Technologies in Agronomic Biofortification with Zinc in Brazil: A Review
Abstract
1. Introduction
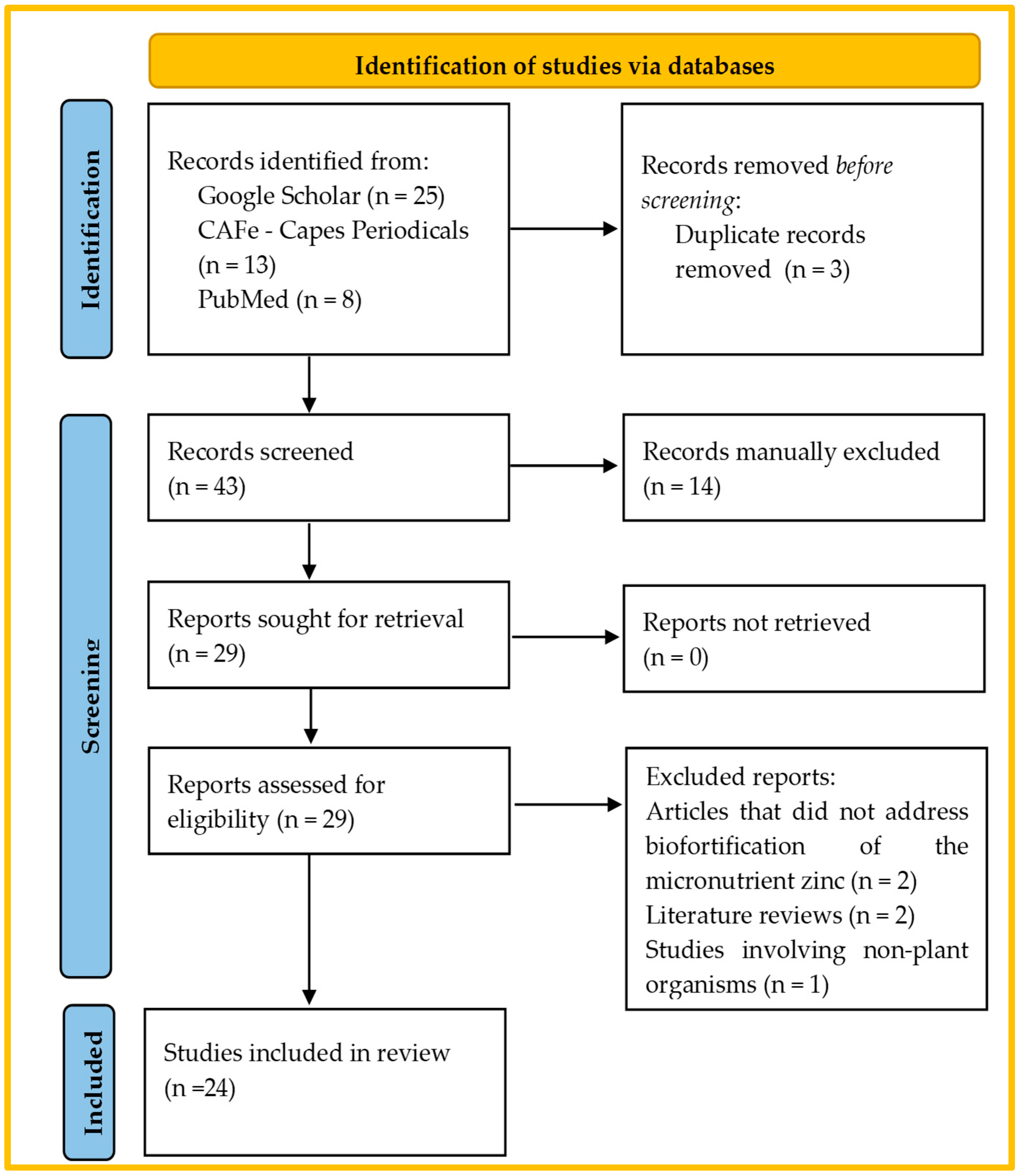
2. Methodology
3. Results and Discussion
3.1. Zinc Application Method
3.2. Fertilizer Types, Doses, and Application Efficiency
3.3. Influence of Genotypes/Phenotypes
3.4. Special Fertilizers (Nanoparticles)
3.5. Biofertilizers (Test with Bacteria)
3.6. Health Impacts
4. Future Perspectives and Strategies
5. Conclusions
Funding
Conflicts of Interest
References
- Estrada-Domínguez, V.; Sanchez-Chavez, E.; Lazaro, E.C.; Márquez-Quiroz, C. Effect of Zinc Chelate and Sulfate on Mineral Content, Antioxidant Activity and Grain Yield of Vigna unguiculata L. Philipp. Agric. Sci. 2020, 103, 47–54. Available online: https://www.researchgate.net/publication/340464423 (accessed on 5 January 2025). [CrossRef]
- Cakmak, I.; McLaughlin, M.J.; White, P. Zinc for better crop production and human health. Plant Soil 2017, 411, 1–4. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Silva, V.M.; Nardeli, A.J.; Mendes, N.A.d.C.; Rocha, M.d.M.; Wilson, L.; Young, S.D.; Broadley, M.R.; White, P.J.; dos Reis, A.R. Agronomic biofortification of cowpea with zinc: Variation in primary metabolism responses and grain nutritional quality among 29 diverse genotypes. Plant Physiol. Biochem. 2021, 162, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H.; Peerson, J.M.; Baker, S.K.; Hess, S.Y. Preventive Zinc Supplementation among Infants, Preschoolers, and Older Prepubertal Children. Food Nutr. Bull. 2009, 30, S12–S40. [Google Scholar] [CrossRef]
- Walker, C.L.F.; Ezzati, M.; Black, R.E. Global and regional child mortality and burden of disease attributable to zinc deficiency. Eur. J. Clin. Nutr. 2009, 63, 591–597. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2017, 69, 172–180. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Ojeh, N.; Murererehe, J.; Atfi, A.; Razzaque, M.S. Zinc Adequacy Is Essential for the Maintenance of Optimal Oral Health. Nutrients 2020, 12, 949. [Google Scholar] [CrossRef]
- Gupta, P.; Singh, R.; Malhotra, S.; Boora, K.S.; Singal, H.R. Cowpea [Vigna unguiculata (L.) walp.] seed proteins: Heterogeneity in total proteins and protein fractions. Legum. Res.-Int. J. 2014, 37, 62–67. [Google Scholar] [CrossRef]
- López-Morales, D.; de la Cruz-Lázaro, E.; Sánchez-Chávez, E.; Preciado-Rangel, P.; Márquez-Quiroz, C.; Osorio-Osorio, R. Impact of Agronomic Biofortification with Zinc on the Nutrient Content, Bioactive Compounds, and Antioxidant Capacity of Cowpea Bean (Vigna unguiculata L. Walpers). Agronomy 2020, 10, 1460. [Google Scholar] [CrossRef]
- Melo, F.D.B.; Bastos, E.A.; Cardoso, M.J.; Ribeiro, V.Q. Cowpea response to phosphorus and zinc. Rev. Caatinga 2018, 31, 240–245. [Google Scholar] [CrossRef][Green Version]
- Kachinski, W.D.; Ávila, F.W.; dos Reis, A.R.; Muller, M.M.L.; Mendes, M.C.; Petranski, P.H. Agronomic biofortification increases concentrations of zinc and storage proteins in common bean (Phaseolus vulgaris L.) grains. Food Res. Int. 2022, 155, 111105. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Jha, A.B.; Warkentin, T.D. Biofortification of Pulse Crops: Status and Future Perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef]
- Cakmak, I.; Marzorati, M.; Abbeele, P.V.D.; Hora, K.; Holwerda, H.T.; Yazici, M.A.; Savasli, E.; Neri, J.; Du Laing, G. Fate and Bioaccessibility of Iodine in Food Prepared from Agronomically Biofortified Wheat and Rice and Impact of Cofertilization with Zinc and Selenium. J. Agric. Food Chem. 2020, 68, 1525–1535. [Google Scholar] [CrossRef]
- Santos, E.F.; Macedo, F.G.; Rodrigues, M.; Pavinato, P.S.; Lavres, J. Low zinc availability limits the response to phosphorus fertilization in cotton. J. Plant Nutr. Soil Sci. 2024, 187, 375–387. [Google Scholar] [CrossRef]
- Silva, V.M.; Lui, A.C.W.; de Carvalho, M.R.; Namorato, F.A.; Fei, Z.; dos Reis, A.R.; Liu, J.; Vatamaniuk, O.K.; Li, L. The selenium-promoted daidzein production contributes to its induced nodulation in soybean plants. Environ. Exp. Bot. 2024, 218, 105591. [Google Scholar] [CrossRef]
- Ditta, A.; Ullah, N.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M.S. Zn Biofortification in Crops Through Zn-Solubilizing Plant Growth-Promoting Rhizobacteria. In Sustainable Plant Nutrition Under Contaminated Environments; Springer: Cham, Switzerland, 2022; pp. 115–133. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.d.S.; Gato, I.M.B.; Moreira, V.d.A.; de Lima, B.H.; Bastos, A.d.C.; Iqbal, B.; Filho, M.C.M.T. Interaction of Mineral Nutrients and Plant Growth-Promoting Microbes for Biofortification of Different Cropping Systems. J. Plant Growth Regul. 2024, 1, 1–17. [Google Scholar] [CrossRef]
- Santos, E.F.; Filho, E.C.; Fontes, L.E.M.F.; Silva, M.A.P.; Silva, G.N.; Oliveira, A.A.; Rocha, M.d.M.; Silva, V.M.; Reis, A.R. Selenium agronomic biofortification and genotypic variability in physiological responses of cowpea plants under field conditions. Acta Physiol. Plant. 2025, 47, 18. [Google Scholar] [CrossRef]
- Prom-U-Thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guilherme, L.R.G.; Corguinha, A.P.B.; Guo, S.; Kaur, C.; Naeem, A.; Yamuangmorn, S.; et al. Simultaneous Biofortification of Rice with Zinc, Iodine, Iron and Selenium Through Foliar Treatment of a Micronutrient Cocktail in Five Countries. Front. Plant Sci. 2020, 11, 589835. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.K.; Chejara, S.; Malik, K.; Kumar, R.; Kumar, A.; Yadav, R.K. Agronomic biofortification of food crops: An emerging opportunity for global food and nutritional security. Front. Plant Sci. 2022, 13, 1055278. [Google Scholar] [CrossRef]
- Silva, V.M.; Wilson, L.; Young, S.D.; Broadley, M.R.; White, P.J.; dos Reis, A.R. Interaction between sulfur and selenium in agronomic biofortification of cowpea plants under field conditions. Plant Soil 2022, 486, 69–85. [Google Scholar] [CrossRef]
- Inacio, K.A.M.; Farfan, N.C.; Azevedo, C.E.X.; Polatto, M.A.G.; Carrion, N.S.; Mendes, P.V.S.; Mateus, N.S.; Santos, E.F. Potential of Cassava Clones for Iron, Zinc, and Selenium Biofortification. Agriculture 2024, 14, 268. [Google Scholar] [CrossRef]
- Santos, E.F.; Figueiredo, C.O.; Rocha, M.A.P.; Lanza, M.G.D.B.; Silva, V.M.; Reis, A.R. Phosphorus and Selenium Interaction Effects on Agronomic Biofortification of Cowpea Plants. J. Soil Sci. Plant Nutr. 2023, 23, 4385–4395. [Google Scholar] [CrossRef]
- Silva, V.M.; Boleta, E.H.; Martins, J.T.; dos Santos, F.L.; Silva, A.C.d.R.; Alcock, T.D.; Wilson, L.; de Sá, M.E.; Young, S.D.; Broadley, M.R.; et al. Agronomic biofortification of cowpea with selenium: Effects of selenate and selenite applications on selenium and phytate concentrations in seeds. J. Sci. Food Agric. 2019, 99, 5969–5983. [Google Scholar] [CrossRef]
- Costa, A.d.S.; Mendes, M.H.A.; de Souza, D.C.; de Páez, B.C.M.; Guerra, T.S.; Costa, P.A.; Ossani, P.C.; Silva, M.L.d.S.; Resende, L.V. Agronomic Biofortification of Unconventional Food Plants with Zinc. ACS Omega 2024, 9, 48416–48426. [Google Scholar] [CrossRef]
- Félix, M.R.; Zauza, S.B.; Namorato, F.A.; Castro, D.G.; Martins, F.A.D.; Lessa, J.H.d.L.; Guilherme, L.R.G.; Botelho, F.B.S. Biofortification of upland rice using selenium-enriched urea: Evaluation of potential genotypes. J. Food Compos. Anal. 2023, 122, 105409. [Google Scholar] [CrossRef]
- Hefferon, K. Biotechnological Approaches for Generating Zinc-Enriched Crops to Combat Malnutrition. Nutrients 2019, 11, 253. [Google Scholar] [CrossRef]
- dos Santos, L.C.; Martins, G.S.; Lima, J.d.S.; da Silva, G.A.M.; Nunes, M.F.P.N.; de Oliveira, I.P.; de Andrade, E.S.; Nascimento, V.d.L.; Guilherme, L.R.G.; Lopes, G. Enhancing Wheat Resilience to Water Deficit through Selenium Biofortification: Perspectives on Physiological, Biochemical and Nutritional Responses. J. Soil Sci. Plant Nutr. 2024, 24, 7418–7435. [Google Scholar] [CrossRef]
- Zauza, S.B.; Namorato, F.A.; Silva, V.M.; de Oliveira, C.; Lopes, G.; Li, L.; Pasqual, M.; Guilherme, L.R.G.; Dória, J. Biofortification of Ora-pro-nobis (Pereskia aculeata Mill.) Through Soil Selenium Application. J. Soil Sci. Plant Nutr. 2023, 23, 5233–5244. [Google Scholar] [CrossRef]
- Cipriano, P.E.; Júnior, M.S.; de Souza, R.R.; da Silva, D.F.; da Silva, R.F.; Faquin, V.; Silva, M.L.d.S.; Guilherme, L.R.G. Macronutrients content of radishes and the influence of biofortification with selenium. Sci. Hortic. 2022, 296, 110908. [Google Scholar] [CrossRef]
- da Silva, D.F.; Cipriano, P.E.; de Souza, R.R.; Siueia, M.; Faquin, V.; Silva, M.L.d.S.; Guilherme, L.R.G. Biofortification with selenium and implications in the absorption of macronutrients in Raphanus sativus L. J. Food Compos. Anal. 2020, 86, 103382. [Google Scholar] [CrossRef]
- Oliveira, C.F.; Silva, M.G.; Silva, G.N.; Ducatti, K.R.; Rocha, M.d.M.; Reis, A.R.; Rabêlo, F.H.S.; Lavres, J.; Santos, E.F. Agronomic Biofortification Increases Concentrations of Zinc and Storage Proteins in Cowpea Grains. Agriculture 2024, 14, 911. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.d.S.; Fernandes, G.C.; da Silva, E.C.; da Costa, K.N.; de Souza, J.S.; Leite, G.d.S.; Biagini, A.L.C.; Galindo, F.S.; Filho, M.C.M.T. Integrated use of plant growth-promoting bacteria and nano-zinc foliar spray is a sustainable approach for wheat biofortification, yield, and zinc use efficiency. Front. Plant Sci. 2023, 14, 1146808. [Google Scholar] [CrossRef]
- Fonseca, J.H.d.S.; Neta, M.N.A.; Pegoraro, R.F.; de Almeida, G.F.; da Costa, C.A.; de Almeida, E.S. Chickpea production in response to fertilization with zinc and doses of phosphorus. Comun. Sci. 2020, 11, e3106. [Google Scholar] [CrossRef]
- Carmona, V.M.V.; Filho, A.B.C.; DE Almeida, H.J.; Silva, G.C.; DOS Reis, A.R. Agronomic biofortification of beet plants with zinc via seed priming1. Rev. Caatinga 2020, 33, 116–123. [Google Scholar] [CrossRef]
- Guevara, J.E.M.; Sanches, A.G.; Pedrosa, V.M.D.; da Cruz, M.C.P.; da Silva, J.A.A.; Teixeira, G.H.d.A. The injection of zinc sulfate into banana tree pseudostem can triple the zinc content and it is an effective method for fruit biofortification. J. Food Compos. Anal. 2021, 102, 104020. [Google Scholar] [CrossRef]
- Tremea, M.G.; Conceição, G.M.; Da Silva, J.A.G.; Schmidt, M.E.; Arnold, L.E.; Bagolin, J.V.; Schalanski, G.R.; Babeski, C.M. Physiological quality of white oat seeds produced with Nitrogen, Iron and Zinc biofortification. Contrib. A LAS Cienc. SOCIALES 2024, 17, e5199. [Google Scholar] [CrossRef]
- Ellis, A.J. The solubility of zinc sulfide in water at high temperatures. Econ. Geol. 1959, 54, 1035–1039. [Google Scholar] [CrossRef]
- Reed, R.B.; Ladner, D.A.; Higgins, C.P.; Westerhoff, P.; Ranville, J.F. Solubility of nano-zinc oxide in environmentally and biologically important matrices. Environ. Toxicol. Chem. 2012, 31, 93–99. [Google Scholar] [CrossRef]
- Kaya, H.; Karabacak, R.B.; Çelik, Y.; Peake, J.; Watkins, S.; Sayer, R.; Suvacı, E. Application of zincon analysis to investigate the zinc speciation in aqueous media for further understanding of ZnO solubility. Microchem. J. 2023, 191, 108772. [Google Scholar] [CrossRef]
- Vattani, H.; Vafaee, N.; Moghadam, A.L.; Keshavarz, N. Research and Comparison of Different Levels of Fertilizer Nano Zinc Chelates and Zinc Sulfate and Its Effect on the Growth Parameters of Parsley (Petroselinum crispum L.). Eur. J. Agric. Food Sci. 2021, 3, 23–27. [Google Scholar] [CrossRef]
- Javid, S.F.; Moravej, H.; Ghaffarzadeh, M.; Esfahani, M.B. Comparison of Zinc Sulfate and Zinc Threonine Based on Zn Bioavailability and Performance of Broiler Chicks. Biol. Trace Element Res. 2021, 199, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Ponce-García, C.O.; Soto-Parra, J.M.; Sánchez, E.; Muñoz-Márquez, E.; Piña-Ramírez, F.J.; Flores-Córdova, M.A.; Pérez-Leal, R.; Yáñez Muñoz, R.M. Efficiency of Nanoparticle, Sulfate, and Zinc-Chelate Use on Biomass, Yield, and Nitrogen Assimilation in Green Beans. Agronomy 2019, 9, 128. [Google Scholar] [CrossRef]
- Jalal, A.; Galindo, F.S.; Freitas, L.A.; Oliveira, C.E.d.S.; de Lima, B.H.; Pereira, Í.T.; Ferraz, G.F.; de Souza, J.S.; da Costa, K.N.; Nogueira, T.A.R.; et al. Yield, zinc efficiencies and biofortification of wheat with zinc sulfate application in soil and foliar nanozinc fertilisation. Crop. Pasture Sci. 2022, 73, 749–759. [Google Scholar] [CrossRef]
- Mahdieh, M.; Sangi, M.R.; Bamdad, F.; Ghanem, A. Effect of seed and foliar application of nano-zinc oxide, zinc chelate, and zinc sulphate rates on yield and growth of pinto bean (Phaseolus vulgaris) cultivars. J. Plant Nutr. 2018, 41, 2401–2412. [Google Scholar] [CrossRef]
- Mahdavi, S.; Karimi, R.; Goudarzi, A.V. Effect of nano zinc oxide, nano zinc chelate and zinc sulfate on vineyard soil Zn- availability and grapevines (Vitis vinifera L.) yield and quality. J. Plant Nutr. 2022, 45, 1961–1976. [Google Scholar] [CrossRef]
- García-Latorre, C.; Velázquez, R.; Hernández, A.; Tejero, P.; Poblaciones, M.J. Combining Zinc Biofortification and Native Trichoderma Inoculation Strategies for Subterranean Clover. Sustainability 2024, 16, 3730. [Google Scholar] [CrossRef]
- Daccak, D.; Lidon, F.C.; Luís, I.C.; Marques, A.C.; Coelho, A.R.F.; Pessoa, C.C.; Caleiro, J.; Ramalho, J.C.; Leitão, A.E.; Silva, M.J.; et al. Zinc Biofortification in Vitis vinifera: Implications for Quality and Wine Production. Plants 2022, 11, 2442. [Google Scholar] [CrossRef]
- García-Latorre, C.; Reynolds-Marzal, M.D.; De la Peña-Lastra, S.; Pinheiro, N.; Poblaciones, M.J. Soil and Foliar Zinc Biofortification of Triticale (x Triticosecale) under Mediterranean Conditions: Effects on Forage Yield and Quality. Plants 2024, 13, 1917. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Iron and zinc biofortification and bioaccessibility in carrot ‘Dordogne’: Comparison between foliar applications of chelate and sulphate forms. Sci. Hortic. 2023, 312, 111851. [Google Scholar] [CrossRef]
- Botoman, L.; Chimungu, J.G.; Bailey, E.H.; Munthali, M.W.; Ander, E.L.; Mossa, A.; Young, S.D.; Broadley, M.R.; Lark, R.M.; Nalivata, P.C. Agronomic biofortification increases grain zinc concentration of maize grown under contrasting soil types in Malawi. Plant Direct 2022, 6, e458. [Google Scholar] [CrossRef]
- Abdu, A.O.; De Groote, H.; Joy, E.J.M.; Kumssa, D.B.; Broadley, M.R.; Gashu, D. Zinc agronomic biofortification of staple crops may be a cost-effective strategy to alleviate zinc deficiency in Ethiopia. Front. Nutr. 2022, 9, 1037161. [Google Scholar] [CrossRef]
- Azeem, A.; Ul-Allah, S.; Azeem, F.; Naeem, M.; Sattar, A.; Ijaz, M.; Sher, A. Effect of foliar applied zinc sulphate on phenotypic variability, association and heritability of yield and zinc biofortification related traits of wheat genotypes. Heliyon 2023, 9, e19643. [Google Scholar] [CrossRef]
- Botoman, L.; Nalivata, P.C.; Chimungu, J.G.; Munthali, M.W.; Bailey, E.H.; Ander, E.L.; Lark, R.M.; Mossa, A.; Young, S.D.; Broadley, M.R. Increasing zinc concentration in maize grown under contrasting soil types in Malawi through agronomic biofortification: Trial protocol for a field experiment to detect small effect sizes. Plant Direct 2020, 4, e00277. [Google Scholar] [CrossRef]
- Grujcic, D.; Yazici, A.M.; Tutus, Y.; Cakmak, I.; Singh, B.R. Biofortification of Silage Maize with Zinc, Iron and Selenium as Affected by Nitrogen Fertilization. Plants 2021, 10, 391. [Google Scholar] [CrossRef]
- Lian, J.; Cheng, L.; Wang, X.; Chen, Y.; Deng, C.; Wang, Y.; Pan, J.; Shohag, J.I.; Xin, X.; He, Z.; et al. Bespoke ZnO NPs Synthesis Platform to Optimize Their Performance for Improving the Grain Yield, Zinc Biofortification, and Cd Mitigation in Wheat. ACS Sustain. Chem. Eng. 2024, 12, 716–727. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, P.K.; Singh, R.P. Growth promotion and zinc biofortification in lettuce (Lactuca sativa L.) by the application of Talaromyces strain as a biostimulant. Sci. Hortic. 2024, 323, 112534. [Google Scholar] [CrossRef]
- Costa, R.M.C.; Grangeiro, L.C.; Gonçalves, F.d.C.; dos Santos, E.C.; de Medeiros, J.F.; Sá, F.V.d.S.; Pereira, D.d.F.; Carmo, L.H.d.A.; Souza, B.d.P. Agronomic Biofortification and Yield of Beet Fertilization with Zinc. Agronomy 2023, 13, 1491. [Google Scholar] [CrossRef]
- Pascoalino, J.A.L.; Thompson, J.A.; Wright, G.; Franco, F.A.; Scheeren, P.L.; Pauletti, V.; Moraes, M.F.; White, P.J. Grain zinc concentrations differ among Brazilian wheat genotypes and respond to zinc and nitrogen supply. PLoS ONE 2018, 13, e0199464. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of edible plants with selenium and iodine—A systematic literature review. Sci. Total. Environ. 2021, 754, 141983. [Google Scholar] [CrossRef]
- Shelford, V.E. Some Concepts of Bioecology. Ecology 1931, 12, 455–467. [Google Scholar] [CrossRef]
- Freitas, T.K.T.; Gomes, F.d.O.; Araújo, M.d.S.; Silva, I.C.V.; Silva, D.J.S.; Damasceno-Silva, K.J.; Rocha, M.d.M. Potential of cowpea genotypes for nutrient biofortification and cooking quality. Rev. Ciência Agronômica 2022, 53, 1. [Google Scholar] [CrossRef]
- Zanotti, R.F.; Lopes, J.C.; Motta, L.B.; Mengarda, L.H.G.; Marçal, T.D.S.; Guilhen, J.H.S.; Paiva, C.E.C. Genetic variability and heritability of biofortified grain beans genotypes. Braz. J. Dev. 2020, 6, 29381–29395. [Google Scholar] [CrossRef]
- Maziero, S.; Ribeiro, N.; Casagrande, C. Genetic dissimilarity of common bean lines for agronomic and biofortification traits. Genet. Mol. Res. 2017, 16, 3. [Google Scholar] [CrossRef]
- Lopes, A.F.d.S.; Jean, A.; Damasceno-Silva, K.J.; Martins, M.D.C.d.C.e.; Rocha, M.d.M. Characterization of cowpea cultivars for grain size, color, and biofortification. Rev. Caatinga 2023, 36, 207–214. [Google Scholar] [CrossRef]
- Zilio, M.; Souza, C.A.; Coelho, C.M.M. Phenotypic diversity of nutrients and anti-nutrients in bean grains grown in different locations. Rev. Bras. De Cienc. Agrar. 2017, 12, 526–534. [Google Scholar] [CrossRef]
- Maziero, S.M.; Ribeiro, N.D.; Facco, H.D.S. Genetic parameters of agronomic and nutritional traits of common bean (Phaseolus vulgaris L.) populations with biofortified grains. Aust. J. Crop Sci. 2016, 10, 824–830. [Google Scholar] [CrossRef]
- Martins, S.; Melo, P.; Faria, L.; Souza, T.; Melo, L.; Pereira, H. Genetic parameters and breeding strategies for high levels of iron and zinc in Phaseolus vulgaris L. Genet. Mol. Res. 2016, 15, 2. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; Oliveira, C.E.d.S.; dos Reis, A.R.; Nogueira, T.A.R.; Neto, M.J.M.; Mortinho, E.S.; Fernandes, G.C.; Filho, M.C.M.T. Common Bean Yield and Zinc Use Efficiency in Association with Diazotrophic Bacteria Co-Inoculations. Agronomy 2021, 11, 959. [Google Scholar] [CrossRef]
- Jalal, A.; Oliveira, C.E.d.S.; Fernandes, H.B.; Galindo, F.S.; da Silva, E.C.; Fernandes, G.C.; Nogueira, T.A.R.; De Carvalho, P.H.G.; Balbino, V.R.; de Lima, B.H.; et al. Diazotrophic Bacteria Is an Alternative Strategy for Increasing Grain Biofortification, Yield and Zinc Use Efficiency of Maize. Plants 2022, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.; Di Gioia, F.; Lambert, J.D.; Connolly, E.L. Zinc biofortification through seed nutri-priming using alternative zinc sources and concentration levels in pea and sunflower microgreens. Front. Plant Sci. 2023, 14, 1177844. [Google Scholar] [CrossRef]
- Philipo, M.; Ndakidemi, P.A.; Mbega, E.R. Importance of common bean genetic zinc biofortification in alleviating human zinc deficiency in sub-Saharan Africa. Cogent Food Agric. 2021, 7, 1907954. [Google Scholar] [CrossRef]
- Mehdaoui, Y.; Yeddes, W.; Selmi, S.; Saidani-Tounsi, M.; Abdelly, C.; Ben Farhat, M. Variations of nutritional and antioxidant contents of Lepidium sativum L. sprouts as affected by zinc biofortification. Sci. Hortic. 2024, 329, 112994. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Zhang, W.; Chen, X.; Zou, C. Agronomic Approach of Zinc Biofortification Can Increase Zinc Bioavailability in Wheat Flour and thereby Reduce Zinc Deficiency in Humans. Nutrients 2017, 9, 465. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- de Sousa, Á.P.; Universidade Federal do Tocantins; Nascimento, I.R.D.; Carline, J.V.G.; de Oliveira, L.B.; Teles, S.P.; Pereira, F.F.; Neto, J.F.d.M.; Kaonrdörfer, D.B. Doses and sources of foliar applied zinc on lettuce seedling quality. Rev. Agrar. Acad. 2022, 5, 111–126. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Scrimgeour, A.G.; Condlin, M.L.; Otieno, L.; Bovill, M.E. Zinc Intervention Strategies: Costs and Health Benefits. In Nutrients, Dietary Supplements, and Nutriceuticals; Humana Press: Totowa, NJ, USA, 2011; pp. 189–214. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ullah, A.; Nadeem, F.; Im, S.Y.; Park, S.K.; Lee, D.-J. Agronomic Biofortification of Zinc in Pakistan: Status, Benefits, and Constraints. Front. Sustain. Food Syst. 2020, 4, 591722. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zou, C.-Q.; Mirza, Z.; Li, H.; Zhang, Z.-Z.; Li, D.-P.; Xu, C.-L.; Zhou, X.-B.; Shi, X.-J.; Xie, D.-T.; et al. Cost of agronomic biofortification of wheat with zinc in China. Agron. Sustain. Dev. 2016, 36, 44. [Google Scholar] [CrossRef]
- Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hejnak, V.; Vachova, P.; Hossain, A. Zinc Biofortification in Food Crops Could Alleviate the Zinc Malnutrition in Human Health. Molecules 2021, 26, 3509. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chakraborty, M.; Padhan, D.; Saha, B.; Murmu, S.; Batabyal, K.; Seth, A.; Hazra, G.; Mandal, B.; Bell, R. Agronomic biofortification of zinc in rice: Influence of cultivars and zinc application methods on grain yield and zinc bioavailability. Field Crop. Res. 2017, 210, 52–60. [Google Scholar] [CrossRef]
- Sida-Arreola, J.; Sánchez, E.; Preciado-Rangel, P.; Márquez-Quiroz, C. Does zinc biofortification affects the antioxidant activity in common bean? Cogent Food Agric. 2017, 3, 1283725. [Google Scholar] [CrossRef]
- Singhal, T.; Satyavathi, C.T.; Singh, S.P.; Mallik, M.; Anuradha, N.; Sankar, S.M.; Bharadwaj, C.; Singh, N. Achieving nutritional security in India through iron and zinc biofortification in pearl millet (Pennisetum glaucum (L.) R. Br.). Physiol. Mol. Biol. Plants 2022, 28, 849–869. [Google Scholar] [CrossRef]
- Teklu, D.; Gashu, D.; Joy, E.J.M.; Lark, R.M.; Bailey, E.H.; Wilson, L.; Amede, T.; Broadley, M.R. Impact of zinc and iron agronomic biofortification on grain mineral concentration of finger millet varieties as affected by location and slope. Front. Nutr. 2023, 10, 1159833. [Google Scholar] [CrossRef]
- Kraisig, A.R.; da Silva, J.A.G.; Carvalho, I.R.; Colet, C.d.F.; Fachinetto, J.; Fraga, D.d.R.; Conceição, G.M.; Peter, C.L.; da Rosa, J.A.; Basso, N.C.F.; et al. Biofortification via foliar application of zinc in oat grains and the effects on nutritional quality and productivity indicators. Aust. J. Crop Sci. 2023, 17, 798–806. [Google Scholar] [CrossRef]
- Ram, H.; Rashid, A.; Zhang, W.; Duarte, A.P.; Phattarakul, N.; Simunji, S.; Kalayci, M.; Freitas, R.; Rerkasem, B.; Bal, R.S.; et al. Biofortification of wheat, rice and common bean by applying foliar zinc fertilizer along with pesticides in seven countries. Plant Soil 2016, 403, 389–401. [Google Scholar] [CrossRef]
- Borymski, S.; Markowicz, A.; Nowak, A.; Matus, K.; Dulski, M.; Sułowicz, S. Copper-oxide nanoparticles exert persistent changes in the structural and functional microbial diversity: A 60-day mesocosm study of zinc-oxide and copper-oxide nanoparticles in the soil-microorganism-nanoparticle system. Microbiol. Res. 2023, 274, 127395. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, K.; Diao, T.; Sun, Y.; Sun, T.; Wang, C. Influence of continuous fertilization on heavy metals accumulation and microorganism communities in greenhouse soils under 22 years of long-term manure organic fertilizer experiment. Sci. Total. Environ. 2025, 959, 178294. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Deng, L.; Narejo, M.-U.; Baloch, I.; Deng, L.; Chachar, S.; Li, Y.; Li, J.; Bozdar, B.; Chachar, Z.; et al. Bridging agro-science and human nutrition: Zinc nanoparticles and biochar as catalysts for enhanced crop productivity and biofortification. Front. Plant Sci. 2024, 15, 1435086. [Google Scholar] [CrossRef]
- Baral, K.; Shivay, Y.S.; Prasanna, R.; Kumar, D.; Srinivasarao, C.; Mandi, S.; Nayak, S.; Reddy, K.S. Enhancing physiological metrics, yield, zinc bioavailability, and economic viability of Basmati rice through nano zinc fertilization and summer green manuring in semi–arid South Asian ecosystem. Front. Plant Sci. 2023, 14, 1283588. [Google Scholar] [CrossRef]
| Culture | Test Conducted | Application Method | Form of Applied Zinc | Zn Concentration | Application Stage | Effect | Side Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Common bean | In the field | Soil: seeding furrow vs. leaf: backpack sprayer | Zinc sulfate heptahydrate | Soil: 4 kg ha−1 incorporated into NPK fertilizer with 1% Zn; leaf: 0, 300, 600, 900, 1200 and 1500 g ha−1 | Soil: planting; leaf: R6, R8 e R9 | The single foliar application of 600 g ha of Zn at the early grain filling stage proved to be the most effective method for improving Zn concentration in the grains, without affecting yield. The agronomic biofortification of bean grains with Zn was more efficient with foliar Zn application than with soil Zn fertilization. | Soil fertilization with zinc resulted in an average increase of 20% to 30% in the concentration of total amino acids and storage proteins, such as albumin, globulin and glutelin, compared to treatments without zinc application. | [14] |
| 2 | Cowpea | Greenhouse | Leaf CO2 pressurized backpack sprayer | Zinc sulfate heptahydrate | 0 and 600 g ha−1 | Full flowering 43 days after sowing (DAS) in the 1st year and 42 DAS in the 2nd year | All genotypes evaluated show zinc enrichment in the grains in response to the foliar application of the micronutrient in the form of zinc sulfate. | There was a significant increase in the concentrations of storage proteins, total free amino acids, sucrose and total sugars. | [36] |
| 3 | Wheat | In the field | Leaf: hand pump | Nano-Zn and bacteria: Azospirillum brasilense, Bacillus subtilis e Pseudomonas fluorescens | 0, 0.75, 1.5, 3 and 6 kg ha−1 | Profiling and grain filling | The foliar application of Nano-Zn increased N, P and Zn concentrations in the plant and grain, in addition to helping wheat growth and yield. | There was an increase in the concentrations of zinc, nitrogen and phosphorus in both the aerial part and the wheat grains. Inoculation with Pseudomonas fluorescens increased phosphorus concentration in grains by up to 32.2% compared to the control. | [38] |
| 4 | Rice | In the field | Leaf: hand pump | Zinc sulfate heptahydrate | 0.5% ZnSO4 7H2O in spray solution. The same concentration was used in a micronutrient cocktail with iodine, iron and selenium | Panicle initiation and early stages of grain milk | The zinc content in brown rice grains increased with the foliar zinc alone and micronutrient cocktail treatments. The increase in zinc content was from 21.4 mg/kg to 28.1 mg/kg with the application of the nutrient and 26.8 mg/kg with the micronutrient cocktail solution, applied via foliar application. | Iodine and selenium showed significant increases. The iron concentration in the grains was not significantly affected by the application of the micronutrient cocktail. There were no significant results for protein levels. | [23] |
| 5 | Beet | Greenhouse | Nutrient solution: seeds immersed in different concentrations of zinc | Zinc sulfate and chloride | 0, 10 and 30 mg mL−1 | Seed priming | The application of zinc, mainly as a sulfate, affected all the parameters evaluated, such as fresh mass (an increase of 70 and 100 g per plant with 10 mg/mL of Zn) and dry root mass, photosynthesis, and zinc concentration in the root (121 and 42 mg/kg) in the years 2015 and 2016, respectively. | The study did not measure protein and amino acid concentrations. | [36] |
| 6 | White oats | In the field | Leaf: backpack sprayer | Zinc sulfate | 0, 1000, 2000 and 4000 g ha−1 | Exposed inflorescence, at the beginning of grain filling | The application of zinc, either alone or in combination with iron via foliar spraying, influenced the physiological quality of white oat seeds. This term refers to the set of attributes that determine the seeds’ potential to germinate and produce vigorous seedlings capable of establishing successfully under varying environmental conditions. Depending on the applied dose and the parameter evaluated—such as germination rate, seedling growth, or membrane integrity—the effects observed were either beneficial or detrimental. | The foliar application of iron and zinc, alone or combined, positively influenced the chemical composition of seeds, including protein and nitrogen content. The use of iron and zinc contributed to improving the nutritional quality of the seeds, resulting in greater vigor and metabolic efficiency during seedling development. | [42] |
| 7 | Banana | In the field | Nutrient solution: injections of solutions with a 9 cm needle to reach the central axis of the pseudostem. | Zinc sulfate | 10.83 g/L (1%), 21.66 g/L (2%) and 43.32 g/L (4%) | 15 days before harvesting the fruits | It was possible to biofortify the banana plant by injecting a solution containing 20 and 40 g/L of zinc sulfate into the pseudostem, tripling the zinc content in the fruits compared to the control, with levels of 3.66 to 3.39 mg/100 g. | Protein levels were not evaluated. However, there was an increase in the soluble solids/acidity ratio, which may improve the perception of sweetness. | [41] |
| 8 | Chickpea | In the field | 50% soil and 50% leaf, and 100% soil. Soil: sowing; sheet: hand sprayer | Zinc sulfate | 2 kg/ha | Sowing and flowering | The zinc fertilization of chickpeas did not influence the crop’s production components. | Zinc played a complementary role in nutritional management, increasing the efficiency of phosphorus use at lower doses. | [39] |
| Culture | Test Conducted | Application Method | Form of Applied Zinc | Zn Concentration | Application Stage | Effect | Side Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Beet | In the field | Nutrient solution: fertigation | Zinc sulfate | 0, 1.5, 3.0, 4.5 and 6.0 kg ha−1 | 23 days after sowing (2019) and 29 days (2021) | In the 2021 experiment, increasing zinc doses promoted the biofortification of beetroot, as it demonstrated a linear increase in the zinc levels contained in the plant’s tuberous root. | There was no evaluation of amino acids and proteins. | [63] |
| 2 | Banana | In the field | Nutrient solution: injections of solutions with a 9 cm needle to reach the central axis of the pseudostem | Zinc sulfate | 10.83 g/L (1%), 21.66 g/L (2%) and 43.32 g/L (4%) | 15 days before harvesting the fruits | It was possible to biofortify the banana plant by injecting a solution containing 20 and 40 g/L of zinc sulfate into the pseudostem, tripling the zinc content in the fruits compared to the control, with levels of 3.66 to 3.39 mg/100 g. | Protein levels were not evaluated. However, there was an increase in the soluble solids/acidity ratio, which may improve the perception of sweetness. | [41] |
| 3 | Common bean | In the field | Soil: seeding furrow vs. leaf: backpack sprayer | Zinc sulfate heptahydrate | Soil: 4 kg ha−1 incorporated into NPK fertilizer with 1% Zn; leaf: 0, 300, 600, 900, 1200 and 1500 g ha−1 | Solo: planting; leaf: R6, R8 and R9 | The single foliar application of 600 g ha of Zn at the early grain filling stage proved to be the most effective method for improving Zn concentration in the grains, without affecting yield. The agronomic biofortification of bean grains with Zn was more efficient with foliar Zn application than with soil Zn fertilization. | Soil fertilization with zinc resulted in an average increase of 20% to 30% in the concentration of total amino acids and storage proteins, such as albumin, globulin and glutelin, compared to treatments without zinc application. | [14] |
| 4 | Wheat | In the field | Nutrient solution: fertigation | Zinc sulfate | 0.15 μM and 2.25 μM | Weekly: from transplanting until plant maturity | The zinc content in wheat grains more than doubled when Zn and N supplies were increased in both genotypes evaluated. | Zinc and nitrogen supply significantly influenced grain zinc concentration, while protein and amino acid content were correlated with zinc uptake and translocation in plants. | [64] |
| Culture | Test Conducted | Application Method | Form of Applied Zinc | Zn Concentration | Application Stage | Effect | Side Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Cowpea | Laboratory | No information | No information | No information | No information | Analyzing 100 cowpea genotypes, it was observed that the zinc content of the grains varied from 2.35 to 4.57 g/100 g, with an average of 3.31 mg/100 g. Among the other criteria evaluated, the grains of the MNC11-1023E-28 lineage presented a better nutritional quality profile, showing potential as a food to meet consumer demands for reversing iron, zinc and protein deficiencies. | The article focuses on the analysis of iron, zinc and protein contents in different cowpea genotypes, using laboratory techniques such as X-ray fluorescence spectrometry for iron and zinc and the Kjeldahl method for proteins. | [67] |
| 2 | Common bean | Laboratory | No information | No information | No information | No information | Analyzing the diversity of the mineralogical composition in 40 common bean genotypes with a focus on the selection of promising parents in the formation of biofortified cultivars, average zinc levels between 2.8 and 4.6 mg/100 g were found. In this study, the minerals studied presented a heritability index of 80%, which suggests a high possibility of success in the selection of genotypes. | The study focuses on the analysis of the mineralogical composition of bean genotypes. | [68] |
| 3 | Common bean | In the field | No information | No information | No information | No information | The study aimed to evaluate 140 genotypes to identify elite lines that combine high levels of zinc and iron in the grains, along with good adaptability (the ability to maintain productive and nutritional performance in different environments), stability (desirable levels of iron and zinc do not vary drastically between the tested environments), and agronomic potential (the plant’s maximum genetic ability to express desirable traits). The heritability of zinc ranged from 41.7% to 95.7%, and the genetic variation coefficient ranged from 8.51% to 9.04%, indicating favorable conditions for nutrient selection. | The article addresses the application of zinc in the context of the genetic improvement of common beans to increase the iron and zinc contents in the grains. | [73] |
| 4 | Common bean | In the field/laboratory | No information | No information | No information | No information | Evaluating genetic parameters in four common bean populations, characteristics such as first pod insertion, grain yield and zinc concentration in the grain showed high heritability, showing that it is possible to select lines biofortified with zinc and with high agronomic performance through line selection. | No information | [72] |
| 5 | Common bean | In the field | No information | No information | No information | No information | Zinc concentration ranged from 2.03 to 3.60 mg/100 g in dry matter. The concentrations of K, P and Zn showed the greatest contribution to the genetic dissimilarity of the evaluated genotypes. Five lines presented zinc concentrations in the grain above 31 mg/kg, which is considered a high value. | The paper focuses mainly on the evaluation of genetic dissimilarity between common bean lines. | [69] |
| 6 | Cowpea | In the field | No information | No information | No information | No information | Evaluating the characteristics of 24 cultivars, it was observed that grain color and size did not influence the iron, zinc and protein contents in the grains. | The focus of the study is on the analysis of zinc concentrations in cowpea grains, using the nitric–perchloric digestion method and atomic absorption spectrophotometry to determine mineral contents. | [70] |
| 7 | Common bean | In the field | No information | No information | No information | No information | Evaluating the influence of different cultivation environments on 26 bean genotypes, it was observed that in relation to the levels of zinc, phosphorus and crude protein, there was no interaction between the genotypes and the environments. | The article explores the variability in protein and micronutrient contents, such as iron and zinc, in different bean genotypes grown in different locations: iron content in beans ranges from 116 mg/kg to 216 mg/kg, and crude protein content varies significantly between genotypes and growing locations. | [71] |
| Culture | Test Conducted | Application Method | Bacteria Used | Form of Applied Zinc | Zn Concentration | Application Stage | Effect | Side Effects | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bean | In the field | Soil: surface of the soil | Rhizobium tropic, Azospirillum brasilense, Bacilo subtilis e Pseudomonas fluorescens | Zinc sulfate | 0 and 8 kg ha−1 | V1 and V2 | The Zn concentration in the common bean plant and grain and the Zn concentration after harvesting the crop were improved. | The study did not measure protein and amino acid concentrations. | [75] |
| 2 | Corn | In the field | Soil: residual fertilization | Azospirillum brasilense, Bacilo subtilis, e Pseudomonas fluorescens | Zinc sulfate | 0 and 8 kg ha−1. | V1 and V2 | The insertion of the first productive ear, plant height, shoot dry matter, grain yield and hundred-grain weight increased. The Zn content in the leaves increased significantly. There was an increase of 15.2 and 15.7% in the Zn concentration in the shoot in the 2019–2020 and 2020–2021 harvests and of 12.7 and 18.2% in the Zn concentration in the grains. | The study did not measure protein and amino acid concentrations. | [76] |
| 3 | Wheat | In the field | Leaf: hand pump | Azospirillum brasilense, Bacillus subtilis e Pseudomonas fluorescens | Nano-Zn | 0, 0.75, 1.5, 3 and 6 kg ha−1 | Profiling and grain filling | The concentrations of zinc (Zn), nitrogen (N) and phosphorus (P) in the shoots and grains of wheat were significantly influenced. The dry matter of the wheat shoot increased. | There was an increase in the concentrations of zinc, nitrogen and phosphorus in both the aerial part and the wheat grains. Inoculation with Pseudomonas fluorescens increased phosphorus concentration in grains by up to 32.2% compared to the control. | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.B.P.; Borges, L.F.S.; Lucini, F.; Silva, G.N.; Santos, E.F. Technologies in Agronomic Biofortification with Zinc in Brazil: A Review. Plants 2025, 14, 1828. https://doi.org/10.3390/plants14121828
Silva ABP, Borges LFS, Lucini F, Silva GN, Santos EF. Technologies in Agronomic Biofortification with Zinc in Brazil: A Review. Plants. 2025; 14(12):1828. https://doi.org/10.3390/plants14121828
Chicago/Turabian StyleSilva, Ana Beatriz Pires, Lidiane Fátima Santos Borges, Fabíola Lucini, Gutierres Nelson Silva, and Elcio Ferreira Santos. 2025. "Technologies in Agronomic Biofortification with Zinc in Brazil: A Review" Plants 14, no. 12: 1828. https://doi.org/10.3390/plants14121828
APA StyleSilva, A. B. P., Borges, L. F. S., Lucini, F., Silva, G. N., & Santos, E. F. (2025). Technologies in Agronomic Biofortification with Zinc in Brazil: A Review. Plants, 14(12), 1828. https://doi.org/10.3390/plants14121828







