Role of Serotonin in Cadmium Mitigation in Plants
Abstract
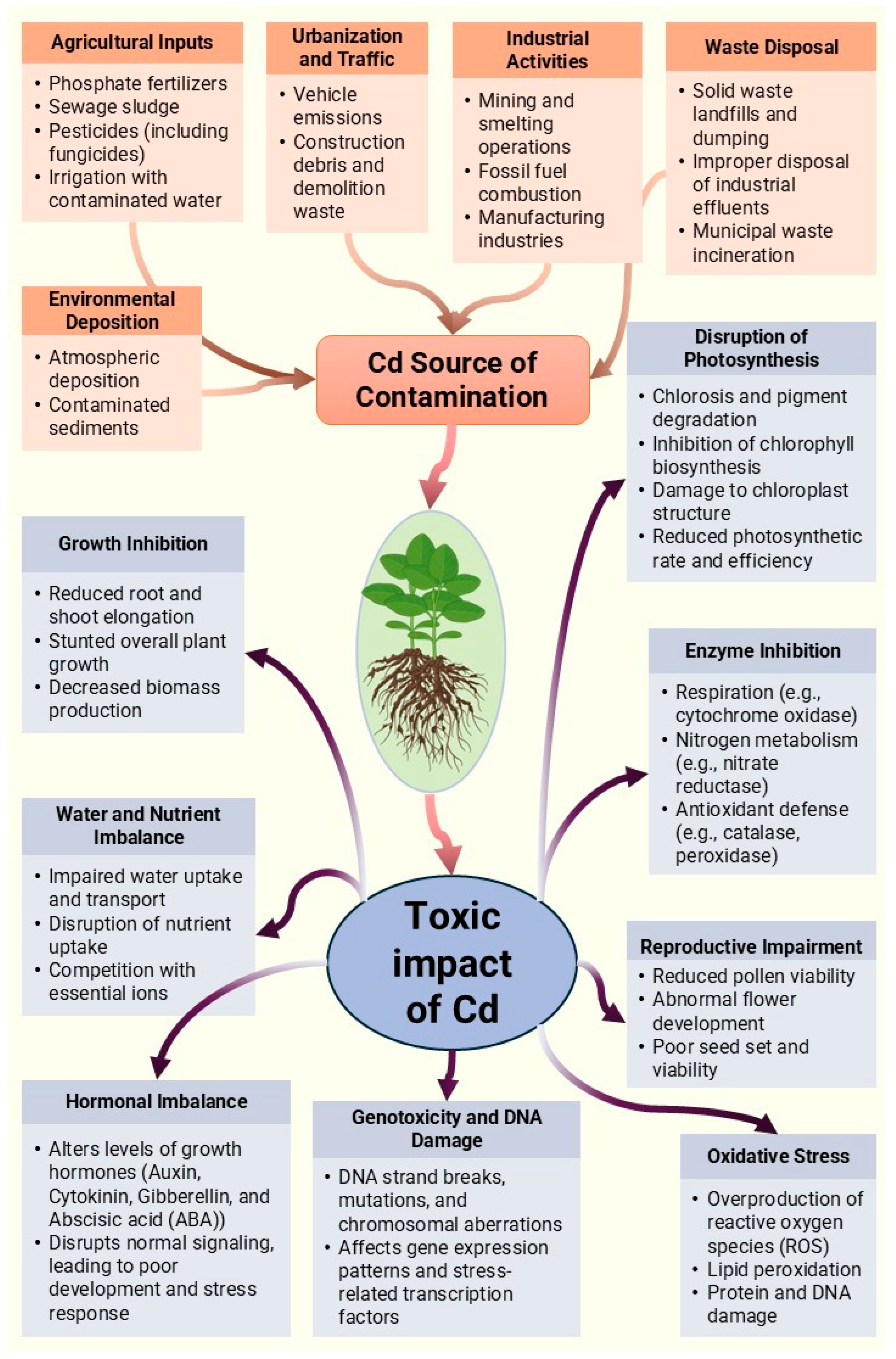
1. The Effect of Cadmium and Its Toxicity in Plants
2. Cadmium Tolerance Mechanisms
3. Serotonin Biosynthesis
4. Serotonin Acts as a Master Regulator in Abiotic Stress in Plants
5. Serotonin Activates Antioxidant Defense Systems in Response to Cd Stress
5.1. The Role of Serotonin and Its Mechanisms of Action
5.2. Transcriptional Regulation
5.3. Post-Translational Modifications
5.4. The Role of Serotonin Transporters
5.5. The Integration of Hormonal Signaling with Growth and Development
5.6. Mitochondrial Protection
5.7. The Interplay with the Secondary Metabolism
5.8. Genetic Variation and Serotonin Regulation
6. Conclusions and Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cd | cadmium |
| Ser | serotonin |
| Pb | lead |
| Hg | mercury |
| As | arsenic |
| RuBP | ribulose-1,5-bisphosphate |
| ROS | reactive oxygen species |
| ER | endoplasmic reticulum |
| UPR | unfolded protein response |
| PAL | phenylalanine ammonialyase |
| POD | peroxidase |
| 4CL | 4-coumarate CoA ligase |
| COMT | caffeic acid 3-O-methyl transferase |
| CAD | cinnamyl alcohol dehydrogenase |
| CCoAOMT | cafeoy1-CoA3-O-methy1transferase |
| PCs | phytochelatins |
| MTs | metallothioneins |
| HMA | heavy metal ATPase |
| NRAMPS | Natural Resistance-Associated Macrophage Proteins |
| CAX | cation exchangers |
| SNAT | acyl-CoA-dependent serotonin N-acyltransferase |
| ASMT | N-acetylserotonin methyltransferase |
| ABA | abscisic acid |
| JA | jasmonic acid |
| SA | salicylic acid |
| SOD | superoxide dismutase |
| CAT | catalase |
| POX | peroxidase |
| APX | ascorbate peroxidase |
| ASDAC | N-acetylserotonin deacetylase |
| SNAT | serotonin N-acetyltransferase |
| ETC | electron transport chain |
References
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Qiu, Z.; Shahzad, S.M.; Nawaz, M.F.; Huang, J.; Naveed, S.; Li, L.; Wang, X.; Cheng, H. Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: A review. Environ. Pollut. 2023, 325, 121433. [Google Scholar] [CrossRef]
- Xuebin, Q.; Yatao, X.; Ahmad, M.I.; Shehzad, M.; Zain, M. Silicon and its application methods improve physiological traits and antioxidants in Triticum aestivum (L.) under cadmium stress. J. Soil Sci. Plant Nutr. 2020, 20, 1110–1121. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Banadka, A.; Rashmi, R.; Nagella, P.; Alessa, F.M.; Almaghasla, M.I. Cadmium toxicity in medicinal plants: An overview of the tolerance strategies, biotechnological and omics approaches to alleviate metal stress. Front. Plant Sci. 2023, 13, 1047410. [Google Scholar] [CrossRef]
- Aslam, M.M.; Okal, E.J.; Waseem, M. Cadmium toxicity impacts plant growth and plant remediation strategies. Plant Growth Regul. 2023, 99, 397–412. [Google Scholar] [CrossRef]
- Joshi, M.K.; Mohanty, P. Chlorophyll a fluorescence as a probe of heavy metal ion toxicity in plants. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2004; pp. 637–661. [Google Scholar]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef]
- Sheoran, I.; Singal, H.; Singh, R. Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeonpea (Cajanus cajan L.). Photosynth. Res. 1990, 23, 345–351. [Google Scholar] [CrossRef]
- Song, X.; Yue, X.; Chen, W.; Jiang, H.; Han, Y.; Li, X. Detection of cadmium risk to the photosynthetic performance of Hybrid Pennisetum. Front. Plant Sci. 2019, 10, 798. [Google Scholar] [CrossRef]
- Vijendra, P.D.; Huchappa, K.M.; Lingappa, R.; Basappa, G.; Jayanna, S.G.; Kumar, V. Physiological and biochemical changes in moth bean (Vigna aconitifolia L.) under cadmium stress. J. Bot. 2016, 2016, 6403938. [Google Scholar] [CrossRef][Green Version]
- Rascio, N.; Dalla Vecchia, F.; La Rocca, N.; Barbato, R.; Pagliano, C.; Raviolo, M.; Gonnelli, C.; Gabbrielli, R. Metal accumulation and damage in rice (cv. Vialone nano) seedlings exposed to cadmium. Environ. Exp. Bot. 2008, 62, 267–278. [Google Scholar] [CrossRef]
- Siedlecka, A.; Samuelsson, G.; Gardeström, P.; Kleczkowslci, L.A.; Krupa, Z. The “activatory model” of plant response to moderate cadmium stress-relationship between carbonic anhydrase and Rubisco. In Proceedings of the Photosynthesis: Mechanisms and Effects: Volume I–V: Proceedings of the XIth International Congress on Photosynthesis, Budapest, Hungary, 17–22 August 1998; Kluwer Academic Pub: Dordrecht, The Netherlands, 1998; pp. 2677–2680. [Google Scholar]
- Cuypers, A.; Vanbuel, I.; Iven, V.; Kunnen, K.; Vandionant, S.; Huybrechts, M.; Hendrix, S. Cadmium-induced oxidative stress responses and acclimation in plants require fine-tuning of redox biology at subcellular level. Free. Radic. Biol. Med. 2023, 199, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Oraby, H.F.; El-Tohamy, M.F.; Kamel, A.M.; Ramadan, M.F. Changes in the concentration of avenanthramides in response to salinity stress in CBF3 transgenic oat. J. Cereal Sci. 2017, 76, 263–270. [Google Scholar] [CrossRef]
- Somashekaraiah, B.; Padmaja, K.; Prasad, A. Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): Involvement of lipid peroxides in chlorphyll degradation. Physiol. Plant. 1992, 85, 85–89. [Google Scholar] [CrossRef]
- Jawad Hassan, M.; Ali Raza, M.; Ur Rehman, S.; Ansar, M.; Gitari, H.; Khan, I.; Wajid, M.; Ahmed, M.; Abbas Shah, G.; Peng, Y. Effect of cadmium toxicity on growth, oxidative damage, antioxidant defense system and cadmium accumulation in two sorghum cultivars. Plants 2020, 9, 1575. [Google Scholar] [CrossRef]
- Goussi, R.; Manaa, A.; Derbali, W.; Ghnaya, T.; Abdelly, C.; Barbato, R. Combined effects of NaCl and Cd2+ stress on the photosynthetic apparatus of Thellungiella salsuginea. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018, 1859, 1274–1287. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- De Benedictis, M.; Gallo, A.; Migoni, D.; Papadia, P.; Roversi, P.; Santino, A. Cadmium treatment induces endoplasmic reticulum stress and unfolded protein response in Arabidopsis thaliana. Plant Physiol. Biochem. 2023, 196, 281–290. [Google Scholar] [CrossRef]
- Martelli, A.; Rousselet, E.; Dycke, C.; Bouron, A.; Moulis, J.-M. Cadmium toxicity in animal cells by interference with essential metals. Biochimie 2006, 88, 1807–1814. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.; Moniuszko-Jakoniuk, J. Interactions between cadmium and zinc in the organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Helbig, K.; Grosse, C.; Nies, D.H. Cadmium toxicity in glutathione mutants of Escherichia coli. J. Bacteriol. 2008, 190, 5439–5454. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef]
- Ren, Q.; Xu, Z.; Xue, Y.; Yang, R.; Ma, X.; Sun, J.; Wang, J.; Lin, S.; Wang, W.; Yang, L. Mechanism of calcium signal response to cadmium stress in duckweed. Plant Signal. Behav. 2022, 17, 2119340. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Yuan, X.; Yuan, M.; Huang, L.; Wang, S.; Liu, C.E.; Duan, C. Effects of heavy metals on stomata in plants: A review. Int. J. Mol. Sci. 2023, 24, 9302. [Google Scholar] [CrossRef]
- Lamoreaux, R.J.; Chaney, W.R. Growth and water movement in silver maple seedlings affected by cadmium. J. Environ. Qual. 1977, 6, 201–205. [Google Scholar] [CrossRef]
- Bashir, A.; Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Ishaque, W.; Atif Riaz, M.; Maqbool, A. Effect of foliar-applied iron complexed with lysine on growth and cadmium (Cd) uptake in rice under Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 20691–20699. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Israr, M.; Mansoor, S.; Hussain, S.A.; Basheer, F.; Azizullah, A.; Ur Rehman, S. Acclimation of cadmium-induced genotoxicity and oxidative stress in mung bean seedlings by priming effect of phytohormones and proline. PLoS ONE 2021, 16, e0257924. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, Y.; Yu, Q.; Liu, J.; Yang, Z.; Chen, Y. Transcriptome analysis reveals the stress tolerance to and accumulation mechanisms of cadmium in Paspalum vaginatum Swartz. Plants 2022, 11, 2078. [Google Scholar] [CrossRef]
- Huang, W.-X.; Zhang, D.-M.; Cao, Y.-Q.; Dang, B.-J.; Jia, W.; Xu, Z.-C.; Han, D. Differential cadmium translocation and accumulation between Nicotiana tabacum L. and Nicotiana rustica L. by transcriptome combined with chemical form analyses. Ecotoxicol. Environ. Saf. 2021, 208, 111412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Q.; Yang, J.; Shen, J.; Chen, T.; Zhu, G.; Chen, H.; Shao, C. Subcellular cadmium distribution and antioxidant enzymatic activities in the leaves of two castor (Ricinus communis L.) cultivars exhibit differences in Cd accumulation. Ecotoxicol. Environ. Saf. 2015, 120, 184–192. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, H.; He, J.; Lyu, D.; Li, H. Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front. Plant Sci. 2017, 8, 966. [Google Scholar] [CrossRef]
- Ai, T.N.; Naing, A.H.; Yun, B.-W.; Lim, S.H.; Kim, C.K. Overexpression of RsMYB1 enhances anthocyanin accumulation and heavy metal stress tolerance in transgenic petunia. Front. Plant Sci. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Akpinar, A.; Cansev, A. Choline supplementation reduces cadmium uptake and alleviates cadmium toxicity in Solanum lycopersicum seedlings. BMC Plant Biol. 2024, 24, 977. [Google Scholar] [CrossRef]
- Behtash, F.; Amini, T.; Mousavi, S.B.; Seyed Hajizadeh, H.; Kaya, O. Efficiency of zinc in alleviating cadmium toxicity in hydroponically grown lettuce (Lactuca sativa L. cv. Ferdos). BMC Plant Biol. 2024, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Xian, P.; Wang, H.; Lin, R.; Lian, T.; Cheng, Y.; Ma, Q.; Nian, H. Transcription factor GmWRKY142 confers cadmium resistance by up-regulating the cadmium tolerance 1-like genes. Front. Plant Sci. 2020, 11, 724. [Google Scholar] [CrossRef]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Farooq, M.A.; Liu, D.; Daud, M.K.; Ali, S.; Zhou, W. Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 2015, 10, e0123328. [Google Scholar] [CrossRef]
- Anjum, N.A.; Aref, I.M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Iqbal, M. Glutathione and proline can coordinately make plants withstand the joint attack of metal (loid) and salinity stresses. Front. Plant Sci. 2014, 5, 662. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, H.; Jiang, H.; Wang, H.; Chen, K.; Duan, J.; Feng, S.; Wu, G. Regulation of cadmium tolerance and accumulation by miR156 in Arabidopsis. Chemosphere 2020, 242, 125168. [Google Scholar] [CrossRef]
- He, M.; Shah Jahan, M.; Wang, Y.; Sun, J.; Shu, S.; Guo, S. Compost amendments based on vinegar residue promote tomato growth and suppress bacterial wilt caused by Ralstonia Solanacearum. Pathogens 2020, 9, 227. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yu, J.; Zhang, H.; Yang, Z. Relationship between the Phenylpropanoid Pathway and Dwarfism of Paspalum seashore Based on RNA-Seq and iTRAQ. Int. J. Mol. Sci. 2021, 22, 9568. [Google Scholar] [CrossRef]
- Sharma, R.; Bhardwaj, R.; Handa, N.; Gautam, V.; Kohli, S.K.; Bali, S.; Kaur, P.; Thukral, A.K.; Arora, S.; Ohri, P. Responses of phytochelatins and metallothioneins in alleviation of heavy metal stress in plants: An overview. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 263–283. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alharbi, B.M.; Alhaithloul, H.A.S.; Abdulmajeed, A.M.; Alghanem, S.M.; Al-Mushhin, A.A.; Jahan, M.S.; Corpas, F.J.; Fang, X.-W.; Soliman, M.H. Spermine-mediated tolerance to selenium toxicity in wheat (Triticum aestivum L.) depends on endogenous nitric oxide synthesis. Antioxidants 2021, 10, 1835. [Google Scholar] [CrossRef]
- Romè, C.; Huang, X.-Y.; Danku, J.; Salt, D.E.; Sebastiani, L. Expression of specific genes involved in Cd uptake, translocation, vacuolar compartmentalisation and recycling in Populus alba Villafranca clone. J. Plant Physiol. 2016, 202, 83–91. [Google Scholar] [CrossRef]
- Li, D.; Xu, X.; Hu, X.; Liu, Q.; Wang, Z.; Zhang, H.; Wang, H.; Wei, M.; Wang, H.; Liu, H. Genome-wide analysis and heavy metal-induced expression profiling of the HMA gene family in Populus trichocarpa. Front. Plant Sci. 2015, 6, 1149. [Google Scholar] [CrossRef]
- Ullah, I.; Wang, Y.; Eide, D.J.; Dunwell, J.M. Evolution, and functional analysis of Natural Resistance-Associated Macrophage Proteins (NRAMPs) from Theobroma cacao and their role in cadmium accumulation. Sci. Rep. 2018, 8, 14412. [Google Scholar] [CrossRef]
- Bucholc, M.; Ciesielski, A.; Goch, G.; Anielska-Mazur, A.; Kulik, A.; Krzywińska, E.; Dobrowolska, G. SNF1-related protein kinases 2 are negatively regulated by a plant-specific calcium sensor. J. Biol. Chem. 2011, 286, 3429–3441. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Qiu, Z.; Hu, B.; Zeng, B.; Zhong, C.; Fan, C. Comprehensive analysis of SnRK gene family and their responses to salt stress in Eucalyptus grandis. Int. J. Mol. Sci. 2019, 20, 2786. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Bhagat, P.K.; Verma, D.; Noryang, S.; Tayyeba, S.; Singh, K.; Sharma, D.; Sinha, A.K. Traversing the links between heavy metal stress and plant signaling. Front. Plant Sci. 2018, 9, 12. [Google Scholar] [CrossRef]
- Li, X.; Mao, X.; Xu, Y.; Li, Y.; Zhao, N.; Yao, J.; Dong, Y.; Tigabu, M.; Zhao, X.; Li, S. Comparative transcriptomic analysis reveals the coordinated mechanisms of Populus × canadensis ‘Neva’ leaves in response to cadmium stress. Ecotoxicol. Environ. Saf. 2021, 216, 112179. [Google Scholar] [CrossRef]
- wa Lwalaba, J.L.; Zvobgo, G.; Gai, Y.; Issaka, J.H.; Mwamba, T.M.; Louis, L.T.; Fu, L.; Nazir, M.M.; Kirika, B.A.; Tshibangu, A.K. Transcriptome analysis reveals the tolerant mechanisms to cobalt and copper in barley. Ecotoxicol. Environ. Saf. 2021, 209, 111761. [Google Scholar] [CrossRef]
- Wu, Y.; An, T.; Gao, Y.; Kuang, Q.; Liu, S.; Liang, L.; Xu, B.; Zhang, S.; Deng, X.; Chen, Y. Genotypic variation in the tolerance to moderate cadmium toxicity among 20 maize genotypes with contrasting root systems. J. Sci. Food Agric. 2023, 103, 2618–2630. [Google Scholar] [CrossRef]
- Pontes, C.V.S.; dos Santos, A.H.A.; Lopes, L.K.C.; Barbosa, M.A.M.; Bajguz, A.; da Silva Lobato, A.K. Exogenous serotonin and 24-epibrassinolide boost root protection and suppress oxidative damages occasioned by severe water deficit in soybean seedlings. J. Plant Growth Regul. 2024, 43, 1833–1843. [Google Scholar] [CrossRef]
- Akcay, U.C.; Okudan, N. Exogenous serotonin improves drought and salt tolerance in tomato seedlings. Plant Growth Regul. 2023, 101, 239–249. [Google Scholar] [CrossRef]
- Murch, S.; KrishnaRaj, S.; Saxena, P. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000, 19, 698–704. [Google Scholar] [CrossRef]
- Schröder, P.; Abele, C.; Gohr, P.; Stuhlfauth-Roisch, U.; Grosse, W. Latest on enzymology of serotonin biosynthesis in walnut seeds. In Tryptophan, Serotonin, and Melatonin: Basic Aspects and Applications; Springer: Boston, MA, USA, 1999; pp. 637–644. [Google Scholar]
- Pelagio-Flores, R.; López-Bucio, J. Serotonin and melatonin in plant growth and development. In Serotonin and Melatonin; CRC Press: Boca Raton, FL, USA, 2016; pp. 119–132. [Google Scholar]
- Erland, L.A.; Turi, C.E.; Saxena, P.K. Serotonin in plants: Origin, functions, and implications. In Serotonin; Academic Press: Cambridge, MA, USA, 2019; pp. 23–46. [Google Scholar] [CrossRef]
- Kang, K.; Kang, S.; Lee, K.; Park, M.; Back, K. Enzymatic features of serotonin biosynthetic enzymes and serotonin biosynthesis in plants. Plant Signal. Behav. 2008, 3, 389–390. [Google Scholar] [CrossRef]
- De Masi, L.; Castaldo, D.; Pignone, D.; Servillo, L.; Facchiano, A. Experimental evidence and in silico identification of tryptophan decarboxylase in Citrus genus. Molecules 2017, 22, 272. [Google Scholar] [CrossRef]
- Bhowal, B.; Bhattacharjee, A.; Goswami, K.; Sanan-Mishra, N.; Singla-Pareek, S.L.; Kaur, C.; Sopory, S. Serotonin and melatonin biosynthesis in plants: Genome-wide identification of the genes and their expression reveal a conserved role in stress and development. Int. J. Mol. Sci. 2021, 22, 11034. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Giebelhaus, R.T.; Erland, L.A.; Murch, S.J. HormonomicsDB: A novel workflow for the untargeted analysis of plant growth regulators and hormones. F1000Research 2024, 11, 1191. [Google Scholar] [CrossRef]
- Roychoudhury, A. Multifaceted roles of serotonin in plants. Young Sci.-Tomorrow’s Sci. Begins Today 2021, 5, 26–35. [Google Scholar]
- Abbasi, B.H.; Younas, M.; Anjum, S.; Ahmad, N.; Ali, M.; Fazal, H.; Hano, C. Serotonin in plant signalling and communication. In Neurotransmitters in Plant Signaling and Communication; Baluška, F., Mukherjee, S., Ramakrishna, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 75–92. [Google Scholar]
- Chen, W.; Zhang, J.; Zheng, S.; Wang, Z.; Xu, C.; Zhang, Q.; Wu, J.; Lou, H. Metabolite profiling and transcriptome analyses reveal novel regulatory mechanisms of melatonin biosynthesis in hickory. Hortic. Res. 2021, 8, 196. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Mishra, V.; Sarkar, A.K. Serotonin: A frontline player in plant growth and stress responses. Physiol. Plant. 2023, 175, e13968. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, X.; Lv, Y.; Cheng, Y.; Li, C.; Yan, L.; Tian, S.; Zou, X. Exogenous serotonin improves salt tolerance in rapeseed (Brassica napus L.) seedlings. Agronomy 2021, 11, 400. [Google Scholar] [CrossRef]
- Akula, R.; Mukherjee, S. New insights on neurotransmitters signaling mechanisms in plants. Plant Signal. Behav. 2020, 15, 1737450. [Google Scholar] [CrossRef]
- Negri, S.; Commisso, M.; Avesani, L.; Guzzo, F. The case of tryptamine and serotonin in plants: A mysterious precursor for an illustrious metabolite. J. Exp. Bot. 2021, 72, 5336–5355. [Google Scholar] [CrossRef]
- Dharmawardhana, P.; Ren, L.; Amarasinghe, V.; Monaco, M.; Thomason, J.; Ravenscroft, D.; McCouch, S.; Ware, D.; Jaiswal, P. A genome scale metabolic network for rice and accompanying analysis of tryptophan, auxin and serotonin biosynthesis regulation under biotic stress. Rice 2013, 6, 15. [Google Scholar] [CrossRef]
- Holloway, T.; González-Maeso, J. Epigenetic mechanisms of serotonin signaling. ACS Chem. Neurosci. 2015, 6, 1099–1109. [Google Scholar] [CrossRef]
- Farrelly, L.A.; Thompson, R.E.; Zhao, S.; Lepack, A.E.; Lyu, Y.; Bhanu, N.V.; Zhang, B.; Loh, Y.-H.E.; Ramakrishnan, A.; Vadodaria, K.C. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature 2019, 567, 535–539. [Google Scholar] [CrossRef]
- Kaur, H.; Mukherjee, S.; Baluska, F.; Bhatla, S.C. Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal. Behav. 2015, 10, e1049788. [Google Scholar] [CrossRef]
- Schardl, C.L.; Young, C.A.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013, 9, e1003323. [Google Scholar] [CrossRef]
- Benincasa, P.; Bravi, E.; Marconi, O.; Lutts, S.; Tosti, G.; Falcinelli, B. Transgenerational effects of salt stress imposed to rapeseed (Brassica napus var. oleifera del.) plants involve greater phenolic content and antioxidant activity in the edible sprouts obtained from offspring seeds. Plants 2021, 10, 932. [Google Scholar] [CrossRef]
- Jacques, C.; Salon, C.; Barnard, R.L.; Vernoud, V.; Prudent, M. Drought stress memory at the plant cycle level: A review. Plants 2021, 10, 1873. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Hwang, O.J.; Lee, H.J.; Lee, K.; Back, K. Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 2015, 58, 470–478. [Google Scholar] [CrossRef]
- Limson, J.; Nyokong, T.; Daya, S. The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: An adsorptive voltammetric study. J. Pineal Res. 1998, 24, 15–21. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef]
- Mukherjee, S.; David, A.; Yadav, S.; Baluška, F.; Bhatla, S.C. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plant. 2014, 152, 714–728. [Google Scholar] [CrossRef]
- Urhan, E.K. Abiotic stress tolerance in plants by genome editing applications. In Applications of Genome Engineering in Plants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2024; pp. 185–221. [Google Scholar] [CrossRef]
- Xu, L.; Lan, Y.; Lin, M.; Zhou, H.; Ying, S.; Chen, M. Genome-wide identification and transcriptional analysis of AP2/ERF Gene Family in Pearl Millet (Pennisetum glaucum). Int. J. Mol. Sci. 2024, 25, 2470. [Google Scholar] [CrossRef]
- Shafi, A.; Singh, A.K.; Zahoor, I. Melatonin: Role in abiotic stress resistance and tolerance. In Plant Growth Regulators: Signalling Under Stress Conditions; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 239–273. [Google Scholar]
- Chen, J.; Zhou, H.; Xie, Y. SnRK2. 6 phosphorylation/persulfidation: Where ABA and H2S signaling meet. Trends Plant Sci. 2021, 26, 1207–1209. [Google Scholar] [CrossRef]
- de Bont, L.; Mu, X.; Wei, B.; Han, Y. Abiotic stress-triggered oxidative challenges: Where does H2S act? J. Genet. Genom. 2022, 49, 748–755. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, L.; Feng, L.; Zhang, M.; Hu, D.; Tie, J.; Liao, W. Hydrogen sulfide promotes adventitious root development in cucumber under salt stress by enhancing antioxidant ability. Plants 2022, 11, 935. [Google Scholar] [CrossRef]
- Sharma, P.; Meyyazhagan, A.; Easwaran, M.; Sharma, M.M.M.; Mehta, S.; Pandey, V.; Liu, W.-C.; Kamyab, H.; Balasubramanian, B.; Baskaran, R. Hydrogen sulfide: A new warrior in assisting seed germination during adverse environmental conditions. Plant Growth Regul. 2022, 98, 401–420. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Zhang, L.; Kong, X.; Zhang, J.; Wang, X.; Pei, Y.; Jin, Z. H2S-mediated balance regulation of stomatal and non-stomatal factors responding to drought stress in Chinese cabbage. Hortic. Res. 2023, 10, uhac284. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef]
- Khan, M.S.S.; Islam, F.; Ye, Y.; Ashline, M.; Wang, D.; Zhao, B.; Fu, Z.Q.; Chen, J. The interplay between hydrogen sulfide and phytohormone signaling pathways under challenging environments. Int. J. Mol. Sci. 2022, 23, 4272. [Google Scholar] [CrossRef]
- Xiang, Z.X.; Li, W.; Lu, Y.T.; Yuan, T.T. Hydrogen sulfide alleviates osmotic stress-induced root growth inhibition by promoting auxin homeostasis. Plant J. 2023, 114, 1369–1384. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Kang, X.; Zhang, L.; Wang, J.; Zheng, S.; Zhang, T. Hydrogen sulfide and nitric oxide are involved in melatonin-induced salt tolerance in cucumber. Plant Physiol. Biochem. 2021, 167, 101–112. [Google Scholar] [CrossRef]
- Wang, X.-N.; Zhang, J.-C.; Zhang, H.-Y.; Wang, X.-F.; You, C.-X. Ectopic expression of MmSERT, a mouse serotonin transporter gene, regulates salt tolerance and ABA sensitivity in apple and Arabidopsis. Plant Physiol. Biochem. 2023, 197, 107627. [Google Scholar] [CrossRef]
- Mukherjee, S. Novel perspectives on the molecular crosstalk mechanisms of serotonin and melatonin in plants. Plant Physiol. Biochem. 2018, 132, 33–45. [Google Scholar] [CrossRef]
- Kang, K.; Kim, Y.-S.; Park, S.; Back, K. Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves. Plant Physiol. 2009, 150, 1380–1393. [Google Scholar] [CrossRef]
- Kanjanaphachoat, P.; Wei, B.-Y.; Lo, S.-F.; Wang, I.-W.; Wang, C.-S.; Yu, S.-M.; Yen, M.-L.; Chiu, S.-H.; Lai, C.-C.; Chen, L.-J. Serotonin accumulation in transgenic rice by over-expressing tryptophan decarboxlyase results in a dark brown phenotype and stunted growth. Plant Mol. Biol. 2012, 78, 525–543. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.Y.; Back, K. Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants. J. Pineal Res. 2018, 64, e12460. [Google Scholar] [CrossRef]
- Murch, S.J.; Campbell, S.S.; Saxena, P.K. The role of serotonin and melatonin in plant morphogenesis: Regulation of auxin-induced root organogenesis in in vitro-cultured explants of St. John’s wort (Hypericum perforatum L.). Vitr. Cell. Dev. Biol.-Plant 2001, 37, 786–793. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. Role of indoleamines in regulation of morphogenesis in in vitro cultures of St. John’s wort (Hypericum perforatum L.). Acta Hortic. 2004, 629, 425–432. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Pacini, A.; Gulisano, M.; Taddei, N.; Fiorillo, C.; Becatti, M. Cadmium-induced cytotoxicity: Effects on mitochondrial electron transport chain. Front. Cell Dev. Biol. 2020, 8, 604377. [Google Scholar] [CrossRef]
- Luo, H.; Li, H.; Zhang, X.; Fu, J. Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress. Ecotoxicology 2011, 20, 770–778. [Google Scholar] [CrossRef]
- Choudhury, S.; Moulick, D.; Mazumder, M.K. Secondary metabolites protect against metal and metalloid stress in rice: An in silico investigation using dehydroascorbate reductase. Acta Physiol. Plant. 2021, 43, 3. [Google Scholar] [CrossRef]
- Chen, X.-X.; Xu, Y.-M.; Lau, A.T. Metabolic effects of long-term cadmium exposure: An overview. Environ. Sci. Pollut. Res. 2022, 29, 89874–89888. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Pathak, S.; Kumar, M.; Dwivedi, P. Role of secondary metabolites for the mitigation of cadmium toxicity in sorghum grown under mycorrhizal inoculated hazardous waste site. In Biotechnological Approaches for Medicinal and Aromatic Plants: Conservation, Genetic Improvement and Utilization; Kumar, N., Ed.; Springer: Singapore, 2018; pp. 199–212. [Google Scholar]
- Mwamba, T.M.; Islam, F.; Ali, B.; Lwalaba, J.; Gill, R.A.; Zhang, F.; Farooq, M.A.; Ali, S.; Ulhassan, Z.; Huang, Q. Comparative metabolomic responses of low-and high-cadmium accumulating genotypes reveal the cadmium adaptive mechanism in Brassica napus. Chemosphere 2020, 250, 126308. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oraby, H.F.; Elnaggar, N.Z.; Omar, A.A.; Mohamed, A.H. Role of Serotonin in Cadmium Mitigation in Plants. Plants 2025, 14, 1738. https://doi.org/10.3390/plants14121738
Oraby HF, Elnaggar NZ, Omar AA, Mohamed AH. Role of Serotonin in Cadmium Mitigation in Plants. Plants. 2025; 14(12):1738. https://doi.org/10.3390/plants14121738
Chicago/Turabian StyleOraby, Hesham F., Nehal Z. Elnaggar, Ahmad A. Omar, and Azza H. Mohamed. 2025. "Role of Serotonin in Cadmium Mitigation in Plants" Plants 14, no. 12: 1738. https://doi.org/10.3390/plants14121738
APA StyleOraby, H. F., Elnaggar, N. Z., Omar, A. A., & Mohamed, A. H. (2025). Role of Serotonin in Cadmium Mitigation in Plants. Plants, 14(12), 1738. https://doi.org/10.3390/plants14121738







