The Auxin Response Factor OsARF25 Negatively Regulates Grain Size and Weight in Rice (Oryza sativa L.) by Activating the Expression of SG1 and OsOFP04
Abstract
1. Introduction
2. Results
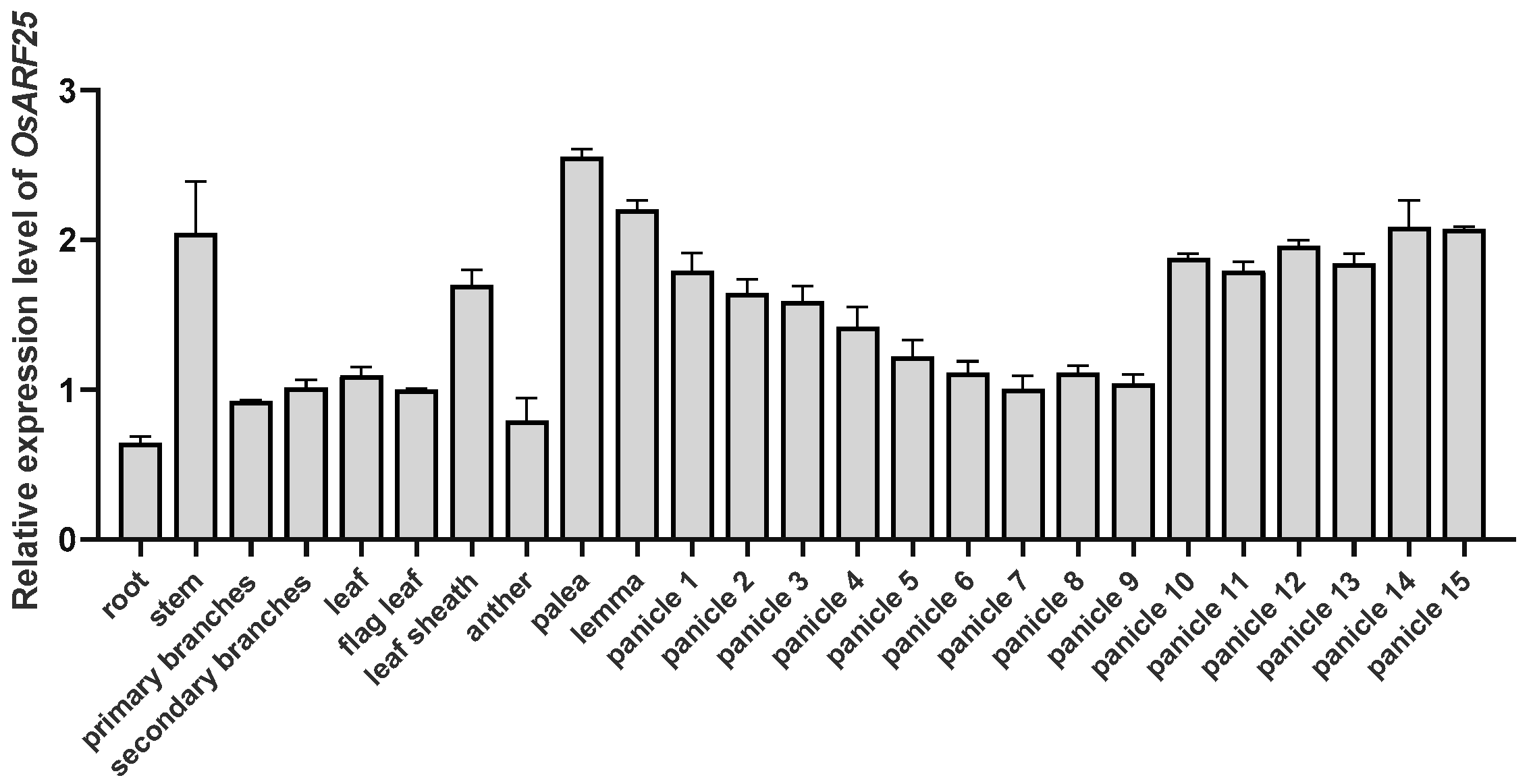
2.1. OsARF25 Is Highly Expressed in Palea, Lemma, and Panicles
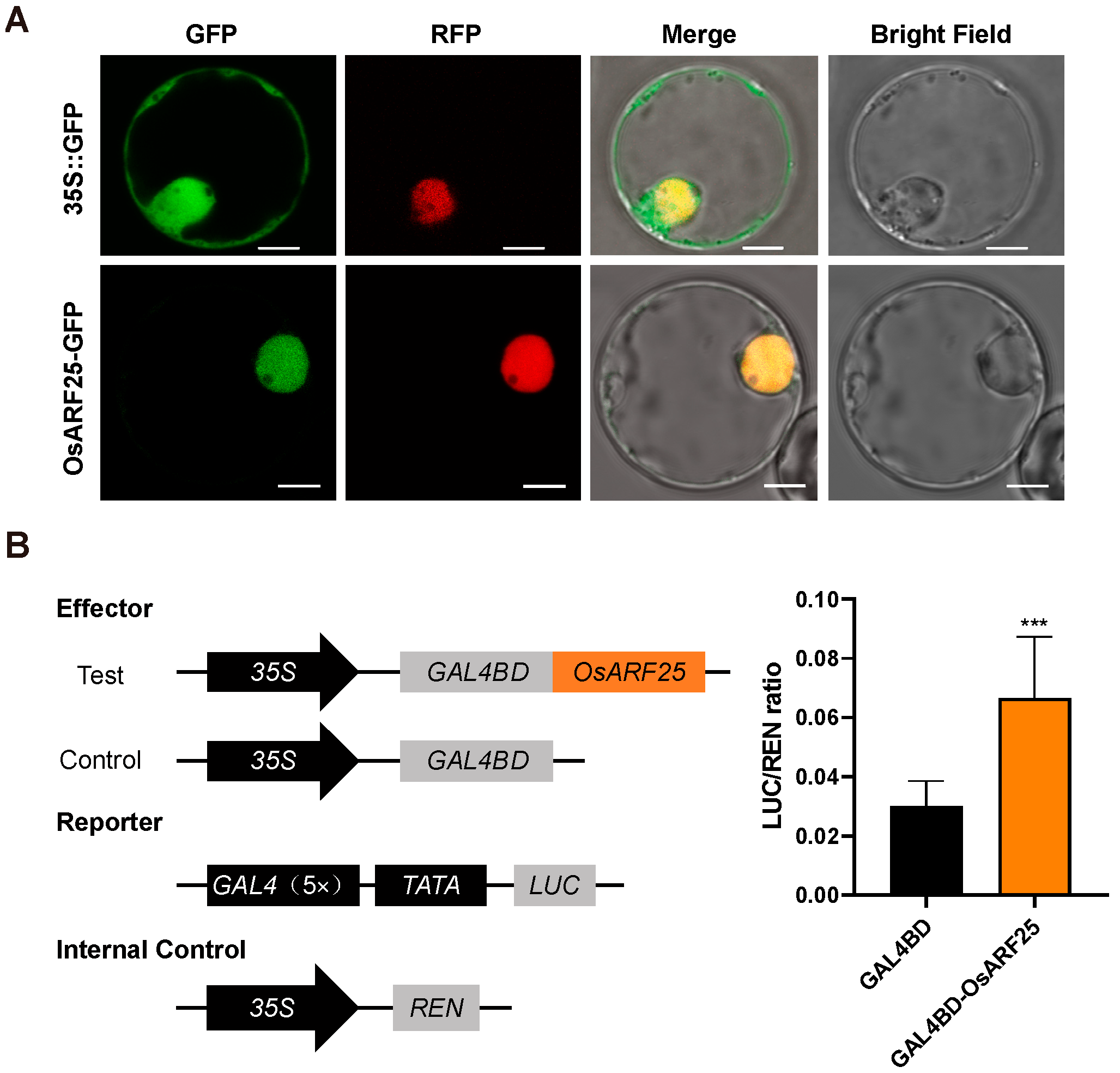
2.2. OsARF25 Localizes into Nuclear and Functions as a Transcriptional Activator
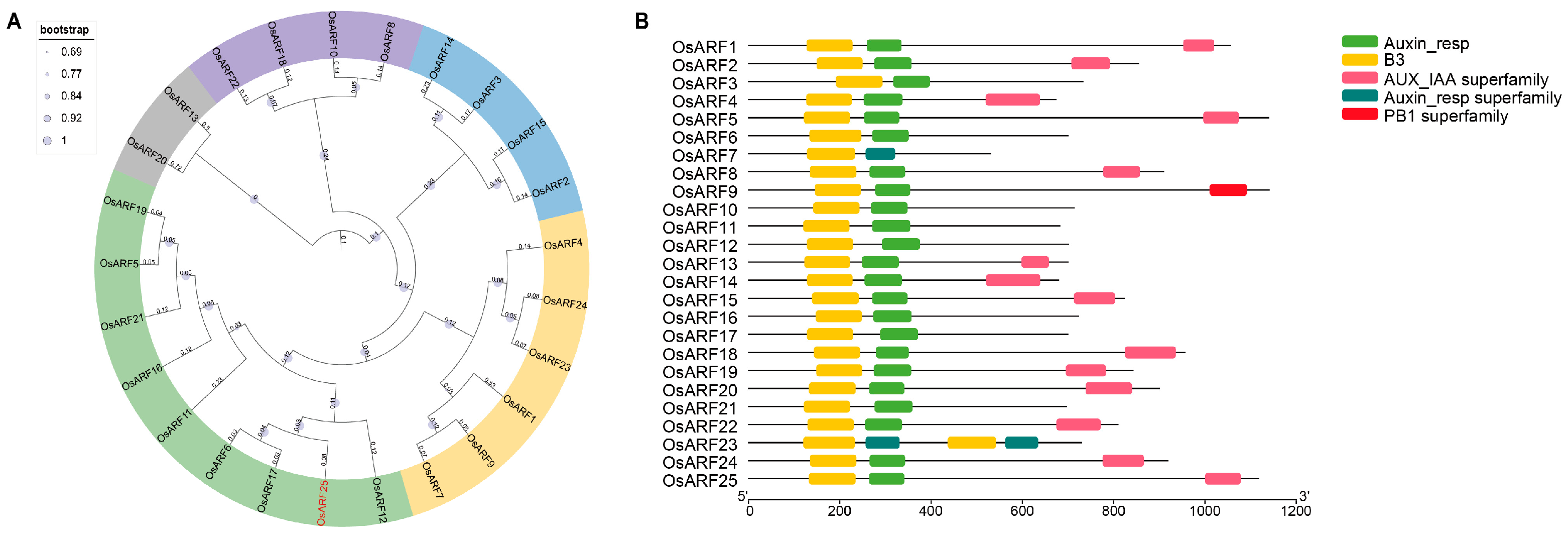
2.3. Bioinformatic Analysis of the OsARFs in Rice
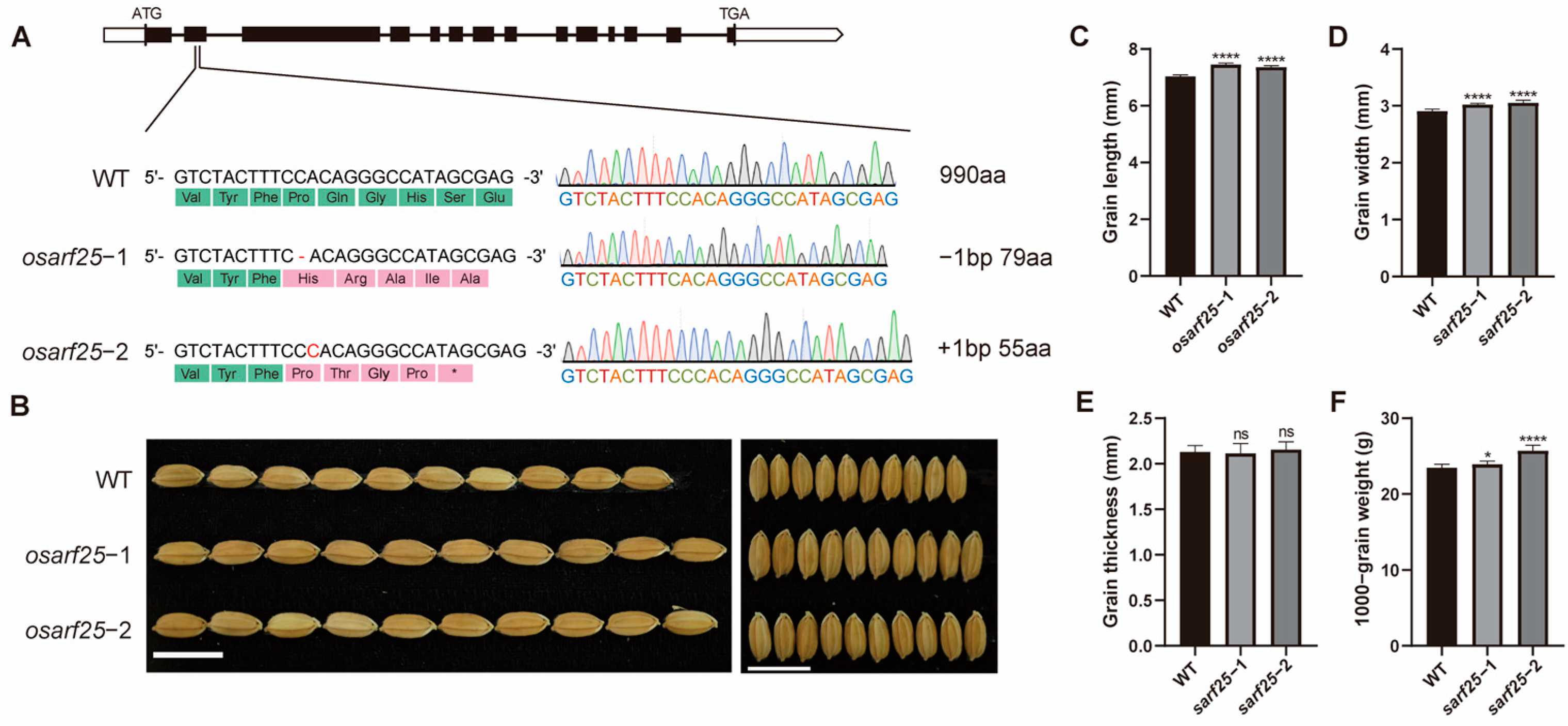
2.4. The osarf25 Mutants Exhibited Increased Rice Grain Size and Weight
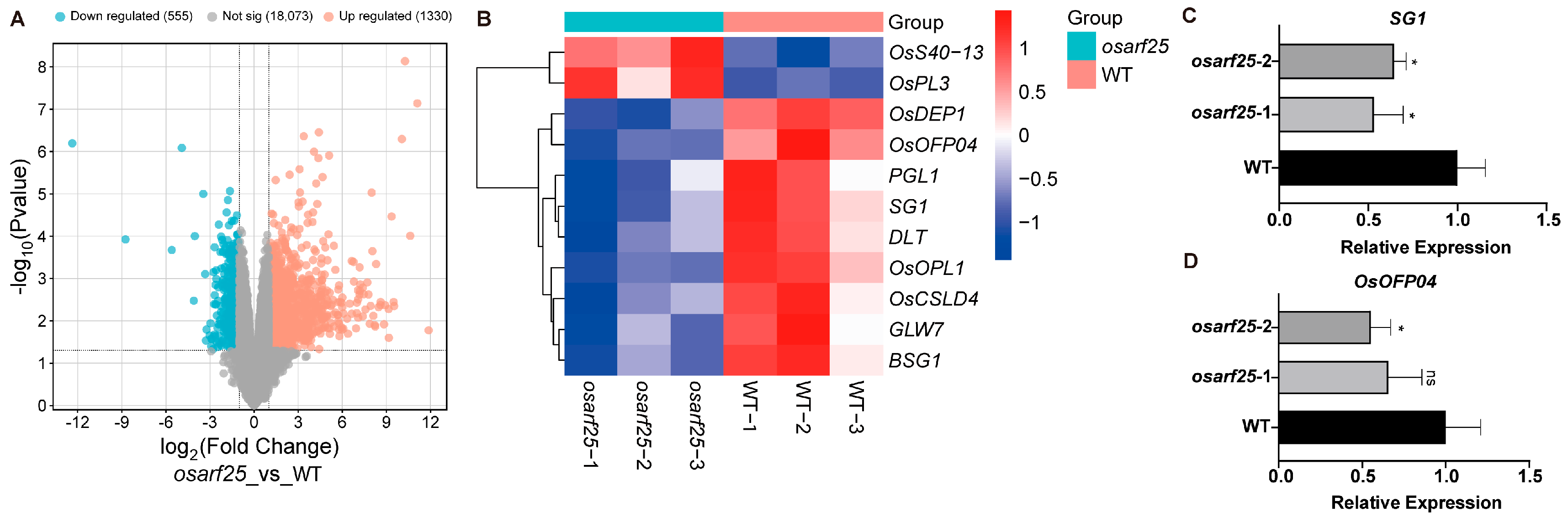
2.5. Transcriptome Analysis of the osarf25 Mutants by RNA-seq
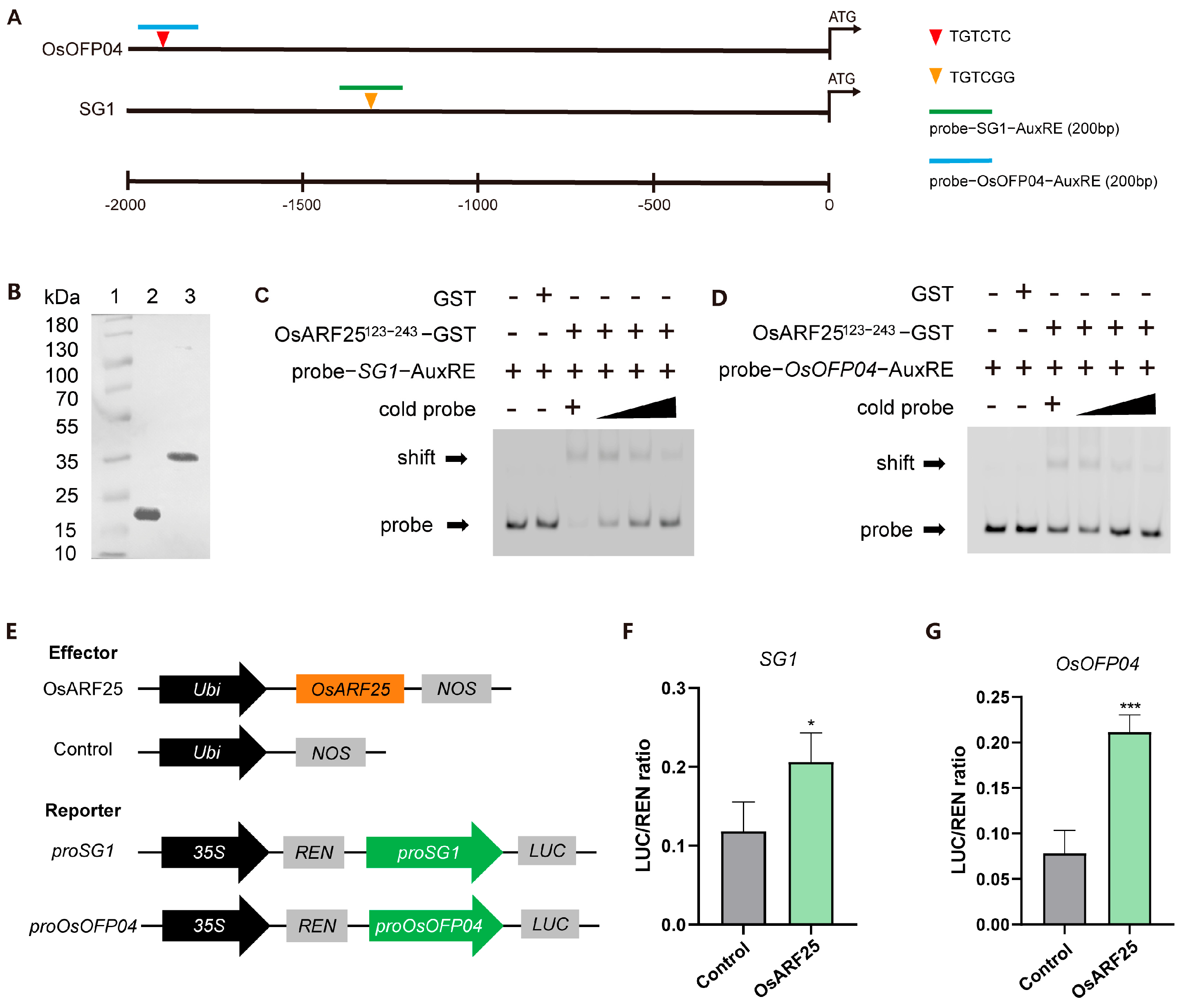
2.6. OsARF25 Directly Binds to the SG1 and OsOFP04 Promoters
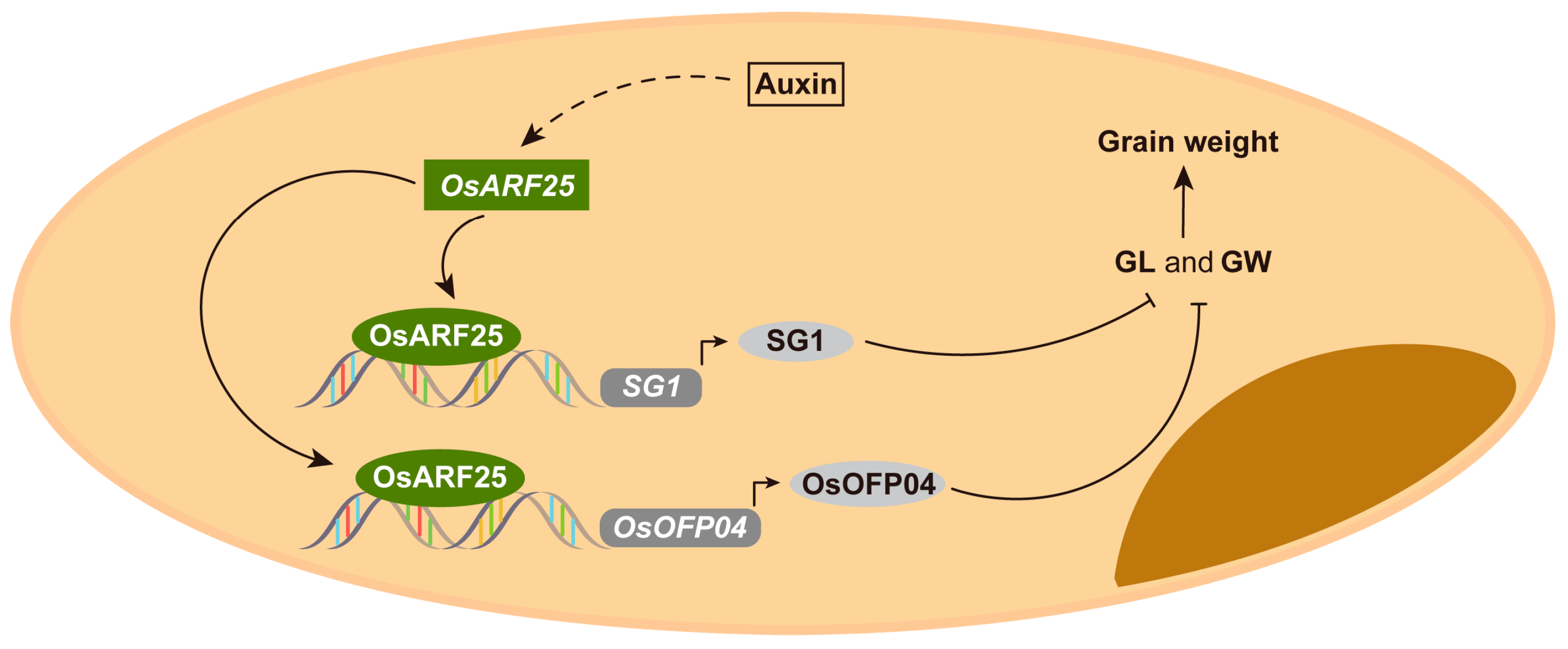
3. Discussion
4. Materials and Methods
4.1. Bioinformatic Analysis
4.2. Plant Materials and Growth Conditions
4.3. Plasmid Construction and Transformation
4.4. Total RNA Extraction and Expression Analysis
4.5. Subcellular Localization Analysis
4.6. Dual-Luciferase Reporter Assay
4.7. Transcriptional Activation Analysis
4.8. RNA Sequencing Analysis
4.9. Western Blotting
4.10. Electrophoretic Mobility Shift Assay
4.11. Lamina Joint Inclination Assay
4.12. Coleoptile Elongation Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA United States Department of Agriculture, Foreign Agricultural Service, Office of Global Analysis. 2020. Available online: https://www.fas.usda.gov/sites/default/files/2025-05/2020-Dec-Grain.pdf (accessed on 18 March 2025).
- McDonald, B. Food Security Emerges as a Complex Global Challenge. J. ERW Mine Action 2012, 16, 13. [Google Scholar]
- Li, N.; Xu, R.; Duan, P.; Li, Y. Control of grain size in rice. Plant Reprod. 2018, 31, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.; Paponov, I.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef]
- Wang, D.; Pei, K.; Fu, Y.; Sun, Z.; Li, S.; Liu, H.; Tang, K.; Han, B.; Tao, Y. Genome-wide analysis of the auxin response factors (ARF) gene family in rice (Oryza sativa). Gene 2007, 394, 13–24. [Google Scholar] [CrossRef]
- Ulmasov, T.; Hagen, G.; Guilfoyle, T.J. ARF1, a transcription factor that binds to auxin response elements. Science 1997, 276, 1865–1868. [Google Scholar] [CrossRef]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. J. Plant Growth Reg. 2001, 10, 281–291. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Qi, Y. Advances in structure and function of auxin response factor in plants. J. Integr. Plant Biol. 2023, 65, 617–632. [Google Scholar] [CrossRef]
- Zhang, R.; Min, Y.; Holappa, L.D.; Walcher-Chevillet, C.L.; Duan, X.; Donaldson, E.; Kong, H.; Kramer, E.M. A role for the Auxin Response Factors ARF6 and ARF8 homologs in petal spur elongation and nectary maturation in Aquilegia. New Phytol. 2020, 227, 1392–1405. [Google Scholar] [CrossRef]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef]
- Kirolinko, C.; Hobecker, K.; Wen, J.; Mysore, K.S.; Niebel, A.; Blanco, F.A.; Zanetti, M.E. Auxin Response Factor 2 (ARF2), ARF3, and ARF4 Mediate Both Lateral Root and Nitrogen Fixing Nodule Development in Medicago truncatula. Front. Plant Sci. 2021, 12, 659061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, F.; Tang, Y.; Yuan, Y.; Deng, W.; Li, Z. Auxin Response Gene SlARF3 Plays Multiple Roles in Tomato Development and is Involved in the Formation of Epidermal Cells and Trichomes. Plant Cell Physiol. 2015, 56, 2110–2124. [Google Scholar] [PubMed]
- Chung, Y.; Zhu, Y.; Wu, M.F.; Simonini, S.; Kuhn, A.; Armenta-Medina, A.; Jin, R.; Østergaard, L.; Gillmor, C.S.; Wagner, D. Auxin Response Factors promote organogenesis by chromatin-mediated repression of the pluripotency gene SHOOTMERISTEMLESS. Nat. Commun. 2019, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Mei, L.; Wu, M.; Wei, W.; Shan, W.; Gong, Z.; Zhang, Q.; Yang, F.; Yan, F.; Zhang, Q.; et al. SlARF10, an auxin response factor, is involved in chlorophyll and sugar accumulation during tomato fruit development. J. Exp. Bot. 2018, 69, 5507–5518. [Google Scholar] [CrossRef]
- Shen, X.; He, J.; Ping, Y.; Guo, J.; Hou, N.; Cao, F.; Li, X.; Geng, D.; Wang, S.; Chen, P.; et al. The positive feedback regulatory loop of miR160-Auxin Response Factor 17-HYPONASTIC LEAVES 1 mediates drought tolerance in apple trees. Plant Physiol. 2022, 188, 1686–1708. [Google Scholar] [CrossRef]
- Qiao, J.; Jiang, H.; Lin, Y.; Shang, L.; Wang, M.; Li, D.; Fu, X.; Geisler, M.; Qi, Y.; Gao, Z.; et al. A novel miR167a-OsARF6-OsAUX3 module regulates grain length and weight in rice. Mol. Plant. 2021, 14, 1683–1698. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, S.J.; Wang, M.J.; He, H.; Sun, L.; Wang, H.; Liu, X.H.; Jiang, L.; Sun, J.L.; Xin, X.; et al. A Novel QTL qTGW3 Encodes the GSK3/SHAGGY-Like Kinase OsGSK5/OsSK41 that Interacts with OsARF4 to Negatively Regulate Grain Size and Weight in Rice. Mol. Plant 2018, 11, 736–749. [Google Scholar] [CrossRef]
- Duan, E.; Lin, Q.; Wang, Y.; Ren, Y.; Xu, H.; Zhang, Y.; Wang, Y.; Teng, X.; Dong, H.; Wang, Y.; et al. The transcriptional hub SHORT INTERNODES1 integrates hormone signals to orchestrate rice growth and development. Plant Cell. 2023, 35, 2871–2886. [Google Scholar] [CrossRef]
- Ma, M.; Shen, S.Y.; Bai, C.; Wang, W.Q.; Feng, X.H.; Ying, J.Z.; Song, X.J. Control of grain size in rice by TGW3 phosphorylation of OsIAA10 through potentiation of OsIAA10-OsARF4-mediated auxin signaling. Cell Rep. 2023, 42, 112187. [Google Scholar] [CrossRef]
- Xian, F.; Liu, S.; Huang, J.; Xie, B.; Zhu, L.; Zhang, Q.; Lv, C.; Xu, Y.; Zhang, X.; Hu, J. The OsIAA3-OsARF16-OsBUL1 auxin signaling module regulates grain size in rice. Plant Physiol. 2025, 197, kiaf122. [Google Scholar] [CrossRef]
- Tian, P.; Liu, J.; Yan, B.; Li, S.; Lei, B.; Shen, R.; Lei, C.; Xu, M. OsBSK3 Positively Regulates Grain Length and Weight by Inhibiting the Phosphatase Activity of OsPPKL1. Plants 2022, 11, 1586. [Google Scholar] [CrossRef] [PubMed]
- Divi, U.K.; Krishna, P. Brassinosteroid: A biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol. 2009, 26, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Ueguchi-Tanaka, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. The Rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell. 2005, 17, 2243–2254. [Google Scholar] [CrossRef]
- Tanabe, S.; Ashikari, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Yano, M.; Yoshimura, A.; Kitano, H.; Matsuoka, M.; Fujisawa, Y.; et al. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell. 2005, 17, 776–790. [Google Scholar] [CrossRef]
- Tong, H.; Liu, L.; Jin, Y.; Du, L.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. DWARF AND LOW-TILLERING acts as a direct downstream target of a GSK3/SHAGGY-like kinase to mediate brassinosteroid responses in rice. Plant Cell. 2012, 24, 2562–2577. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.Q.; Zhang, X.; Zhou, J.; Jiang, Z.; Huang, P.; Tang, Z.; Bao, Y.; Cheng, J.; Tang, H.; et al. Rice qGL3/OsPPKL1 Functions with the GSK3/SHAGGY-Like Kinase OsGSK3 to Modulate Brassinosteroid Signaling. Plant Cell. 2019, 31, 1077–1093. [Google Scholar] [CrossRef]
- Che, R.; Tong, H.; Shi, B.; Liu, Y.; Fang, S.; Liu, D.; Xiao, Y.; Hu, B.; Liu, L.; Wang, H.; et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat. Plants 2015, 2, 15195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Cheng, Y.; Du, X.; Teotia, S.; Miao, C.; Sun, H.; Fan, G.; Tang, G.; Xue, H.; et al. The miR167-OsARF12 module regulates rice grain filling and grain size downstream of miR159. Plant Comm. 2023, 4, 100604. [Google Scholar] [CrossRef]
- Shen, C.; Wang, S.; Bai, Y.; Wu, Y.; Zhang, S.; Chen, M.; Guilfoyle, T.J.; Wu, P.; Qi, Y. Functional analysis of the structural domain of ARF proteins in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 3971–3981. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Boer, D.R.; Freire-Rios, A.; Van Den Berg, W.A.; Saaki, T.; Manfield, I.W.; Kepinski, S.; López-Vidrieo, I.; Franco-Zorrilla, J.M.; de Vries, S.C.; Solano, R.; et al. Structural Basis for DNA Binding Specificity by the Auxin-Dependent ARF Transcription Factors. Cell 2014, 156, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Tanaka, A.; Tanabata, T.; Ohtake, M.; Fujioka, S.; Nakamura, H.; Ichikawa, H.; Mori, M. Short grain1 decreases organ elongation and brassinosteroid response in rice. Plant Physiol. 2012, 158, 1208–1219. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, G.; Liu, D.; Niu, M.; Tong, H.; Chu, C. GSK2 stabilizes OFP3 to suppress brassinosteroid responses in rice. Plant J. 2020, 102, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; E, Z.; Zhang, D.; Yun, Q.; Zhou, Y.; Niu, B.; Chen, C. OsYUC11-mediated auxin biosynthesis is essential for endosperm development of rice. Plant Physiol. 2021, 185, 934–950. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, M.; Zhou, Y.; Wang, Y.; Shen, J.; Chen, H.; Zhang, L.; Lü, B.; Liang, G.; Liang, J. The Rice G Protein γ Subunit DEP1/qPE9-1 Positively Regulates Grain-Filling Process by Increasing Auxin and Cytokinin Content in Rice Grains. Rice 2019, 12, 91. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, W.; Guo, X.; Ling, S.; Li, W.; Wang, X.; Yao, J. Heat Stress Responsive Aux/IAA Protein, OsIAA29 Regulates Grain Filling Through OsARF17 Mediated Auxin Signaling Pathway. Rice 2024, 17, 16. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Xu, Y.; Yu, C.; Shen, C.; Qian, Q.; Geisler, M.; Jiang, d.A.; Qi, Y. The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ. 2015, 38, 638–654. [Google Scholar] [CrossRef]
- Song, Y.; You, J.; Xiong, L. Characterization of OsIAA1 gene, a member of rice Aux/IAA family involved in auxin and brassinosteroid hormone responses and plant morphogenesis. Plant Mol. Biol. 2009, 70, 297–309. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, Y.; Han, S.; Chang, S.; Gao, Z.; Qi, Y.; Qian, Q. OsARF4 regulates leaf inclination via auxin and brassinosteroid pathways in rice. Front. Plant Sci. 2022, 13, 979033. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.Y.; Miao, R.; Zhou, C.L.; Cao, P.H.; Lan, J.; Zhu, X.J.; Mou, C.L.; Huang, Y.S.; Liu, S.J.; et al. DS1/OsEMF1 interacts with OsARF11 to control rice architecture by regulation of brassinosteroid signaling. Rice 2018, 11, 46. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Chen, Z.; Wei, Y.; Qi, Y.; Wu, C. OsmiR167a-targeted auxin response factors modulate tiller angle via fine-tuning auxin distribution in rice. Plant Biotechnol. J. 2020, 18, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zheng, C.; Wang, Y.; Xu, Y.; Wu, J.; Wang, L.; Liu, X.; Chen, Z. OsmiRNA5488 Regulates the Development of Embryo Sacs and Targets OsARF25 in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2023, 24, 16240. [Google Scholar] [CrossRef]
- Wu, D.; Cao, Y.; Wang, D.; Zong, G.; Han, K.; Zhang, W.; Qi, Y.; Xu, G.; Zhang, Y. Auxin receptor OsTIR1 mediates auxin signaling during seed filling in rice. Plant Physiol. 2024, 194, 2434–2448. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; He, J.; Liu, L.; Deng, Q.; Yao, X.; Liu, C.; Qiao, Y.; Li, P.; Ming, F. OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development. Plant Biotechnol. J. 2020, 18, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Marchler-Bauer, A.; Bo, Y. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xu, Y.; Xian, F.; Liu, S.; Huang, J.; Xie, B.; Hu, J. The Auxin Response Factor OsARF25 Negatively Regulates Grain Size and Weight in Rice (Oryza sativa L.) by Activating the Expression of SG1 and OsOFP04. Plants 2025, 14, 1808. https://doi.org/10.3390/plants14121808
Zhang X, Xu Y, Xian F, Liu S, Huang J, Xie B, Hu J. The Auxin Response Factor OsARF25 Negatively Regulates Grain Size and Weight in Rice (Oryza sativa L.) by Activating the Expression of SG1 and OsOFP04. Plants. 2025; 14(12):1808. https://doi.org/10.3390/plants14121808
Chicago/Turabian StyleZhang, Xinrong, Yimeng Xu, Fengjun Xian, Shuya Liu, Jishuai Huang, Bin Xie, and Jun Hu. 2025. "The Auxin Response Factor OsARF25 Negatively Regulates Grain Size and Weight in Rice (Oryza sativa L.) by Activating the Expression of SG1 and OsOFP04" Plants 14, no. 12: 1808. https://doi.org/10.3390/plants14121808
APA StyleZhang, X., Xu, Y., Xian, F., Liu, S., Huang, J., Xie, B., & Hu, J. (2025). The Auxin Response Factor OsARF25 Negatively Regulates Grain Size and Weight in Rice (Oryza sativa L.) by Activating the Expression of SG1 and OsOFP04. Plants, 14(12), 1808. https://doi.org/10.3390/plants14121808





