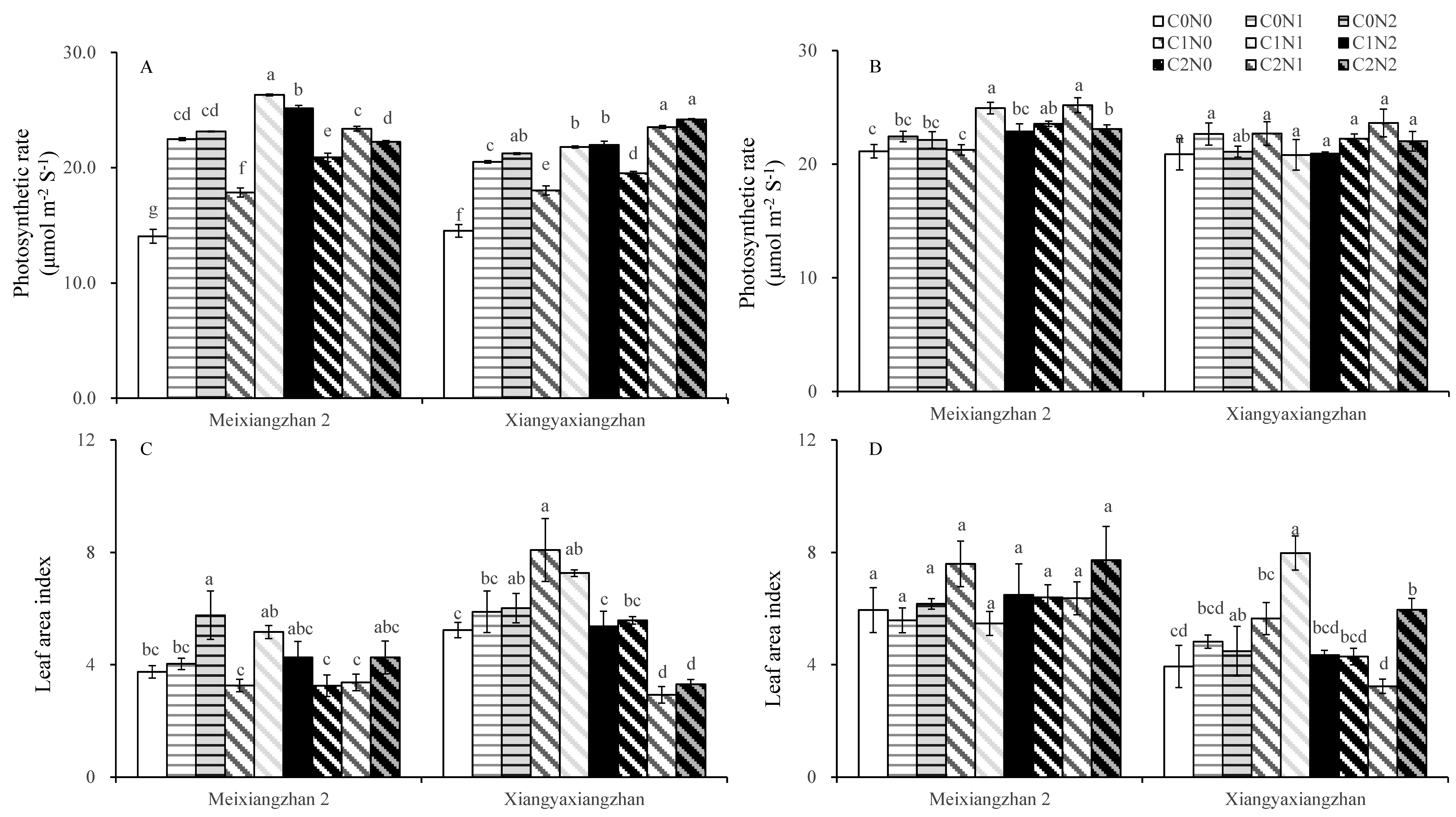
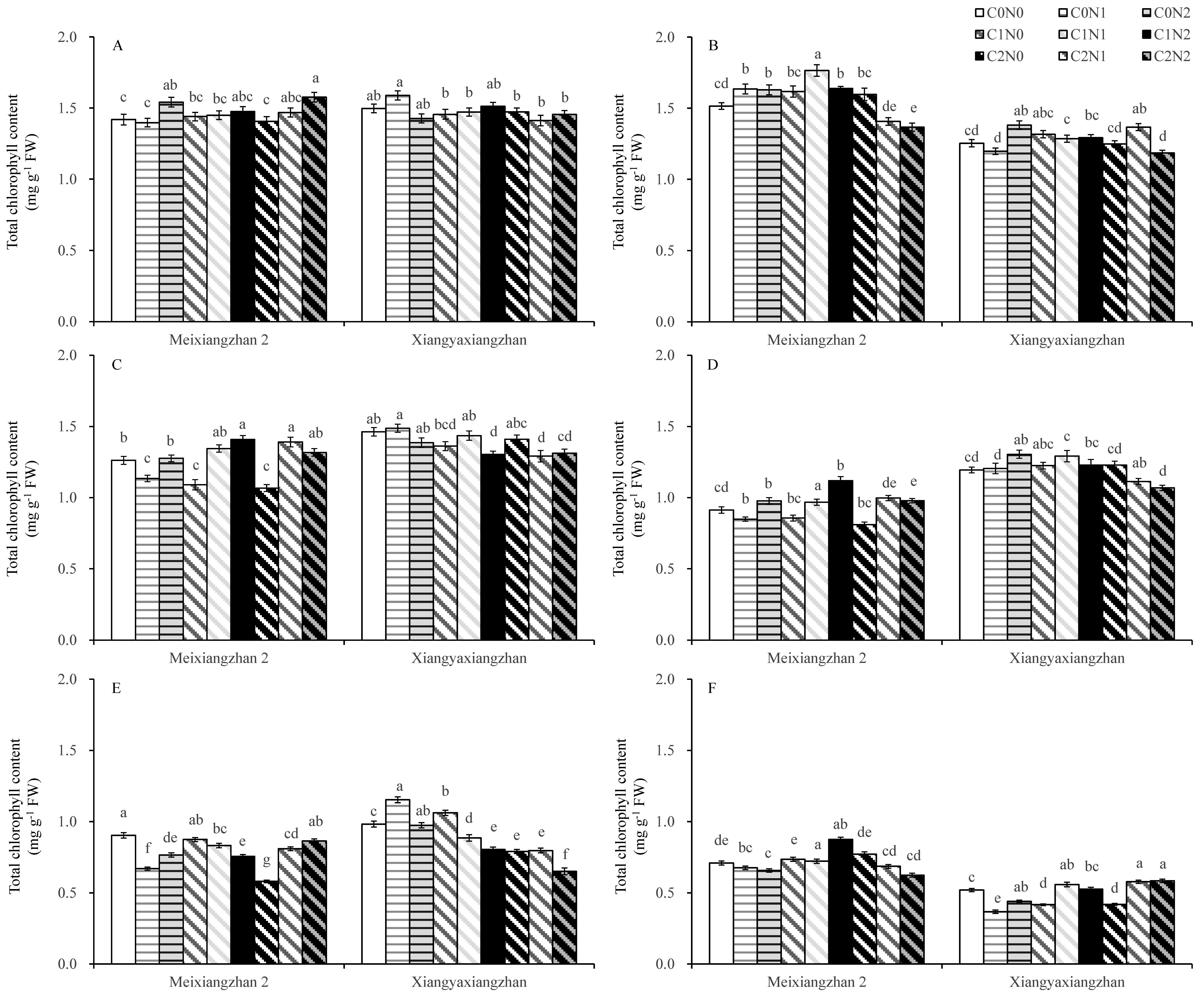
3.3. Photosynthesis Parameters
3.4. Non-Structural Carbohydrate Content and Soluble Protein Content
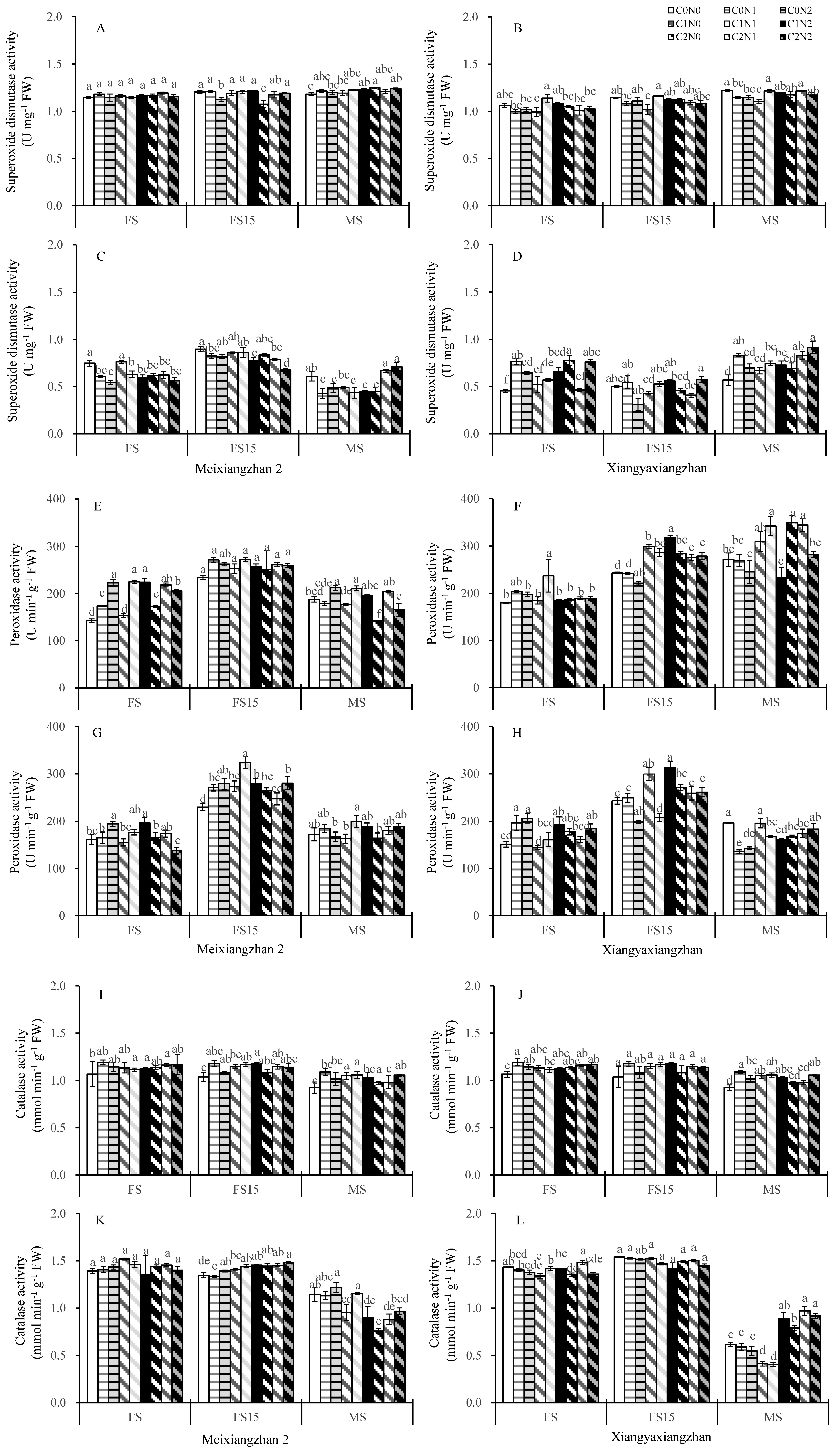
3.5. Antioxidant Defense and Osmoregulation
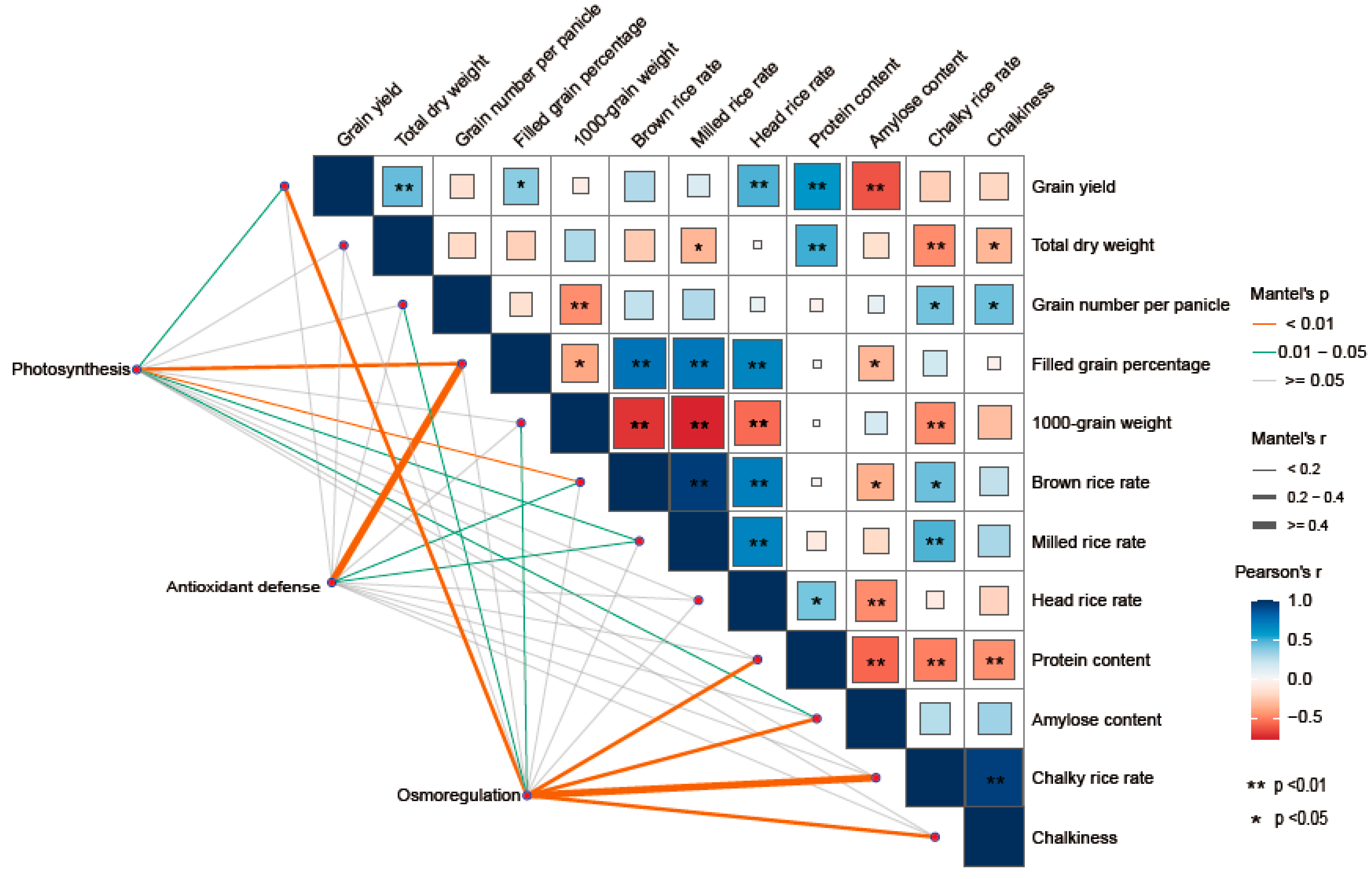
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gui, R.F.; Wang, Z.M.; Pan, S.G.; Zhang, M.H.; Tang, X.R.; Mo, Z.W. Effects of nitrogen-reducing side deep application of liquid fertilizer at tillering stage on yield and nitrogen utilization of fragrant rice. Sci. Agric. Sin. 2022, 55, 1529–1545. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; He, Z.Z.; Xing, P.P.; Luo, H.W.; Yan, Z.S.; Tang, X.R. Effects of paclobutrazol seed priming on seedling quality, photosynthesis, and physiological characteristics of fragrant rice. BMC Plant Biol. 2024, 24, 53. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Ke, L.Y.; Du, X.L.; Li, X.X.; Xu, J.L. Magnesium application improves 2-acetyl-1-pyrroline biosynthesis and overall quality properties in fragrant rice. J. Plant Growth Regul. 2024, 43, 4062–4073. [Google Scholar] [CrossRef]
- Lau, W.C.P.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.; Latif, M.A.; Asfaliza, R.; Miah, G. Development of advanced fragrant rice lines from MR269 x Basmati 370 through marker-assisted backcrossing. Euphytica 2017, 213, 11. [Google Scholar] [CrossRef]
- Li, Y.X.; Lai, R.F.; Li, W.; Liu, J.Q.; Huang, M.Z.; Tang, Y.J.; Tang, X.R.; Pan, S.G.; Duan, M.Y.; Tian, H.; et al. γ-Aminobutyric acid regulates grain yield formation in different fragrant rice genotypes under different nitrogen levels. J. Plant Growth Regul. 2020, 39, 738–750. [Google Scholar] [CrossRef]
- Fei, L.W.; Yang, S.C.; Ma, A.L.Y.; Lun, C.; Wang, M.; Wang, G.J.; Guo, S.W. Grain chalkiness is reduced by coordinating the biosynthesis of protein and starch in fragrant rice (Oryza sativa L.) grain under nitrogen fertilization. Field Crops Res. 2023, 302, 109098. [Google Scholar] [CrossRef]
- Qiu, X.F.; Zhang, X.C.; Mo, Z.W.; Pan, S.G.; Tian, H.; Duan, M.Y.; Tang, X.R. Effects of different tillage and fertilization methods on the yield and nitrogen leaching of fragrant rice. Agronomy 2023, 13, 2773. [Google Scholar] [CrossRef]
- Chen, Y.J.; Dai, L.; Cheng, S.R.; Ren, Y.; Deng, H.Z.; Wang, X.Y.; Li, Y.Z.; Tang, X.R.; Wang, Z.M.; Mo, Z.W. Regulation of 2-acetyl-1-pyrroline and grain quality in early-season indica fragrant rice by nitrogen and silicon fertilization under different plantation methods. J. Integr. Agric. 2024, 23, 511–535. [Google Scholar] [CrossRef]
- Hong, W.Y.; Chen, Y.J.; Huang, S.H.; Li, Y.Z.; Wang, Z.M.; Tang, X.R.; Pan, S.G.; Tian, H.; Mo, Z.W. Optimization of nitrogen–silicon (N-Si) fertilization for grain yield and lodging resistance of early-season indica fragrant rice under different planting methods. Eur. J. Agron. 2022, 136, 126508. [Google Scholar] [CrossRef]
- Tian, H.; Pan, S.G.; Mo, Z.W.; Duan, M.Y.; Tang, X.R. Effects of soil moisture and fertilization on fragrance, quality and yield of fragrant rice. J. Irrig. Drain. 2018, 37, 36–41. [Google Scholar] [CrossRef]
- Wang, W.X.; Jiang, S.C.; Xing, D.Y.; Du, B. Effect of planting density and irrigation management on the growth, yield, and 2-acetyl-o1-pyrroline content of fragrant rice. J. Soil Sci. Plant Nutr. 2021, 22, 1000–1008. [Google Scholar] [CrossRef]
- Pan, S.G.; Liu, H.D.; Mo, Z.W.; Patterson, B.; Duan, M.Y.; Tian, H.; Hu, S.J.; Tang, X.R. Effects of nitrogen and shading on root morphologies, nutrient accumulation, and photosynthetic parameters in different rice genotypes. Sci. Rep. 2017, 7, 45611. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Sun, M.X.; Zhou, X.; Li, J.; Xie, G.X.; Yang, X.D.; Peng, J.W. Increase in yield and nitrogen use efficiency of double rice with long-term application of controlled-release urea. J. Integr. Agric. 2022, 21, 2106–2118. [Google Scholar] [CrossRef]
- Yang, G.T.; Wang, Q.; Zhang, G.H.; Jiang, C.Y.; Ma, P.; Hu, Y.G. Effect of nitrogen and phosphorus application on starch characteristics and quality of rice with different nitrogen efficiency. Front. Nutr. 2024, 11, 1462689. [Google Scholar] [CrossRef]
- Tang, J.C.; Sun, Z.G.; Chen, Q.H.; Damaris, R.N.; Lu, B.L.; Hu, Z.R. Nitrogen fertilizer induced alterations in the root proteome of two rice cultivars. Int. J. Mol. Sci. 2019, 20, 3674. [Google Scholar] [CrossRef]
- Wang, W.L.; Huang, L.Y.; Zhu, G.L.; Zhang, H.; Wang, Z.Q.; Adnan, M.; Saud, S.; Hayat, Z.; Fahad, S. Screening of Rice Cultivars for Nitrogen Use Efficiency and Yield Stability under Varying Nitrogen Levels. J. Plant Growth Regul. 2021, 41, 1808–1819. [Google Scholar] [CrossRef]
- Xu, H.G.; Zhong, G.R.; Lin, J.J.; Ding, Y.F.; Li, G.H.; Wang, S.H.; Liu, Z.H.; Tang, S.; Ding, C.Q. Effect of nitrogen management during the panicle stage in rice on the nitrogen utilization of rice and succeeding wheat crops. Eur. J. Agron. 2015, 70, 41–47. [Google Scholar] [CrossRef]
- Jiang, M.J.; Xu, W.B.; Li, L.J.; Zhang, J.F.; Wang, R.J.; Ji, G.M.; Luo, D.Q.; Jiang, X.H.; Tian, J.Y.; Li, M. Balanced Nitrogen Reduction for Improved Grain Yield and Eating Quality in Mechanically Transplanted Hybrid Indica Rice. Agriculture 2024, 14, 1313. [Google Scholar] [CrossRef]
- Sui, B.; Feng, X.M.; Tian, G.L.; Hu, X.Y.; Shen, Q.R.; Guo, S.W. Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crops Res. 2013, 150, 99–107. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, K.J.; Zhao, C.J.; Li, Z.T.; Wang, Y.F.; Fu, J.; Guo, L.; Li, W.S. Alleviation of the adverse effects of salt stress by regulating photosynthetic system and active oxygen metabolism in maize seedlings. Sci. Agric. Sin. 2014, 47, 3962–3972. [Google Scholar] [CrossRef]
- Wu, T.T.; Chen, J.B.; Yuan, H.W.; Zheng, B.S.; Yan, D.L. Effects of foliar spraying organic carbon on carbohydrate metabolism and Fe, Zn content of dendrobium officinale. Ecol. Sci. 2021, 40, 31–36. [Google Scholar] [CrossRef]
- Li, J.; He, C.C.; Liu, S.H.; Guo, Y.T.; Zhang, Y.X.; Zhang, L.J.; Zhou, X.; Xu, D.Y.; Luo, X.; Liu, H.Y.; et al. Research progress and application strategies of sugar transport mechanisms in rice. Front. Plant Sci. 2024, 15, 1454615. [Google Scholar] [CrossRef] [PubMed]
- Oiestad, A.J.; Martin, J.M.; Giroux, M.J. Yield increases resulting from AGPase overexpression in rice are reliant on plant nutritional status. Plant Growth Regul. 2019, 89, 179–190. [Google Scholar] [CrossRef]
- Ishimaru, K.; Hirotsu, N.; Madoka, Y.; Murakami, N.; Hara, N.; Onodera, H.; Kashiwagi, T.; Ujiie, K.; Shimizu, B.; Onishi, A.; et al. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 2013, 45, 707–711. [Google Scholar] [CrossRef]
- Smidansky, E.D.; Meyer, F.D.; Blakeslee, B.; Weglarz, T.E.; Greene, T.W.; Giroux, M.J. Expression of a modified ADP-glucose pyrophosphorylase large subunit in wheat seeds stimulates photosynthesis and carbon metabolism. Planta 2007, 225, 965–976. [Google Scholar] [CrossRef]
- Hou, J.; Li, T.; Wang, Y.M.; Hao, C.Y.; Liu, H.X.; Zhang, X.Y. ADP-glucose pyrophosphorylase genes, associated with kernel weight, underwent selection during wheat domestication and breeding. Plant Biotechnol. J. 2017, 15, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Sami, F.; Hayat, S. Glucose: Sweet or bitter effects in plants-a review on current and future perspective. Carbohydr. Res. 2020, 487, 107884. [Google Scholar] [CrossRef]
- Prasanna, R.; Pabby, A.; Singh, P.K. Effect of glucose and light-dark environment on pigmentation profiles in the cyanobacterium Calothrix elenkenii. Folia Microbiol. 2004, 49, 26–30. [Google Scholar] [CrossRef]
- Yusuf, M.; Almehrzi, A.S.S.; Alnajjar, A.J.N.; Alam, P.; Elsayed, N.; Khalil, R.; Hayat, S. Glucose modulates copper induced changes in photosynthesis, ion uptake, antioxidants and proline in Cucumis sativus plants. Carbohydr. Res. 2021, 501, 108271. [Google Scholar] [CrossRef]
- Ma, Q.X.; Cao, X.C.; Xie, Y.A.; Xiao, H.; Tan, X.L.; Wu, L.H. Effects of glucose on the uptake and metabolism of glycine in pakchoi (Brassica chinensis L.) exposed to various nitrogen sources. BMC Plant Biol. 2017, 17, 58. [Google Scholar] [CrossRef]
- Jiang, N.; Fu, W.M.; Fu, G.F.; Tao, L.X. Sucrose alleviates inhibition on the kernel weight and assimilation distribution caused by heat stress at grain filling stage of rice. China Rice 2020, 26, 13–17. [Google Scholar] [CrossRef]
- Lyu, T.F.; Shen, J.; Ma, P.; Dai, Z.; Yang, Z.Y.; Xu, H.; Zheng, C.G.; Ma, J. Effects of combined application of slow release nitrogen fertilizer and urea on the nitrogen utilization characteristics in mechane—transplanted hybrid rice. Scientia Agric. Sin. 2021, 54, 1410–1423. [Google Scholar] [CrossRef]
- Guo, W.; Li, J.W.; Zou, C.L.; Yu, L.H. Effect of spraying urea and sucrose on dry matter accumulation and grain yield at post-anthesis of spring wheat. Soil Fertil. Sci. China 2017, 5, 17–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, P.L.; Wang, F.; Zhao, L.M.; Qian, K.Y.; Zhang, Y.D.; Fan, X.R. Effects of carbon and nitrogen fertilisers on rice quality of the OsNRT2.3b-overexpressing line. Agriculture 2022, 12, 802. [Google Scholar] [CrossRef]
- Yoneyama, T.; Ito, O.; Engelaar, W.M.H.G. Uptake, metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15N: Progress over the last 30 years. Phytochem. Rev. 2003, 2, 121–132. [Google Scholar] [CrossRef]
- Wu, K.; Wang, S.S.; Song, W.Z.; Zhang, J.Q.; Wang, Y.; Liu, Q.; Yu, J.P.; Ye, Y.F.; Li, S.; Chen, J.F.; et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 367, 6478. [Google Scholar] [CrossRef]
- Yoneyama, T.; Tanno, F.; Tatsumi, J.; Mae, T. Whole-Plant Dynamic System of Nitrogen Use for Vegetative Growth and Grain Filling in Rice Plants (Oryza sativa L.) as Revealed through the Production of 350 Grains from a Germinated Seed Over 150 Days: A Review and Synthesis. Front. Plant Sci. 2016, 7, 1151. [Google Scholar] [CrossRef]
- Arai, K.; Kono, Y. Development of the rice panicle. I. Characteristics of the growth of spikelets at different positions on panicle. Jpn. J. Crop Sci. 1978, 47, 699–706. [Google Scholar] [CrossRef]
- Takebe, M.; Oikawa, T.; Matsuno, K.; Shimizu, E.; Yoneyama, T. Influence of nitrogen application on the contents of glutelin and prolamin of polished rice grains (Oryza sativa L.). Jpn. J. Soil Sci. Plant Nutr. 1996, 67, 139–146. [Google Scholar]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Mathan, J.; Singh, A.; Ranjan, A. Sucrose transport and metabolism control carbon partitioning between stem and grain in rice. J. Exp. Bot. 2021, 72, 4355–4372. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.F.; Huang, A.B.; Sang, Y.Y.; Fu, Y.; Yang, Z.B. Carbon-Nitrogen Interaction Modulates Plant Growth and Expression of Metabolic Genes in Rice. J. Plant Growth Regul. 2013, 32, 575–584. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Akiyama, A.; Kisaka, H.; Uchimiya, H.; Miwa, T. Metabolic engineering with Dof1 transcription factor in plants: Improved nitrogen assimilation and growth under low-nitrogen conditions. Proc. Natl. Acad. Sci. USA 2004, 101, 7833–7838. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Hao, D.L.; Jin, M.; Li, Y.; Liu, Z.T.; Huang, Y.N.; Chen, T.X.; Su, Y.H. Internal ammonium excess induces ROS-mediated reactions and causes carbon scarcity in rice. BMC Plant Biol. 2020, 20, 143. [Google Scholar] [CrossRef]
- Jiang, Z.; Yao, X.B.; Du, B.; Wang, X.Y.; Tang, X.R.; Pan, S.G.; Mo, Z.W. The biosynthesis of 2-acetyl-1-pyrroline is physiologically driven by carbon-nitrogen metabolism in fragrant rice. Eur. J. Agron 2025, 164, 127476. [Google Scholar] [CrossRef]
- Mo, Z.W.; Li, W.; Pan, S.G.; Fitzgerald, T.L.; Xiao, F.; Tang, Y.J.; Wang, Y.L.; Duan, M.Y.; Tian, H.; Tang, X.R. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice 2015, 8, 9. [Google Scholar] [CrossRef]
- Zhou, N.; Jing, L.Q.; Wang, Y.X.; Zhu, J.G.; Yang, L.X.; Wang, Y.L. Effects of elevated atmospheric CO2 and temperature on dynamics of leaf chlorophyll contents and SPAD value of rice in open-air field conditions. Chin. J. Rice Sci. 2017, 31, 524–532. [Google Scholar] [CrossRef]
- Li, Y.Z.; Liang, L.X.; Fu, X.M.; Gao, Z.F.; Liu, H.C.; Tan, J.T.; Potcho, M.P.; Pan, S.G.; Tian, H.; Duan, M.Y.; et al. Light and water treatment during the early grain filling stage regulates yield and aroma formation in aromatic rice. Sci. Rep. 2020, 10, 13. [Google Scholar] [CrossRef]
- Pan, S.G.; Rasul, F.; Li, W.; Tian, H.; Mo, Z.W.; Duan, M.Y.; Tang, X.R. Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 2013, 6, 10. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, S.H.; Ma, L.; Kong, L.L.; Pan, S.G.; Tang, X.R.; Tian, H.; Duan, M.Y.; Mo, Z.W. Effect of exogenous melatonin application on the grain yield and antioxidant capacity in aromatic rice under combined lead-cadmium stress. Antioxidants 2022, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.T.; Chen, X.Y.; Ren, Y.; Qing, B.W.; Zhang, M.H.; Mo, Z.W.; Wang, S.L. Effects of iron oxide nanocoatings on the seed germination, seedling growth, and antioxidant response of aromatic rice grown in the presence of different concentrations of rice straw extracts. J. Nanopart. Res. 2024, 26, 78. [Google Scholar] [CrossRef]
- Mo, Z.W.; Li, Y.H.; Nie, J.; He, L.X.; Pan, S.G.; Duan, M.Y.; Tian, H.; Xiao, L.Z.; Zhong, K.Y.; Tang, X.R. Nitrogen application and different water regimes at booting stage improved yield and 2-acetyl-1-pyrroline (2AP) formation in fragrant rice. Rice 2019, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Potcho, P.M.; Imran, M.; Korohou, T.; Kamara, N.; Tang, X.R. Fertilizer deep placement significantly affected yield, rice quality, 2-AP biosynthesis and physiological characteristics of the fragrant rice cultivars. Agronomy 2022, 12, 162. [Google Scholar] [CrossRef]
- Zhang, J.F.; Li, X.; He, Y.F.; Xie, Y.F. Physiological mechanism on drought tolerance enhanced by exogenous glucose in C4 -pepc rice. Acta Agron. Sin. 2018, 44, 82–94. [Google Scholar] [CrossRef]
- Cong, X.H.; Shi, F.Z.; Ruan, X.M.; Luo, Y.X.; Wang, Y.L.; Xu, Y.Z.; Luo, Z.X. Effects of nitrogen application rate on nitrogen use efficiency and key enzymes for carbon and nitrogen metabolism in different rice varieties. J. Henan Agric. Univ. 2019, 53, 325–330. [Google Scholar] [CrossRef]
- Xiao, D.; Hu, R.; Han, T.; Zhang, W.; Hou, J.; Ren, K. Effects of nitrogen fertilizer consumption and operation on rice yield and its components in China: A meta-analysis. Chin. J. Rice Sci. 2023, 37, 529–542. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, D.; Li, Q.; Gu, M.; Liu, Q. Progresses in research on cloning and functional analysis of key genes involving in rice grain quality. Scientia Agric. Sin. 2016, 49, 4267–4283. [Google Scholar] [CrossRef]
- Ma, Z.H.; Gao, M.H.; Cheng, H.T.; Song, W.W.; Lu, L.J.; Lyu, W.Y. Differences in rice component distribution across layers and their relationship with taste. J. Sci. Food Agric. 2023, 104, 1824–1832. [Google Scholar] [CrossRef]
- Shi, H.; Yun, P.; Zhu, Y.; Wang, L.; Wang, Y.P.; Li, P.B.; Zhou, H.; Cheng, S.Y.; Liu, R.J.; Gao, G.J.; et al. Natural variation of WBR7 confers rice high yield and quality by modulating sucrose supply in sink organs. Plant Biotechnol. J. 2024, 22, 2985–2999. [Google Scholar] [CrossRef]
- Ding, C.; Xu, C.; Lu, B.; Zhu, X.; Luo, X.; He, B.; Elidio, C.; Liu, Z.; Ding, Y.; Yang, J.; et al. Comprehensive evaluation of rice qualities under different nitrogen levels in south China. Foods 2023, 12, 697. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.J.; Wang, E.T.; Li, C.X.; Cai, M.L.; Cheng, B.; Cao, C.G.; Jiang, Y. Use of protein content, amylose content, and rva parameters to evaluate the taste quality of rice. Front. Nutr. 2022, 8, 758547. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Tang, S.; Li, G.H.; Liu, Z.H.; Ding, C.Q.; Chen, L.; Wang, S.H.; Ding, Y.F. Application of nitrogen fertilizer at heading stage improves rice quality under elevated temperature during grain-filling stage. Crop Sci. 2017, 57, 2183–2192. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Deng, X.Y.; Pan, X.H.; Liao, P.; Xiong, Q.Q.; Gao, H.; Wei, H.Y.; Dai, Q.G.; Zeng, Y.J.; et al. Effects of biochar on rice yield, grain quality and starch viscosity attributes. J. Sci. Food Agric. 2023, 103, 5747–5753. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Ouwerkerk, P.B.F. Molecular and environmental factors determining grain quality in rice. Food Energy Secur. 2012, 1, 111–132. [Google Scholar] [CrossRef]
- Qin, J.; Yang, Z.Y.; Sun, Y.J.; Xu, H.; Lu, T.F.; Dai, Z.; Zheng, J.K.; Jiang, K.F.; Ma, J. Effects of nitrogen topdressing for panicle initiation on leaf morphology, photosynthetic production and grain yield of two middle-season hybrid rice. Chin. J. Rice Sci. 2017, 31, 391–399. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, T.; Zhang, C.L.; He, X.E.; Shi, A.L.; Tan, W.J.; Yi, Z.X.; Wang, X.H. Optimizing irrigation and nitrogen management to increase yield and nitrogen recovery efficiency in double-cropping rice. Agronomy 2022, 12, 1190. [Google Scholar] [CrossRef]
- Yin, X.H.; Chen, J.N.; Cao, F.B.; Tao, Z.; Huang, M. Short-term application of biochar improves post-heading crop growth but reduces pre-heading biomass translocation in rice. Plant Prod. Sci. 2020, 23, 522–528. [Google Scholar] [CrossRef]
- Liu, F.; Shi, A.; Zhu, H.; Duan, Y.; Guan, C.; Luo, L.; Peng, T.; Wang, X. Effects of nitrogen application rate and fertilizer ratio on population growth and yield of rice. Hybr. Rice 2024, 39, 117–126. [Google Scholar] [CrossRef]
- Mondal, S.; Hasan, M.J.; Ahmed, T.; Miah, M.G.; Cruz, P.C.Z.; Ismail, A.M. Effects of AG1 and AG2 QTLs on nonstructural carbohydrate and seed management options for rice seedling growth and establishment under flooding stress. Rice Sci. 2020, 27, 515–528. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Z.H.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Pre-anthesis non-structural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crops Res. 2011, 123, 170–182. [Google Scholar] [CrossRef]
- Wei, H.H.; Ge, J.L.; Zhang, X.B.; Zhu, W.; Deng, F.; Ren, W.J.; Chen, Y.L.; Meng, T.Y.; Dai, Q.G. Decreased panicle N application alleviates the negative effects of shading on rice grain yield and grain quality. J. Integr. Agric. 2023, 22, 2041–2053. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, J.D. Delaying or promoting? Manipulation of leaf senescence to improve crop yield and quality. Planta 2023, 258, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.T.; Long, W.F.; Wang, H.R.; Long, P.; Xu, Y.; Fu, Z.Q. Matter Production Characteristics and Nitrogen Use Efficiency under Different Nitrogen Application Patterns in Chinese Double-Cropping Rice Systems. Agronomy 2022, 12, 1165. [Google Scholar] [CrossRef]
- Wang, J.; Lu, K.; Nie, H.P.; Zeng, Q.S.; Wu, B.W.; Qian, J.J.; Fang, Z.M. Rice nitrate transporter OsNPF7.2 positively regulates tiller number and grain yield. Rice 2018, 11, 12. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhou, J. Effects of N, K supply at initial stage of panicle emerging on physiological traits in flag leaf of hybrid rice shanyou 63. Chin. Rice Sci. 1997, 3, 165–169. [Google Scholar] [CrossRef]
- Zhou, S.M.; Ma, H.Y.; Zheng, S.L.; Huang, Q.; Xiong, H.; Gu, W.Z.; Xiao, R.; Yuan, J.C.; He, W. Effects of organic carbon fertilizer on the resistance of potato leaves to anthesis under phenolic acid stress. J. Sichuan Agric. Univ. 2019, 37, 836–841. [Google Scholar] [CrossRef]
- Feng, D.B.; Huang, J.C.; Peng, Z.P.; Xu, P.Z.; Zhang, R.Z. Effects of different organic carbon(nitrogen) nutritions application on main stress-resistant physiological indexes of waterlogged rice. J. South. Agric. 2015, 46, 2106–2111. [Google Scholar] [CrossRef]
- Ding, Y.M.; Chen, M.Q.; Ding, W.R.; Ren, Q.M.; Shen, W.Y.; Liu, D.T.; Lu, C.B.; Xiong, F. Research advances on the carbon-nitrogen metabolic interaction mechanisms in cereal crops. Plant Physiol. 2024, 60, 753–761. [Google Scholar] [CrossRef]
- Li, Q.S.; Wang, D.Q.; Du, C.Y.; Li, X.D.; Zhang, G.C.; Wang, S.K.; Guan, E.S.; Wang, S.Q. Effects of exogenous carbon sources on nitrogen transformation and N2O emissions in Tobacco-planting soil. Chin. Tob. Sci. 2020, 41, 13–19. [Google Scholar] [CrossRef]
- Guo, W.; Li, J.W. Effects of exogenous nitrogen and carbon on the balance of carbon-nitrogen metabolism between source and sink in wheat. Crops 2015, 6, 70–75. [Google Scholar] [CrossRef]
- Ruan, Y.L. Signaling role of sucrose metabolism in development. Mol. Plant 2012, 5, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Li, C.S.; Tang, Y.L.; Liu, Y.B.; Li, B.Q.; Fan, G.Q.; Xiong, T. Effect of nitrogen management modes on grain yield, nitrogen use efficiency and light use efficiency of wheat. Chin. J. Appl. Ecol. 2017, 28, 1889–1898. [Google Scholar] [CrossRef]


| Grain Yield | (t hm−2) | Total Dry Weight | (t hm−2) | ||
|---|---|---|---|---|---|
| Cultivar | Treatment | 2021 | 2022 | 2021 | 2022 |
| Meixiangzhan 2 | C0N0 | 3.57 b | 5.26 d | 6.29 d | 10.04 e |
| C0N1 | 4.23 ab | 6.24 c | 8.77 cd | 14.67 abcd | |
| C0N2 | 4.30 ab | 6.38 bc | 9.33 bc | 11.8 cde | |
| C1N0 | 4.34 ab | 5.98 c | 13.13 a | 15.42 abc | |
| C1N1 | 4.88 ab | 6.40 bc | 10.05 bc | 15.74 ab | |
| C1N2 | 5.28 ab | 7.22 a | 10.77 abc | 16.15 a | |
| C2N0 | 3.83 b | 6.17 c | 10.19 bc | 14.36 abcd | |
| C2N1 | 6.07 a | 6.89 ab | 11.76 ab | 12.31 bcde | |
| C2N2 | 5.01 ab | 6.98 ab | 11.6 ab | 11.64 de | |
| Xiangyaxiangzhan | C0N0 | 4.30 a | 4.24 e | 11.22 e | 10.20 d |
| C0N1 | 4.48 a | 5.21 c | 12.32 cde | 15.09 ab | |
| C0N2 | 4.42 a | 5.69 bc | 14.49 b | 13.43 abc | |
| C1N0 | 4.63 a | 4.71 de | 12.20 de | 14.11 abc | |
| C1N1 | 4.50 a | 5.56 bc | 17.95 a | 16.14 a | |
| C1N2 | 4.82 a | 6.58 a | 14.29 bc | 14.01 abc | |
| C2N0 | 4.63 a | 5.20 cd | 13.93 bcd | 12.12 bcd | |
| C2N1 | 4.76 a | 6.51 a | 14.95 b | 15.27 a | |
| C2N2 | 4.50 a | 5.72 b | 11.54 e | 11.31 cd | |
| ANOVA | Y | ** | ns | ||
| C | ** | ** | |||
| T | ** | ** | |||
| Y × C | ** | ** | |||
| Y × T | ** | ns | |||
| C × T | ns | ** | |||
| Y × C × T | * | ns |
| Cultivar | Treatment | Grain Number per Panicle | Filled-Grain Percentage (%) | 1000-Grain Weight (g) | |||
|---|---|---|---|---|---|---|---|
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | ||
| Meixiangzhan 2 | C0N0 | 132.25 abc | 126.18 abc | 83.84 a | 81.61 ab | 17.66 bc | 18.09 a |
| C0N1 | 132.72 abc | 114.11 c | 76.10 abcd | 86.33 a | 17.45 c | 17.88 ab | |
| C0N2 | 147.92 a | 141.12 ab | 80.68 abc | 86.25 a | 18.19 ab | 17.71 abc | |
| C1N0 | 125.97 bc | 116.15 bc | 78.89 abc | 78.73 bc | 17.41 c | 17.47 bc | |
| C1N1 | 131.77 abc | 118.96 bc | 67.31 d | 81.26 ab | 17.52 c | 18.14 a | |
| C1N2 | 139.28 ab | 147.32 a | 73.51 bcd | 79.95 abc | 17.68 bc | 18.12 a | |
| C2N0 | 116.03 c | 126.96 abc | 82.82 ab | 81.70 ab | 18.58 a | 17.70 abc | |
| C2N1 | 126.00 bc | 127.99 abc | 81.58 abc | 76.77 bc | 17.68 bc | 17.31 c | |
| C2N2 | 139.73 ab | 132.29 abc | 72.63 cd | 73.56 c | 17.64 bc | 17.64 abc | |
| Xiangyaxiangzhan | C0N0 | 144.90 a | 108.56 cd | 52.81 bc | 66.92 a | 18.35 a | 19.14 a |
| C0N1 | 130.30 a | 126.25 a | 49.15 c | 69.33 a | 17.96 a | 19.13 a | |
| C0N2 | 144.33 a | 113.59 abcd | 56.20 bc | 64.93 a | 18.41 a | 19.05 a | |
| C1N0 | 139.47 a | 103.17 d | 54.18 bc | 71.04 a | 18.21 a | 19.14 a | |
| C1N1 | 133.63 a | 112.20 bcd | 53.09 bc | 66.08 a | 18.26 a | 19.09 a | |
| C1N2 | 127.27 a | 124.69 ab | 69.10 a | 68.33 a | 18.35 a | 19.14 a | |
| C2N0 | 130.82 a | 112.75 abcd | 52.80 bc | 64.29 a | 18.37 a | 19.30 a | |
| C2N1 | 141.48 a | 105.12 d | 58.02 b | 68.46 a | 18.54 a | 19.06 a | |
| C2N2 | 137.85 a | 118.75 abc | 60.11 b | 68.38 a | 18.41 a | 19.24 a | |
| ANOVA | Y | ** | ** | ** | |||
| C | ns | ** | ** | ||||
| T | ** | ns | ns | ||||
| Y × C | * | ** | ** | ||||
| Y × T | ns | * | ns | ||||
| C × T | ns | ** | ns | ||||
| Y × C × T | ns | * | ns |
| Year/Cultivar | Treatment | Brown Rice Rate (%) | Milled Rice Rate (%) | Head Rice Rate (%) | Protein Content (%) | Amylose Content (%) | Chalky Rice Rate (%) | Chalkiness (%) |
|---|---|---|---|---|---|---|---|---|
| 2021 | ||||||||
| Meixiangzhan 2 | C0N0 | 74.10 a | 66.07 a | 57.82 a | 7.68 d | 18.80 abc | 14.29 a | 3.56 b |
| C0N1 | 74.83 a | 66.85 a | 54.03 a | 7.78 cd | 19.05 ab | 21.77 a | 6.91 a | |
| C0N2 | 75.73 a | 67.41 a | 49.51 a | 7.85 c | 18.35 cd | 17.94 a | 5.32 ab | |
| C1N0 | 74.19 a | 67.02 a | 53.10 a | 7.83 c | 19.10 a | 18.02 a | 5.40 ab | |
| C1N1 | 74.83 a | 66.45 a | 53.88 a | 7.98 ab | 18.58 bc | 17.41 a | 4.95 ab | |
| C1N2 | 75.69 a | 67.54 a | 56.85 a | 8.03 a | 18.39 cd | 21.22 a | 6.51 a | |
| C2N0 | 74.72 a | 66.75 a | 50.36 a | 7.68 d | 18.90 ab | 22.23 a | 6.67 ab | |
| C2N1 | 74.91 a | 66.40 a | 50.38 a | 7.85 c | 18.95 ab | 18.67 a | 5.27 ab | |
| C2N2 | 75.02 a | 66.92 a | 51.57 a | 7.88 bc | 17.93 d | 20.42 a | 6.01 ab | |
| Xiangyaxiangzhan | C0N0 | 71.22 a | 61.41 ab | 49.73 ab | 8.23 abc | 18.40 c | 13.79 a | 4.67 a |
| C0N1 | 71.89 a | 62.39 ab | 48.93 bc | 8.15 bc | 18.55 bc | 14.79 a | 5.53 a | |
| C0N2 | 72.23 a | 64.97 a | 51.62 ab | 8.13 c | 19.00 a | 14.88 a | 5.28 a | |
| C1N0 | 69.94 a | 59.93 b | 44.63 c | 8.15 bc | 18.63 abc | 18.02 a | 5.40 a | |
| C1N1 | 71.21 a | 62.32 ab | 52.71 ab | 8.28 ab | 18.75 abc | 9.68 a | 3.01 a | |
| C1N2 | 72.47 a | 63.75 a | 54.39 a | 8.33 a | 18.85 ab | 17.16 a | 5.86 a | |
| C2N0 | 71.62 a | 62.77 ab | 48.70 bc | 8.20 abc | 18.88 ab | 11.23 a | 3.53 a | |
| C2N1 | 72.88 a | 63.87 a | 48.80 bc | 7.90 d | 18.80 abc | 14.63 a | 5.32 a | |
| C2N2 | 72.15 a | 63.43 ab | 53.60 ab | 7.85 d | 18.85 ab | 12.73 a | 3.79 a | |
| 2022 | ||||||||
| Meixiangzhan 2 | C0N0 | 74.76 b | 67.26 a | 59.32 a | 8.60 a | 18.10 ab | 12.59 ab | 3.41 ab |
| C0N1 | 75.07 ab | 66.80 a | 60.14 a | 8.33 cd | 18.25 a | 10.51 ab | 2.71 b | |
| C0N2 | 73.50 c | 65.75 a | 58.55 a | 8.45 b | 18.13 ab | 10.73 ab | 3.43 ab | |
| C1N0 | 75.66 ab | 66.83 a | 59.07 a | 8.38 bc | 17.85 b | 10.87 ab | 2.83 b | |
| C1N1 | 75.70 a | 66.06 a | 59.81 a | 8.33 cd | 18.15 ab | 9.70 b | 2.82 b | |
| C1N2 | 75.18 ab | 65.44 a | 59.13 a | 8.63 a | 18.08 ab | 13.17 ab | 4.11 ab | |
| C2N0 | 75.61 ab | 65.64 a | 57.95 a | 8.25 de | 18.05 ab | 16.84 a | 4.73 ab | |
| C2N1 | 75.25 ab | 66.80 a | 59.71 a | 8.28 d | 18.10 ab | 15.77 ab | 5.27 a | |
| C2N2 | 75.40 ab | 66.63 a | 58.45 a | 8.18 e | 18.03 ab | 16.98 a | 5.51 a | |
| Xiangyaxiangzhan | C0N0 | 72.30 ab | 62.74 a | 49.35 cd | 8.25 a | 18.48 ab | 10.45 a | 2.98 a |
| C0N1 | 72.69 a | 62.14 ab | 50.30 bc | 7.95 c | 18.48 ab | 12.49 a | 4.14 a | |
| C0N2 | 71.22 bcd | 61.64 ab | 48.37 d | 8.13 b | 18.33 b | 11.28 a | 3.62 a | |
| C1N0 | 70.43 d | 61.24 b | 51.49 ab | 8.00 c | 18.50 ab | 8.43 a | 2.60 a | |
| C1N1 | 70.86 cd | 61.95 ab | 48.28 d | 8.25 a | 18.40 ab | 14.04 a | 4.64 a | |
| C1N2 | 71.45 bcd | 62.05 ab | 50.18 bc | 8.20 ab | 18.58 ab | 12.83 a | 4.37 a | |
| C2N0 | 71.98 abc | 61.58 ab | 50.26 bc | 8.00 c | 18.43 ab | 10.99 a | 3.93 a | |
| C2N1 | 71.44 bcd | 62.06 ab | 50.50 bc | 8.20 ab | 18.38 ab | 11.57 a | 4.12 a | |
| C2N2 | 71.33 bcd | 62.55 ab | 53.17 a | 8.03 c | 18.68 a | 13.29 a | 4.41 a | |
| ANOVA | ||||||||
| Y | ns | ns | ns | ** | ** | * | * | |
| C | ** | ** | ** | ns | ** | ** | ns | |
| T | ns | ns | ns | ** | ns | ns | ns | |
| Y × C | ns | ns | * | ** | ** | * | ns | |
| Y × T | ns | ns | ns | ** | * | ns | ns | |
| C × T | ns | ns | ns | ** | ** | ns | ns | |
| Y × C × T | ns | ns | ns | ** | ** | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Jiang, Z.; Lin, L.; Wang, X.; Zhang, L.; Mo, Z. Optimized Carbon–Nitrogen Fertilization Boosts Fragrant Rice (Oryza sativa L.) Yield and Quality via Enhanced Photosynthesis, Antioxidant Defense, and Osmoregulation. Plants 2025, 14, 1832. https://doi.org/10.3390/plants14121832
Xie W, Jiang Z, Lin L, Wang X, Zhang L, Mo Z. Optimized Carbon–Nitrogen Fertilization Boosts Fragrant Rice (Oryza sativa L.) Yield and Quality via Enhanced Photosynthesis, Antioxidant Defense, and Osmoregulation. Plants. 2025; 14(12):1832. https://doi.org/10.3390/plants14121832
Chicago/Turabian StyleXie, Wenjun, Zhe Jiang, Li Lin, Xinyi Wang, Lihe Zhang, and Zhaowen Mo. 2025. "Optimized Carbon–Nitrogen Fertilization Boosts Fragrant Rice (Oryza sativa L.) Yield and Quality via Enhanced Photosynthesis, Antioxidant Defense, and Osmoregulation" Plants 14, no. 12: 1832. https://doi.org/10.3390/plants14121832
APA StyleXie, W., Jiang, Z., Lin, L., Wang, X., Zhang, L., & Mo, Z. (2025). Optimized Carbon–Nitrogen Fertilization Boosts Fragrant Rice (Oryza sativa L.) Yield and Quality via Enhanced Photosynthesis, Antioxidant Defense, and Osmoregulation. Plants, 14(12), 1832. https://doi.org/10.3390/plants14121832







