The Fiber Cell-Specific Overexpression of COMT2 Modulates Secondary Cell Wall Biosynthesis in Poplar
Abstract
1. Introduction
2. Results
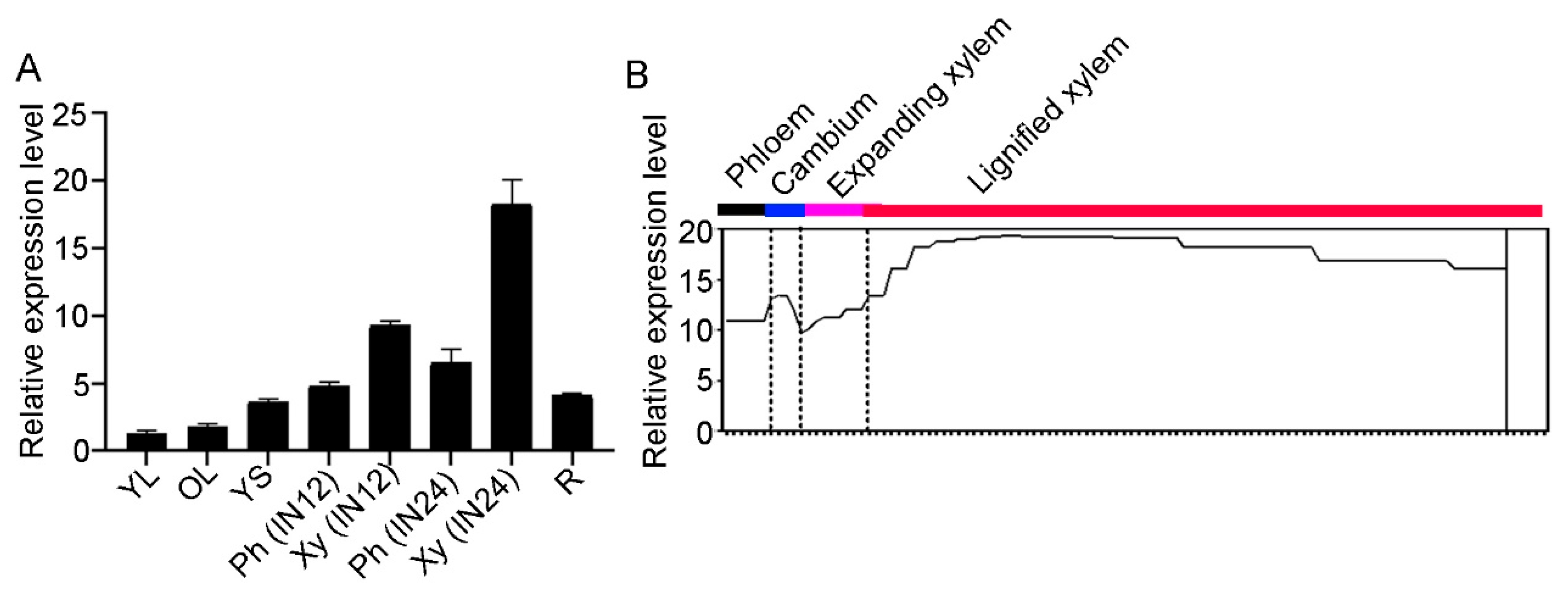
2.1. Characterization of COMT2 from Populus Tomentosa
2.2. Generation of Fiber Cells Specific Overexpression of COMT2 Transgenic Lines in P. tomentosa
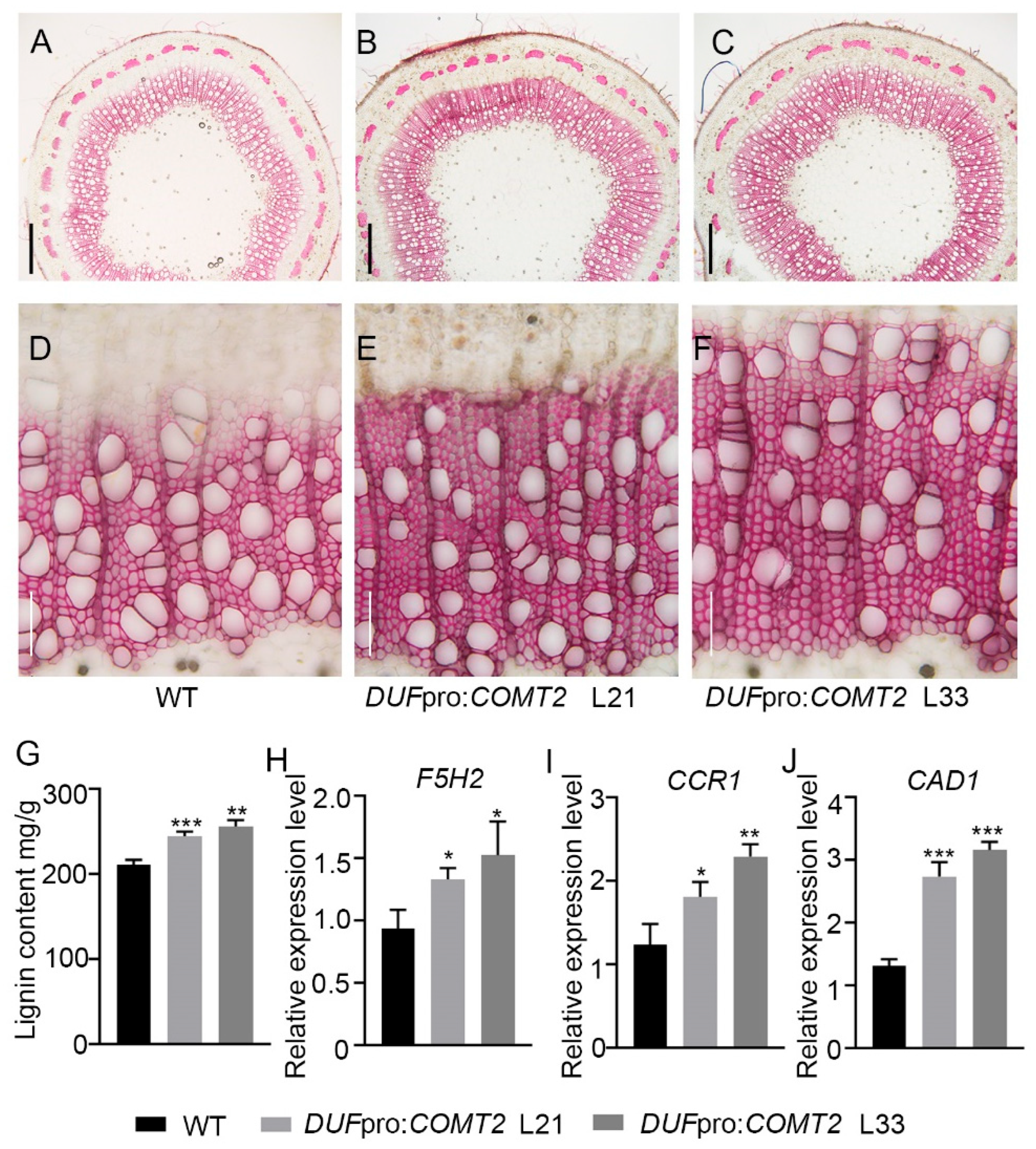
2.3. COMT2 Positively Regulates Xylem Development During Wood Formation in P. tomentosa
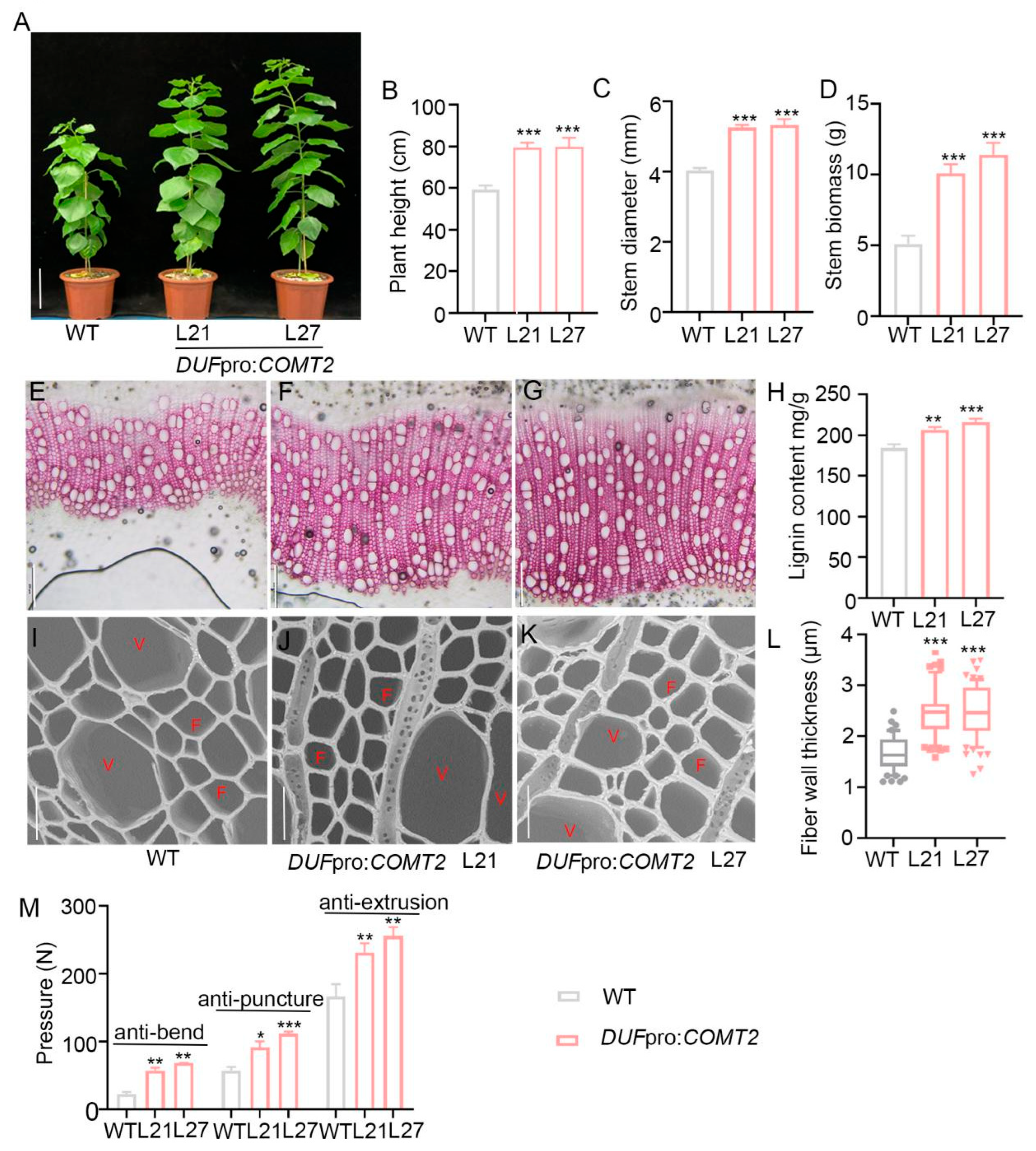
2.4. Fiber Cells Specific Overexpression of COMT2 Promotes SCW Deposition and Stem Strength in P. tomentosa
2.5. Generation of Fiber Cells Specific Overexpression of COMT2 Transgenic Lines in P. deltoides × P. euramericana cv ‘Nanlin895’
3. Discussion
3.1. Genetic Functional Conservation of the COMT Family and Its Effects on Plant Growth
3.2. Specific Overexpression of COMT2 in Poplar Fiber Cells Promotes Secondary Wall Synthesis and Stem Strength
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Phylogenetic Tree Construction and Sequence Analysis
4.3. Vector Construction and Plant Transformation
4.4. RNA Extraction and Quantitative RT-PCR
4.5. Cross-Sectioning and Histological Staining
4.6. Scanning Electron Micrograph (SEM) Observation
4.7. Lignin Content Determination
4.8. Stem Strength Determination
4.9. Accession Numbers
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidhendi, A.J.; Geitmann, A. Relating the Mechanics of the Primary Plant Cell Wall to Morphogenesis. J. Exp. Bot. 2016, 67, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Ye, Z.H. Regulation of Cell Wall Biosynthesis. Curr. Opin. Plant Biol. 2007, 10, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Ye, Z.H. Secondary Cell Walls: Biosynthesis, Patterned Deposition and Transcriptional Regulation. Plant Cell Physiol. 2015, 56, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Meents, M.J.; Watanabe, Y.; Samuels, A.L. The Cell Biology of Secondary Cell Wall Biosynthesis. Ann. Bot. 2018, 121, 1107–1125. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, M.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Recent Advances in the Transcriptional Regulation of Secondary Cell Wall Biosynthesis in the Woody Plants. Front. Plant Sci. 2018, 871, 1535. [Google Scholar] [CrossRef]
- Service, R.F. Biofuel researchers prepare to reap a new harvest. Science 2007, 315, 1488–1491. [Google Scholar] [CrossRef]
- Zhong, R.; Lee, C.; Ye, Z.H. Global Analysis of Direct Targets of Secondary Wall NAC Master Switches in Arabidopsis. Mol. Plant 2010, 3, 1087–1103. [Google Scholar] [CrossRef]
- Taylor-Teeples, M.; Lin, L.; de Lucas, M.; Turco, G.; Toal, T.W.; Gaudinier, A.; Young, N.F.; Trabucco, G.M.; Veling, M.T.; Lamothe, R.; et al. An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 2015, 517, 571–575. [Google Scholar] [CrossRef]
- McCarthy, R.L.; Zhong, R.; Ye, Z.H. MYB83 Is a Direct Target of SND1 and Acts Redundantly with MYB46 in the Regulation of Secondary Cell Wall Biosynthesis in Arabidopsis. Plant Cell Physiol. 2009, 50, 1950–1964. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of Lignin in Seaweed Reveals Convergent Evolution of Cell-Wall Architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef]
- Campbell, M.M.; Sederoff, R.R. Variation in Lignin Content and Composition. Plant Physiol. 1996, 110, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, M.; Benske, A.; Smith, R.A.; Watanabe, Y.; Tobimatsu, Y.; Ralph, J.; Demura, T.; Ellis, B.; Samuels, A.L. Laccases Direct Lignification in the Discrete Secondary Cell Wall Domains of Protoxylem. Plant Physiol. 2014, 166, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Ran, L.; Tian, Q.; Fan, D.; Luo, K. PtoMYB92 is a transcriptional activator of the lignin biosynthetic pathway during secondary cell wall formation in Populus tomentosa. Plant Cell Physiol. 2015, 56, 2436–2446. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of Phenylpropanoid Metabolism to Plant Development and Plant–Environment Interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Voelker, S.L.; Lachenbruch, B.; Meinzer, F.C.; Jourdes, M.; Ki, C.; Patten, A.M.; Davin, L.B.; Lewis, N.G.; Tuskan, G.A.; Gunter, L.; et al. Antisense Down-Regulation of 4CL Expression Alters Lignification, Tree Growth, and Saccharification Potential of Field-Grown Poplar. Plant Physiol. 2010, 154, 874–886. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Kong, L.; Song, Q.; Wei, H.; Wang, Y.; Lin, M.; Sun, K.; Zhang, Y.; Yang, J.; Li, C.; Luo, K. The AP2/ERF Transcription Factor PtoERF15 Confers Drought Tolerance via JA-Mediated Signaling in Populus. New Phytol. 2023, 240, 1848–1867. [Google Scholar] [CrossRef]
- Ibrahim, L.; Proe, M.F.; Cameron, A.D. Interactive Effects of Nitrogen and Water Availabilities on Gas Exchange and Whole-Plant Carbon Allocation in Poplar. Tree Physiol. 1998, 18, 481–487. [Google Scholar] [CrossRef]
- La Camera, S.; Gouzerh, G.; Dhondt, S.; Hoffmann, L.; Fritig, B.; Legrand, M.; Heitz, T. Metabolic Reprogramming in Plant Innate Immunity: The Contributions of Phenylpropanoid and Oxylipin Pathways. Immunol. Rev. 2004, 198, 267–284. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Muzac, I. Chapter Eleven The Methyltransferase Gene Superfamily: A Tree with Multiple Branches. Recent Adv. Phytochem. 2000, 34, 349–384. [Google Scholar] [CrossRef]
- Jouanin, L.; Goujon, T.; de Nadaı, V.; Martin, M.T.; Mila, I.; Vallet, C.; Pollet, B.; Yoshinaga, A.; Chabbert, B.; Petit-Conil, M.; et al. Lignification in transgenic poplars with extremely reduced caffeic acid O-methyltransferase activity. Plant Physiol. 2000, 123, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Ma, W.; Han, D.; Liu, Y.; Wang, Z.; Wang, N.; Yang, G.; Qu, G.; Wang, Q.; Zhao, K.; et al. Genome-Wide Analysis of the Catalpa Bungei Caffeic Acid O-Methyltransferase (COMT) Gene Family: Identification and Expression Profiles in Normal, Tension, and Opposite Wood. PeerJ 2019, 7, e6520. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Sun, Y.H.; Li, Q.; Heber, S.; Sederoff, R.; Chiang, V.L. Towards a Systems Approach for Lignin Biosynthesis in Populus Trichocarpa: Transcript Abundance and Specificity of the Monolignol Biosynthetic Genes. Plant Cell Physiol. 2010, 51, 144–163. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pu, Y.; Yoo, C.G.; Gjersing, E.; Decker, S.R.; Doeppke, C.; Shollenberger, T.; Tschaplinski, T.J.; Engle, N.L.; Sykes, R.W.; et al. Study of Traits and Recalcitrance Reduction of Field-Grown COMT down-Regulated Switchgrass. Biotechnol. Biofuels 2017, 10, 12. [Google Scholar] [CrossRef]
- Yuan, L.; Dang, J.; Zhang, J.; Wang, L.; Zheng, H.; Li, G.; Li, J.; Zhou, F.; Khan, A.; Zhang, Z.; et al. A Glutathione S-Transferase Regulates Lignin Biosynthesis and Enhances Salt Tolerance in Tomato. Plant Physiol. 2024, 196, 2989–3006. [Google Scholar] [CrossRef]
- Baxter, H.L.; Mazarei, M.; Fu, C.; Cheng, Q.; Turner, G.B.; Sykes, R.W.; Windham, M.T.; Davis, M.F.; Dixon, R.A.; Wang, Z.Y.; et al. Time Course Field Analysis of COMT-Downregulated Switchgrass: Lignification, Recalcitrance, and Rust Susceptibility. Bioenergy Res. 2016, 9, 1087–1100. [Google Scholar] [CrossRef]
- Van Doorsselaere, J.; Baucher, M.; Chognot, E.; Chabbert, B.; Tollier, M.T.; Petit-Conil, M.; Leplé, J.C.; Pilate, G.; Cornu, D.; Monties, B.; et al. A novel lignin in poplar trees with a reduced caffeic acid/5-hydroxyferulic acid O-methyltransferase activity. Plant J. 2003, 8, 855–864. [Google Scholar] [CrossRef]
- Liang, S.; Xu, S.; Qu, D.; Yang, L.; Wang, J.; Liu, H.; Xin, W.; Zou, D.; Zheng, H. Identification and Functional Analysis of the Caffeic Acid O-Methyltransferase (COMT) Gene Family in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 8491. [Google Scholar] [CrossRef]
- Li, Q.; Fu, C.; Liang, C.; Ni, X.; Zhao, X.; Chen, M.; Ou, L. Crop Lodging and The Roles of Lignin, Cellulose, and Hemicellulose in Lodging Resistance. Agronomy 2022, 12, 1795. [Google Scholar] [CrossRef]
- Jia, Z.; Sun, Y.; Yuan, L.; Tian, Q.; Luo, K. The Chitinase Gene (Bbchit1) from Beauveria Bassiana Enhances Resistance to Cytospora Chrysosperma in Populus Tomentosa Carr. Biotechnol. Lett. 2010, 32, 1325–1332. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, H.; Zhao, Z.; Pan, J.; Yao, Y.; Wang, Y.; Luo, K.; Song, Q. The Fiber Cell-Specific Overexpression of COMT2 Modulates Secondary Cell Wall Biosynthesis in Poplar. Plants 2025, 14, 1739. https://doi.org/10.3390/plants14121739
Chen H, Wang H, Zhao Z, Pan J, Yao Y, Wang Y, Luo K, Song Q. The Fiber Cell-Specific Overexpression of COMT2 Modulates Secondary Cell Wall Biosynthesis in Poplar. Plants. 2025; 14(12):1739. https://doi.org/10.3390/plants14121739
Chicago/Turabian StyleChen, Hanyu, Hong Wang, Zhengjie Zhao, Jiarui Pan, Yao Yao, Yihan Wang, Keming Luo, and Qin Song. 2025. "The Fiber Cell-Specific Overexpression of COMT2 Modulates Secondary Cell Wall Biosynthesis in Poplar" Plants 14, no. 12: 1739. https://doi.org/10.3390/plants14121739
APA StyleChen, H., Wang, H., Zhao, Z., Pan, J., Yao, Y., Wang, Y., Luo, K., & Song, Q. (2025). The Fiber Cell-Specific Overexpression of COMT2 Modulates Secondary Cell Wall Biosynthesis in Poplar. Plants, 14(12), 1739. https://doi.org/10.3390/plants14121739





