Spontaneous Flora as Reservoir for the Survival and Spread of the Almond Anthracnose Pathogen (Colletotrichum godetiae) in Intensive Almond Orchards
Abstract
1. Introduction
2. Results
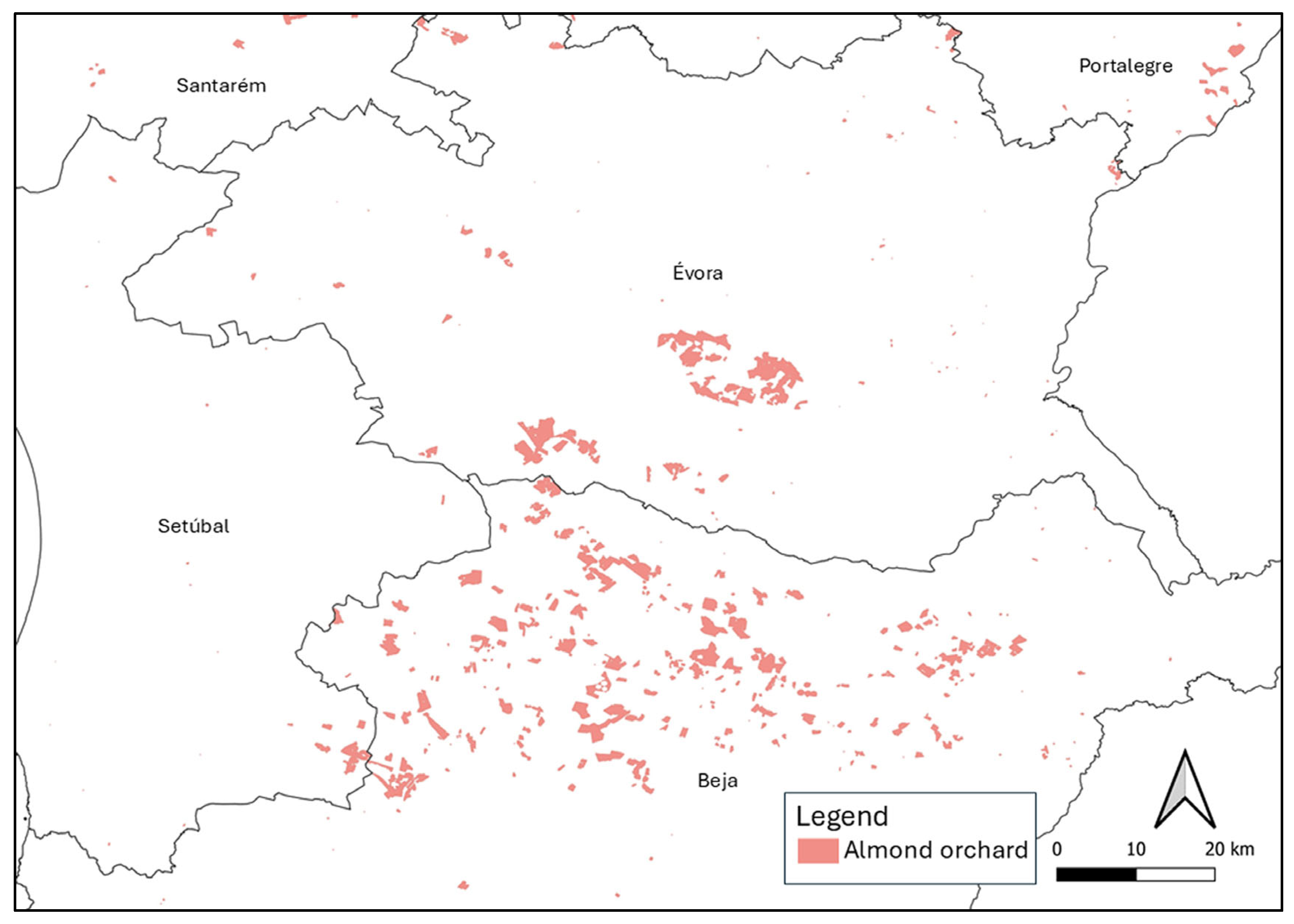
2.1. Identification of Plant Species Present in Almond Orchards in Alentejo
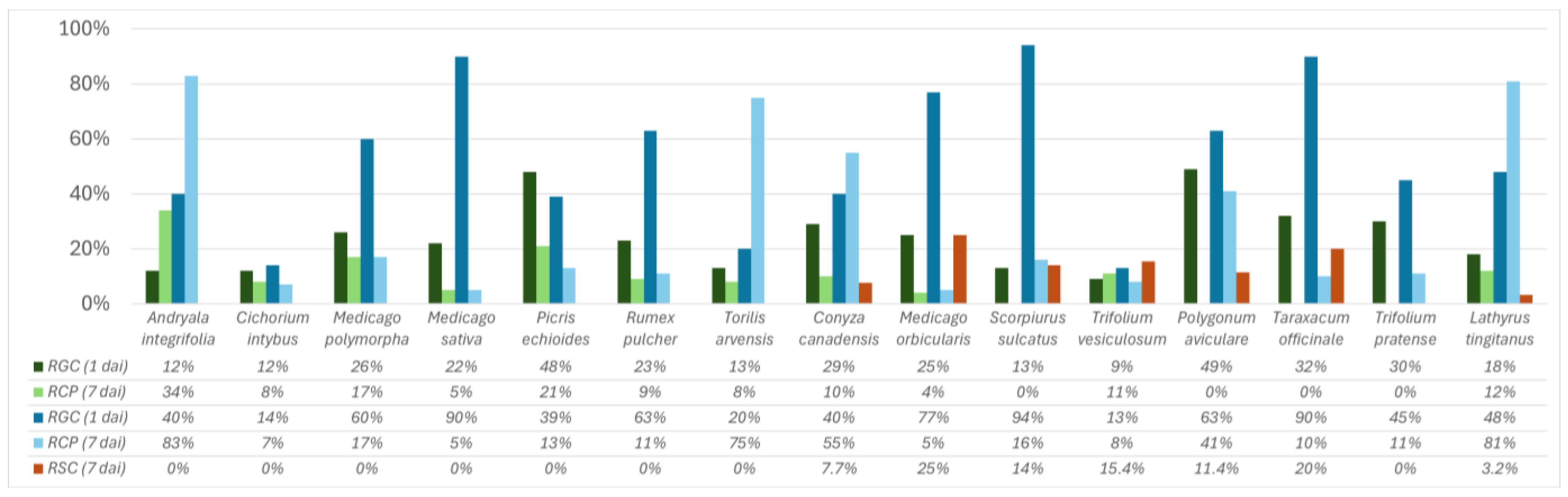
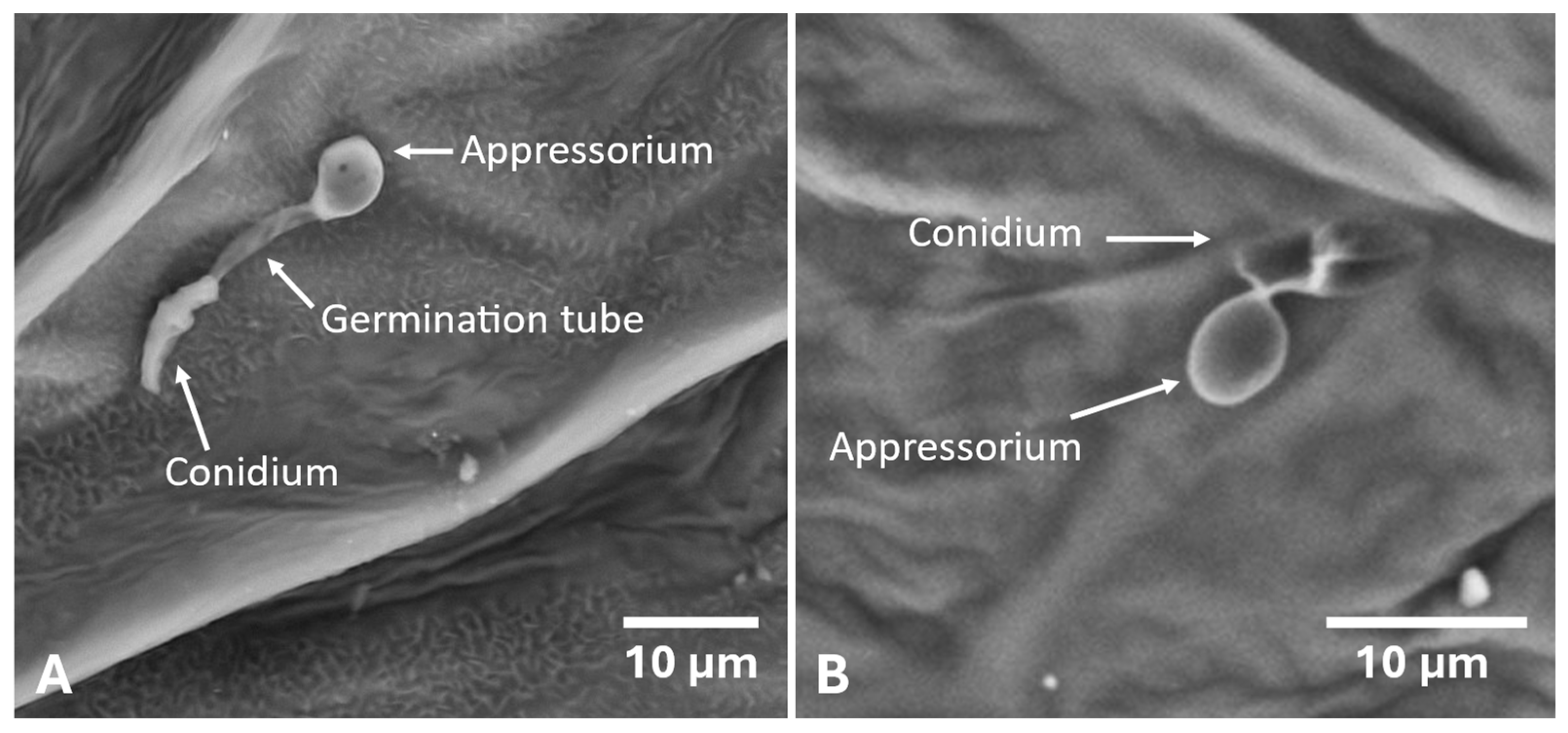
2.2. Development of the Colletotrichum godetiae Infection Process and Symptoms on Inoculated Leaves
3. Discussion
4. Materials and Methods
4.1. Identification of Plant Species Present in Almond Orchards in Alentejo
4.2. Cultivation of Selected Plants
4.3. Inoculation of Plant Material with Colletotrichum godetiae
4.4. Germination of Conidia and Fungal Development on Inoculated Leaves
4.5. Symptomatic Development and Confirmation of the Presence of Colletotrichum godetiae
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gradziel, T.M. History of cultivation. In Almonds. Botany, Production and Uses; Socias i Company, R., Gradziel, T.M., Eds.; CAB International: Boston, MA, USA, 2017; pp. 43–71. [Google Scholar]
- López-Moral, A.; Agustí-Brisach, C.; Lovera, M.; Luque, F.; Roca, L.F.; Arquero, O.; Trapero, A. Effects of cultivar susceptibility, fruit maturity, leaf age, fungal isolate, and temperature on infection of almond by Colletotrichum spp. Plant Dis. 2019, 103, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Ollero-Lara, A.; Agustí-Brisach, C.; Lovera, M.; Roca, L.F.; Arquero, O.; Trapero, A. Field susceptibility of almond cultivars to the four most common aerial fungal diseases in southern Spain. Crop Prot. 2019, 121, 12–27. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Freeman, S.; Shabi, E. Anthracnose. In Compendium of Nut Crop Diseases in Temperate Zones; Teviotdale, B.L., Michailides, T.J., Pscheidt, J.W., Eds.; APS Press: St. Paul, MN, USA, 2002. [Google Scholar]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 31–113. [Google Scholar] [CrossRef]
- López-Moral, A.; Raya-Ortega, M.C.; Agustí-Brisach, C.; Roca, L.F.; Lovera, M.; Luque, F.; Arquero, O.; Trapero, A. Morphological, pathogenic and molecular characterization of Colletotrichum acutatum isolates causing almond anthracnose in Spain. Plant Dis. 2017, 101, 2034–2045. [Google Scholar] [CrossRef]
- de Silva, D.D.; Mann, R.C.; Kaur, J.; Ekanayake, P.N.; Sawbridge, T.I.; McKay, S.; Taylor, P.W.J.; Edwards, J. Revisiting the Colletotrichum species causing anthracnose of almond in Australia. Australas. Plant Pathol. 2021, 50, 267–279. [Google Scholar] [CrossRef]
- Varjas, V.; Szilágyi, S.; Lakatos, T. First report of Colletotrichum nymphaeae causing anthracnose on almond in Hungary. Plant Dis. 2022, 106, 1527. [Google Scholar] [CrossRef] [PubMed]
- Guerber, J.C.; Liu, B.; Correll, J.C.; Johnston, P.R. Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 2003, 95, 872–895. [Google Scholar] [CrossRef]
- Hall, B.H.; Jones, M.K.; Wicks, T.J. First report of anthracnose of almond in South Australia. Australas. Plant Pathol. 1998, 27, 127. [Google Scholar] [CrossRef]
- McKay, S.F.; Freeman, S.; Minz, D.; Maymon, M.; Sedgley, M.; Collins, G.C.; Scott, E.S. Morphological, genetic and pathogenic characterization of Colletotrichum acutatum, the cause of anthracnose of almond in Australia. Phytopathology 2009, 99, 985–995. [Google Scholar] [CrossRef]
- Ramos, M.; Arsénio, P.; Talhinhas, P.; Baroncelli, R. Caracterização genética e epidemiológica da antracnose da amendoeira no Alentejo. Agrotec 2023, 49, 33–37. [Google Scholar]
- Moral, J.; Trapero, A. Mummified fruit as a source of inoculum and disease dynamics of olive anthracnose caused by Colletotrichum spp. Phytopathology 2012, 102, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, V. The role of epidemiology data in developing integrated management of anthracnose in olives: A review. Acta Hortic. 2014, 1057, 163–168. [Google Scholar] [CrossRef]
- Madden, L.V.; Yang, X.; Wilson, L.L. Effect of rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology 1996, 86, 864–874. [Google Scholar] [CrossRef]
- Talhinhas, P.; Loureiro, A.; Oliveira, H. Olive anthracnose: A yield-and oil quality-degrading disease caused by several species of Colletotrichum that differ in virulence, host preference and geographical distribution. Mol. Plant Pathol. 2018, 19, 1797–1807. [Google Scholar] [CrossRef]
- López-Moral, A.; Agustí-Brisach, C.; Lovera, M.; Arquero, O.; Trapero, A. Almond anthracnose: Current knowledge and future perspectives. Plants 2020, 9, 945. [Google Scholar] [CrossRef]
- Talhinhas, P.; Mota-Capitão, C.; Martins, S.; Ramos, A.P.; Neves-Martins, L.; Guerra-Guimarães, L.; Várzea, V.; Silva, M.C.; Sreenivasaprasad, S.; Oliveira, H. Epidemiology, histopathology and aetiology of olive anthracnose caused by Colletotrichum acutatum and C. gloeosporioides in Portugal. Plant Pathol. 2011, 60, 483–495. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Förster, H.; Soto-Estrada, A.; Adaskaveg, J.E. Subcuticular-intracellular hemibiotrophic and intercellular necrotrophic development of Colletotrichum acutatum on almond. Phytopathology 2005, 95, 751–758. [Google Scholar] [CrossRef]
- Freeman, S.; Katan, T.; Shabi, E. Characterization of Colletotrichum species responsible for anthracnose diseases of various fruits. Plant Dis. 1998, 82, 596–605. [Google Scholar] [CrossRef]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar]
- Jelev, Z.J.; Bobev, S.G.; Minz, D.; Maymon, M.; Freeman, S. Characterization of Colletotrichum species causing strawberry anthracnose in Bulgaria. J. Phytopathol. 2008, 156, 668–677. [Google Scholar] [CrossRef]
- Penet, L.; Guyader, S.; Pétro, D.; Salles, M.; Bussière, F. Direct splash dispersal prevails over indirect and subsequent spread during rains in Colletotrichum gloeosporioides infecting yams. PLoS ONE 2014, 9, e115757. [Google Scholar] [CrossRef] [PubMed]
- Arquero, O.; Belmonte, A.; Casado, B.; Cruz-Blanco, M.; Espadafor, M.; Fernández, J.L.; Gallego, J.C.; García, A.; Lorite, I.; Lovera, M. Manual del Almendro; Junta de Andaluzia, Ministério da Agricultura, Pescas e Desenvolvimento Rural: Sevilla, Spain, 2013; p. 80. [Google Scholar]
- Aguiar, C.; Pereira, J.A.; Arrobas, M.; Almeida, A.; Bento, A.; Cortés, I.L.; Rodrigues, N.; Rodrigues, M.A.; Ribeiro, I.C.; Santos, S.A.P. Manual Técnico Amendoeira: Estado da Produção; CNCFS: Bragança, Portugal, 2017; pp. 1–475. [Google Scholar]
- Moreira, I.; Vasconcelos, T.; Caixinhas, M.L.; Espírito Santo, D. Ervas Daninhas das Vinhas e Pomares, 2nd ed.; Direção Geral de Proteção das Culturas: Oeiras, Portugal, 2000; p. 209. [Google Scholar]
- García-Franco, N.; Albaladejo, J.; Almagro, M.; Martínez-Mena, M. Beneficial effects of reduced tillage and green manure on soil aggregation and stabilization of organic carbon in a Mediterranean agroecosystem. Soil Till. Res. 2015, 153, 66–75. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Ferreira, I.; Arrobas, M. Ensaios com cultivares de colza de inverno, doses de azoto e profundidades de sementeira em Trás-os-Montes. Rev. Ciênc. Agr. 2010, 33, 27–39. [Google Scholar]
- Almagro, M.; Vente, J.; Boix-Fayos, C.; García-Franco, N.; Aguilar, J.M.; González, D.; Solé-Benet, A.; Martínez-Mena, M. Sustainable land management practices as providers of several ecosystem services under rainfed Mediterranean agroecosystems. Mitig. Adapt. Strat. Glob. Change 2016, 21, 1029–1043. [Google Scholar] [CrossRef]
- Forte, P.; Caixinhas, M.L.; Sousa, M.E. Trevos, anafes e luzernas de Portugal; Verbo: Lisboa, Portugal, 2016; p. 184. [Google Scholar]
- Freeman, S.; Horowitz, S.; Sharon, A. Pathogenic and nonpathogenic lifestyles in Colletotrichum acutatum from strawberry and other plants. Phytopathology 2001, 91, 986–992. [Google Scholar] [CrossRef]
- Karimi, K.; Arzanlou, M.; Pertot, I. Weeds as potential inoculum reservoir for Colletotrichum nymphaeae causing strawberry anthracnose in Iran and Rep-PCR fingerprinting as useful marker to differentiate C. Acutatum Complex strawberry. Front. Microbiol. 2019, 10, 129. [Google Scholar] [CrossRef]
- INE. Instituto Nacional de Estatística. Estatísticas Agrícolas 2023. Available online: https://www.ine.pt/xurl/pub/439500127 (accessed on 3 May 2024).
- IFAP. Instituto de Financiamento da Agricultura e Pescas. Serviços de Dados Geográficos Disponibilizados Pelo IFAP (WMS/WFS). 2023. Available online: https://www.ifap.pt/isip/ows/ (accessed on 3 November 2024).
- Carranca, C. O Coberto Vegetal em Pomares e Vinha: Efeitos na Produção, Qualidade dos Frutos e Qualidade do Solo; Atena Editora: São Paulo, Brazil, 2022; p. 199. [Google Scholar]
- Peres, N.A.; Timmer, L.W.; Adaskaveg, J.E.; Correll, J.C. Lifestyles of Colletotrichum acutatum. Plant Dis. 2005, 89, 784–796. [Google Scholar] [CrossRef]
- Prusky, D.; Alkan, N.; Mengiste, T.; Fluhr, R. Quiescent and nectrophic lifestyle choice during postharvest disease development. Annu. Rev. Phytopathol. 2013, 51, 155–176. [Google Scholar] [CrossRef]
- Silva, D.D.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Colletotrichum species associated with chili anthracnose in Australia. Plant Pathol. 2017, 66, 254–267. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Bhunjun, C.S.; Hyde, K.D.; Gentekaki, E.; Itthayakorn, P. Colletotrichum: Lifestyles, biology, morpho-species, species complexes and accepted species. Mycosphere 2021, 12, 519–669. [Google Scholar] [CrossRef]
- Leandro, L.F.S.; Gleason, M.L.; Nutter, F.W.; Wegulo, S.N.; Dixon, P.M. Strawberry plant extracts stimulate secondary conidiation by Colletotrichum acutatum on symptomless strawberry leaves. Phytopathology 2004, 93, 513–520. [Google Scholar] [CrossRef]
- Felipe, A. Almendro. Estado fenológicos. Inf. Tec. Econ. Agr. 1977, 27, 8–9. [Google Scholar]
- Flora de Portugal Interactiva. Sociedade Portuguesa de Botânica. 2025. Available online: https://flora-on.pt/ (accessed on 3 November 2024).
- Loureiro, A.; Azinheira, H.G.; Silva, M.C.; Talhinhas, P. A method for obtaining RNA from Hemileia vastatrix appressoria produced in planta, suitable for transcriptomic analyses. Fungal Biol. 2015, 119, 1093–1099. [Google Scholar] [CrossRef]

| Botanical Family | Species | Beja (%) | Évora (%) | Portalegre (%) | Setúbal (%) | Total Number of Reports (%) |
|---|---|---|---|---|---|---|
| Apiaceae | Torilis arvensis | 18.3 | 23.9 | 58.3 | 17.6 | 24.7 |
| Asteraceae | Andryala integrifolia | 33.8 | 43.5 | 37.5 | 35.3 | 37.3 |
| Cichorium intybus | 22.5 | 21.7 | 45.8 | 41.2 | 27.8 | |
| Conyza canadensis | 2.8 | 2.2 | 4.2 | 11.8 | 3.8 | |
| Picris echioides | 23.9 | 8.7 | 12.5 | 17.6 | 16.5 | |
| Taraxacum officinale | 30.3 | 0.0 | 0.0 | 27.8 | 13.5 | |
| Fabaceae | Lathyrus tingitanus | 4.2 | 34.8 | 8.3 | 11.8 | 14.6 |
| Medicago orbicularis | 31.0 | 2.2 | 0.0 | 11.8 | 15.2 | |
| Medicago polymorpha | 43.7 | 39.1 | 45.8 | 29.4 | 38.6 | |
| Medicago sativa | 0.0 | 2.2 | 0.0 | 5.9 | 1.3 | |
| Scorpiurus sulcatus | 39.4 | 10.9 | 12.5 | 17.6 | 24.1 | |
| Trifolium pratense | 1.4 | 2.2 | 16.7 | 11.8 | 4.4 | |
| Trifolium vesiculosum | 1.4 | 6.5 | 0.0 | 5.9 | 3.2 | |
| Polygonaceae | Polygonum aviculare | 5.6 | 2.2 | 20.8 | 17.6 | 8.2 |
| Rumex pulcher | 19.7 | 13.0 | 33.3 | 17.6 | 19.0 |
| Species | 7 Dai | ||
|---|---|---|---|
| Average Germ Tube Size (µm) | Average Number of Germ Tubes/Conidium | Rate of Sessile Appressoria | |
| Torilis arvensis | 218.3 ± 0.4 a * | 1.1 ± 0.3 ab * | 31.3% |
| Andryala integrifolia | 183.0 ± 0.2 a | 1.0 ± 0.0 a | 20.0% |
| Cichorium intybus | 314.4 ± 0.2 a | 1.0 ± 0.0 a | 0% |
| Conyza canadensis | 327.7 ± 0.4 a | 1.0 ± 0.0 a | 19.2% |
| Picris echioides | 658.1 ± 0.2 ab | 1.0 ± 0.0 a | 0% |
| Taraxacum officinale | 438.0 ± 0.3 a | 1.4 ± 0.5 ab | 0% |
| Lathyrus tingitanus | 208.3 ± 0.4 a | 1.0 ± 0.0 a | 48.4% |
| Medicago orbicularis | 439.8 ± 0.4 a | 1.7 ± 0.5 c | 0% |
| Medicago polymorpha | 519.3 ± 0.4 a | 1.1 ± 0.4 ab | 0% |
| Medicago sativa | 348.5 ± 0.3 a | 1.3 ± 0.5 ab | 0% |
| Scorpiurus sulcatus | 237.9 ± 0.2 a | 1.4 ± 0.5 b | 4.7% |
| Trifolium pratense | 373.4 ± 0.3 a | 1.2 ± 0.4 ab | 0% |
| Trifolium vesiculosum | 1049.7 ± 0.8 b | 1.0 ± 0.0 a | 0% |
| Polygonum aviculare | 354.1 ± 0.3 a | 1.0 ± 0.0 a | 8.6% |
| Rumex pulcher | 530.3 ± 0.4 a | 1.0 ± 0.0 a | 10.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, M.; Maurício, R.; Sousa, V.; Talhinhas, P. Spontaneous Flora as Reservoir for the Survival and Spread of the Almond Anthracnose Pathogen (Colletotrichum godetiae) in Intensive Almond Orchards. Plants 2025, 14, 1762. https://doi.org/10.3390/plants14121762
Ramos M, Maurício R, Sousa V, Talhinhas P. Spontaneous Flora as Reservoir for the Survival and Spread of the Almond Anthracnose Pathogen (Colletotrichum godetiae) in Intensive Almond Orchards. Plants. 2025; 14(12):1762. https://doi.org/10.3390/plants14121762
Chicago/Turabian StyleRamos, Madalena, Rodrigo Maurício, Vicelina Sousa, and Pedro Talhinhas. 2025. "Spontaneous Flora as Reservoir for the Survival and Spread of the Almond Anthracnose Pathogen (Colletotrichum godetiae) in Intensive Almond Orchards" Plants 14, no. 12: 1762. https://doi.org/10.3390/plants14121762
APA StyleRamos, M., Maurício, R., Sousa, V., & Talhinhas, P. (2025). Spontaneous Flora as Reservoir for the Survival and Spread of the Almond Anthracnose Pathogen (Colletotrichum godetiae) in Intensive Almond Orchards. Plants, 14(12), 1762. https://doi.org/10.3390/plants14121762









