On the Precipice of Extinction: Genetic Data in the Conservation Management of In Situ and Ex Situ Collections of the Critically Endangered Muehlenbeckia tuggeranong (Tuggeranong Lignum)
Abstract
1. Introduction
2. Results
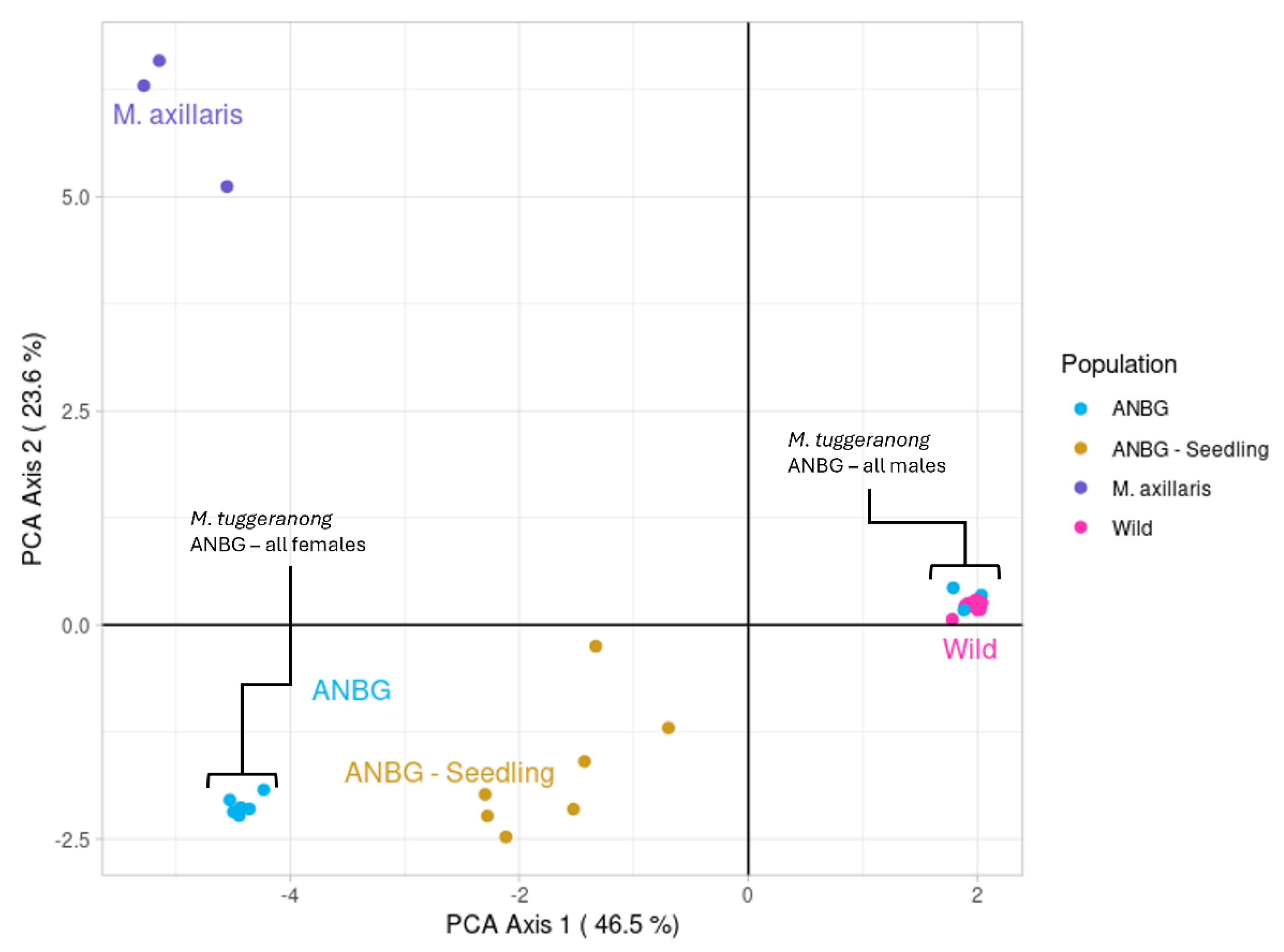
2.1. Assessment of Potential Hybridisation and Population Genetic Structure
2.2. Analysis of Unique and Clonal Genotypes
2.2.1. In Situ Population
2.2.2. Ex Situ Population
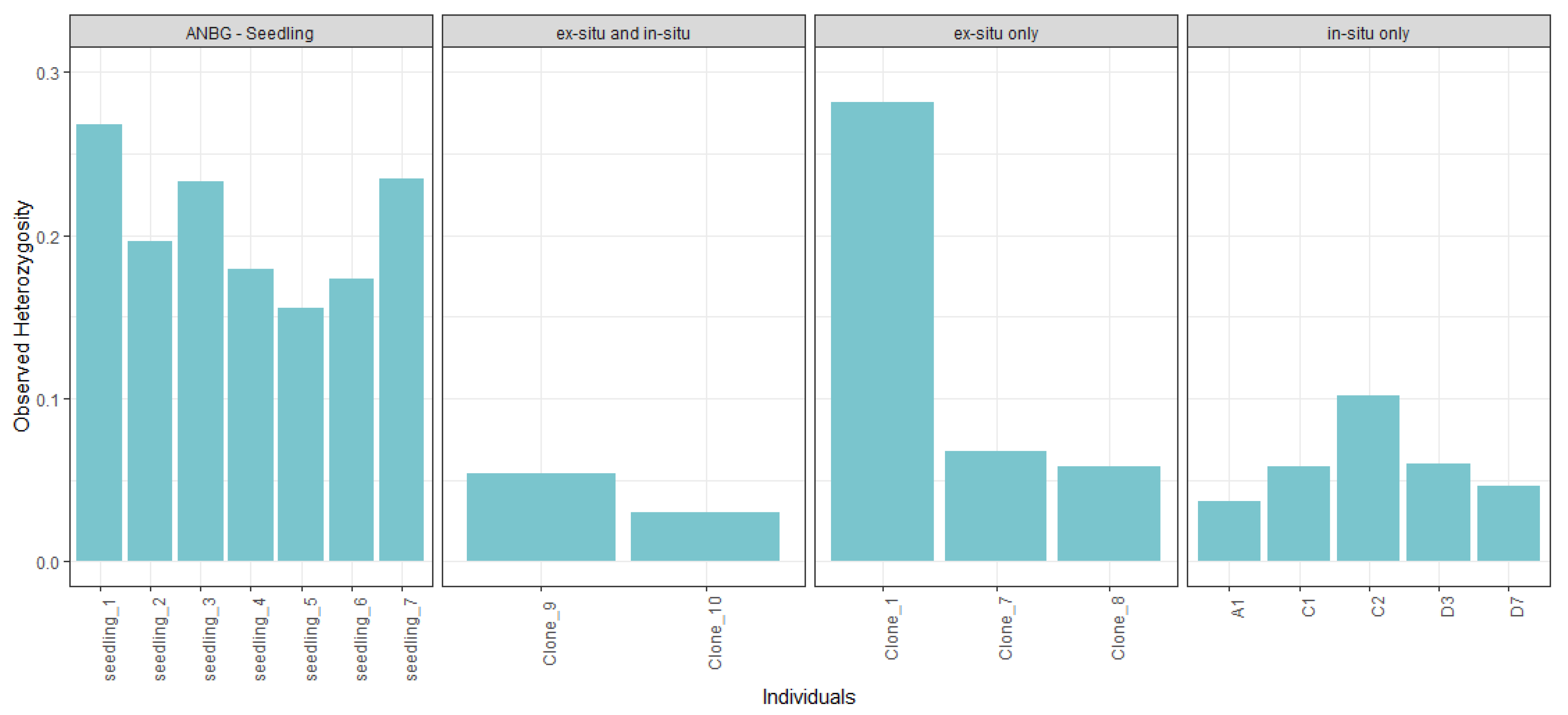
2.3. Genetic Diversity
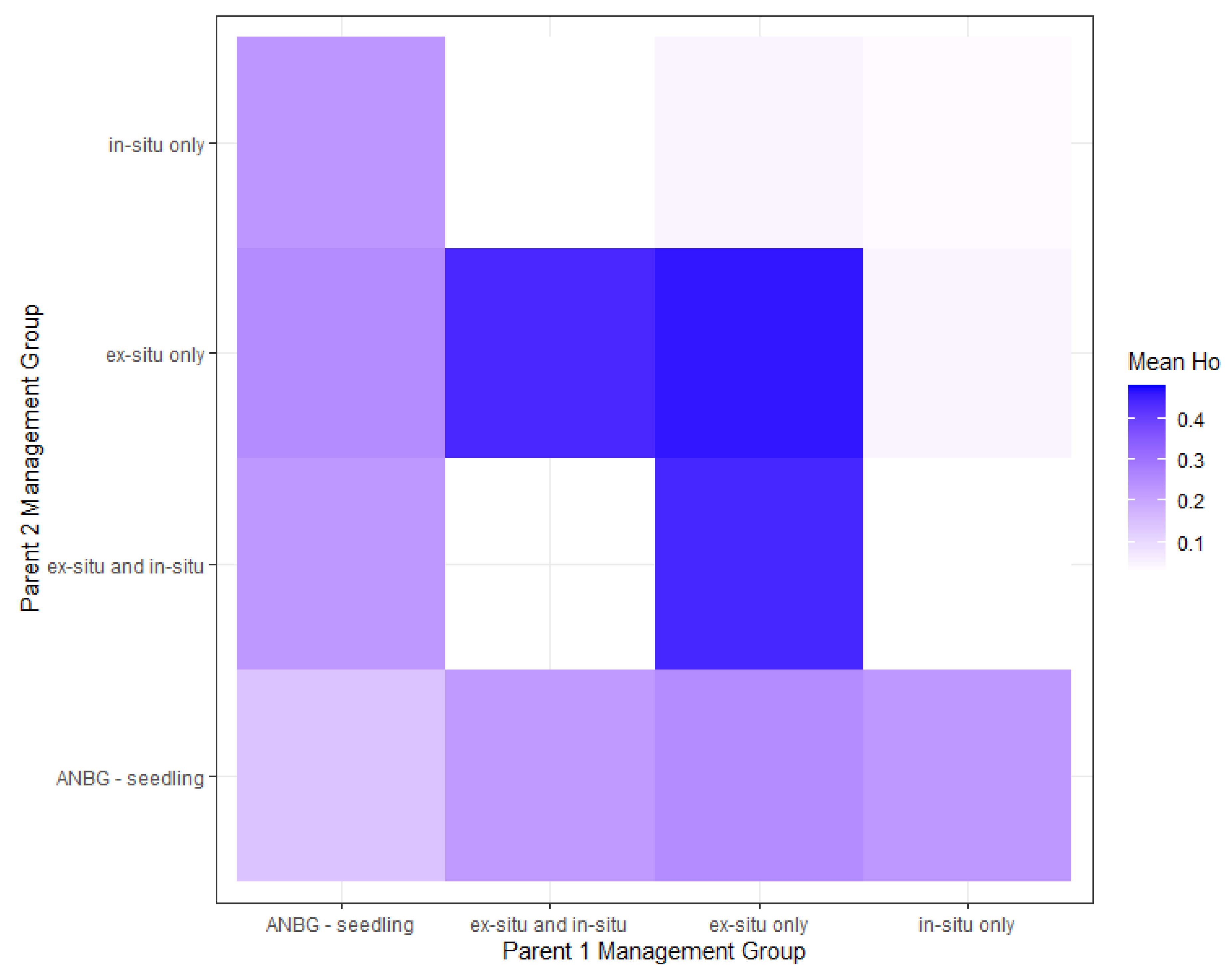
2.4. Breeding Scenarios to Maximise Genetic Diversity
3. Discussion
3.1. Management of Living Collections
3.2. Muehlenbeckia Tuggeranong Case Study
4. Materials and Methods
4.1. Target Species
4.2. DNA Extraction, Amplification, Sequencing and Genotyping
4.3. Genetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ANBG | Australian National Botanic Gardens. |
| PCoA | Principal Co-ordinate Analysis. |
References
- Brito, D.; Fernandez, F.A.S. Dealing with extinction is forever: Understanding the risks faced by small populations. Cienc. E Cult. J. Braz. Assoc. Adv. Sci. 2000, 52, 161–170. [Google Scholar]
- Frankham, R. Genetics and Extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar] [CrossRef]
- Hoban, S.; Bruford, M.W.; Funk, W.C.; Galbusera, P.; Griffith, M.P.; Grueber, C.E.; Heuertz, M.; Hunter, M.E.; Hvilsom, C.; Stroil, B.K.; et al. Global Commitments to Conserving and Monitoring Genetic Diversity Are Now Necessary and Feasible. BioScience 2021, 71, 964–976. [Google Scholar] [CrossRef]
- Schafer, D.; Vincent, H.; Fischer, M.; Kempel, A. The importance of genetic diversity for the translocation of eight threatened plant species into the wild. Glob. Ecol. Conserv. 2020, 24, e01240. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between Fitness and Genetic Diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Turelli, M.; Ginzburg, L.R. Should individual fitness increase with heterozygosity? Genetics 1983, 104, 191–209. [Google Scholar] [CrossRef]
- Furlan, E.; Stoklosa, J.; Griffiths, J.; Gust, N.; Ellis, R.; Huggins, R.M.; Weeks, A.R. Small population size and extremely low levels of genetic diversity in island populations of the platypus, Ornithorhynchus anatinus. Ecol. Evol. 2012, 2, 844–857. [Google Scholar] [CrossRef]
- Wright, L.I.; Tregenza, T.; Hosken, D.J. Inbreeding, inbreeding depression and extinction. Conserv. Genet. 2008, 9, 833–843. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B.W.; Frankham, R. Most species are not driven to extinction before genetic factors impact them. Proc. Natl. Acad. Sci. 2004, 101, 15261–15264. [Google Scholar] [CrossRef]
- Whiteley, A.R.; Fitzpatrick, S.W.; Funk, W.C.; Tallmon, D.A. Genetic rescue to the rescue. Trends Ecol. & Evol. 2015, 30, 42–49. [Google Scholar] [CrossRef]
- Sinclair, J.P.; Korte, J.L.; Freeman, D.C. The pattern of dioecy in terrestrial, temperate plant succession. Evol. Ecol. Res. 2013, 15, 545–556. [Google Scholar]
- Grayson, K.L.; Mitchell, N.J.; Monks, J.M.; Keall, S.N.; Wilson, J.N.; Nelson, N.J. Sex ratio bias and extinction risk in an isolated population of Tuatara (Sphenodon punctatus). PLoS ONE 2014, 9, e94214. [Google Scholar] [CrossRef]
- Volis, S. Living Collections of Threatened Plants in Botanic Gardens: When Is Ex Situ Cultivation Less Appropriate than Quasi In Situ Cultivation? J. Zool. Bot. Gard. 2023, 4, 462–475. [Google Scholar] [CrossRef]
- Mounce, R.; Smith, P.; Brockington, S. Ex situ conservation of plant diversity in the world’s botanic gardens. Nat. Plants 2017, 3, 795–802. [Google Scholar] [CrossRef]
- Foster, J.A.; Walsh, S.K.; Havens, K.; Kramer, A.T.; Fant, J.B. Supporting long-term sustainability of ex situ collections using a pedigree-based population management approach. Appl. Plant Sci. 2021, 10, e11491. [Google Scholar] [CrossRef]
- Lozada-Gobilard, S.; Pánková, H.; Zhu, J.; Stojanova, B.; Münzbergová, Z. Potential risk of interspecific hybridization in ex situ collections. J. Nat. Conserv. 2020, 58, 125912. [Google Scholar] [CrossRef]
- Fant, J.B.; Havens, K.; Kramer, A.T.; Walsh, S.K.; Callicrate, T.; Lacy, R.C.; Maunder, M.; Meyer, A.H.; Smith, P.P. What to do when we can’t bank on seeds: What botanic gardens can learn from the zoo community about conserving plants in living collections. Am. J. Bot. 2016, 103, 1541–1543. [Google Scholar] [CrossRef]
- Thomas, W.J.W.; Anthony, J.M.; Dobrowolski, M.P.; Krauss, S.L. Optimising the conservation of genetic diversity of the last remaining population of a critically endangered shrub. AoB PLANTS 2021, 13, plab005. [Google Scholar] [CrossRef]
- Frankham, R. Genetics and conservation biology. Comptes Rendus Biol. 2003, 326, 22–29. [Google Scholar] [CrossRef]
- Wu, F.Q.; Shen, S.K.; Zhang, X.J.; Wang, Y.H.; Sun, W.B. Genetic diversity and population structure of an extremely endangered species: The world’s largest Rhododendron. AoB PLANTS 2015, 7, plu082. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Guan, S.-M.; Yang, S.-Z.; Luo, Y.; Chen, X.-Y. Genetic decline and inbreeding depression in an extremely rare tree. Conserv. Genet. 2012, 13, 343–347. [Google Scholar] [CrossRef]
- Kramer, A.T.; Havens, K. Plant conservation genetics in a changing world. Trends Plant Sci. 2009, 14, 599–607. [Google Scholar] [CrossRef]
- Finger, A.; Kettle, C.J.; Kaiser-Bunbury, C.N.; Valentin, T.; Doudee, D.; Matatiken, D.; Ghazoul, J. Back from the brink: Potential for genetic rescue in a critically endangered tree. Mol. Ecol. 2011, 20, 3773–3784. [Google Scholar] [CrossRef]
- Penas, J.; Barrios, S.; Bobo-Pinilla, J.; Lorite, J.; Martinez-Ortega, M.M. Designing conservation strategies to preserve the genetic diversity of Astragalus edulis Bunge, an endangered species from western Mediterranean region. PeerJ 2016, 4, e1474. [Google Scholar] [CrossRef]
- Wawrzyczek, S.; Holmes, G.D.; Hoebee, S.E. Reproductive biology and population structure of the endangered shrub Grevillea bedggoodiana (Proteaceae). Conserv. Genet. 2023, 24, 7–23. [Google Scholar] [CrossRef]
- Jusaitis, M.; Adams, M. Conservation implications of clonality and limited sexual reproduction in the endangered shrub Acanthocladium dockeri (Asteraceae). Aust. J. Bot. 2005, 53, 535–544. [Google Scholar] [CrossRef]
- Barrett, S.C.H. Influences of clonality on plant sexual reproduction. Proc. Natl. Acad. Sci. 2015, 112, 8859–8866. [Google Scholar] [CrossRef]
- Mallinson, D.; Armstrong, B.; Baker, K.; Bogie, L.; Docherty, L.; Gilmore, S.; Hopkins, E.; Indhamusika, S.; Jewell, M.; Kivshar, V. Ecology and conservation status of Muehlenbeckia tuggeranong (Polygonaceae) near Canberra. Cunningham 1998, 5, 773–778. [Google Scholar]
- Makinson, R.; Mallinson, D. Muehlenbeckia tuggeranong (Polygonaceae): A new species from the Canberra district. Telopea 1997, 7, 215–219. [Google Scholar] [CrossRef]
- Desjardins, S.D.; Bailey, J.P.; Zhang, B.; Zhao, K.; Schwarzacher, T. New insights into the phylogenetic relationships of Japanese knotweed (Reynoutria japonica) and allied taxa in subtribe Reynoutriinae (Polygonaceae). PhytoKeys 2023, 220, 83–108. [Google Scholar] [CrossRef]
- Buckley, S.J.; Brauer, C.; Lamin, C.; Rose, P.; Vornicu, D.-E.; Beheregaray, L.B. A community-driven captive-breeding and reintroduction program maintains genetic diversity in a threatened freshwater fish. Conserv. Sci. Pract. 2024, 6, e13054. [Google Scholar] [CrossRef]
- Nistelberger, H.M.; Roycroft, E.; Macdonald, A.J.; McArthur, S.; White, L.C.; Grady, P.G.S.; Pierson, J.; Sims, C.; Cowen, S.; Moseby, K.; et al. Genetic mixing in conservation translocations increases diversity of a keystone threatened species, Bettongia lesueur. Mol. Ecol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Robiansyah, I.; Dodo. The Reintroduction of Threatened Plants by Bogor Botanic Gardens: Lessons Learned from Ujung Kulon National Park. Floresta E Ambiente 2022, 29. [Google Scholar] [CrossRef]
- Godefroid, S.; Le Pajolec, S.; Van Rossum, F. Pre-translocation considerations in rare plant reintroductions: Implications for designing protocols. Plant Ecol. 2016, 217, 169–182. [Google Scholar] [CrossRef]
- Willoughby, J.R.; Fernandez, N.B.; Lamb, M.C.; Ivy, J.A.; Lacy, R.C.; DeWoody, J.A. The impacts of inbreeding, drift and selection on genetic diversity in captive breeding populations. Mol. Ecol. 2015, 24, 98–110. [Google Scholar] [CrossRef]
- Hogg, C.J.; McLennan, E.A.; Wise, P.; Lee, A.V.; Pemberton, D.; Fox, S.; Belov, K.; Grueber, C.E. Preserving the demographic and genetic integrity of a single source population during multiple translocations. Biol. Conserv. 2020, 241, 108318. [Google Scholar] [CrossRef]
- Orsenigo, S.; Gentili, R.; Smolders, A.J.P.; Efremov, A.; Rossi, G.; Ardenghi, N.M.G.; Citterio, S.; Abeli, T. Reintroduction of a dioecious aquatic macrophyte (Stratiotes aloides L.) regionally extinct in the wild. Interesting answers from genetics. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 10–23. [Google Scholar] [CrossRef]
- Maschinski, J.; Albrecht, M.A. Center for Plant Conservation’s Best Practice Guidelines for the reintroduction of rare plants. Plant Divers. 2017, 39, 390–395. [Google Scholar] [CrossRef]
- Jones, O.R.; Wang, J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol. Ecol. Resour. 2010, 10, 551–555. [Google Scholar] [CrossRef]
- Leakey, R.R.B.; Newton, A.C.; Dick, J.M.P. Capture of Genetic Variation by Vegetative Propoagation: Processes Deermining Sucess. In Tropical Trees: The Potential for Domestication and the Rebuilding of Forest Resources; Leakey, R.R.B., Newton, A.C., Eds.; HMSO: London, UK, 1994; Volume 29. [Google Scholar]
- Willi, Y.; Van Buskirk, J.; Hoffmann, A.A. Limits to the Adaptive Potential of Small Populations. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 433–458. [Google Scholar] [CrossRef]
- Reichel, K.; Masson, J.-P.; Malrieu, F.; Arnaud-Haond, S.; Stoeckel, S. Rare sex or out of reach equilibrium? The dynamics of F IS in partially clonal organisms. BMC Genet. 2016, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; DeWoody, A. Genetic load has potential in large populations but is realized in small populations. Authorea Prepr. 2020, 14, 1540–1557. [Google Scholar] [CrossRef] [PubMed]
- Department of the Environment. Muehlenbeckia Tuggeranong in Species Profile and Threats Database; Department of the Environment: Canberra, Australia, 2025. Available online: https://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=64934 (accessed on 11 March 2025).
- ACT Government. Tuggeranong Lignum (Muehlenbeckia tuggeranong) Threatened Species Listing; ACT Government: Canberra, Australia, 2025. Available online: https://www.act.gov.au/environment/animals-and-plants/act-threatened-species/tuggeranong-lignum-muehlenbeckia-tuggeranong (accessed on 11 March 2025).
- Schuster, T.M.; Wilson, K.L.; Kron, K.A. Phylogenetic relationships of Muehlenbeckia, Fallopia, and Reynoutria (Polygonaceae) investigated with chloroplast and nuclear sequence data. Int. J. Plant Sci. 2011, 172, 1053–1066. [Google Scholar] [CrossRef]
- Hoyle, G.L.; Bredell, P.; Stevens, A.; Cook, E.; Knapp, Z.; Guja, L.K. ’Ex situ’ conservation breakthrough at the Australian national botanic gardens. Australas. Plant Conserv. J. Aust. Netw. Plant Conserv. 2023, 32, 3–7. [Google Scholar]
- Gruber, B.; Unmack, P.J.; Berry, O.F.; Georges, A. dartr: An r package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Mol. Ecol. Resour. 2018, 18, 691–699. [Google Scholar] [CrossRef]
- Mijangos, J.L.; Gruber, B.; Berry, O.; Pacioni, C.; Georges, A. dartR v2: An accessible genetic analysis platform for conservation, ecology and agriculture. Methods Ecol. Evol. 2022, 13, 2150–2158. [Google Scholar] [CrossRef]
- Jombart, T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Higgisson, W.; Broadhurst, L.; Shams, F.; Gruber, B.; Dyer, F. Reproductive Strategies and Population Genetic Structure in Two Dryland River Floodplain Plants, Marsilea drummondii and Eleocharis acuta. Genes 2022, 13, 1506. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Chauhan, B.S. Genetic Diversity, Conservation, and Utilization of Plant Genetic Resources. Genes 2023, 14, 174. [Google Scholar] [CrossRef]
- Pregler, K.; Clemento, A.; Grill, M.; Adelizi, P.; Carlson, S.; Garza, J. Reintroduction of spring-run Chinook salmon in the San Joaquin River: Evaluating genetic and phenotypic effects of captive breeding. Conserv. Sci. Pract. 2024, 6, e13176. [Google Scholar] [CrossRef]
- Richdon, S.; Menchaca Rodriguez, A.; Price, E.; Wormell, D.; McCabe, G.; Jones, G. Thirty years of conservation breeding: Assessing the genetic diversity of captive Livingstone’s fruit bats. Zoo Biol. 2024, 43, 395–404. [Google Scholar] [CrossRef]
- Diaz-Martin, Z.; Fant, J.; Havens, K.; Cinea, W.; Lima, J.M.T.; Griffith, M.P. Current management practices do not adequately safeguard endangered plant species in conservation collections. Biol. Conserv. 2023, 280, 109955. [Google Scholar] [CrossRef]
| ANBG—Seedling | ANBG | Wild | M. axillaris | M. gunnii | |
|---|---|---|---|---|---|
| ANBG (n = 12) | 0.073 | NA | NA | NA | NA |
| Wild (n = 21) | 0.373 | 0.171 | NA | NA | NA |
| M. axillaris (n = 3) | 0.501 | 0.452 | 0.629 | NA | NA |
| M. gunnii (n = 1) | 0.905 | 0.897 | 0.941 | 0.875 | NA |
| M. complexa (n = 2) | 0.885 | 0.867 | 0.950 | 0.836 | 0.936 |
| Management Group | HO | HE | FIS |
|---|---|---|---|
| ANBG seedlings | 0.200 | 0.233 | 0.198 |
| ANBG | 0.135 | 0.244 | 0.438 |
| Wild | 0.059 | 0.051 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walcott, I.; Lanspeary, A.; Shams, F.; Bredell, P.; Cook, E.; Higgisson, W. On the Precipice of Extinction: Genetic Data in the Conservation Management of In Situ and Ex Situ Collections of the Critically Endangered Muehlenbeckia tuggeranong (Tuggeranong Lignum). Plants 2025, 14, 1812. https://doi.org/10.3390/plants14121812
Walcott I, Lanspeary A, Shams F, Bredell P, Cook E, Higgisson W. On the Precipice of Extinction: Genetic Data in the Conservation Management of In Situ and Ex Situ Collections of the Critically Endangered Muehlenbeckia tuggeranong (Tuggeranong Lignum). Plants. 2025; 14(12):1812. https://doi.org/10.3390/plants14121812
Chicago/Turabian StyleWalcott, Isobel, Angela Lanspeary, Foyez Shams, Peter Bredell, Emma Cook, and William Higgisson. 2025. "On the Precipice of Extinction: Genetic Data in the Conservation Management of In Situ and Ex Situ Collections of the Critically Endangered Muehlenbeckia tuggeranong (Tuggeranong Lignum)" Plants 14, no. 12: 1812. https://doi.org/10.3390/plants14121812
APA StyleWalcott, I., Lanspeary, A., Shams, F., Bredell, P., Cook, E., & Higgisson, W. (2025). On the Precipice of Extinction: Genetic Data in the Conservation Management of In Situ and Ex Situ Collections of the Critically Endangered Muehlenbeckia tuggeranong (Tuggeranong Lignum). Plants, 14(12), 1812. https://doi.org/10.3390/plants14121812






