Cesium Accumulation Patterns and Stress Response in Hydroponic Radish (Raphanus sativus L.): A Physiological–Transcriptomic Study
Abstract
1. Introduction
2. Results
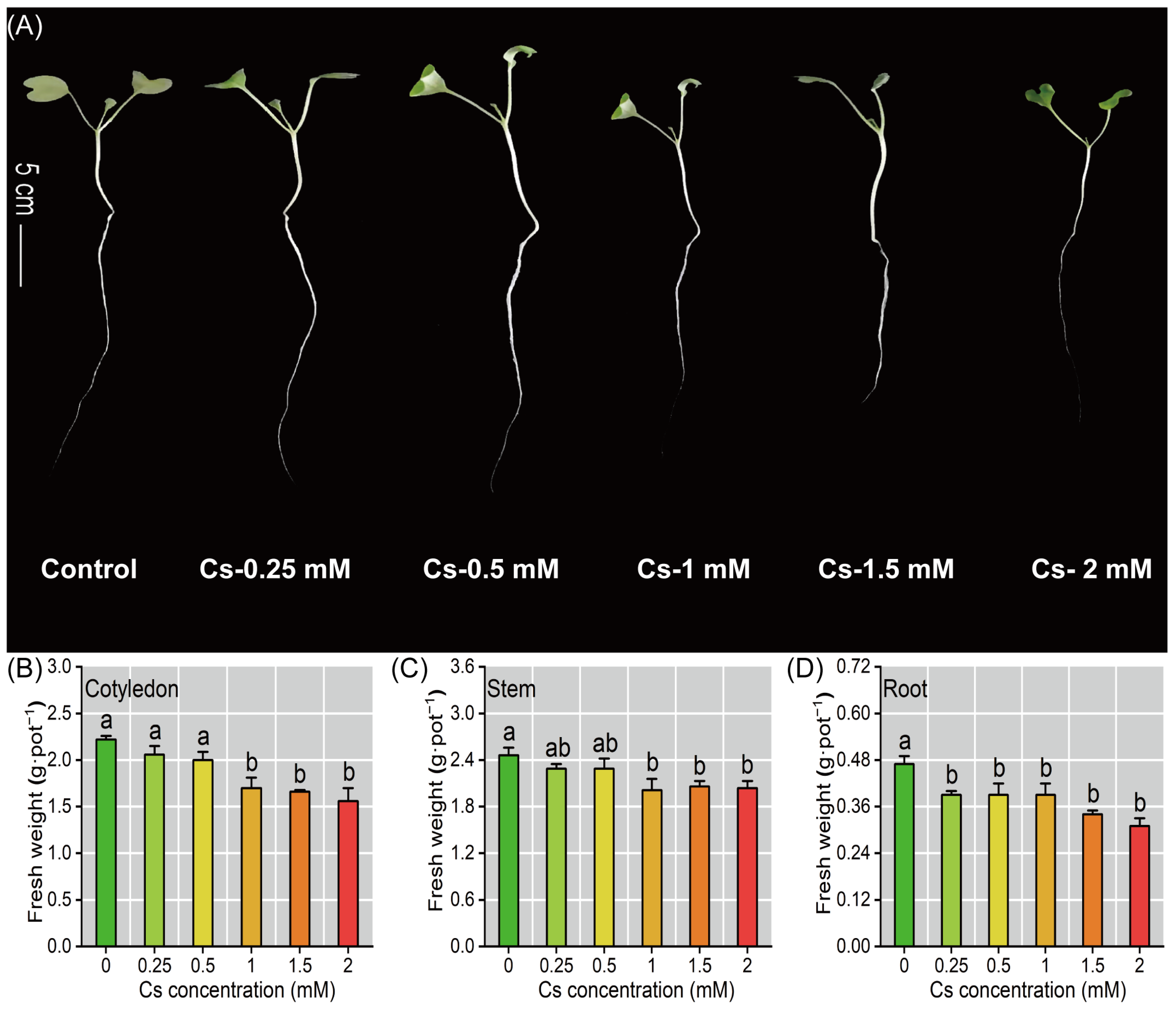
2.1. The Radish Seedlings Growth Under Cs Stress
2.2. Cs Accumulation Characteristics in Radish Seedlings
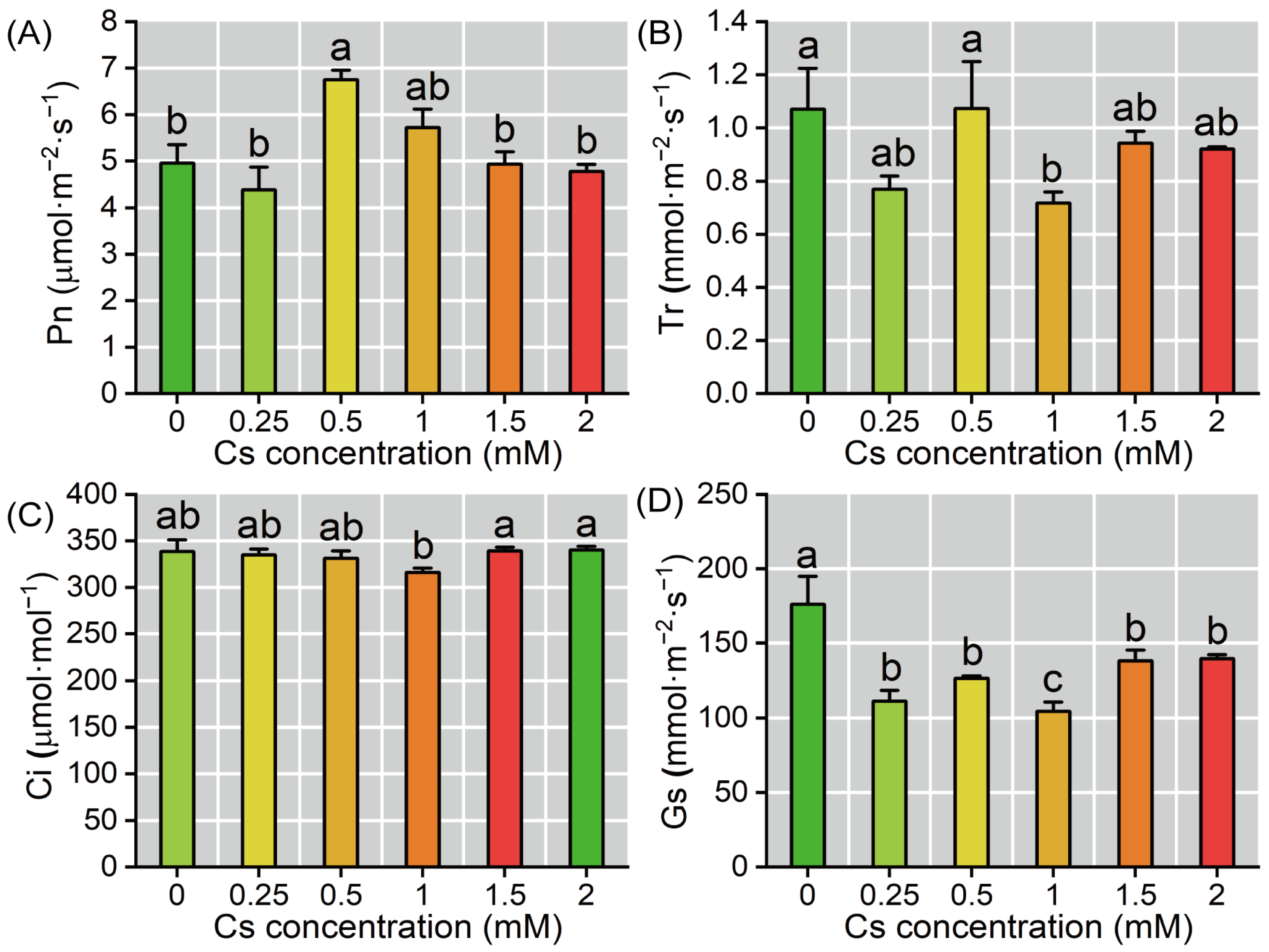
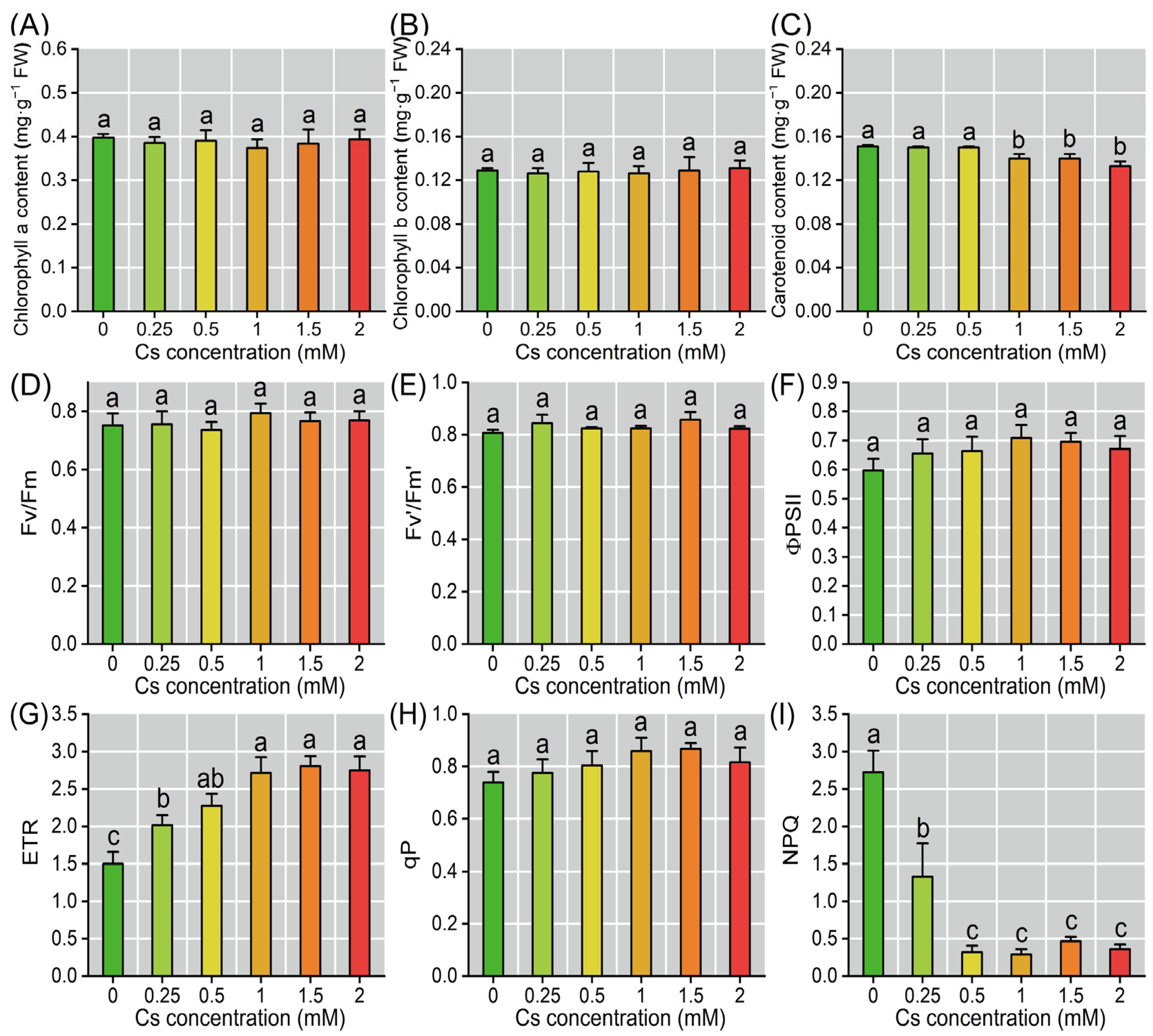
2.3. Response of Photosynthetic Parameters of Radish Seedlings to Cs Stress in the Cotyledon
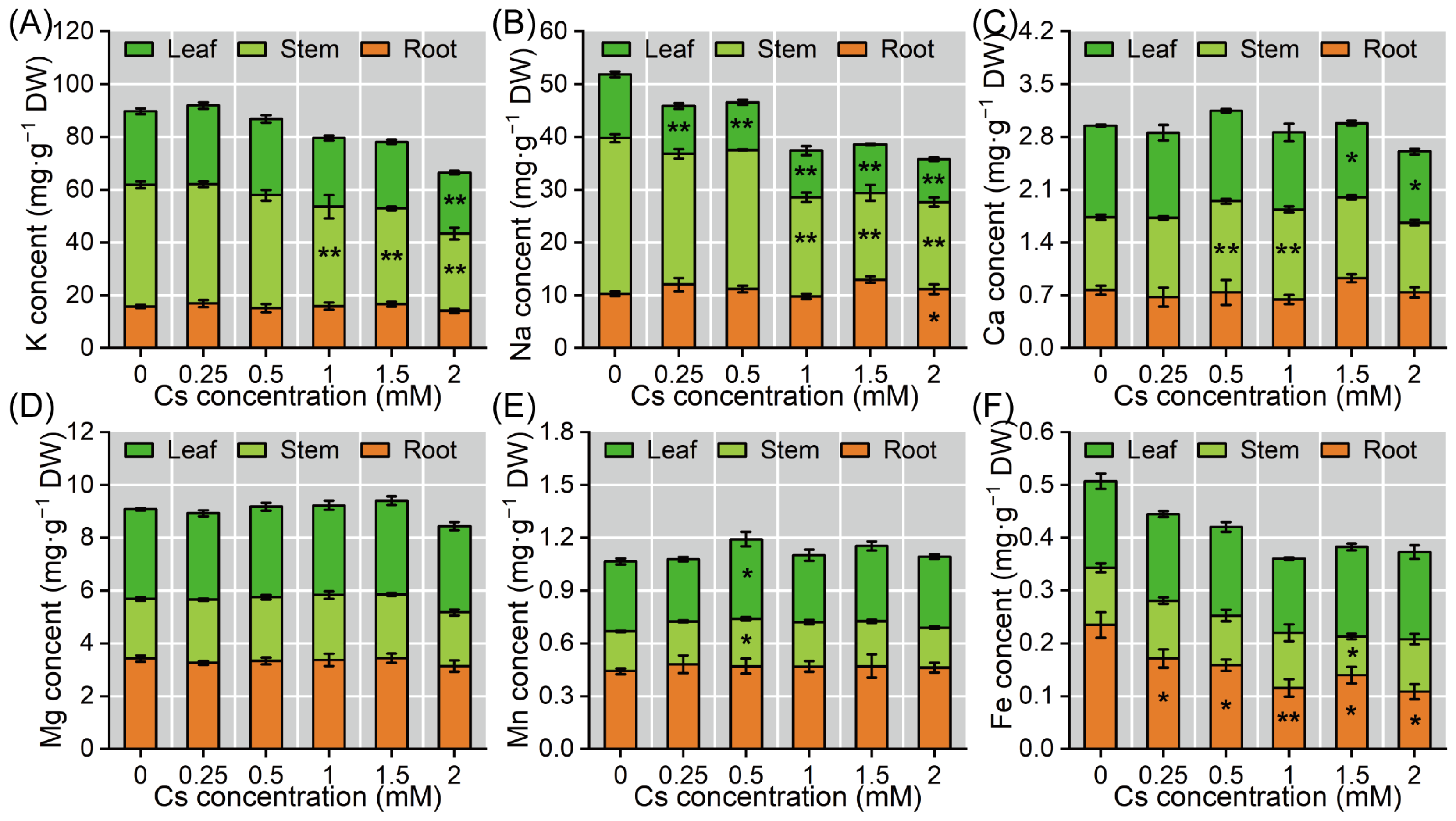
2.4. The Mineral Element Content Changes of Radish Seedlings Under Cs Stress
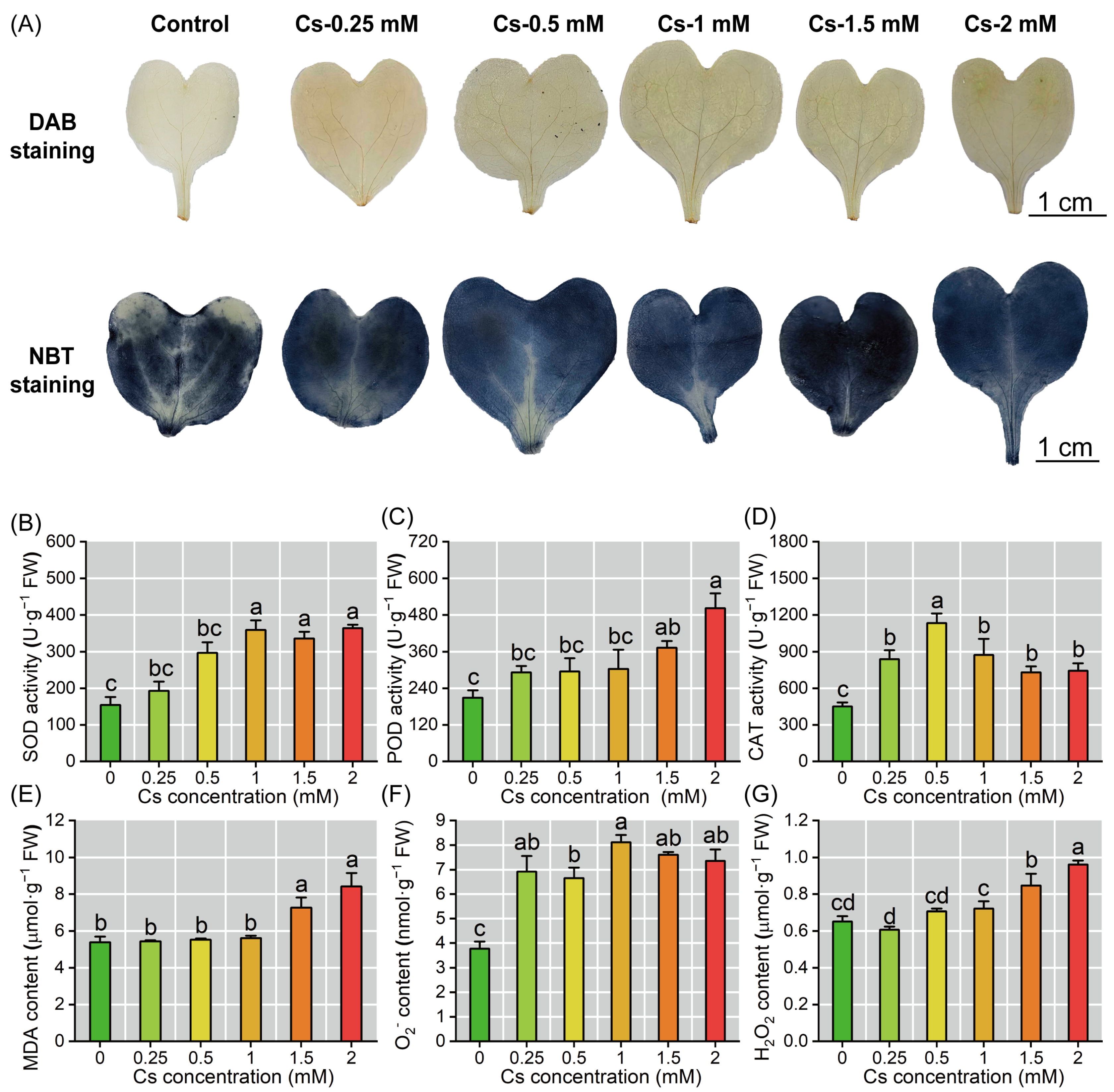
2.5. Cs Induced Oxidative Stress in Radish Seedlings
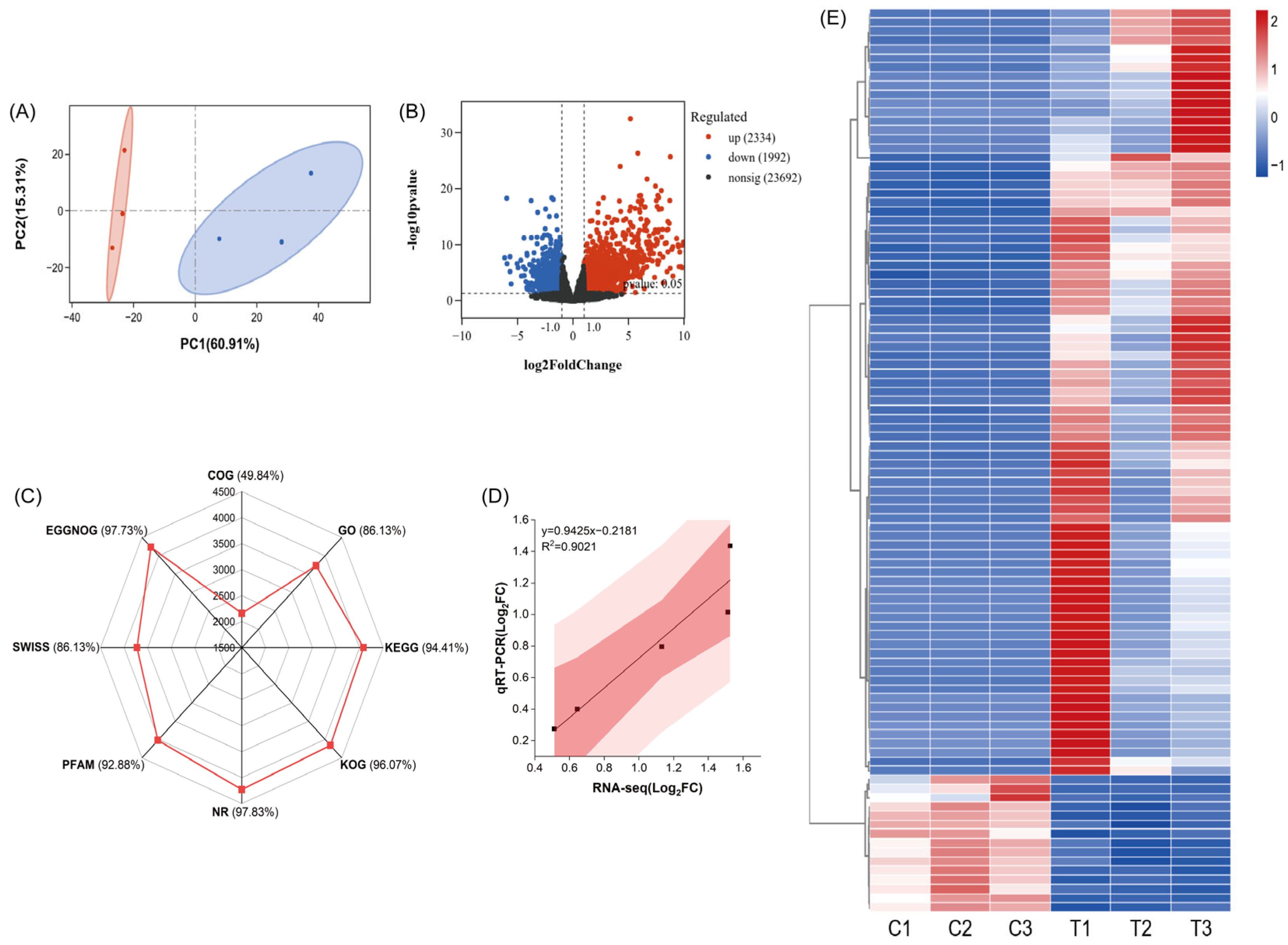
2.6. Transcriptome Analysis of Radish Seedlings
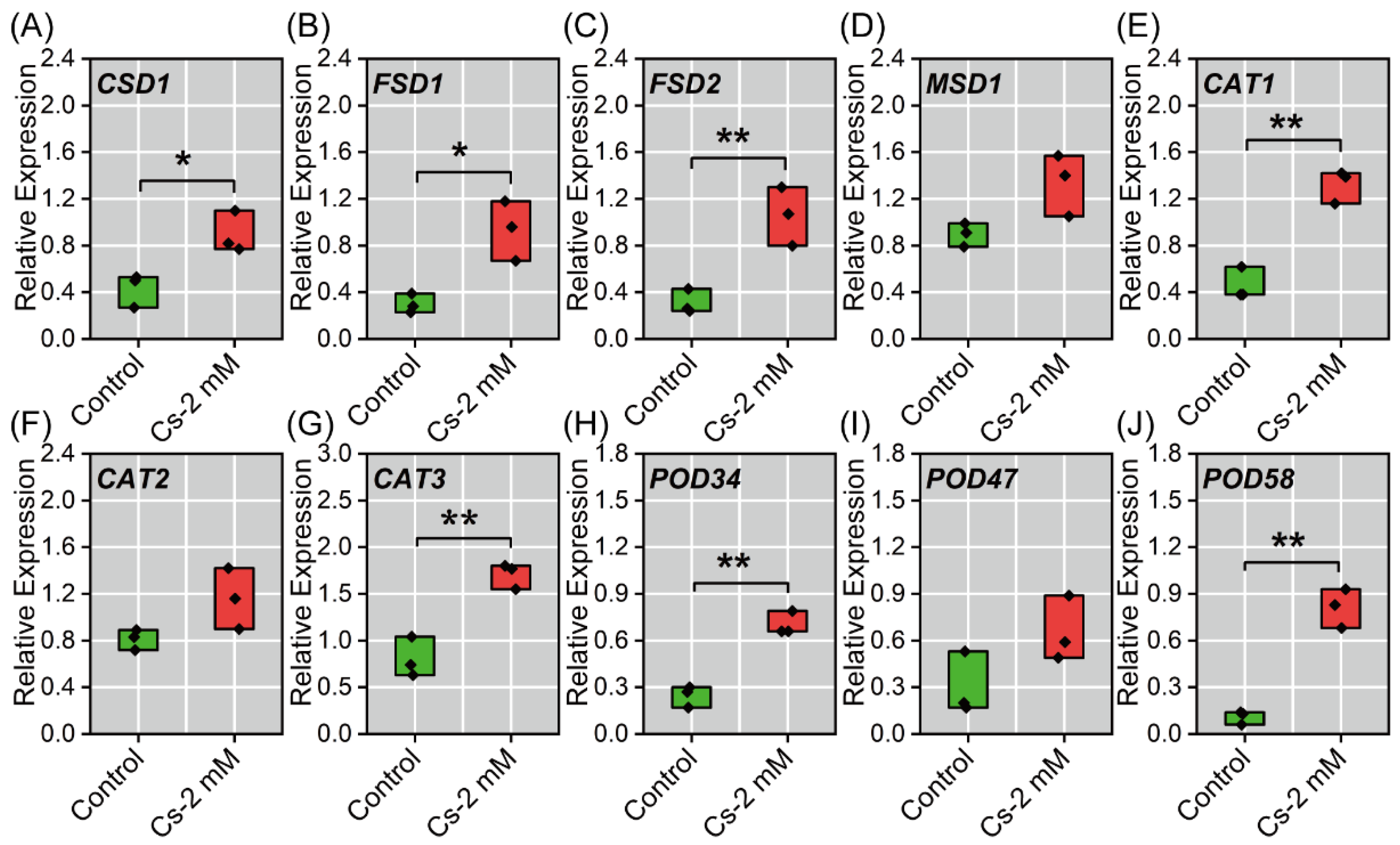
2.7. Antioxidant Enzyme-Associated Gene Expression in Radish Seedlings to Cs Stress
3. Discussion
4. Materials and Methods
4.1. Plant Culture and Cs Treatment
4.2. Determination of Physiological Parameters
4.2.1. Determination of Element Content and Photosynthetic Parameters
4.2.2. Determination of the ROS Metabolism
4.3. Transcriptome and Gene Expression Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.G.; Smolders, E. Plant uptake of radiocaesium: A review of mechanisms, regulation and application. J. Exp. Bot. 2000, 51, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Chen, J.; Wu, Y.; Zheng, C. Research on the background values of the Chinese soil environment. Environ. Sci. 1991, 12, 5–19. [Google Scholar] [CrossRef]
- Ammar, A.; Nouira, A.; El Mouridi, Z.; Boughribil, S. Recent trends in the phytoremediation of radionuclide contamination of soil by cesium and strontium: Sources, mechanisms and methods: A comprehensive review. Chemosphere 2024, 359, 142273. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akib, S.; Maah, M.J.; Yusoff, I.; Balkhair, K.S. Cesium-137: Radio-Chemistry, Fate, and Transport, Remediation, and Future Concerns. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1740–1793. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Liu, Q.; Li, J.; Qiao, Z.; Sun, P.; Yang, Y. A critical review on soil washing during soil remediation for heavy metals and organic pollutants. Int. J. Environ. Sci. Technol. 2021, 19, 601–624. [Google Scholar] [CrossRef]
- Xie, L.; van Zyl, D. The geochemical processes affecting the mobility of Cu, Pb, Zn during phytostabilization on sulfidic mine tailings revealed by a greenhouse study. J. Clean. Prod. 2023, 418, 138119. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Shen, Z.; Zhu, J.; Jia, X.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J. Field trials of phytomining and phytoremediation: A critical review of influencing factors and effects of additives. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2724–2774. [Google Scholar] [CrossRef]
- Xie, C.; Pu, S.; Xiong, X.; Chen, S.; Peng, L.; Fu, J.; Sun, L.; Guo, B.; Jiang, M.; Li, X. Melatonin-assisted phytoremediation of Pb-contaminated soil using bermudagrass. Environ. Sci. Pollut. Res. 2021, 28, 44374–44388. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.O.; Maier, R.M. Phytostabilization of Mine Tailings in Arid and Semiarid Environments—An Emerging Remediation Technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef]
- Ali, S.; Wang, D.; Kaleri, A.R.; Baloch, S.B.; Brtnicky, M.; Kucerik, J.; Mustafa, A. Physiological Responses and Phytoremediation Abilities of Cucumber (Cucumis sativus L.) under Cesium and Strontium Contaminated Soils. Agronomy 2022, 12, 1311. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, J.-l.; Tao, Z.-Y.; Han, N.; Wu, G. Characterizations of bio-accumulations, subcellular distribution and chemical forms of cesium in Brassica juncea, and Vicia faba. J. Environ. Radioact. 2016, 154, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-l.; Luo, X.-G. Comparative transcriptomics analysis of potassium uptake pathways mediated cesium accumulation differences and related molecular mechanisms in Brassica juncea and Vicia faba. Ecotoxicol. Environ. Saf. 2019, 179, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, C.; Hu, Y.; Wang, J. Response of Amaranthus tricolor to cesium stress in hydroponic system: Growth, photosynthesis and cesium accumulation. Chemosphere 2022, 307, 135754. [Google Scholar] [CrossRef]
- Adams, E.; Miyazaki, T.; Saito, S.; Uozumi, N.; Shin, R. Cesium Inhibits Plant Growth Primarily Through Reduction of Potassium Influx and Accumulation in Arabidopsis. Plant Cell Physiol. 2019, 60, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-l.; Tao, Z.-G.; Fu, Q.; Han, N.; Wu, G.; Zhang, H.; Lu, H.; Luo, X.-G. The chemical toxicity of cesium in Indian mustard (Brassica juncea L.) seedlings. J. Environ. Radioact. 2016, 160, 93–101. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akihiro, T.; Ito, K.; Kumagai, S.; Sugita, R.; Tanoi, K.; Rahman, A. ATP binding cassette proteins ABCG37 and ABCG33 function as potassium-independent cesium uptake carriers in Arabidopsis roots. Mol. Plant 2021, 14, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Weidinger, M.; Adlassnig, W.; Puschenreiter, M.; Lichtscheidl, I. Response of Plantago major to cesium and strontium in hydroponics: Absorption and effects on morphology, physiology and photosynthesis. Environ. Pollut. 2019, 254, 113084. [Google Scholar] [CrossRef]
- Kondo, M.; Makino, T.; Eguchi, T.; Goto, A.; Nakano, H.; Takai, T.; Arai-Sanoh, Y.; Kimura, T. Comparative analysis of the relationship between Cs and K in soil and plant parts toward control of Cs accumulation in rice. Soil Sci. Plant Nutr. 2014, 61, 144–151. [Google Scholar] [CrossRef]
- Shinano, T.; Watanabe, T.; Chu, Q.; Osaki, M.; Kobayashi, D.; Okouchi, T.; Matsunami, H.; Nagata, O.; Okazaki, K.; Nakamura, T. Varietal difference in radiocesium uptake and transfer from radiocesium deposited soils in the genus Amaranthus. Soil Sci. Plant Nutr. 2014, 60, 809–817. [Google Scholar] [CrossRef]
- Tsukada, H.; Hasegawa, H.; Hisamatsu, S.; Yamasaki, S. Rice uptake and distributions of radioactive 137Cs, stable 133Cs and K from soil. Environ. Pollut. 2002, 117, 403–409. [Google Scholar] [CrossRef]
- Atapaththu, K.S.S.; Rashid, M.H.; Asaeda, T. Growth and Oxidative Stress of Brittlewort (Nitella pseudoflabellata) in Response to Cesium Exposure. Bull. Environ. Contam. Toxicol. 2016, 96, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Weidinger, M.; Adlassnig, W.; Puschenreiter, M.; Lichtscheidl, I. Response of Arabidopsis halleri to cesium and strontium in hydroponics: Extraction potential and effects on morphology and physiology. Ecotoxicol. Environ. Saf. 2019, 184, 109625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.-J. Effects of cesium accumulation on chlorophyll content and fluorescence of Brassica juncea L. J. Environ. Radioact. 2018, 195, 26–32. [Google Scholar] [CrossRef]
- Carvalho, C.; Mosquera, B.; Anjos, R.M.; Sanches, N.; Bastos, J.; Macario, K.; Veiga, R. Accumulation and long-term behavior of radiocaesium in tropical plants. Braz. J. Phys. 2006, 36, 1345–1348. [Google Scholar] [CrossRef]
- Anjos, R.; Mosquera, B.; Sanches, N.; Cambui, C.; Mercier, H. Caesium, potassium and ammonium distributions in different organs of tropical plants. Environ. Exp. Bot. 2009, 65, 111–118. [Google Scholar] [CrossRef]
- Lai, J.-l.; Luo, X.-G. High-efficiency antioxidant system, chelating system and stress-responsive genes enhance tolerance to cesium ionotoxicity in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2019, 181, 491–498. [Google Scholar] [CrossRef]
- Stanojković, J.N.; Ćosić, M.V.; Božović, D.P.; Sabovljević, A.D.; Sabovljević, M.S.; Čučulović, A.A.; Vujičić, M.M. Effects of Cesium on Physiological Traits of the Catherine’s Moss Atrichum undulatum Hedw. Plants 2023, 13, 54. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, H.; He, Y.; Chen, Z.; Yao, L.; Han, H. Improving radish phosphorus utilization efficiency and inhibiting Cd and Pb uptake by using heavy metal-immobilizing and phosphate-solubilizing bacteria. Sci. Total Environ. 2023, 868, 161685. [Google Scholar] [CrossRef]
- Sun, X.; Wang, S.; Tian, J.; Xiang, X.; Zheng, H.; Liu, H.; Fang, Z.; Tian, Z.; Liu, L.; Zhu, Y.; et al. Synergistic interplay between ABA-generating bacteria and biochar in the reduction of heavy metal accumulation in radish, pakchoi, and tomato. Environ. Pollut. 2023, 333, 122084. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.; Tang, M.; Wang, Y.; Dong, J.; Ying, J.; Chen, Y.; Hu, B.; Li, C.; Liu, L. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef]
- Shah, S.S.H.; Nakagawa, K.; Yokoyama, R.; Berndtsson, R. Heavy metal immobilization and radish growth improvement using Ca(OH)2-treated cypress biochar in contaminated soil. Chemosphere 2024, 360, 142385. [Google Scholar] [CrossRef]
- Adams, E.; Abdollahi, P.; Shin, R. Cesium Inhibits Plant Growth through Jasmonate Signaling in Arabidopsis thaliana. Int. J. Mol. Sci. 2013, 14, 4545–4559. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yu, L.; Li, B.; Du, N.; Guo, S. The effect of exogenous calcium on cucumber fruit quality, photosynthesis, chlorophyll fluorescence, and fast chlorophyll fluorescence during the fruiting period under hypoxic stress. BMC Plant Biol. 2018, 18, 180. [Google Scholar] [CrossRef]
- Guo, H.; Dong, Q.; Li, S.; Cha, X.; Sun, L.; Duan, H.; Li, S.; Jin, Y.; Zhang, M. Effects of exogenous calcium on growth, chlorophyll fluorescence characteristics and antioxidant system of Fraxinus malacophylla seedlings. Plant Physiol. Biochem. 2023, 201, 107860. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, G.; Xiao, P.; Ma, Q.; Li, Y.; Lai, J.; Luo, X.; Ji, X.; Xia, J.; Yang, X. A new perspective on the inhibition of plant photosynthesis by uranium: Decrease of root activity and stomatal closure. Int. J. Phytoremediation 2021, 24, 1071–1080. [Google Scholar] [CrossRef]
- Bragina, T.V.; Ponomareva, Y.V.; Drozdova, I.S.; Grinieva, G.M. Photosynthesis, respiration, and transpiration in maize seedlings under hypoxia induced by complete flooding. Dokl. Biol. Sci. 2002, 384, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, N.; Luo, Y.; Ni, Y.; Liu, Z.; Wu, G.; Zheng, T.; Dang, Y.; Chen, H.; Li, W. Effects of strontium on the morphological and photosynthetic physiological characteristics of Vicia faba seedlings. Int. J. Phytoremediation 2022, 25, 811–821. [Google Scholar] [CrossRef]
- Chen, M.; Tang, Y.L.; Ao, J.; Wang, D. Effects of strontium on photosynthetic characteristics of oilseed rape seedlings. Russ. J. Plant Physiol. 2012, 59, 772–780. [Google Scholar] [CrossRef]
- Lokdarshi, A.; Guan, J.; Urquidi Camacho, R.A.; Cho, S.K.; Morgan, P.W.; Leonard, M.; Shimono, M.; Day, B.; von Arnim, A.G. Light Activates the Translational Regulatory Kinase GCN2 via Reactive Oxygen Species Emanating from the Chloroplast. Plant Cell 2020, 32, 1161–1178. [Google Scholar] [CrossRef]
- Pospíšil, P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 2012, 1817, 218–231. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Genies, L.; Martin, L.; Kanno, S.; Chiarenza, S.; Carasco, L.; Camilleri, V.; Vavasseur, A.; Henner, P.; Leonhardt, N. Disruption of AtHAK/KT/KUP9 enhances plant cesium accumulation under low potassium supply. Physiol. Plant. 2020, 173, 1243–1290. [Google Scholar] [CrossRef]
- Isaure, M.P.; Fraysse, A.; Devès, G.; Le Lay, P.; Fayard, B.; Susini, J.; Bourguignon, J.; Ortega, R. Micro-chemical imaging of cesium distribution in Arabidopsis thaliana plant and its interaction with potassium and essential trace elements. Biochimie 2006, 88, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Lichtscheidl, I. Stable and radioactive cesium: A review about distribution in the environment, uptake and translocation in plants, plant reactions and plants’ potential for bioremediation. Sci. Total Environ. 2018, 618, 1459–1485. [Google Scholar] [CrossRef] [PubMed]
- Rai, H.; Yokoyama, S.; Satoh-Nagasawa, N.; Furukawa, J.; Nomi, T.; Ito, Y.; Fujimura, S.; Takahashi, H.; Suzuki, R.; Yousra, E.; et al. Cesium Uptake by Rice Roots Largely Depends Upon a Single Gene, HAK1, Which Encodes a Potassium Transporter. Plant Cell Physiol. 2017, 58, 1486–1493. [Google Scholar] [CrossRef]
- Ródenas, R.; Nieves-Cordones, M.; Rivero, R.M.; Martinez, V.; Rubio, F. Pharmacological and gene regulation properties point to the SlHAK5 K+ transporter as a system for high-affinity Cs+ uptake in tomato plants. Physiol. Plant. 2017, 162, 455–466. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Mechanisms of caesium uptake by plants. New Phytol. 2008, 147, 241–256. [Google Scholar] [CrossRef]
- Lucena, J.J.; Hernandez-Apaolaza, L. Iron nutrition in plants: An overview. Plant Soil 2017, 418, 1–4. [Google Scholar] [CrossRef]
- Kroh, G.E.; Pilon, M. Regulation of Iron Homeostasis and Use in Chloroplasts. Int. J. Mol. Sci. 2020, 21, 3395. [Google Scholar] [CrossRef]
- Ravet, K.; Pilon, M. Copper and Iron Homeostasis in Plants: The Challenges of Oxidative Stress. Antioxid. Redox Signal. 2013, 19, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Sun, W.; Wang, T. The Adaptive Mechanism of Plants to Iron Deficiency via Iron Uptake, Transport, and Homeostasis. Int. J. Mol. Sci. 2019, 20, 2424. [Google Scholar] [CrossRef] [PubMed]
- Berthet, S.; Villiers, F.; Alban, C.; Serre, N.B.C.; Martin-Laffon, J.; Figuet, S.; Boisson, A.M.; Bligny, R.; Kuntz, M.; Finazzi, G.; et al. Arabidopsis thaliana plants challenged with uranium reveal new insights into iron and phosphate homeostasis. New Phytol. 2017, 217, 657–670. [Google Scholar] [CrossRef]
- Chen, X.; Dang, Y.-X.; Li, Q.-L.; Li, W.; Xie, M.-T.; Wang, M.-X.; Tao, M.-F.; Zhao, S.-S.; Lai, J.-L.; Wu, G. Uranium affects nitrogen metabolism and endoplasmic reticulum protein homeostasis in plants. Environ. Exp. Bot. 2023, 213, 105444. [Google Scholar] [CrossRef]
- Borgstahl, G.E.O.; Oberley-Deegan, R.E. Superoxide Dismutases (SODs) and SOD Mimetics. Antioxidants 2018, 7, 156. [Google Scholar] [CrossRef]
- Lai, J.-L.; Liu, Z.-W.; Li, C.; Luo, X.-G. Analysis of accumulation and phytotoxicity mechanism of uranium and cadmium in two sweet potato cultivars. J. Hazard. Mater. 2021, 409, 124997. [Google Scholar] [CrossRef]
- Choi, D.; Ko, D.K.; Kim, D.-H. Transcriptome analysis revealed that Arabidopsis model plant invokes the activation of heat shock proteins and ER stress response against cesium stress. Plant Biotechnol. Rep. 2024, 18, 385–399. [Google Scholar] [CrossRef]
- Ong, W.-D.; Makita, Y.; Miyazaki, T.; Matsui, M.; Shin, R. Arabidopsis transcriptomic analysis reveals cesium inhibition of root growth involves abscisic acid signaling. Planta 2024, 259, 36. [Google Scholar] [CrossRef]
- Fukai, T.; Gallie, D.R.; Chen, Z. Chloroplast-localized iron superoxide dismutases FSD2 and FSD3 are functionally distinct in Arabidopsis. PLoS ONE 2019, 14, e0220078. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, W.-h.; Jia, W.; Zhang, J.; Foyer, C. Mitogen-activated protein kinase kinase 5 (MKK5)-mediated signalling cascade regulates expression of iron superoxide dismutase gene in Arabidopsis under salinity stress. J. Exp. Bot. 2015, 66, 5971–5981. [Google Scholar] [CrossRef]
- Lai, J.-L.; Liu, Z.-W.; Luo, X.-G. A metabolomic, transcriptomic profiling, and mineral nutrient metabolism study of the phytotoxicity mechanism of uranium. J. Hazard. Mater. 2020, 386, 121437. [Google Scholar] [CrossRef] [PubMed]
- Min, X.U.; Jun, L.I.U.; Abuduwayiti, A. Comparative Methods on Chlorophyll Extraction in Plant Physiology Experiment Teaching. Exp. Sci. Technol. 2018, 16, 129–133. [Google Scholar]
- Lai, J.-L.; Deng, Z.-X.; Ji, X.-H.; Luo, X.-G. Absorption and interaction mechanisms of uranium & cadmium in purple sweet potato (Ipomoea batatas L.). J. Hazard. Mater. 2020, 400, 123264. [Google Scholar] [CrossRef] [PubMed]
- Vanhoudt, N.; Horemans, N.; Biermans, G.; Saenen, E.; Wannijn, J.; Nauts, R.; Van Hees, M.; Vandenhove, H. Uranium affects photosynthetic parameters in Arabidopsis thaliana. Environ. Exp. Bot. 2014, 97, 22–29. [Google Scholar] [CrossRef]
- Vanhoudt, N.; Vandenhove, H.; Horemans, N.; Wannijn, J.; Bujanic, A.; Vangronsveld, J.; Cuypers, A. Study of oxidative stress related responses induced in Arabidopsis thaliana following mixed exposure to uranium and cadmium. Plant Physiol. Biochem. 2010, 48, 879–886. [Google Scholar] [CrossRef]
- Serre, N.B.C.; Alban, C.; Bourguignon, J.; Ravanel, S. Uncovering the physiological and cellular effects of uranium on the root system of Arabidopsis thaliana. Environ. Exp. Bot. 2019, 157, 121–130. [Google Scholar] [CrossRef]
- Gomes, B.R.; Siqueira-Soares, R.d.C.; Santos, W.D.d.; Marchiosi, R.; Soares, A.R.; Ferrarese-Filho, O. The effects of dopamine on antioxidant enzymes activities and reactive oxygen species levels in soybean roots. Plant Signal. Behav. 2014, 9, e977704. [Google Scholar] [CrossRef]
- Alves, L.R.; Monteiro, C.C.; Carvalho, R.F.; Ribeiro, P.C.; Tezotto, T.; Azevedo, R.A.; Gratão, P.L. Cadmium stress related to root-to-shoot communication depends on ethylene and auxin in tomato plants. Environ. Exp. Bot. 2017, 134, 102–115. [Google Scholar] [CrossRef]
- Sekulska-Nalewajko, J.; Gocławski, J.; Chojak-Koźniewska, J.; Kuźniak, E. Automated image analysis for quantification of reactive oxygen species in plant leaves. Methods 2016, 109, 114–122. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Zhao, H.; Wang, Y.; Wu, C.; Zhao, Y.; Xue, H.; Zhu, Q.; Zhang, J.; Ou, X. The 14-3-3 Protein BdGF14a Increases the Transcriptional Regulation Activity of BdbZIP62 to Confer Drought and Salt Resistance in Tobacco. Plants 2024, 13, 245. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, W.; Zheng, D.; Xiong, H.; Feng, X.; Zhang, X.; Wang, Q.; Wu, F.; Xu, J.; Lu, Y. ZmLBD5 Increases Drought Sensitivity by Suppressing ROS Accumulation in Arabidopsis. Plants 2022, 11, 1382. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, M.-T.; Li, Q.-L.; Dang, Y.-X.; Peng, S.; Tan, Y.-Y.; Wang, M.-Y.; Fan, Y.-M.; Lai, J.-L.; Wu, G. New insights into plant physiological responses to uranium: An integrative analysis of autophagy, DNA repair, and antioxidant systems in radish. Plant Physiol. Biochem. 2025, 221, 109641. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and Detection of Reactive Oxygen Species (ROS), Lipid Peroxidation, and Electrolyte Leakage in Plants. In Plant Stress Tolerance; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–297. [Google Scholar] [CrossRef]
- Provero, P.; Zhang, Z.H.; Jhaveri, D.J.; Marshall, V.M.; Bauer, D.C.; Edson, J.; Narayanan, R.K.; Robinson, G.J.; Lundberg, A.E.; Bartlett, P.F.; et al. A Comparative Study of Techniques for Differential Expression Analysis on RNA-Seq Data. PLoS ONE 2014, 9, e103207. [Google Scholar] [CrossRef]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. WIREs RNA 2016, 8. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, G.; Dang, Y.-X.; Li, Q.-l.; Xie, M.-T.; Li, W.; Zhang, H.; Lai, J.-L. Uranium triggers ferroptosis-like cell death in Vicia faba roots by increasing iron accumulation and inhibiting glutathione peroxidase activity. Environ. Exp. Bot. 2023, 205, 105122. [Google Scholar] [CrossRef]
- Ganger, M.T.; Dietz, G.D.; Ewing, S.J. A common base method for analysis of qPCR data and the application of simple blocking in qPCR experiments. BMC Bioinform. 2017, 18, 534. [Google Scholar] [CrossRef]
- Pfaffl, M.W. The ongoing evolution of qPCR. Methods 2010, 50, 215–216. [Google Scholar] [CrossRef]
- Moberly, J.G.; Bernards, M.T.; Waynant, K.V. Key features and updates for Origin 2018. J. Cheminform 2018, 10, 5. [Google Scholar] [CrossRef]
- Tanavalee, C.; Luksanapruksa, P.; Singhatanadgige, W. Limitations of Using Microsoft Excel Version 2016 (MS Excel 2016) for Statistical Analysis for Medical Research. Clin. Spine Surg. 2016, 29, 203–204. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.-H.; Chen, X.; Sun, M.; Yang, C.-H.; Xu, M.-Y.; Lai, F.-X.; Fu, S.-Q.; Fan, Y.-M.; Guo, X.-P.; Li, Q.; et al. Cesium Accumulation Patterns and Stress Response in Hydroponic Radish (Raphanus sativus L.): A Physiological–Transcriptomic Study. Plants 2025, 14, 1802. https://doi.org/10.3390/plants14121802
Wen Y-H, Chen X, Sun M, Yang C-H, Xu M-Y, Lai F-X, Fu S-Q, Fan Y-M, Guo X-P, Li Q, et al. Cesium Accumulation Patterns and Stress Response in Hydroponic Radish (Raphanus sativus L.): A Physiological–Transcriptomic Study. Plants. 2025; 14(12):1802. https://doi.org/10.3390/plants14121802
Chicago/Turabian StyleWen, Yu-Han, Xi Chen, Ming Sun, Chao-Hui Yang, Meng-Yuan Xu, Feng-Xiang Lai, Si-Qi Fu, Yu-Meng Fan, Xin-Peng Guo, Qun Li, and et al. 2025. "Cesium Accumulation Patterns and Stress Response in Hydroponic Radish (Raphanus sativus L.): A Physiological–Transcriptomic Study" Plants 14, no. 12: 1802. https://doi.org/10.3390/plants14121802
APA StyleWen, Y.-H., Chen, X., Sun, M., Yang, C.-H., Xu, M.-Y., Lai, F.-X., Fu, S.-Q., Fan, Y.-M., Guo, X.-P., Li, Q., & Wu, G. (2025). Cesium Accumulation Patterns and Stress Response in Hydroponic Radish (Raphanus sativus L.): A Physiological–Transcriptomic Study. Plants, 14(12), 1802. https://doi.org/10.3390/plants14121802






