Ecophysiological and Biochemical Responses of Lessonia spicata to Solar Eclipse-Induced Light Deprivation
Abstract
1. Introduction
2. Results
2.1. Environmental Conditions
2.2. Photosynthetic Performance
2.3. Biochemical Responses
3. Discussion
4. Methods and Materials
4.1. Sampling and Experimental Design
4.2. Abiotic Parameters
4.3. Physiological Responses
4.4. Mathematical Model
4.5. Biochemical Responses
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Littmann, M.; Espenak, F.; Wilcox, K. Totality: Eclipses of the Sun; Oxford University Press Inc.: New York, NY, USA, 2008; Volume 340. [Google Scholar]
- Haberle, K.H.; Reiter, I.; Patzner, K.; Heyne, C.; Matyssek, R. Switching the light off: A break in photosynthesis and sap flow of forest trees under total solar eclipse. Meteorol. Z. 2001, 10, 201–206. [Google Scholar] [CrossRef]
- Bischof, K.; Gómez, I.; Molis, M.; Hanelt, D.; Karsten, U.; Lüder, U.; Roleda, M.Y.; Zacher, K.; Wiencke, C. Ultraviolet radiation shapes seaweed communities. Rev. Environ. Sci. Biotechnol. 2006, 5, 141–166. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Moenne, F.; Rodríguez-Rojas, F.; Pardo, D.; Lavergne, C.; Moenne, A.; Brown, M.T.; Huovinen, P.; Gómez, I.; Navarro, N.; et al. Antarctic intertidal macroalgae under predicted increased temperatures mediated by global climate change: Would they cope? Sci. Total Environ. 2020, 740, 140379. [Google Scholar] [CrossRef] [PubMed]
- Sambandan, K.; Devi, K.S.; Kumar, S.S.; Nancharaiah, M.; Dhatchanamoorthy, N. Effects of solar eclipse on photosynthesis of Portulaca oleracea and Phyla nodiflora in coastal wild conditions. J. Phytol. 2012, 2012, 34–40. [Google Scholar]
- Celis-Plá, P.S.M.; Trabal, A.; Navarrete, C.; Troncoso, M.; Moenne, F.; Zúñiga, A.; Figueroa, F.L.; Sáez, C.A. Daily changes on seasonal ecophysiological responses of the intertidal brown macroalga Lessonia spicata: Implications of climate change. Front. Plant Sci. 2022, 13, 941061. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, A.; Sáez, C.A.; Trabal, A.; Figueroa, F.L.; Pardo, D.; Navarrete, C.; Rodríguez-Rojas, F.; Moenne, F.; Celis-Plá, P.S.M. Seasonal Photoacclimation and Vulnerability Patterns in the Brown Macroalga Lessonia spicata (Ochrophyta). Water 2020, 13, 6. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef]
- Gómez, I.; Huovinen, P. Morpho-functional patterns and zonation of South Chilean seaweeds: The importance of photosynthetic and bio-optical traits. Mar. Ecol. Prog. Ser. 2011, 422, 77–91. [Google Scholar] [CrossRef]
- Abdala-Díaz, R.T.; Cabello-Pasini, A.; Pérez-Rodríguez, E.; Conde Álvarez, R.M.; Figueroa, F.L. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol. 2006, 148, 459–465. [Google Scholar] [CrossRef]
- Gómez, I.; Español, S.; Véliz, K.; Huovinen, P. Spatial distribution of phlorotannins and its relationship with photosynthetic UV tolerance and allocation of storage carbohydrates in blades of the kelp Lessonia spicata. Mar. Biol. 2016, 163, 1–14. [Google Scholar] [CrossRef]
- Huovinen, P.; Gómez, I.; Lovengreen, C. A five-year study of solar ultraviolet radiation in Southern Chile (39° S): Potential impact on physiology of coastal marine algae? Photochem. Photobiol. 2006, 82, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, D. Capability of dynamic photoinhibition in Arctic macroalgae is related to their depth distribution. Mar. Biol. 1998, 131, 361–369. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Atala, C.; Carrasco-Urra, F. Differential impact of an eclipse on photosynthetic performance of trees with different degrees of shade tolerance. Forests 2021, 12, 1353. [Google Scholar] [CrossRef]
- Beverly, D.P.; Guadagno, C.R.; Bretfeld, M.; Speckman, H.N.; Albeke, S.E.; Ewers, B.E. Hydraulic and photosynthetic responses of big sagebrush to the 2017 total solar eclipse. Sci. Rep. 2019, 9, 8839. [Google Scholar] [CrossRef]
- Gómez, I.; López-Figueroa, F.; Ulloa, N.; Morales, V.; Lovengreen, C.; Huovinen, P.; Hess, S. Patterns of photosynthesis in 18 species of intertidal macroalgae from southern Chile. Mar. Ecol. Prog. Ser. 2004, 270, 103–116. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Bonomi-Barufi, J.; Celis-Plá, P.S.M.; Nitschke, U.; Arenas, F.; Connan, S.; Abreu, M.H.; Malta, E.J.; Conde-Álvarez, R.; Chow, F.; et al. Corrigendum to: Short-term effects of increased CO2, nitrate and temperature on photosynthetic activity in Ulva rigida (Chlorophyta) estimated by different pulse amplitude modulated fluorometers and oxygen evolution. J. Exp. Bot. 2021, 72, 4591. [Google Scholar] [CrossRef]
- Cabello-Pasini, A.; Aguirre-Von-Wobeser, E.; Figueroa, F.L. Photoinhibition of photosynthesis in Macrocystis pyrifera (Phaeophyceae), Chondrus crispus (Rhodophyceae) and Ulva lactuca (Chlorophyceae) in outdoor culture systems. J. Photochem. Photobiol. B 2000, 57, 169–178. [Google Scholar] [CrossRef]
- Gévaert, F.; Créach, A.; Davoult, D.; Migné, A.; Levavasseur, G.; Arzel, P.; Holl, A.C.; Lemoine, Y. Laminaria saccharina photosynthesis measured in situ: Photoinhibition and xanthophyll cycle during a tidal cycle. Mar. Ecol. Prog. Ser. 2003, 247, 43–50. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Korbee, N.; Gómez-Garreta, A.; Figueroa, F.L. Seasonal photoacclimation patterns in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Sci. Mar. 2014, 78, 377–388. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Bouzon, Z.L.; Hall-Spencer, J.M.; Schmidt, E.C.; Korbee, N.; Figueroa, F.L. Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Mar. Environ. Res. 2016, 115, 89–97. [Google Scholar] [CrossRef]
- Franklin, L.A.; Levavasseur, G.; Osmond, C.B.; Henley, W.J.; Ramus, J. Two components of onset and recovery during photoinhibition of Ulva rotundata. Planta 1992, 186, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Osmond, C.B.; Ramus, J.; Levavasseur, G.; Franklin, L.A.; Henley, W.J. Fluorescence quenching during photosynthesis and photoinhibition of Ulva rotundata blid. Planta 1993, 190, 97–106. [Google Scholar] [CrossRef]
- Lopez-Figueroa, F.; Niell, F.X. Red-light and blue-light photoreceptors controlling chlorophyll a synthesis in the red alga Porphyra umbilicalis and in the green alga Ulva rigida. Physiol. Plant 1989, 76, 391–397. [Google Scholar] [CrossRef]
- López-Figueroa, F. Diurnal variation in pigment content in Porphyra laciniata and Chondrus crispus and its relation to the diurnal changes of underwater light quality and quantity. Mar. Ecol. 1992, 13, 285–305. [Google Scholar] [CrossRef]
- Jiménez, C.; Salles, S.; Mercado, J.; Viñegla, B.; Altamirano, M.; Aguilera, J.; Flores-Moya, A.; Figueroa, F. Effects of solar radiation on photoinhibition and pigmentation in the red alga Porphyra leucosticta. Mar. Ecol. Prog. Ser. 1997, 151, 81–90. [Google Scholar]
- Figueroa, F.L.; Conde-Álvarez, R.; Gómez, I. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynth. Res. 2003, 75, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Borum, J.; Pedersen, M.F.; Krause-Jensen, D.; Christensen, P.B.; Nielsen, K. Biomass, photosynthesis and growth of Laminaria saccharina in a high-arctic fjord, NE Greenland. Mar. Biol. 2002, 141, 11–19. [Google Scholar] [CrossRef]
- Garcia-Mendoza, E.; Ocampo-Alvarez, H. Photoprotection in the brown alga Macrocystis pyrifera: Evolutionary implications. J. Photochem. Photobiol. B Biol. 2011, 104, 377–385. [Google Scholar] [CrossRef]
- Roach, T.; Miller, R.; Aigner, S.; Kranner, I. Diurnal changes in the xanthophyll cycle pigments of freshwater algae correlate with the environmental hydrogen peroxide concentration rather than non-photochemical quenching. Ann. Bot. 2015, 116, 519–527. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, L.Y.; Kong, F.Y.; Deng, Y.S.; Li, B.; Meng, Q.W. Constitutive accumulation of zeaxanthin in tomato alleviates salt stress-induced photoinhibition and photooxidation. Physiol. Plant. 2012, 146, 363–373. [Google Scholar] [CrossRef]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghassempour, A.R. Seasonal variation of fucoxanthin content in four species of brown seaweeds from Qeshm Island, Persian Gulf and evaluation of their antibacterial and antioxidant activities. Iran J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar] [CrossRef]
- Swanson, A.K.; Druehl, L.D. Induction, exudation and the UV protective role of kelp phlorotannins. Aquat. Bot. 2002, 73, 241–253. [Google Scholar] [CrossRef]
- Bischof, K.; Janknegt, P.J.; Buma, A.G.; Rijstenbil, J.W.; Peralta, G.; Breeman, A.M. Oxidative stress and enzymatic scavenging of superoxide radicals induced by solar UV-B radiation in Ulva canopies from southern Spain. Sci. Mar. 2003, 67, 353–359. [Google Scholar] [CrossRef]
- Shiu, C.T.; Lee, T.M. Ultraviolet-B-induced oxidative stress and responses of the ascorbate–glutathione cycle in a marine macroalga Ulva fasciata. J. Exp. Bot. 2005, 56, 2851–2865. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Zhang, R.; Lee, K.H.; Chae, S.; Kim, B.J.; Kwak, Y.S.; Park, J.W.; Lee, N.H.; Hyun, J.W. Protective effect of triphlorethol-A from Ecklonia cava against ionizing radiation in vitro. J. Radiat. Res. 2006, 47, 61–68. [Google Scholar] [CrossRef]
- Harnita, A.N.I.; Santosa, I.E.; Martono, S.; Sudarsono Widyarini, S.; Harren, F.J.M. Inhibition of Lipid Peroxidation Induced by Ultraviolet Radiation by Crude Phlorotannins Isolated from Brown Algae Sargassum hystrix v. buxifolium C. Agardh. Indones. J. Chem. 2013, 13, 14–20. [Google Scholar] [CrossRef]
- Quintano, E.; Celis-Plá, P.S.M.; Martínez, B.; Díez, N.; Muguerza, N.; Figueroa, F.L.; Gorostiaga, J.M. Ecophysiological responses of a threatened red alga to increased irradiance in an in-situ transplant experiment. Mar. Environ. Res. 2019, 144, 166–177. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Parsons, T.R.; Strickland, J.D. Discussion of spectrophotometric determination of marine-plant pigments, with revised equations for ascertaining chlorophylls and carotenoids. J. Mar. Res. 1963, 21, 155–163. [Google Scholar]
- Álvarez-Gómez, F.; Bouzon, Z.L.; Korbee, N.; Celis-Plá, P.; Schmidt, C.; Figueroa, F.L. Combined effects of UVR and nutrients on cell ultrastructure, photosynthesis and biochemistry in Gracilariopsis longissima (Gracilariales, Rhodophyta). Algal Res. 2017, 26, 190–202. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology, Experiments in Ecology; Cambridge University Press: Cambridge, NY, USA, 1997. [Google Scholar] [CrossRef]
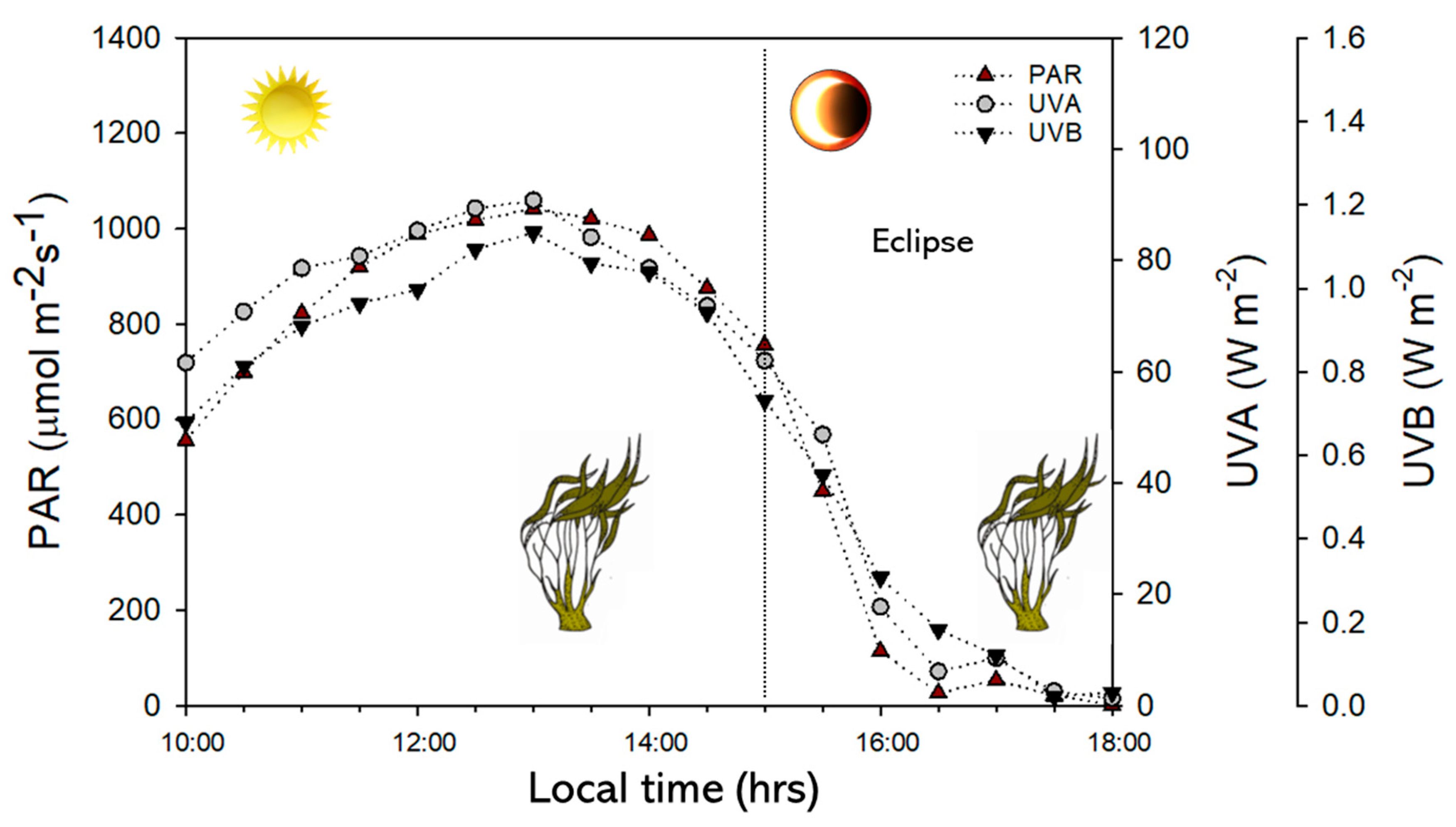


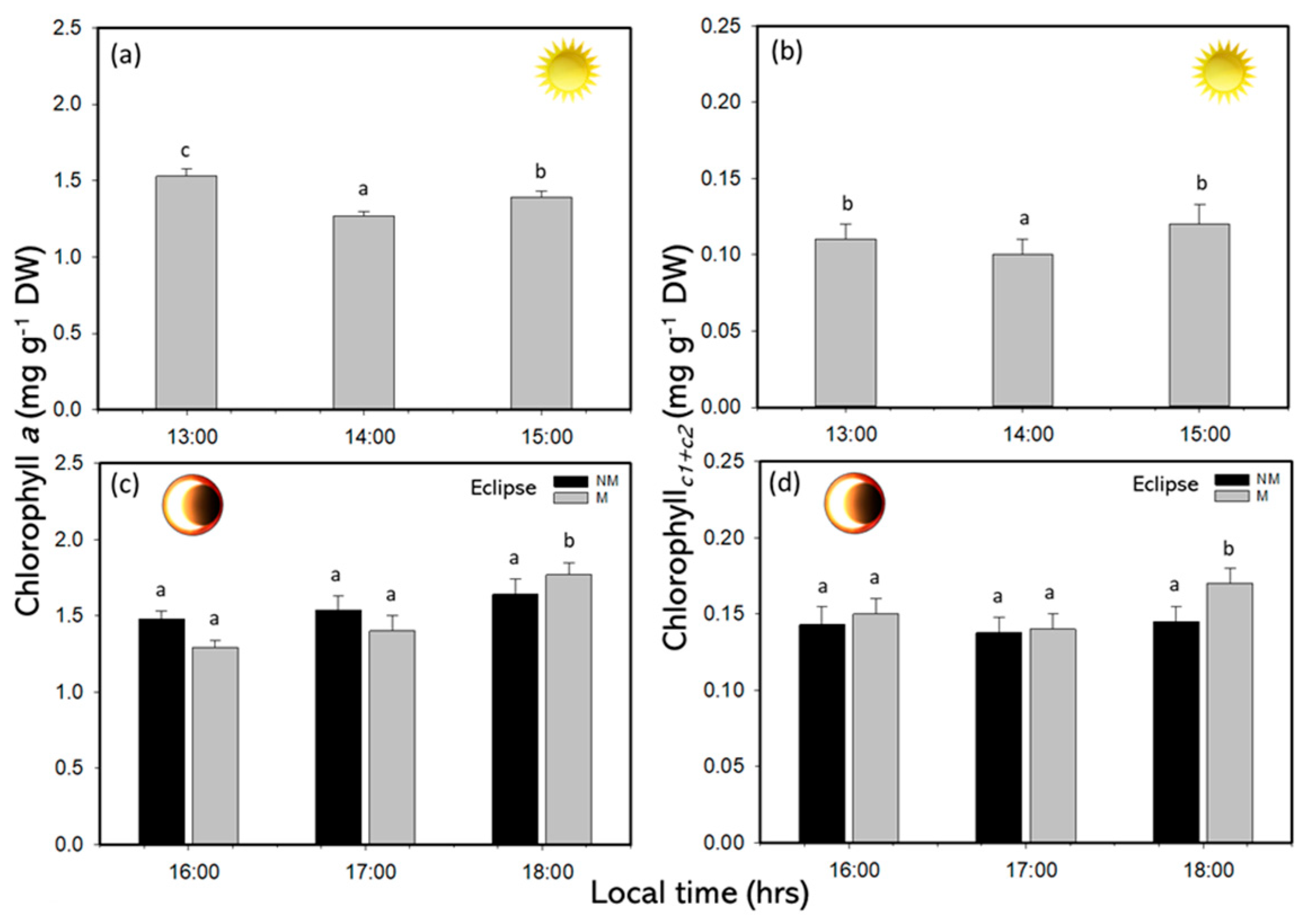


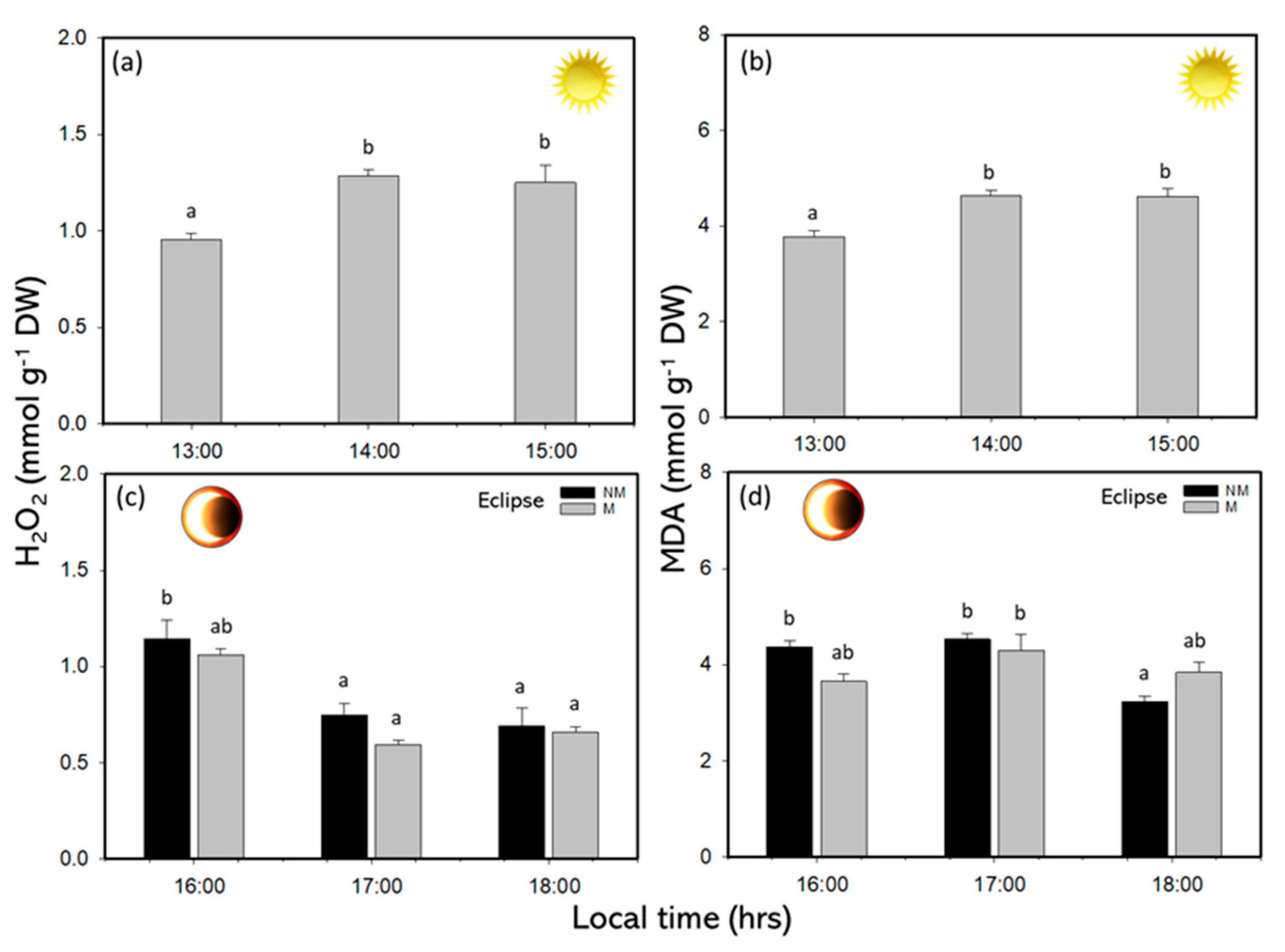

| Variables | Values |
|---|---|
| PAR (KJ m−2) | 2380.1 ± 10.8 |
| UVA (KJ m−2) | 745.2 ± 1.3 |
| UVB (KJ m−2) | 11.3 ± 0.5 |
| Temperature (°C) | 12.3 ± 0.1 |
| pH | 7.9 ± 0.1 |
| Salinity (PSU) | 31.9 ± 0.4 |
| Pfast | Kfast | Pslow | Kslow | |
|---|---|---|---|---|
| Exposure | 0.30 ± 0.06 | 0.01 ± 0.00 | 0.15 ± 0.07 | 0.01 ± 0.00 |
| Non-mesh | 0.63 ± 0.11 a,* | 0.02 ± 0.00 | 2.04 ± 0.13 * | 0.02 ± 0.00 |
| Mesh | 1.19 ± 0.10 b,* | 0.02 ± 0.00 | 2.16 ± 0.03 * | 0.02 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celis-Plá, P.S.M.; Navarrete, C.E.; Trabal, A.; Castro-Varela, P.A.; Figueroa, F.L.; Troncoso, M.; Sáez, C.A. Ecophysiological and Biochemical Responses of Lessonia spicata to Solar Eclipse-Induced Light Deprivation. Plants 2025, 14, 1810. https://doi.org/10.3390/plants14121810
Celis-Plá PSM, Navarrete CE, Trabal A, Castro-Varela PA, Figueroa FL, Troncoso M, Sáez CA. Ecophysiological and Biochemical Responses of Lessonia spicata to Solar Eclipse-Induced Light Deprivation. Plants. 2025; 14(12):1810. https://doi.org/10.3390/plants14121810
Chicago/Turabian StyleCelis-Plá, Paula S. M., Camilo E. Navarrete, Andrés Trabal, Pablo A. Castro-Varela, Félix L. Figueroa, Macarena Troncoso, and Claudio A. Sáez. 2025. "Ecophysiological and Biochemical Responses of Lessonia spicata to Solar Eclipse-Induced Light Deprivation" Plants 14, no. 12: 1810. https://doi.org/10.3390/plants14121810
APA StyleCelis-Plá, P. S. M., Navarrete, C. E., Trabal, A., Castro-Varela, P. A., Figueroa, F. L., Troncoso, M., & Sáez, C. A. (2025). Ecophysiological and Biochemical Responses of Lessonia spicata to Solar Eclipse-Induced Light Deprivation. Plants, 14(12), 1810. https://doi.org/10.3390/plants14121810









