Vegetation Analysis and Environmental Relationships of Qatar’s Depression Habitat
Abstract
1. Introduction
2. Results
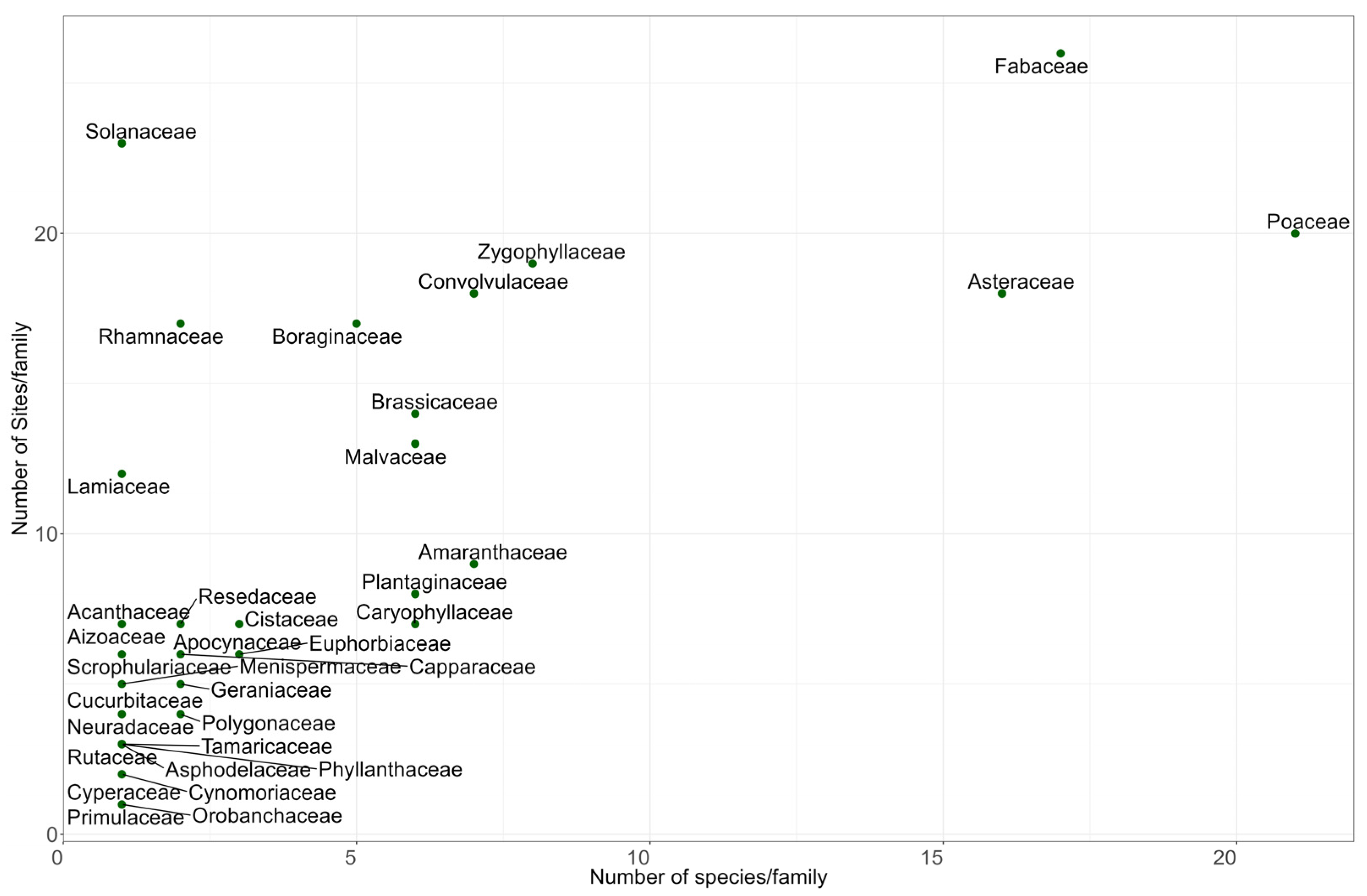
2.1. Floristic Composition
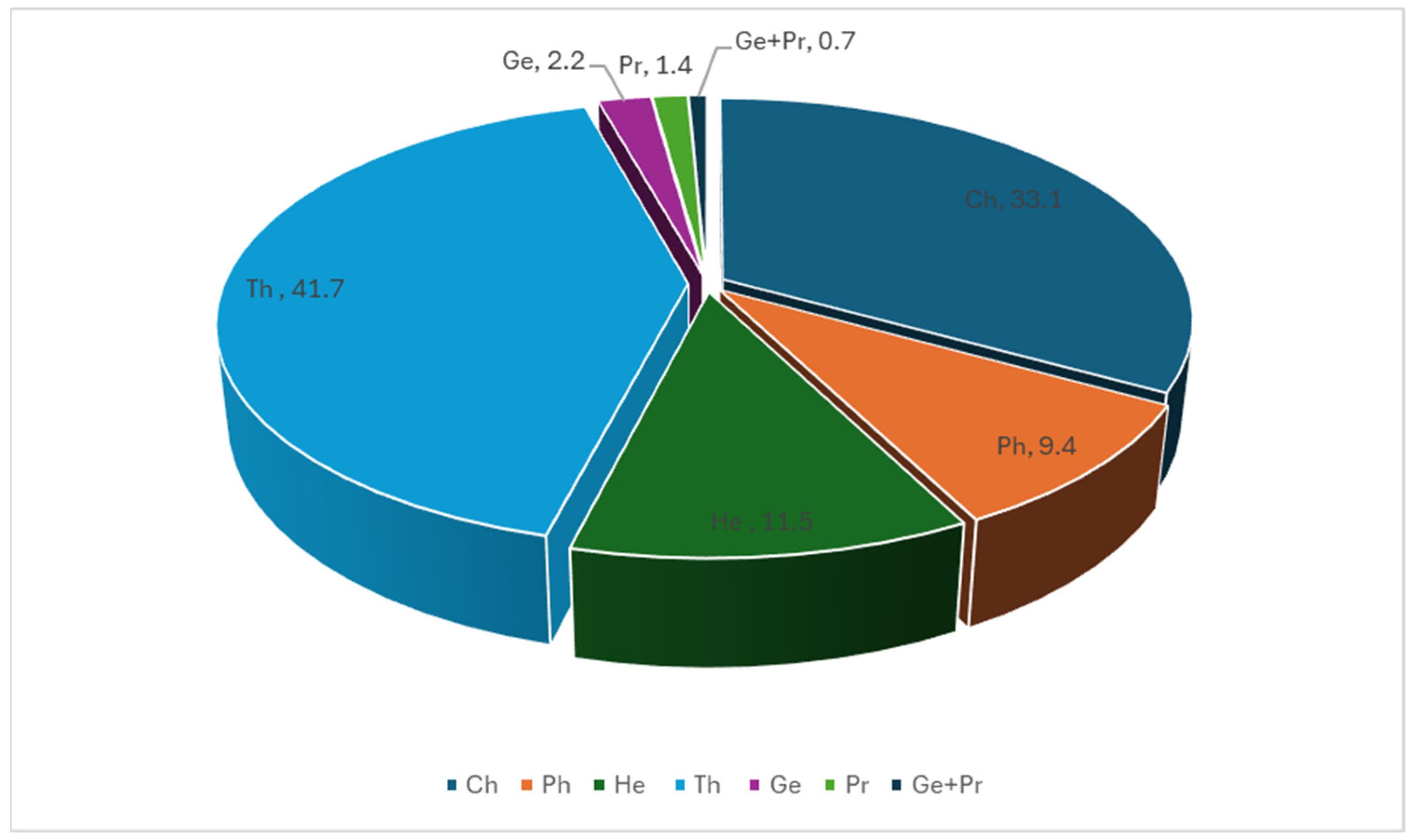
2.2. Life Forms in Qatar’s Depression Habitat
2.3. Phytogeographical Affinities
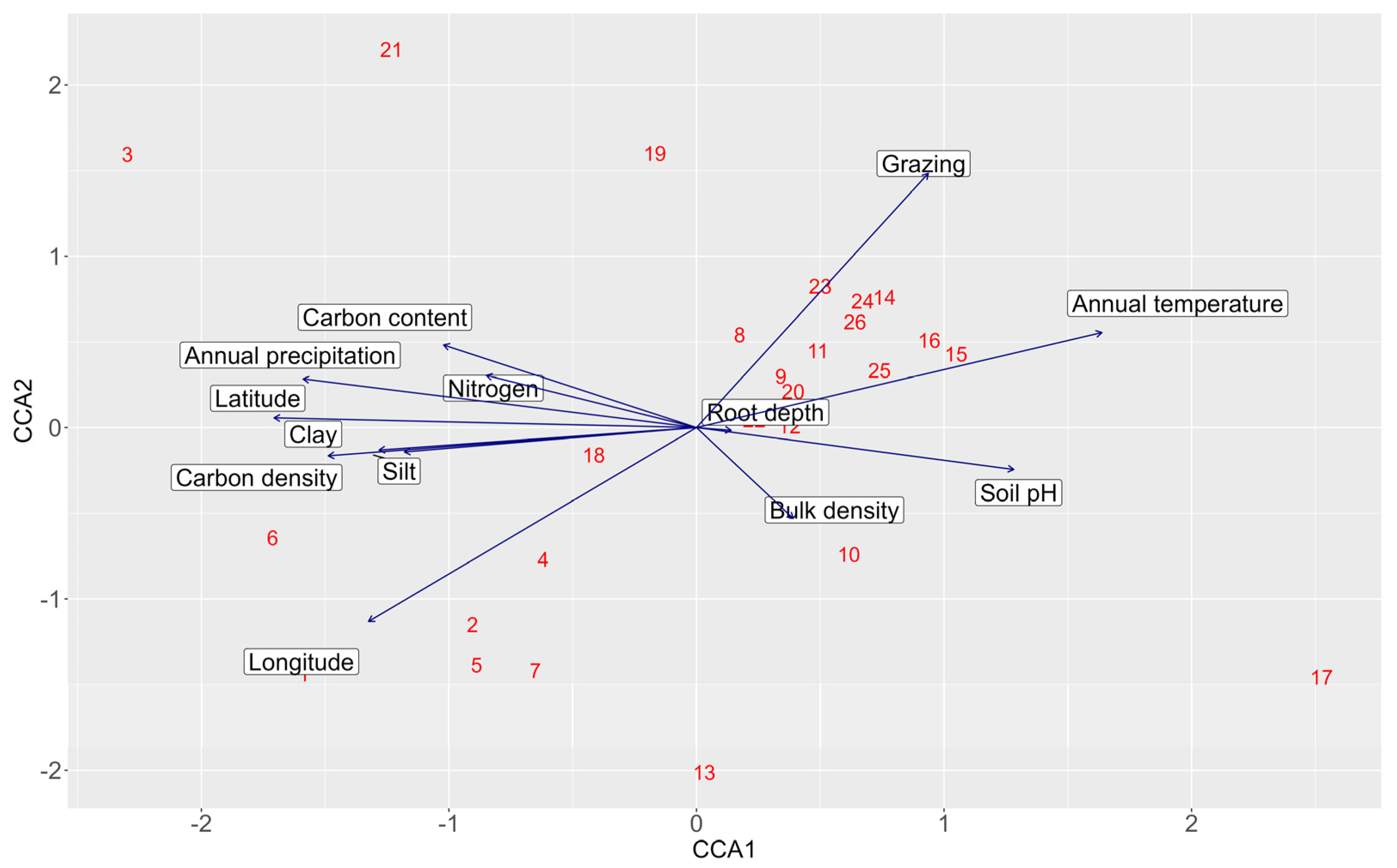
2.4. Vegetation Analysis
2.5. Alpha Diversity
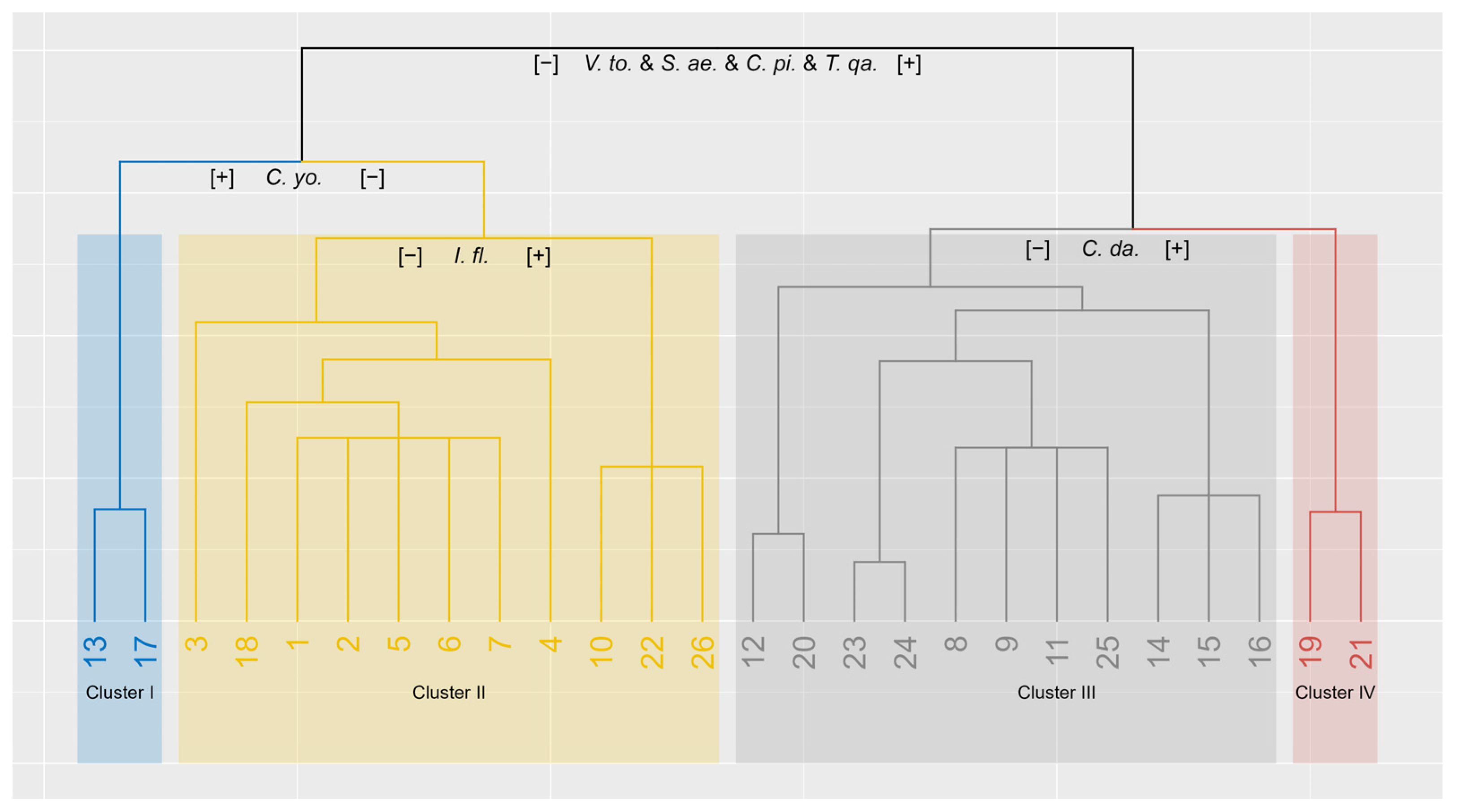
2.6. TWINSPAN
3. Discussion
3.1. Floristic Composition
3.2. Environmental Gradients and Vegetation Patterns
4. Materials and Methods
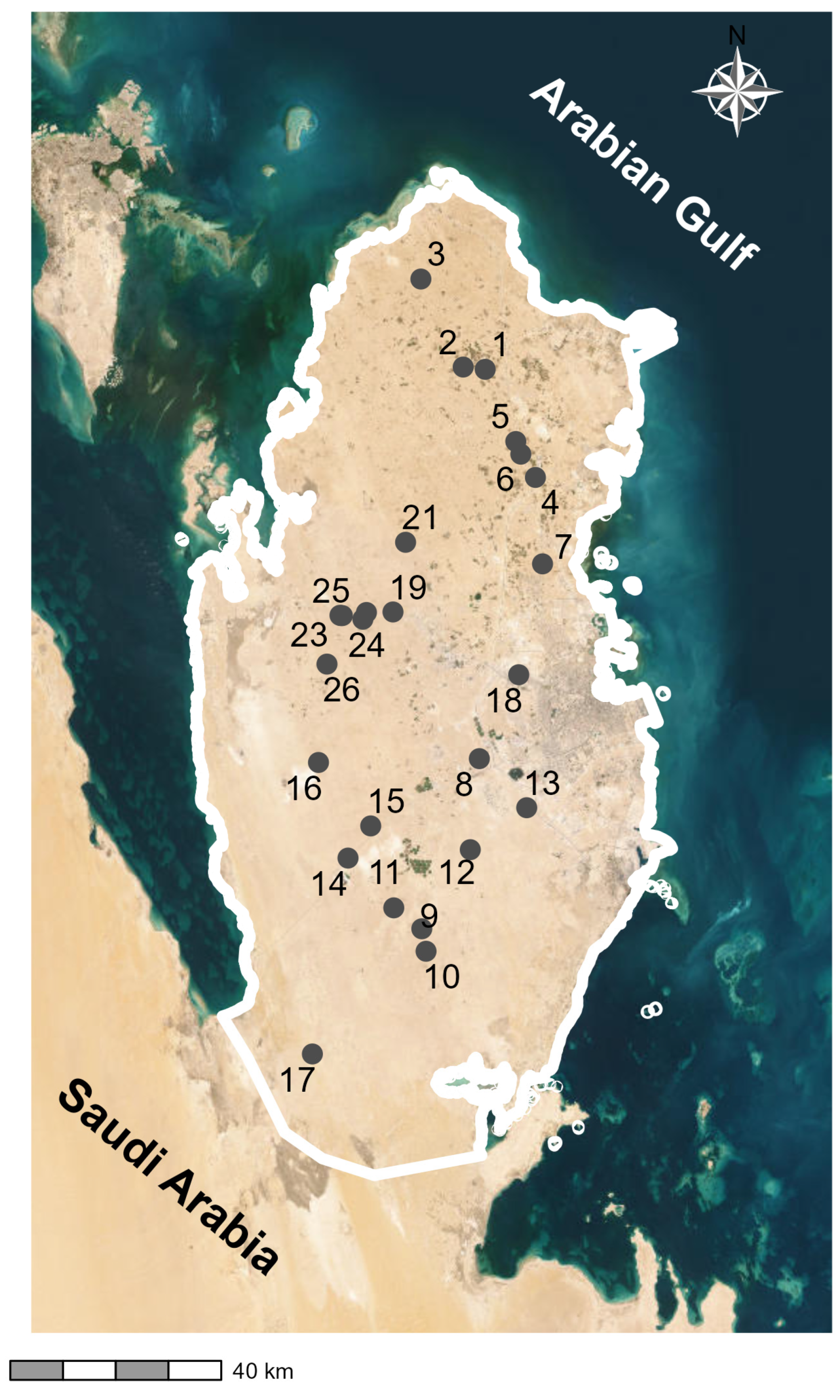
4.1. Study Area
4.2. Vegetation Sampling and Floristic Survey
4.3. Plant Life Form and Phytogeographical Analysis
4.4. Multivariate Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babiker, A.A. The Vegetation of the State of Qatar as Related to Landform and Soil. J. Doc. Humanit Res. Cent. 1990, 462–478. Available online: http://hdl.handle.net/10576/8409 (accessed on 9 June 2025).
- Batanouny, K.H. Ecology and Flora of Qatar; Alden Press Ltd. for the Centre for Scientific and Applied Research, University of Qatar: Doha, Qatar, 1981. [Google Scholar]
- Babikir, A.A.A. The Vegetation of Natural Depressions in Qatar in Relation to Climate and Soil. J. Arid. Environ. 1986, 10, 165–173. [Google Scholar] [CrossRef]
- Norton, J.; Majid, S.A.; Allan, D.; Al Safran, M.; Böer, B.; Richer, R. An Illustrated Checklist of the Flora of Qatar; Browndown Publications: Gosport, UK, 2009; ISBN 0956396100. [Google Scholar]
- De Steven, D.; Toner, M.M. Vegetation of Upper Coastal Plain Depression Wetlands: Environmental Templates and Wetland Dynamics within a Landscape Framework. Wetlands 2004, 24, 23–42. [Google Scholar] [CrossRef]
- Batanouny, K.H. Plants in the Deserts of the Middle East; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2000; ISBN 3540525726. [Google Scholar]
- Abdel Bary, E.M.M. Flora of Qatar: The Dicotyledons Volume 1. Environmental Studies Center, Qatar University. 2012. Available online: https://www.qu.edu.qa/en-us/research/esc/documents/books/the-flora-of-qatar-the-dicotyledons.pdf (accessed on 25 December 2023).
- Warner, T.T. Desert Meteorology; Cambridge University Press: Cambridge, UK, 2009; ISBN 113944963X. [Google Scholar]
- Ghazanfar, S.A. Vegetation of the Plains. In Vegetation of the Arabian Peninsula; Springer: Berlin/Heidelberg, Germany, 1998; pp. 175–190. [Google Scholar]
- Sillitoe, P.; Alshawi, A.A.; Al-Amir Hassan, A.K. Challenges to Conservation: Land Use Change and Local Participation in the Al Reem Biosphere Reserve, West Qatar. J. Ethnobiol. Ethnomed. 2010, 6, 28. [Google Scholar] [CrossRef]
- Fisher, M.; Ghazanfar, S.A.; Chaudhary, S.A.; Seddon, P.J.; Robertson, E.F.; Omar, S.; Abbas, J.A.; Böer, B. Diversity and Conservation. In Vegetation of the Arabian Peninsula; Ghazanfar, S.A., Fisher, M., Eds.; Springer: Dordrecht, The Netherlands, 1998; pp. 265–302. ISBN 978-94-017-3637-4. [Google Scholar]
- Buckley, R. Environmental Impacts of Motorized Off-Highway Vehicles. In Environmental Impacts of Ecotourism; Buckley, R.C., Ed.; CAB International: Wallingford, UK, 2004; pp. 83–97. [Google Scholar] [CrossRef]
- Osman, K.T.; Osman, K.T. Soil Resources and Soil Degradation. Soils Princ. Prop. Manag. 2013, 175–213. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated Dryland Expansion under Climate Change. Nat. Clim. Change 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Rodriguez, L.F. Can Invasive Species Facilitate Native Species? Evidence of How, When, and Why These Impacts Occur. Biol. Invasions 2006, 8, 927–939. [Google Scholar] [CrossRef]
- Hereher, M.; Alghmdi, A.; Mseddi, K.; El Kenawy, A. Remote Sensing of Vegetation Prolonged Drought at the Salt Playas of Hail—Saudi Arabia. Egypt. J. Remote Sens. Space Sci. 2022, 25, 135–145. [Google Scholar] [CrossRef]
- El-Ghanim, W.M.; Hassan, L.M.; Galal, T.M.; Badr, A. Floristic Composition and Vegetation Analysis in Hail Region North of Central Saudi Arabia. Saudi J. Biol. Sci. 2010, 17, 119–128. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Al-Harbi, H.F.; Alowaifeer, A.M.; El-Sheikh, M.A.; Assaeed, A.M.; Alsaleem, T.S.; Kassem, H.S.; Azab, O.M.; Dar, B.A.; Malik, J.A.; et al. Correlation between Aboveground Vegetation Composition and Soil Seed Bank of Raudhat Desert Habitat: A Case Study of Raudhat Alkhafs, Saudi Arabia. BMC Plant Biol. 2025, 25, 136. [Google Scholar] [CrossRef]
- Ahmed, M.; Al Saleh, A.; Dousari, N. Al Ecological Study on Native Plant Diversity in Kuwait Desert Ecosystem. IOP Conf. Ser. Earth Environ. Sci. 2022, 1087, 012029. [Google Scholar] [CrossRef]
- Abul Fatih, H.A.; Abdel Bari, E.M.; Alsubaey, A.; Ibrahim, Y.M. Halophytes and Soil Salinity in Qatar. Qatar Univ. Sci. J. 2002, 22, 119–135. [Google Scholar]
- Zohary, M. A Vegetation Map of Western Palestine. J. Ecol. 1947, 34, 1–19. [Google Scholar] [CrossRef]
- Zohary, M. The Segetal Plant Communities of Palestine. Vegetatio 1950, 2, 387–411. [Google Scholar] [CrossRef]
- Eig, A.; Zohary, M.; Feinbrun, N. The Plants of Palestine, an Analytical Key by A. Eig, M. Zohary, N. Feinbrun…; Jerusalem University Press: Jerusalem, Israel, 1931. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiær. 1934. Available online: https://archive.org/details/in.ernet.dli.2015.271790 (accessed on 24 September 2024).
- Al Shaye, N.A.; Masrahi, Y.S.; Thomas, J. Ecological Significance of Floristic Composition and Life Forms of Riyadh Region, Central Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Collenette, S. Wildflowers of Saudi Arabia; National Commission for Wildlife Conservation and Development (NCWCD): Riyadh, Saudi Arabia, 1999; ISBN 9960614093. [Google Scholar]
- Osman, A.K.E.; Abdein, M.A.E.-H. Floristic Diversity of Wadi Ar’ar, Saudi Arabia. J. Taibah Univ. Sci. 2019, 13, 772–789. [Google Scholar] [CrossRef]
- Abbas, A.M.; Al-Kahtani, M.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Badry, M.O. Floristic Diversity and Phytogeography of JABAL Fayfa: A Subtropical Dry Zone, South-West Saudi Arabia. Diversity 2020, 12, 345. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Sheded, M.G.; Salem, A.I. Vegetation Spatial Heterogeneity in a Hyper Arid Biosphere Reserve Area in North Africa. Acta Bot. Croat. 2010, 69, 31–46. [Google Scholar]
- Almutawa, A.A. Native and Xeric Plant Recommendations for Urban Landscapes in Kuwait. Technol. Hortic. 2022, 2, 7. [Google Scholar] [CrossRef]
- Wickens, G.E. Ecophysiology of Economic Plants in Arid and Semi-Arid Lands; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; ISBN 3540521712. [Google Scholar]
- AlNafie, A.H. Phytogeography of Saudi Arabia. Saudi J. Biol. Sci. 2008, 15, 159–176. [Google Scholar]
- Rousta, I.; Olafsson, H.; Zhang, H.; Moniruzzaman, M.; Baranowski, P.; Krzyszczak, J. Anthropogenic Factors Affecting the Vegetation Dynamics in the Arid Middle East. Environ. Clim. Technol. 2022, 26, 774–805. [Google Scholar] [CrossRef]
- Abd El-Ghani, M.M.; Huerta-Martínez, F.M.; Hongyan, L.; Qureshi, R. Plant Responses to Hyperarid Desert Environments; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 3319591355. [Google Scholar]
- Batanouny, K.H. Halophytes and Halophytic Plant Communities in the Arab Region: Their Potential as a Rangeland Resource. Halophytes A Resour. Livest. Rehabil. Degrad. Lands 1994, 32, 139–163. [Google Scholar]
- Hussein, E.A.; El-Ghani, M.M.A.; Hamdy, R.S.; Shalabi, L.F. Do Anthropogenic Activities Affect Floristic Diversity and Vegetation Structure More than Natural Soil Properties in Hyper-Arid Desert Environments? Diversity 2021, 13, 157. [Google Scholar] [CrossRef]
- Alzamel, N.M. Floristic Composition, Diversity, and Vegetation Structure in Wadi Al-Quwayiyah, Riyadh Region, Saudi Arabia. J. Environ. Stud. 2024, 34, 1–9. [Google Scholar] [CrossRef]
- Mayor, Á.G.; Bautista, S.; Small, E.E.; Dixon, M.; Bellot, J. Measurement of the Connectivity of Runoff Source Areas as Determined by Vegetation Pattern and Topography: A Tool for Assessing Potential Water and Soil Losses in Drylands. Water Resour Res 2008, 44, W10423. [Google Scholar] [CrossRef]
- Sallam, H.; Alzain, M.N.; Abuzaid, A.O.; Loutfy, N.; Badry, M.O.; Osman, A.K.; Hammad, S.A. Wild Plant Diversity and Soil Characteristics of Desert Roadside Vegetation in the Eastern Desert. Diversity 2023, 15, 874. [Google Scholar] [CrossRef]
- Khan, W.; Khan, S.M.; Ahmad, H.; Alqarawi, A.A.; Shah, G.M.; Hussain, M.; Abd Allah, E.F. Life Forms, Leaf Size Spectra, Regeneration Capacity and Diversity of Plant Species Grown in the Thandiani Forests, District Abbottabad, Khyber Pakhtunkhwa, Pakistan. Saudi J. Biol. Sci. 2018, 25, 94–100. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, K.; Li, J.; Li, Q.; Na, W.; Gao, Y.; Gao, Z. Response of Soil Water in Deep Dry Soil Layers to Monthly Precipitation, Plant Species, and Surface Mulch in a Semi-Arid Hilly Loess Region of China. Agric. Water Manag. 2024, 291, 108612. [Google Scholar] [CrossRef]
- Alsobeai, S.M. FLORISTIC DIVERSITY, LIFE FORM, AND PLANT LIFE CHOROLOGY AT AL-FAWZAN RESERVE, KINGDOM OF SAUDI ARABIA. Appl. Ecol. Env. Res. 2024, 22, 3709–3726. [Google Scholar] [CrossRef]
- Kargar-Chigani, H.; Javadi, S.A.; Zahedi-Amiri, G.; Khajeddin, S.J.; Jafari, M. Vegetation Composition Differentiation and Species-Environment Relationships in the Northern Part of Isfahan Province, Iran. J. Arid. Land. 2017, 9, 161–175. [Google Scholar] [CrossRef]
- Elrys, A.S.; Zhang, J.; Meng, L.; Nardi, P.; Müller, C. Clay-to-Carbon Ratio: An Overlooked but Pivotal Mediator of Soil Nitrogen Mineralization and Availability. Soil. Tillage Res. 2025, 251, 106533. [Google Scholar] [CrossRef]
- Wan, H.; Bai, Y.; Hooper, D.U.; Schönbach, P.; Gierus, M.; Schiborra, A.; Taube, F. Selective Grazing and Seasonal Precipitation Play Key Roles in Shaping Plant Community Structure of Semi-Arid Grasslands. Landsc. Ecol. 2015, 30, 1767–1782. [Google Scholar] [CrossRef]
- Noumi, Z.; Mezghani, I.; Mseddi, K. Relative Contributions of Short-Term Canopy and Long-Term Soil Effects of a Native Shrub under Arid Bioclimate: A Case Study from Tunisia. Wood Res. 2023, 68, 141–153. [Google Scholar] [CrossRef]
- Gallacher, D. Effects of Camel vs Oryx and Gazelle Grazing on the Plant Ecology of the Dubai Desert Conservation Reserve. 2006. Available online: https://camed.cirad.fr/content/download/4391/32240/version/1/file/Gallacher+2006+Effects+of+camel+vs+oryx+and+gazelle+grazing+on+the+plant+ecology+of+the+Dubai+Desert+Conservation+Reserve.pdf (accessed on 20 February 2025).
- French, M.H. The Use and Misuse of Shrubs and Trees as Fodder. East. Afr. Agric. J. 1949, 14, 157–166. [Google Scholar] [CrossRef]
- Bi, X.; Li, B.; Xu, X.; Zhang, L. Response of Vegetation and Soil Characteristics to Grazing Disturbance in Mountain Meadows and Temperate Typical Steppe in the Arid Regions of Central Asian, Xinjiang. Int. J. Env. Res. Public Health 2020, 17, 4572. [Google Scholar] [CrossRef]
- Fenetahun, Y.; Yuan, Y.; Xinwen, X.; Fentahun, T.; Nzabarinda, V.; Yong-dong, W. Impact of Grazing Intensity on Soil Properties in Teltele Rangeland, Ethiopia. Front Env. Sci 2021, 9, 664104. [Google Scholar] [CrossRef]
- Alsafran, M.H. Environmental Determinants of the Ecology and Distribution of ACACIA. Ph.D. Thesis, Newcastle University, Tyne, UK, 2014. [Google Scholar]
- Khafaga, T. Impact of Grazing on Floral Diversity in High Altitude Mountains in St. Katherine, South Sinai, Egypt; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Böer, B.; Sargeant, D. Desert Perennials as Plant and Soil Indicators in Eastern Arabia. Plant Soil. 1998, 199, 261–266. [Google Scholar] [CrossRef]
- Bagheri, H.; Shahmoradi, A.; Adnani, M. Autecology of Stipagrostis Plumosa in Rangelands of Qom Province. Iran. J. Range Desert Res. 2011, 18, 187–201. [Google Scholar]
- Cunningham, P.L. Observations of the Seasonal Dietary Preference of Male Gazella Subgutturosa Marica Thomas, 1897 (Cetartiodactyla: Bovidae) along Foraging Trails of Central Saudi Arabia. J. Threat. Taxa 2009, 1, 445–449. [Google Scholar] [CrossRef]
- Mohamed, E.E.; Rashed, A.-M.; Maryam, A.-Q.; Nafeesath, S.; Aisha, A.-K.; Masoud, A.-M. Current Status of ‘Ghaf Trees’ Prosopis Cineraria (Sw.) Dc in the State of Qatar; Hamad bin Khalifa University Press (HBKU Press): Doha, Qatar, 2016; Volume 2016, p. 21. [Google Scholar]
- Hofland, N.; Hatim, M.Z.; Thomas, J.; Janssen, J.A.M.; Alharti, A.S.; Alfarhan, A.H.; Alatar, A.A.; Schaminée, J.H.J.; El-Sheikh, M.A. The Vegetation around the Foothills of the Hijaz Mountains, Saudi Arabia. Rend. Lincei 2024, 35, 437–449. [Google Scholar] [CrossRef]
- Singh, K.; Pandey, V.C.; Singh, R.P. Cynodon Dactylon: An Efficient Perennial Grass to Revegetate Sodic Lands. Ecol. Eng. 2013, 54, 32–38. [Google Scholar] [CrossRef]
- Ellenberg, D.; Mueller-Dombois, D. Aims and Methods of Vegetation Ecology; Wiley: New York, NY, USA, 1974; Volume 547. [Google Scholar]
- FAO & IIASA. Harmonized World Soil Database Version 2.0; International Institute for Applied Systems Analysis (IIASA): Rome, Italy; Laxenburg, Austria, 2023; ISBN 978-92-5-137499-3. [Google Scholar]
- Hengl, T.; Mendes de Jesus, J.; Heuvelink, G.B.M.; Ruiperez Gonzalez, M.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global Gridded Soil Information Based on Machine Learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef]
- Hengl, T.; Leenaars, J.G.B.; Shepherd, K.D.; Walsh, M.G.; Heuvelink, G.B.M.; Mamo, T.; Tilahun, H.; Berkhout, E.; Cooper, M.; Fegraus, E.; et al. Soil Nutrient Maps of Sub-Saharan Africa: Assessment of Soil Nutrient Content at 250 m Spatial Resolution Using Machine Learning. Nutr. Cycl. Agroecosyst. 2017, 109, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, P.; Liu, N.; Zhang, L.; Yu, Z.; Feng, Q.; Smettem, K.; Liu, S. Alternating Dominant Effects of Temperature and Precipitation along Elevational Gradient on the Alpine and Subalpine Vegetation Activities in Southwestern China. Ecol. Manag. 2024, 554, 121668. [Google Scholar] [CrossRef]
- Karger, D.N.; Zimmermann, N.E. CHELSAcruts—High Resolution Temperature and Precipitation Timeseries for the 20th Century and Beyond. EnviDat 2018. [Google Scholar] [CrossRef]
- Hijmans, R.J. Terra: Spatial Data Analysis, R Package Version 1.7-71; 2024. Available online: https://CRAN.R-project.org/package=terra (accessed on 15 February 2025).
- Bonham, C.D. Measurements for Terrestrial Vegetation; Wiley-Blackwell: Hoboken, NJ, USA, 2013; ISBN 9780470972588. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. The Interrelations of Certain Analytic and Synthetic Phytosociological Characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Zelený, D. Methods of Vegetation Survey. Available online: https://www.davidzeleny.net/wiki/doku.php/vegsurvey:materials:how_to_calculate_ivi (accessed on 22 March 2025).
- Pearse, K. An Area-List Method of Measuring Range Plant Populations. Ecology 1935, 16, 573–579. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, S.M.; Jaremko, M.; Jahangir, S.; Ullah, Z.; Ali, I.; Ahmad, Z.; Badshah, H. Vegetation Assessments under the Influence of Environmental Variables from the Yakhtangay Hill of the Hindu-Himalayan Range, North Western Pakistan. Sci. Rep. 2022, 12, 20973. [Google Scholar] [CrossRef]
- Bhadra, A.K.; Pattanayak, S.K. Dominance Is More Justified than Abundance to Calculate Importance Value Index (IVI) of Plant Species. Asian J. Sci. Technol. 2017, 8, 4304–4326. [Google Scholar]
- Mustapha, Y.; Adamu, S.; Inuwa, A. Importance Value Index (IVI) of Tree Species and Diversity of Baturiya Hadejia Wetland National Park, Jigawa State, Nigeria. Int. J. Trend Sci. Res. Dev. 2022, 6, 876–883. [Google Scholar]
- Li, C.; Chytrý, M.; Zelený, D.; Chen, M.; Chen, T.; Chiou, C.; Hsia, Y.; Liu, H.; Yang, S.; Yeh, C. Classification of T Aiwan Forest Vegetation. Appl. Veg. Sci. 2013, 16, 698–719. [Google Scholar] [CrossRef]
- Ellenberg, H.; Mueller-Dombois, D. A Key to Raunkiaer Plant Life Forms with Revised Subdivisions. Ber. Geobot. Inst. Rubel. 1967, 37, 56–73. [Google Scholar]
- Aljedaani, G.S.; Fakhry, A.M. Floristic Diversity of Jeddah: An Arid Desert, Western Region of Saudi Arabia. Egypt. J. Bot. 2023, 63, 57–68. [Google Scholar] [CrossRef]
- Al-Malki, M.A.; Osman, H.E.; El-Morsy, M.H.M. Ecological and Nutritional Values of Halophytes in the Al-Qunfudhah, Saudi Arabia. Umm Al-Qura Univ. J. Appl. Sci. 2021, 7, 27–33. [Google Scholar]
- Thomas, J.; El-Sheikh, M.A.; Alatar, A.A. Endemics and Endangered Species in the Biodiversity Hotspot of the Shada Mountains, Saudi Arabia. J. Arid. Land. 2017, 9, 109–121. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24, ISBN 0444538690. [Google Scholar]
- Ter Braak, C.J.F. The Analysis of Vegetation-Environment Relationships by Canonical Correspondence Analysis. Vegetatio 1987, 69, 69–77. [Google Scholar] [CrossRef]
- Yang, Y.-S.; Shao, H.-B.; Wu, S.-F.; Bu, C.-F.; Wang, C.; Zhang, P. Spatial Distribution of Biological Soil Crusts on the Slope of the Chinese Loess Plateau Based on Canonical Correspondence Analysis. Catena 2016, 137, 373–381. [Google Scholar] [CrossRef]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; ISBN 9780521814096. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Canonical Ordination. In Numerical Ecology with R.; Use R! Springer Nature: Berlin/Heidelberg, Germany, 2018; pp. 203–297. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-4. 2017. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 March 2025).
- ter Braak, C.J.F. Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R.; Use R! Springer International Publishing: Cham, Germany, 2018; ISBN 978-3-319-71403-5. [Google Scholar]
- Carleton, T.J.; Stitt, R.H.; Nieppola, J. Constrained Indicator Species Analysis (COINSPAN): An Extension of TWINSPAN. J. Veg. Sci. 1996, 7, 125–130. [Google Scholar] [CrossRef]
- Oksanen, J.; Hill, M.O. Twinspan: Two-Way Indicator Species Analysis, R Package Version 0.9-5; 2025. Available online: https://jarioksa.r-universe.dev/twinspan (accessed on 15 March 2025).
- Willis, A.D. Rarefaction, Alpha Diversity, and Statistics. Front. Microbiol. 2019, 10, 2407. [Google Scholar] [CrossRef]
- Janczyk, M.; Pfister, R. One-Way Analysis of Variance (ANOVA). In Understanding Inferential Statistics; Springer: Berlin, Heidelberg, 2023; pp. 97–125. [Google Scholar]

| Chorotype * | No. of Species per Category | Group | Presence of Floristic Category in North | Presence of Floristic Category in South | Presence of Floristic Category in Centre |
|---|---|---|---|---|---|
| SA + IR-TR | 27 | Biregional | 20 | 21 | 14 |
| SA | 26 | Monoregional | 15 | 20 | 8 |
| ME + SA | 15 | Biregional | 11 | 6 | 5 |
| SA + SU | 9 | Biregional | 6 | 7 | 9 |
| SU | 6 | Monoregional | 4 | 2 | 5 |
| PAN | 5 | Pluriregional | 5 | 3 | 1 |
| SA-SI + S-Z | 4 | Biregional | 3 | 3 | 3 |
| ME | 4 | Monoregional | 4 | 1 | 2 |
| ME + SA-SI | 4 | Biregional | 3 | 2 | 1 |
| COSM | 4 | Pluriregional | 3 | 0 | 1 |
| SA + IR-TR + SU | 4 | Pluriregional | 4 | 2 | 2 |
| SA-SI + IR-TR | 3 | Biregional | 3 | 2 | 1 |
| ME + SA + IR-TR | 3 | Pluriregional | 2 | 3 | 1 |
| ME + SA-SI + IR-TR | 3 | Pluriregional | 2 | 1 | 0 |
| SA-SI + IR-TR | 3 | Biregional | 2 | 2 | 2 |
| TR | 3 | Monoregional | 2 | 0 | 2 |
| ME + IR-TR | 2 | Biregional | 2 | 0 | 0 |
| S-Z + SA-SI | 2 | Biregional | 2 | 1 | 1 |
| Other minor chorotypes | 1 | Mixed | 7 | 6 | 4 |
| Species | Life Form * | Cluster I | Cluster II | Cluster III | Cluster IV | All Sites |
|---|---|---|---|---|---|---|
| Convolvulus pilosellifolius Desr. (C. pi) | He | 0 | 1.82 | 0.79 | 0 | 0.77 |
| Cynodon dactylon (L.) Pers. (C. da) | Ge | 0 | 2.4 | 0 | 38.35 | 4.08 |
| Cyperus conglomeratus Rottb. (C. yo) | He | 1.69 | 0 | 0 | 0 | 0.13 |
| Ifloga spicata (Forssk.) Sch. Bip. (I. fl) | Th | 0.35 | 0.68 | 0.25 | 0 | 0.42 |
| Lycium shawii Roem. & Schult. (L. sh) | Ph | 0 | 5.93 | 11.65 | 5.72 | 8 |
| Plantago boissieri Hausskn. & Bornm. (P. bo) | Th | 9.66 | 0 | 0 | 0 | 0.74 |
| Salvia aegyptiaca L. (S. ae) | Ch | 3.12 | 5.66 | 1.32 | 0 | 2.97 |
| Schismus arabicus Nees (S. ar) | Th | 9.67 | 0.28 | 0 | 0 | 0.86 |
| Stipa capensis Thunb. (S. ca) | Th | 2.08 | 6.46 | 0 | 0 | 2.89 |
| Stipagrostis plumosa Munro ex T.Anderson (S. pl) | He | 10.65 | 5.66 | 0 | 0 | 3.21 |
| Tetraena qatarensis (Hadidi) Beier & Thulin (T. qa) | Ch | 3.64 | 1.55 | 1.52 | 0 | 1.51 |
| Vachellia tortilis (Forssk.) Galasso & Banfi (V. to) | Ph | 0 | 8.42 | 31.07 | 12.72 | 18.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgharib, A.; Trigo, M.d.M.; Moursy, M.M.; Soultan, A. Vegetation Analysis and Environmental Relationships of Qatar’s Depression Habitat. Plants 2025, 14, 1807. https://doi.org/10.3390/plants14121807
Elgharib A, Trigo MdM, Moursy MM, Soultan A. Vegetation Analysis and Environmental Relationships of Qatar’s Depression Habitat. Plants. 2025; 14(12):1807. https://doi.org/10.3390/plants14121807
Chicago/Turabian StyleElgharib, Ahmed, María del Mar Trigo, Mohamed M. Moursy, and Alaaeldin Soultan. 2025. "Vegetation Analysis and Environmental Relationships of Qatar’s Depression Habitat" Plants 14, no. 12: 1807. https://doi.org/10.3390/plants14121807
APA StyleElgharib, A., Trigo, M. d. M., Moursy, M. M., & Soultan, A. (2025). Vegetation Analysis and Environmental Relationships of Qatar’s Depression Habitat. Plants, 14(12), 1807. https://doi.org/10.3390/plants14121807






