Vascular Flora on Croatian Historic Structures: Drivers of Biodeterioration and Conservation Implications
Abstract
1. Introduction
2. Results
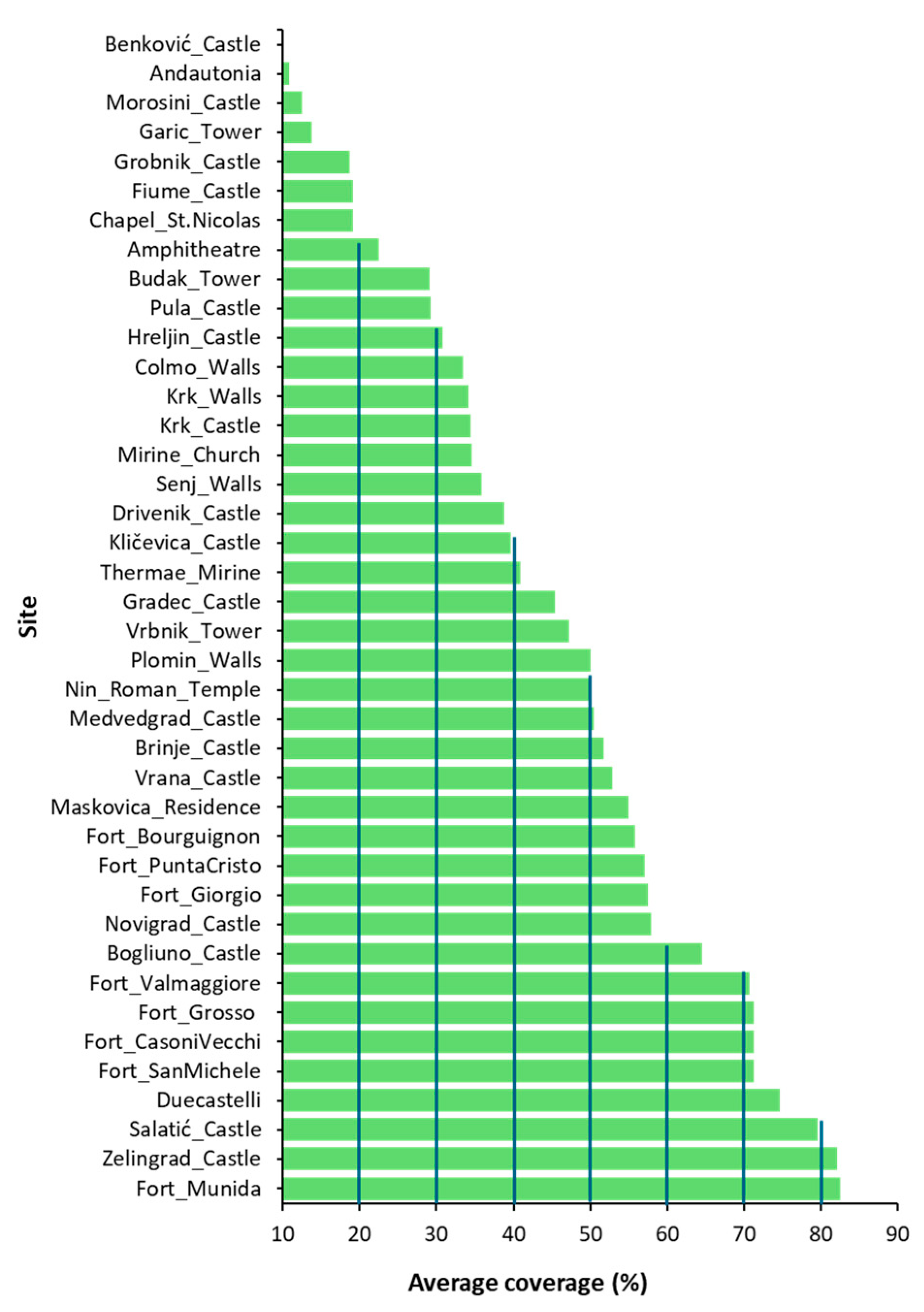
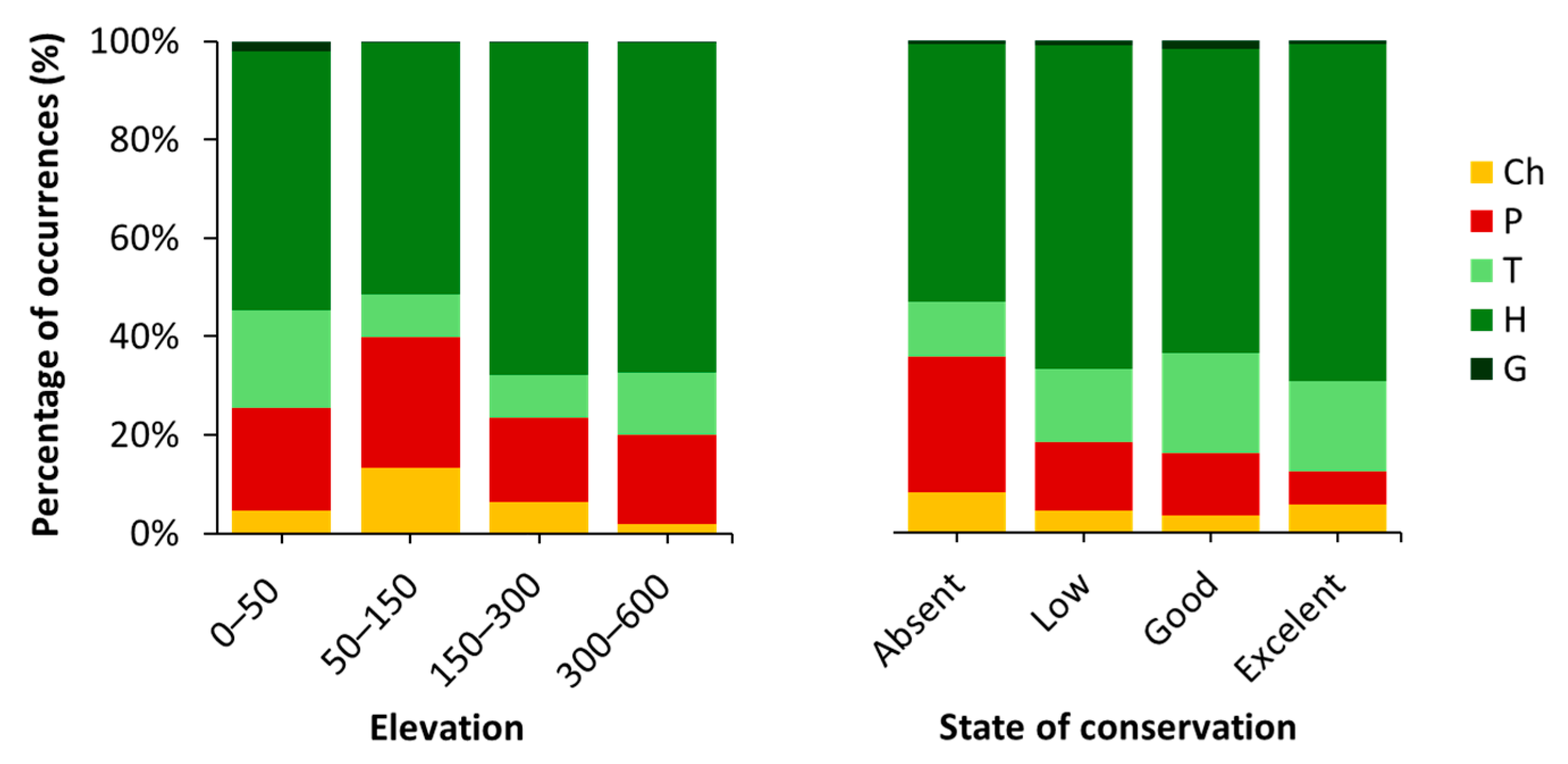
2.1. Floristic Surveys
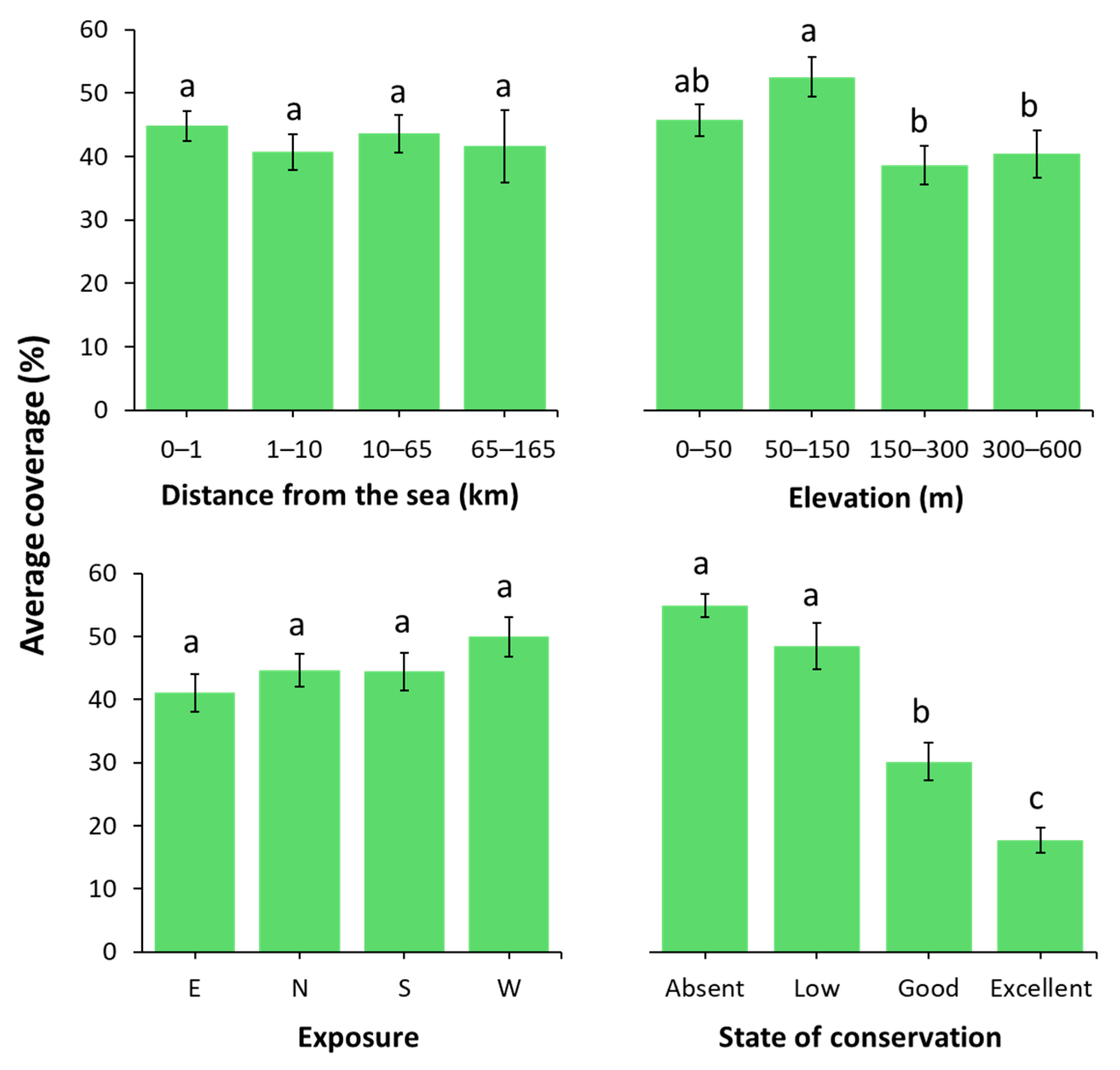
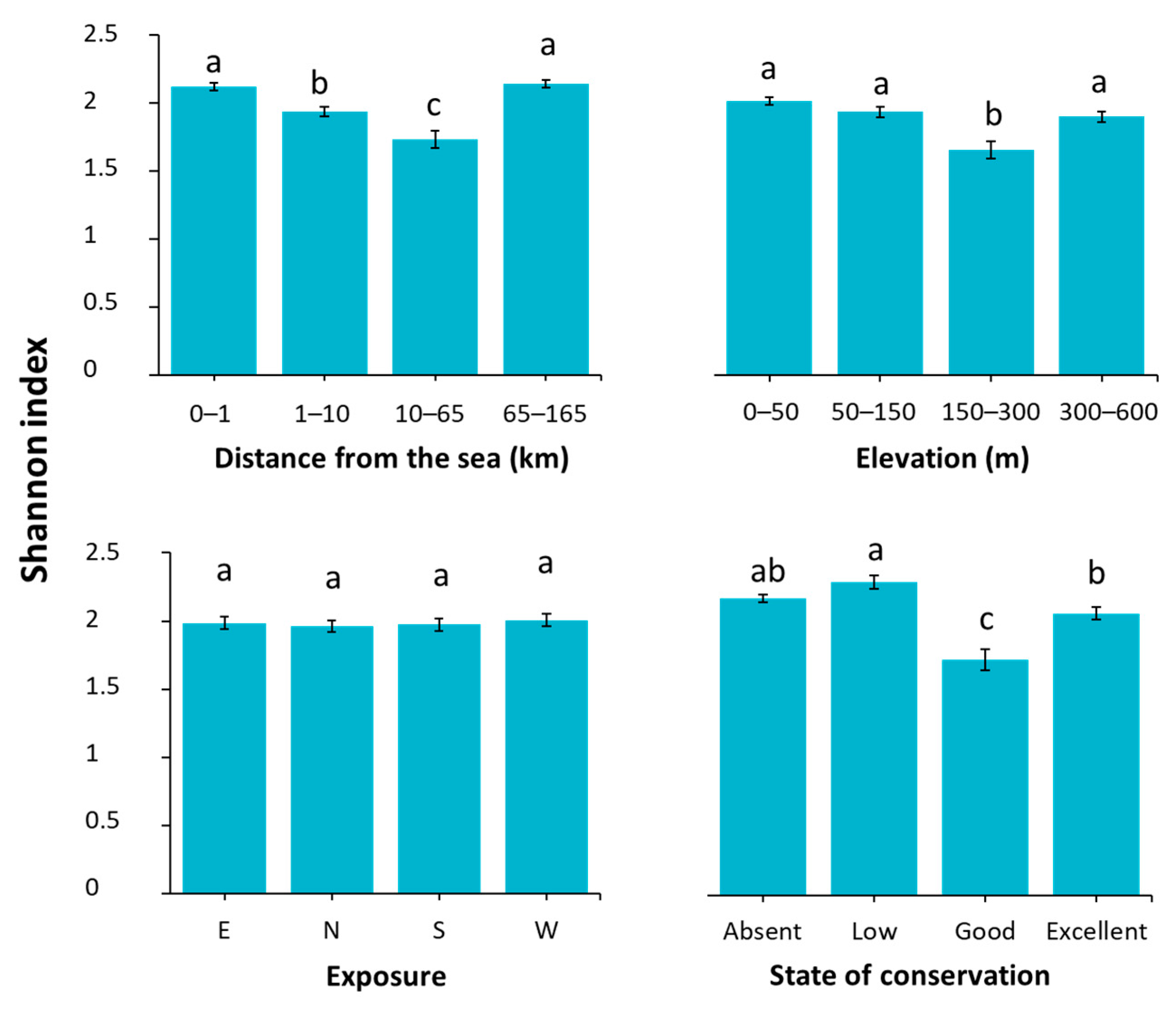
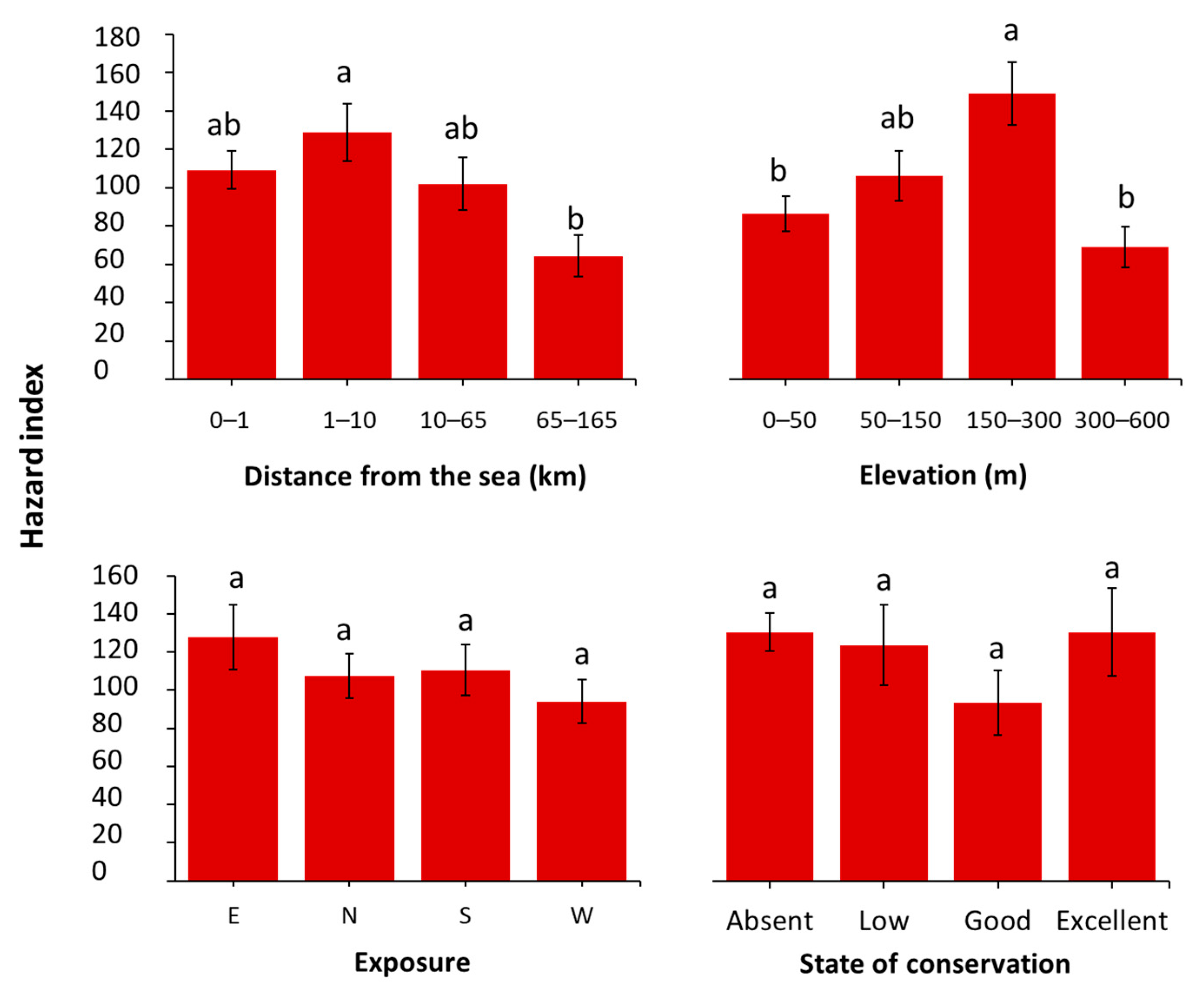
2.2. Vegetation Response to Selected Variables
2.3. Hazard Index
3. Discussions
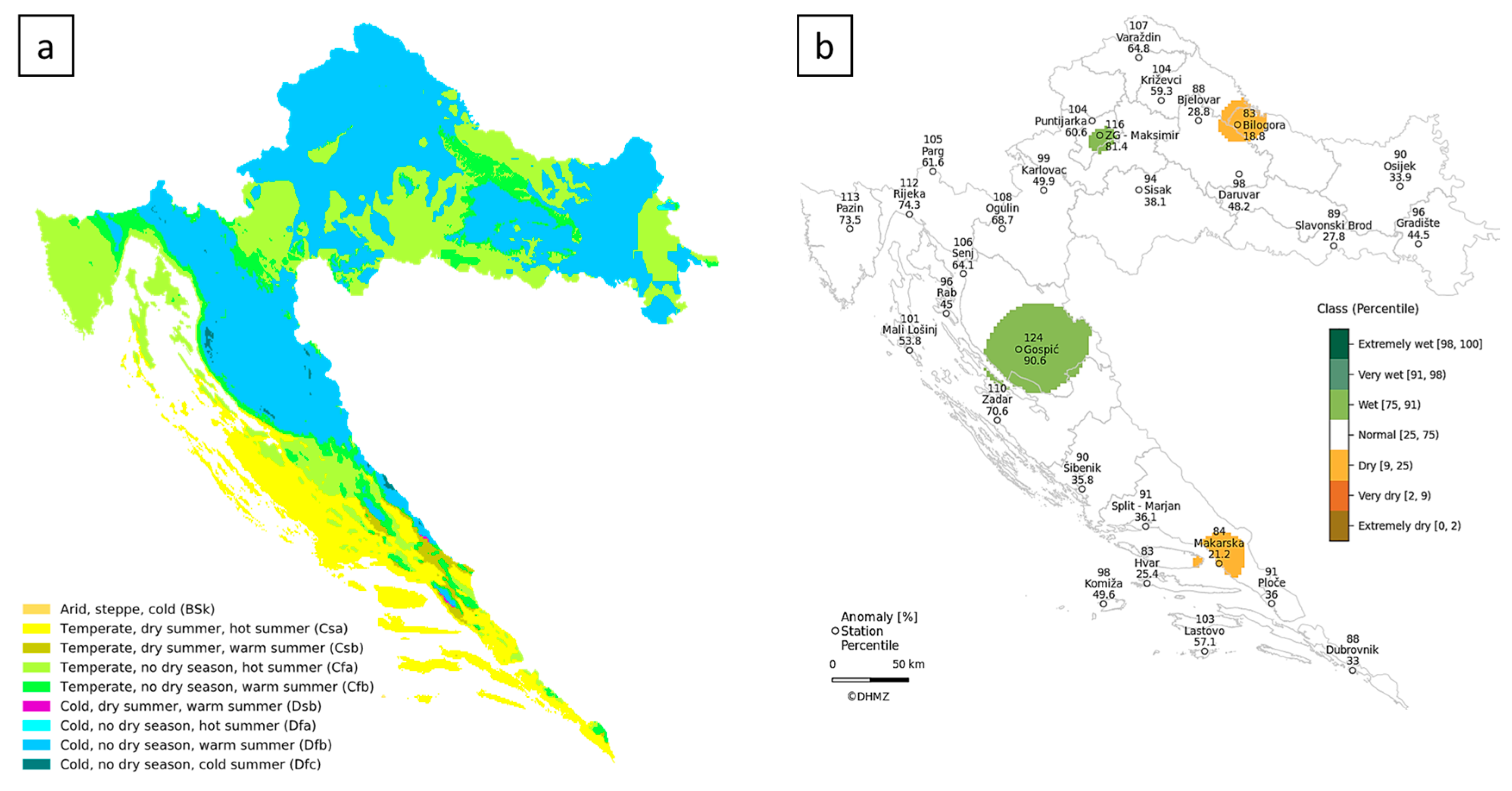
4. Materials and Methods
4.1. Study Sites
4.2. Data Collection and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Qian, Y.; Wu, F.; Wang, Y.; Wang, W.; Gu, J.D. Biofilms on Stone Monuments: Biodeterioration or Bioprotection? Trends Microbiol. 2022, 30, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.; Plets, G. Cultural Heritage as a Strategy for Social Needs and Community Identity. In A Companion to Heritage Studies; Wiley: Hoboken, NJ, USA, 2015; pp. 203–214. [Google Scholar]
- Zaidi, M.; Baghdad, B.; Chakiri, S.; Taleb, A. Characterization of the Biodegradation of Kasbahs of the Gharb Region (Mehdia and Kenitra Kasbahs, Morocco). Open J. Ecol. 2016, 6, 753–766. [Google Scholar] [CrossRef]
- Gunasdi, Y.; Aksakal, O.; Kemaloglu, L. Biodeterioration of Some Historical Monuments in Erzurum by Vascular Plants. Int. Biodeterior. Biodegrad. 2023, 176, 105530. [Google Scholar] [CrossRef]
- Beata, G. The Use of -Omics Tools for Assessing Biodeterioration of Cultural Heritage: A Review. J. Cult. Herit. 2020, 45, 351–361. [Google Scholar] [CrossRef]
- Caneva, G.; Salvadori, O.; Ricci, S.; Ceschin, S. Ecological Analysis and Biodeterioration Processes over Time at the Hieroglyphic Stairway in the Copàn (Honduras) Archaeological Site. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2005, 139, 295–310. [Google Scholar] [CrossRef]
- Sterflinger, K.; Piñar, G. Microbial Deterioration of Cultural Heritage and Works of Art—Tilting at Windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef]
- Guillitte, O. Bioreceptivity: A New Concept for Building Ecology Studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Paiva, D.S.; Fernandes, L.; Trovão, J.; Pereira, E.; Mesquita, N.; Tiago, I.; Gil, F.; Portugal, A. Black Mold on a White Limestone: The Role of Stachybotrys Chartarum in Stone Heritage Deterioration. NPJ Herit. Sci. 2025, 13, 29. [Google Scholar] [CrossRef]
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and Reanalysing the Concept of Bioreceptivity 25 Years On. Sci. Total Environ. 2021, 770, 145314. [Google Scholar] [CrossRef]
- Stohl, L.; Manninger, T.; von Werder, J.; Dehn, F.; Gorbushina, A.; Meng, B. Bioreceptivity of Concrete: A Review. J. Build. Eng. 2023, 76, 107201. [Google Scholar] [CrossRef]
- Lisci, M.; Monte, M.; Pacini, E. Lichens and Higher Plants on Stone: A Review. Int. Biodeterior. Biodegrad. 2003, 51, 1–17. [Google Scholar] [CrossRef]
- Elgohary, Y.M.; Mansour, M.M.A.; Salem, M.Z.M. Assessment of the Potential Effects of Plants with Their Secreted Biochemicals on the Biodeterioration of Archaeological Stones. Biomass Convers. Biorefin. 2024, 14, 12069–12083. [Google Scholar] [CrossRef]
- Li, J.; Deng, M.; Gao, L.; Yen, S.; Katayama, Y.; Gu, J.D. The Active Microbes and Biochemical Processes Contributing to Deterioration of Angkor Sandstone Monuments under the Tropical Climate in Cambodia—A Review. J. Cult. Herit. 2021, 47, 218–226. [Google Scholar] [CrossRef]
- Ceschin, S.; Caneva, G. Plants as Bioindicators for Archaeological Prospection: A Case of Study from Domitian’s Stadium in the Palatine (Rome, Italy). Environ. Monit. Assess. 2013, 185, 5317–5326. [Google Scholar] [CrossRef]
- Cozzolino, A.; Adamo, P.; Bonanomi, G.; Motti, R. The Role of Lichens, Mosses, and Vascular Plants in the Biodeterioration of Historic Buildings: A Review. Plants 2022, 11, 3429. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G. Vascular Plant Colonisation of Four Castles in Southern Italy: Effects of Substrate Bioreceptivity, Local Environment Factors and Current Management. Int. Biodeterior. Biodegrad. 2018, 133, 26–33. [Google Scholar] [CrossRef]
- Mascaro, M.E.; Pellegrino, G.; Palermo, A.M. Analysis of Biodeteriogens on Architectural Heritage. An Approach of Applied Botany on a Gothic Building in Southern Italy. Sustainability 2021, 14, 34. [Google Scholar] [CrossRef]
- Prieto, B.; Paz-Bermúdez, G.; López de Silanes, M.E.; Montojo, C.; Pérez-Velón, D. Current Knowledge Regarding Biological Recolonization of Stone Cultural Heritage after Cleaning Treatments. J. Build. Eng. 2024, 87, 109091. [Google Scholar] [CrossRef]
- Zangari, G.; Bartoli, F.; Lucchese, F.; Caneva, G. Plant Diversity in Archaeological Sites and Its Bioindication Values for Nature Conservation: Assessments in the UNESCO Site Etruscan Necropolis of Tarquinia (Italy). Sustainability 2023, 15, 16469. [Google Scholar] [CrossRef]
- Caneva, G.; Ceschin, S.; De Marco, G. Mapping the Risk of Damage from Tree Roots for the Conservation of Archaeological Sites: The Case of the Domus Aurea, Rome. Conserv. Manag. Archaeol. Sites 2006, 7, 163–170. [Google Scholar] [CrossRef]
- Caneva, G.; Hosseini, Z.; Bartoli, F. Risk, Hazard, and Vulnerability for Stone Biodeterioration Due to Higher Plants: The Methodological Proposal of a Multifactorial Index (RHV). J. Cult. Herit. 2023, 62, 217–229. [Google Scholar] [CrossRef]
- Krigas, N.; Lagiou, E.; Hanlidou, E.; Kokkini, S. The Vascular Flora of the Byzantine Walls of Thessaloniki (N Greece). Willdenowia 1999, 29, 77–94. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Stinca, A. Deteriogenic Flora of the Phlegraean Fields Archaeological Park: Ecological Analysis and Management Guidelines. Nord. J. Bot. 2020, 38. [Google Scholar] [CrossRef]
- Šimundić, B.; Škokić, V.; Čaušević, S. Cultural Heritage Tourism in EU Policy Discourse: A Case Study of Croatia. In Tourism Planning and Development in Eastern Europe; CABI: Wallingford, UK, 2022; pp. 100–116. [Google Scholar]
- Kanellou, E.; Papafotiou, M.; Saitanis, C.; Economou, G. Ecological Analysis and Opportunities for Enhancement of the Archaeological Landscape: The Vascular Flora of Seven Archaeological Sites in Greece. Environments 2024, 11, 16. [Google Scholar] [CrossRef]
- Hosseini, Z.; Caneva, G. Evaluating Hazard Conditions of Plant Colonization in Pasargadae World Heritage Site (Iran) as a Tool of Biodeterioration Assessment. Int. Biodeterior. Biodegrad. 2021, 160, 105216. [Google Scholar] [CrossRef]
- Baričević, D.; Bakšić, D.; Vukelić, J.; Perković, I.; Pernar, N.; Šapić, I. Vegetational-pedological relationships on Mount Medvednica (Croatia). In 23rd International Workshop of the European Vegetation Survey, Ljubljana, Slovenia, 8–12 May 2014; Book of Abstracts; ZRC Publishing House: Ljubljana, Slovenia, 2014; pp. 64–65. [Google Scholar]
- Medvenica Nature Park Official Website. Available online: https://www.pp-medvednica.hr/en/nature-preservation/forests/ (accessed on 18 March 2025).
- Casavecchia, S.; Biscotti, N.; Pesaresi, S.; Biondi, E. The Paliurus Spina-Christi Dominated Vegetation in Europe. Biologia 2015, 70, 879–892. [Google Scholar] [CrossRef]
- Staska, B.; Essl, F.; Samimi, C. Density and Age of Invasive Robinia Pseudoacacia Modulate Its Impact on Floodplain Forests. Basic. Appl. Ecol. 2014, 15, 551–558. [Google Scholar] [CrossRef]
- Wilkaniec, A.; Borowiak-Sobkowiak, B.; Irzykowska, L.; Breś, W.; Świerk, D.; Pardela, Ł.; Durak, R.; Środulska-Wielgus, J.; Wielgus, K. Biotic and Abiotic Factors Causing the Collapse of Robinia pseudoacacia L. Veteran Trees in Urban Environments. PLoS ONE 2021, 16, e0245398. [Google Scholar] [CrossRef]
- Vojnović, N. The Potential of Memorial Tourism Development in Istria (Croatia). Hrvat. Geogr. Glas./Croat. Geogr. Bull. 2021, 82, 107–129. [Google Scholar] [CrossRef]
- Džin, K. Two incomplete sculptures from Cavae Romanae quarries (Istria, Croatia). In Interdisciplinary Studies on Ancient Stone: Proceedings of the IX Association for the Study of Marbles and Other Stones in Antiquity (ASMOSIA) Conference, Tarragona, Spain, 8–13 June 2009; Institut Català d’Arqueologia Clàssica (ICAC): Tarragona, Spain, 2012; pp. 89–92. [Google Scholar]
- Istria Culture Official Website. Available online: https://www.istria-culture.com/it (accessed on 18 March 2025).
- Girardi-Jurkic, V. The Cavae Romanae quarry: Properties and use of the stone for the amphitheatre in Pula (Croatia). In Interdisciplinary Studies on Ancient Stone: Proceedings of the IX Association for the Study of Marbles and Other Stones in Antiquity (ASMOSIA) Conference, Tarragona, Spain, 8–13 June 2009; Institut Català d’Arqueologia Clàssica (ICAC): Tarragona, Spain, 2012; pp. 640–644. [Google Scholar]
- Ávila, S.P.; Ramalho, R.S.; Habermann, J.M.; Quartau, R.; Kroh, A.; Berning, B.; Johnson, M.; Kirby, M.X.; Zanon, V.; Titschack, J.; et al. Palaeoecology, Taphonomy, and Preservation of a Lower Pliocene Shell Bed (Coquina) from a Volcanic Oceanic Island (Santa Maria Island, Azores). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 430, 57–73. [Google Scholar] [CrossRef]
- Arizmendi Sanchez, A.; Hudyma, N. Engineering Properties of Coquina: An Interesting and Historic Building Stone. 2023 Undergraduate Research Showcase, Boise State University. 2023. Available online: https://scholarworks.boisestate.edu/under_showcase_2023/91 (accessed on 18 March 2025).
- Novellino, M.D.; Fontana, A.; Bertuletti, P.; Furlanetto, G.; Pini, R.; Felja, I.; Juračić, M.; Ravazzi, C. Vegetation and Climate History during the Last Interglacial on the Istrian Coast (Northern Adriatic Sea). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2025, 659, 112671. [Google Scholar] [CrossRef]
- Meyer, S.E.; Callaham, M.A.; Stewart, J.E.; Warren, S.D. Invasive Species Response to Natural and Anthropogenic Disturbance. In Invasive Species in Forests and Rangelands of the United States; Springer International Publishing: Cham, Switzerland, 2021; pp. 85–110. [Google Scholar]
- Montserrat-Martí, G.; Palacio, S.; Milla, R.; Giménez-Benavides, L. Meristem Growth, Phenology, and Architecture in Chamaephytes of the Iberian Peninsula: Insights into a Largely Neglected Life Form. Folia Geobot. 2011, 46, 117–136. [Google Scholar] [CrossRef]
- Midolo, G.; Axmanová, I.; Divíšek, J.; Dřevojan, P.; Lososová, Z.; Večeřa, M.; Karger, D.N.; Thuiller, W.; Bruelheide, H.; Aćić, S.; et al. Diversity and Distribution of Raunkiær’s Life Forms in European Vegetation. J. Veg. Sci. 2024, 35, e13229. [Google Scholar] [CrossRef]
- Paradiso, A.; Durante, M.; Caretto, S.; De Paolis, A. Establishment of Dittrichia Viscosa L. Hairy Roots and Improvement of Bioactive Compound Production. Plants 2024, 13, 3236. [Google Scholar] [CrossRef]
- Cozzolino, A.; Bonanomi, G.; Motti, R. The Role of Stone Materials, Environmental Factors, and Management Practices in Vascular Plant-Induced Deterioration: Case Studies from Pompeii, Herculaneum, Paestum, and Velia Archaeological Parks (Italy). Plants 2025, 14, 514. [Google Scholar] [CrossRef]
- Jasprica, N.; Milović, M. Flora of the Cobbled Streets and Pavements in the Mediterranean Old City of Dubrovnik during the COVID-19 Lockdown. Nat. Croat. 2020, 29, 19–28. [Google Scholar] [CrossRef]
- Jasprica, N.; Škvorc, Ž.; Pandža, M.; Milović, M.; Purger, D.; Krstonošić, D.; Kovačić, S.; Sandev, D.; Lasić, A.; Caković, D.; et al. Phytogeographic and Syntaxonomic Diversity of Wall Vegetation (Cymbalario-Parietarietea Diffusae) in Southeastern Europe. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2021, 155, 622–631. [Google Scholar] [CrossRef]
- Szczęśniak, E.; Świerkosz, K. Cymbalaria muralis P. Gaertn., B. Mey. & Schreb. and Cymbalarietum muralis Görs 1966 in Lower Silesia—Expansion or regression? In Phytogeographical Problems of Synanthropic Plants; Institute of Botany Jagiellonian University: Krakow, Poland, 2003; pp. 185–193. [Google Scholar]
- Lewandowski, D.; Robain, H.; Clergeau, P.; Le Roy, R. Bioreceptivity of Living Walls: Interactions between Building Materials and Substrates, and Effect on Plant Growth. Urban. For. Urban. Green 2023, 83, 127912. [Google Scholar] [CrossRef]
- Caneva, G.; Pacini, A.; Celesti Grapow, L.; Ceschin, S. The Colosseum’s Use and State of Abandonment as Analysed through Its Flora. Int. Biodeterior. Biodegrad. 2003, 51, 211–219. [Google Scholar] [CrossRef]
- Motti, R.; Zotti, M.; Bonanomi, G.; Cozzolino, A.; Stinca, A.; Migliozzi, A. Climatic and Anthropogenic Factors Affect Ailanthus Altissima Invasion in a Mediterranean Region. Plant Ecol. 2021, 222, 1347–1359. [Google Scholar] [CrossRef]
- Trotta, G.; Savo, V.; Cicinelli, E.; Carboni, M.; Caneva, G. Colonization and Damages of Ailanthus Altissima (Mill.) Swingle on Archaeological Structures: Evidence from the Aurelian Walls in Rome (Italy). Int. Biodeterior. Biodegrad. 2020, 153, 105054. [Google Scholar] [CrossRef]
- Dabghi, A.; Achoual, K.; Benharbit, M.; Magri, N.; Belahbib, N.; Dahmani, J. Contribution to the Study of the Vascular Flora of the Archaeological Site of Volubilis (Morocco). Plant Arch. 2020, 20, 7519–7527. [Google Scholar]
- Fassar, M.; Dahmani, J.; Benharbit, M.; Belahbib, N. Vascular Plants Colonization of the Historical Old Medina of Safi, Morocco. In International Conference on Advanced Intelligent Systems for Sustainable Development, 15–17 October; Marrakech, Morocco; Springer Nature: Cham, Switzerland, 2023; pp. 190–202. [Google Scholar]
- McCain, C.M.; Grytnes, J.A. Elevational gradients in species richness. In Encyclopedia of Life Sciences (eLS); John Wiley & Sons: Chichester, UK, 2010; pp. 1–10. [Google Scholar]
- Di Musciano, M.; Calvia, G.; Ruggero, A.; Farris, E.; Ricci, L.; Frattaroli, A.R.; Bagella, S. Elevational Patterns of Plant Species Richness and Phylogenetic Diversity in a Mediterranean Island. Perspect. Plant Ecol. Evol. Syst. 2024, 65, 125815. [Google Scholar] [CrossRef]
- Fenu, G.; Carboni, M.; Acosta, A.T.R.; Bacchetta, G. Environmental Factors Influencing Coastal Vegetation Pattern: New Insights from the Mediterranean Basin. Folia Geobot. 2013, 48, 493–508. [Google Scholar] [CrossRef]
- Acosta, A.; Carranza, M.L.; Izzi, C.F. Are There Habitats That Contribute Best to Plant Species Diversity in Coastal Dunes? Biodivers. Conserv. 2009, 18, 1087–1098. [Google Scholar] [CrossRef]
- Chen, J.; Blume, H.P.; Beyer, L. Weathering of Rocks Induced by Lichen Colonization—A Review. Catena 2000, 39, 121–146. [Google Scholar] [CrossRef]
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.D. Microbial Deterioration and Sustainable Conservation of Stone Monuments and Buildings. Nat. Sustain. 2020, 3, 991–1004. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Ricotta, C. Plant Invasion as an Emerging Challenge for the Conservation of Heritage Sites: The Spread of Ornamental Trees on Ancient Monuments in Rome, Italy. Biol. Invasions 2021, 23, 1191–1206. [Google Scholar] [CrossRef]
- Kumbaric, A.; Ceschin, S.; Zuccarello, V.; Caneva, G. Main Ecological Parameters Affecting the Colonization of Higher Plants in the Biodeterioration of Stone Embankments of Lungotevere (Rome). Int. Biodeterior. Biodegrad. 2012, 72, 31–41. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Zhu, S.; Wang, B. Experimental Study on Mechanical and Porous Characteristics of Limestone Affected by High Temperature. Appl. Therm. Eng. 2017, 110, 356–362. [Google Scholar] [CrossRef]
- Dahmani, J.; Benharbit, M.; Fassar, M.; Hajila, R.; Zidane, L.; Magri, N.; Belahbib, N. Vascular Plants Census Linked to the Biodeterioration Process of the Portuguese City of Mazagan in El Jadida, Morocco. J. King Saud. Univ. Sci. 2020, 32, 682–689. [Google Scholar] [CrossRef]
- Dabghi, A.; Magri, N.; Achoual, K.; Belahbib, N.; Benharbit, M.; Dahmani, J. Floristic Diversity And Its Biodeteriogenic Effect On The Archaeological Site Of Volubilis (Morocco). Orig. Res. Artic. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 53–70. [Google Scholar]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Control of biodeterioration and bioremediation techniques. In Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Getty Publication: Los Angeles, CA, USA, 2008; pp. 309–345. [Google Scholar]
- Panitsa, M.; Tsakiri, M.; Kampiti, D.; Skotadi, M. Archaeological Areas as Habitat Islands: Plant Diversity of Epidaurus UNESCO World Heritage Site (Greece). Diversity 2024, 16, 403. [Google Scholar] [CrossRef]
- Zangari, G.; Hosseini, Z.; Caneva, G. Vegetation Analysis in the Archaeological Area of Pasargadae WHS (Iran) Enhancing the Naturalistic Value of the Site within the Occurring Environmental Changes. Sustainability 2024, 16, 3784. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- DHMZ—Croatian Meteorological and Hydrological Service. Available online: https://meteo.hr/index_en.php (accessed on 27 May 2025).
- Bustillo Revuelta, M. Geological Occurrence. In The Basics of Aggregates; Springer Textbooks in Earth Sciences, Geography and Environment; Springer: Cham, Switzerland, 2024; pp. 53–100. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Plant Sociology; McGraw-Hill Book Company: New York, NY, USA, 1932. [Google Scholar]
- Signorini, M.A. Lo Studio e il Controllo della Vegetazione Infestante nei Siti Archeologici: Una Proposta Metodologica. In L’area Archeologica di Fiesole. Rilievi e Ricerche per la Conservazione; Signorini, M.A., Ed.; Alinea: Florence, Italy, 1995; pp. 41–46. [Google Scholar]
- Signorini, M.A. L’indice di pericolosità: Un contributo del botanico al controllo della vegetazione infestante nelle aree monumentali. Inf. Bot. Ital. 1996, 28, 7–14. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Pignatti, S. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2018. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1964; Volumes 1–5. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Nikolić, T. Flora Croatica Vol. 1-4–Errāta corrige 2. Glas. Hrvat. Bot. Društva 2021, 9, 47–48. [Google Scholar]
- FCD (Flora Croatica Database). Available online: https://hirc.botanic.hr/fcd/ (accessed on 15 October 2024).
- WFO (World Flora Online). Available online: https://www.worldfloraonline.org/ (accessed on 16 October 2024).
- Chase, M.W. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants:APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Brummitt, P.K.; Powell, C.E. Authors of Plant Names; Royal Botanic Gardens: Kew, UK, 1992. [Google Scholar]
- Raunkiaer, C. The Life Forms and Statistical Plant Geography; Oxford Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, Volume 1; Edagricole: Bologna, Itaily, 2017. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, Volume 2; Edagricole: Bologna, Itaily, 2017. [Google Scholar]


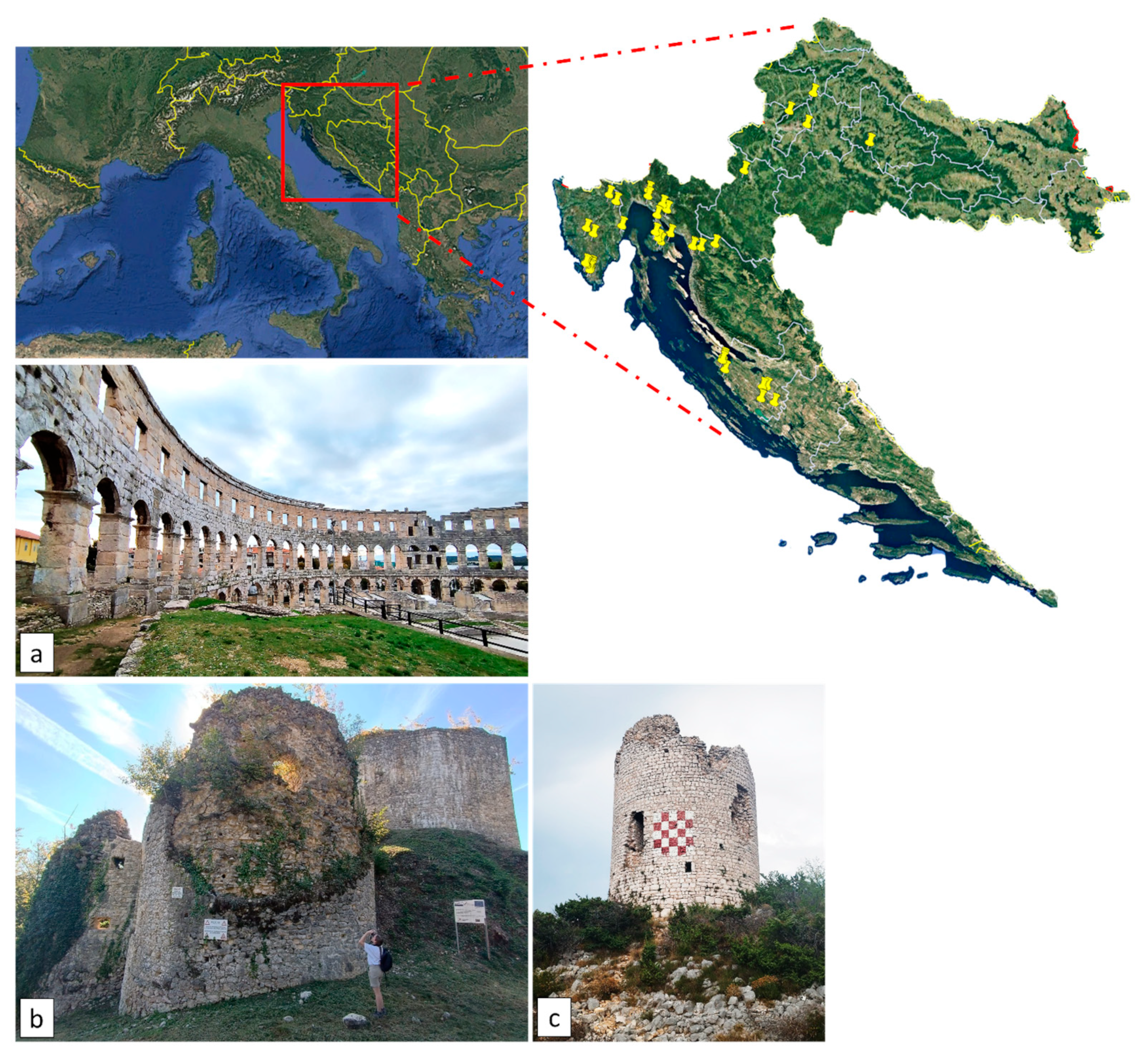
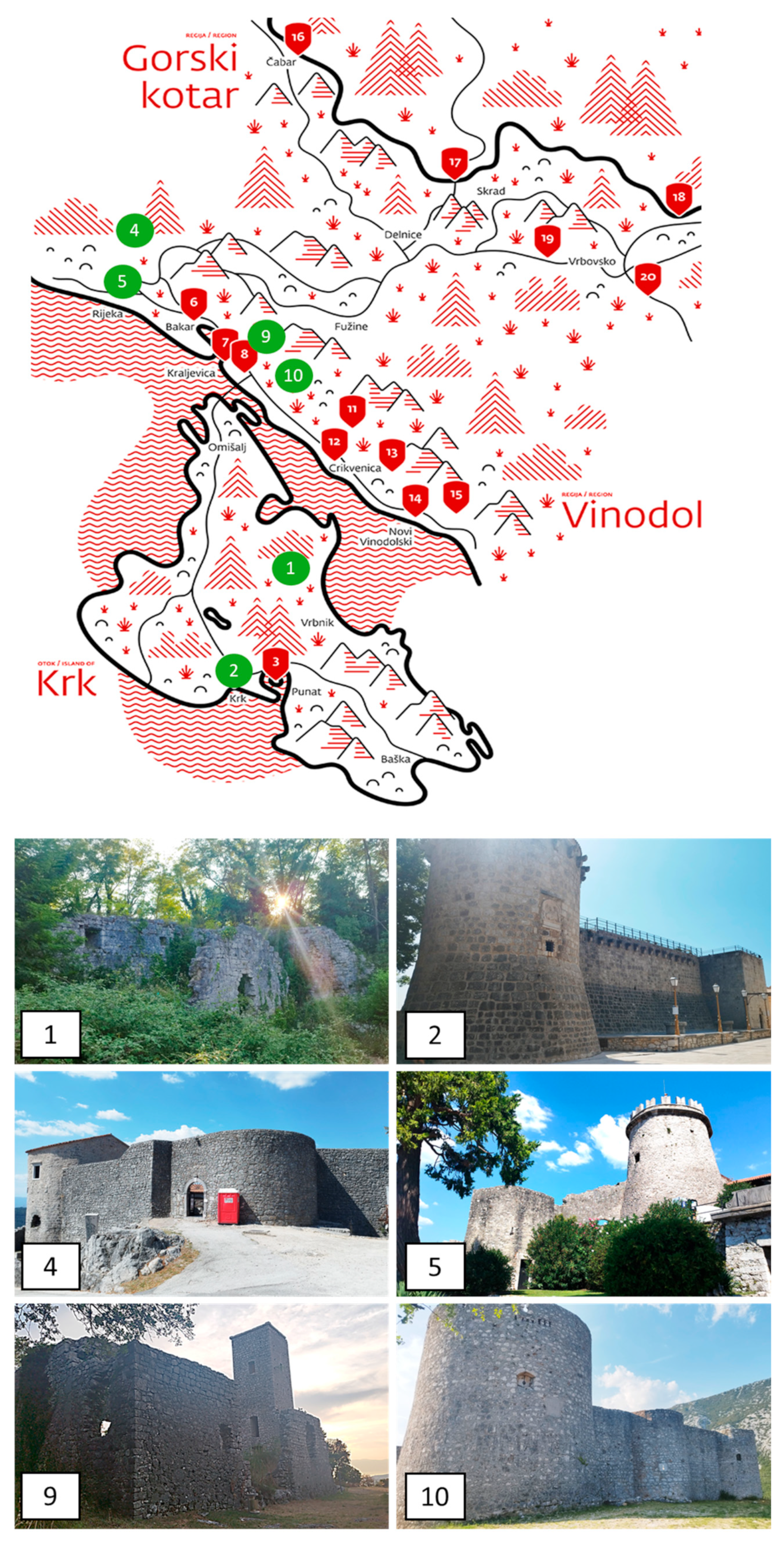
| N. | Study Sites | Type | N. of Plots | Coordinates (WGS 84) |
|---|---|---|---|---|
| 1 | Church Fulfinium Mirine (island Krk) | Archaeological site | 12 | 45.20386, 14.54395 |
| 2 | Mirinski češalj (Thermae Mirine, island Krk) | Archaeological site | 12 | 45.20539, 14.54362 |
| 3 | Nin Roman Temple | Archaeological site | 9 | 44.24364, 15.18398 |
| 4 | Frankopanski Kaštel (Town Krk) | Castle | 8 | 45.0257, 14.57641 |
| 5 | Pula Amphitheatre | Archaeological site | 12 | 44.87323, 13.85024 |
| 6 | Town Senj | Ancient city walls | 12 | 44.99131, 14.90279 |
| 7 | Town Krk walls | Ancient city walls | 6 | 45.02839, 14.57579 |
| 8 | Maškovića Han (Vrana) | Castle | 9 | 43.95412, 15.5489 |
| 9 | Vrana | Castle | 12 | 43.9557, 15.54961 |
| 10 | Vrbnik (Krk) | Tower | 9 | 45.0776, 14.6759 |
| 11 | Pulski Kaštel | Castle | 12 | 44.87022, 13.84549 |
| 12 | Fort SanMichele (Pula) | Fortress | 12 | 44.8662, 13.8538 |
| 13 | Fort PuntaCristo (Pula) | Fortress | 12 | 44.8922, 13.79762 |
| 14 | Fort Valmaggiore (Pula) | Fortress | 7 | 44.88361, 13.80775 |
| 15 | Fort Bourguignon (Pula) | Fortress | 12 | 44.84683, 13.8335 |
| 16 | Fort CasoniVecchi (Pula) | Fortress | 12 | 44.85268, 13.84598 |
| 17 | Fort Giorgio (Pula) | Fortress | 12 | 44.8819, 13.85554 |
| 18 | Fort Munida (Pula) | Fortress | 10 | 44.88203, 13.81502 |
| 19 | Fort Grosso (Pula) | Fortress | 12 | 44.88646, 13.81028 |
| 20 | Salatić (island Krk) | Castle | 12 | 45.04904, 14.54879 |
| 21 | Andautonia (Zagreb) | Archaeological site | 12 | 45.77357, 16.11724 |
| 22 | Trsat (Rijeka) | Castle | 12 | 45.33252, 14.4554 |
| 23 | Dvigrad (Duecastelli) | Castle | 15 | 45.12708, 13.81173 |
| 24 | Kličevica (Benkovac) | Castle | 15 | 44.03357, 15.56764 |
| 25 | Drivenik | Castle | 12 | 45.238, 14.6467 |
| 26 | Plomin | Ancient city walls | 12 | 45.1378, 14.1788 |
| 27 | Kapela sv. Nikole (Nin) | Archaeological site | 12 | 44.23091, 15.17853 |
| 28 | Novigrad na Dobri | Castle | 12 | 45.48236, 15.45296 |
| 29 | Gradec (island Krk) | Castle | 14 | 45.09989, 14.62847 |
| 30 | Benković (Benkovac) | Castle | 12 | 44.03389, 15.6097 |
| 31 | Budak (Stankovci) | Tower | 12 | 43.92441, 15.68013 |
| 32 | Hreljin | Castle | 12 | 45.27466, 14.60233 |
| 33 | Boljun (Bogliuno) | Castle | 12 | 45.30163, 14.12177 |
| 34 | Morosini (Svetvinčenat) | Castle | 8 | 45.08814, 13.88235 |
| 35 | Hum (Colmo) | Ancient city walls | 10 | 45.34828, 14.05054 |
| 36 | Zelingrad | Castle | 12 | 45.98154, 16.19656 |
| 37 | Garić grad (Podgarić) | Tower | 8 | 45.63118, 16.75696 |
| 38 | Grobnik | Castle | 12 | 45.37133, 14.4606 |
| 39 | Brinje | Castle | 12 | 44.99834, 15.13162 |
| 40 | Medvedgrad (Zagreb) | Castle | 12 | 45.86909, 15.94023 |
| Tot | 452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozzolino, A.; Motti, R.; Vitasović-Kosić, I. Vascular Flora on Croatian Historic Structures: Drivers of Biodeterioration and Conservation Implications. Plants 2025, 14, 1773. https://doi.org/10.3390/plants14121773
Cozzolino A, Motti R, Vitasović-Kosić I. Vascular Flora on Croatian Historic Structures: Drivers of Biodeterioration and Conservation Implications. Plants. 2025; 14(12):1773. https://doi.org/10.3390/plants14121773
Chicago/Turabian StyleCozzolino, Alessia, Riccardo Motti, and Ivana Vitasović-Kosić. 2025. "Vascular Flora on Croatian Historic Structures: Drivers of Biodeterioration and Conservation Implications" Plants 14, no. 12: 1773. https://doi.org/10.3390/plants14121773
APA StyleCozzolino, A., Motti, R., & Vitasović-Kosić, I. (2025). Vascular Flora on Croatian Historic Structures: Drivers of Biodeterioration and Conservation Implications. Plants, 14(12), 1773. https://doi.org/10.3390/plants14121773








