Assessment of Different Irrigation Thresholds to Optimize the Water Use Efficiency and Yield of Potato (Solanum tuberosum L.) Under Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
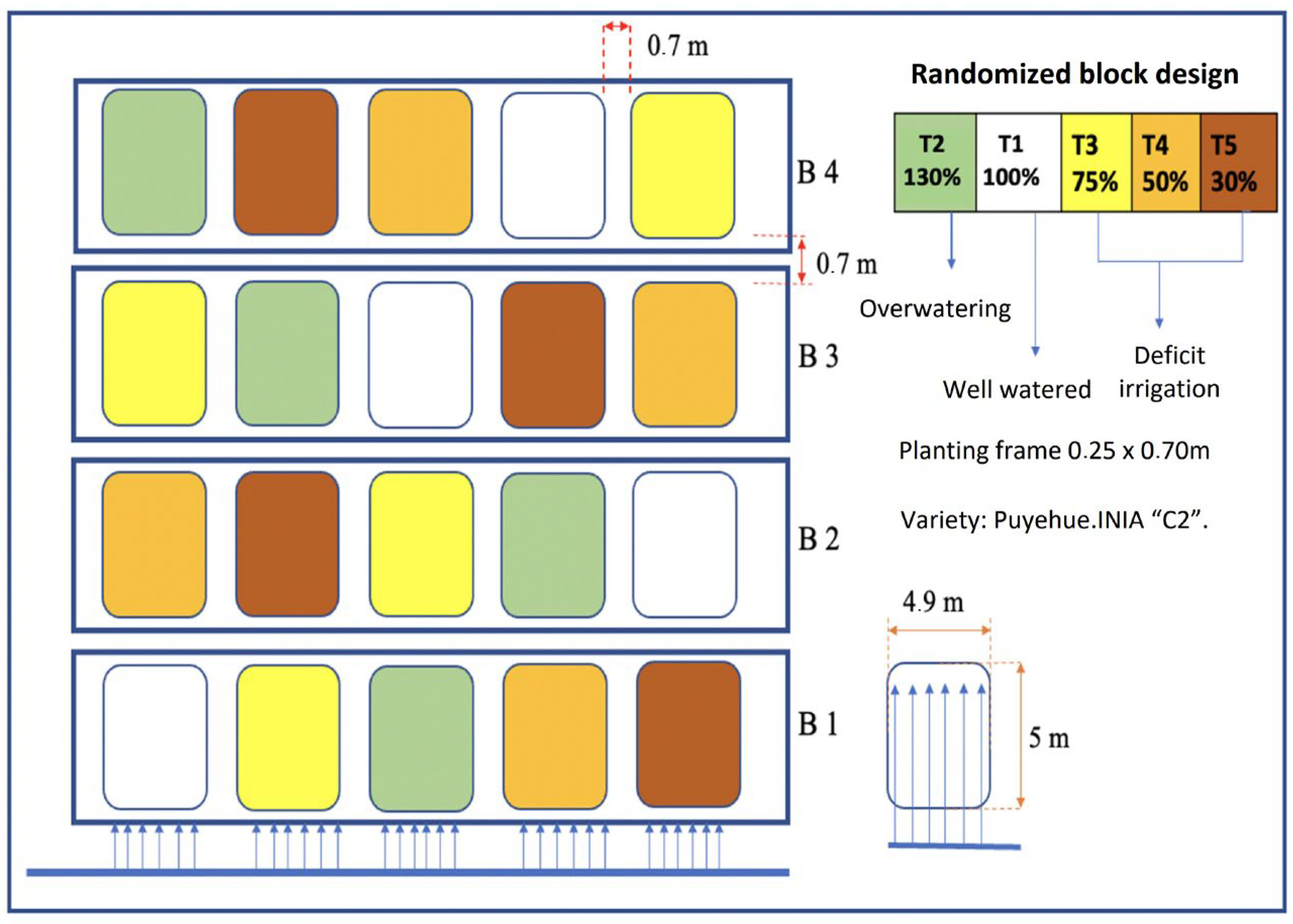
2.2. Irrigation Water Treatments and Water Management
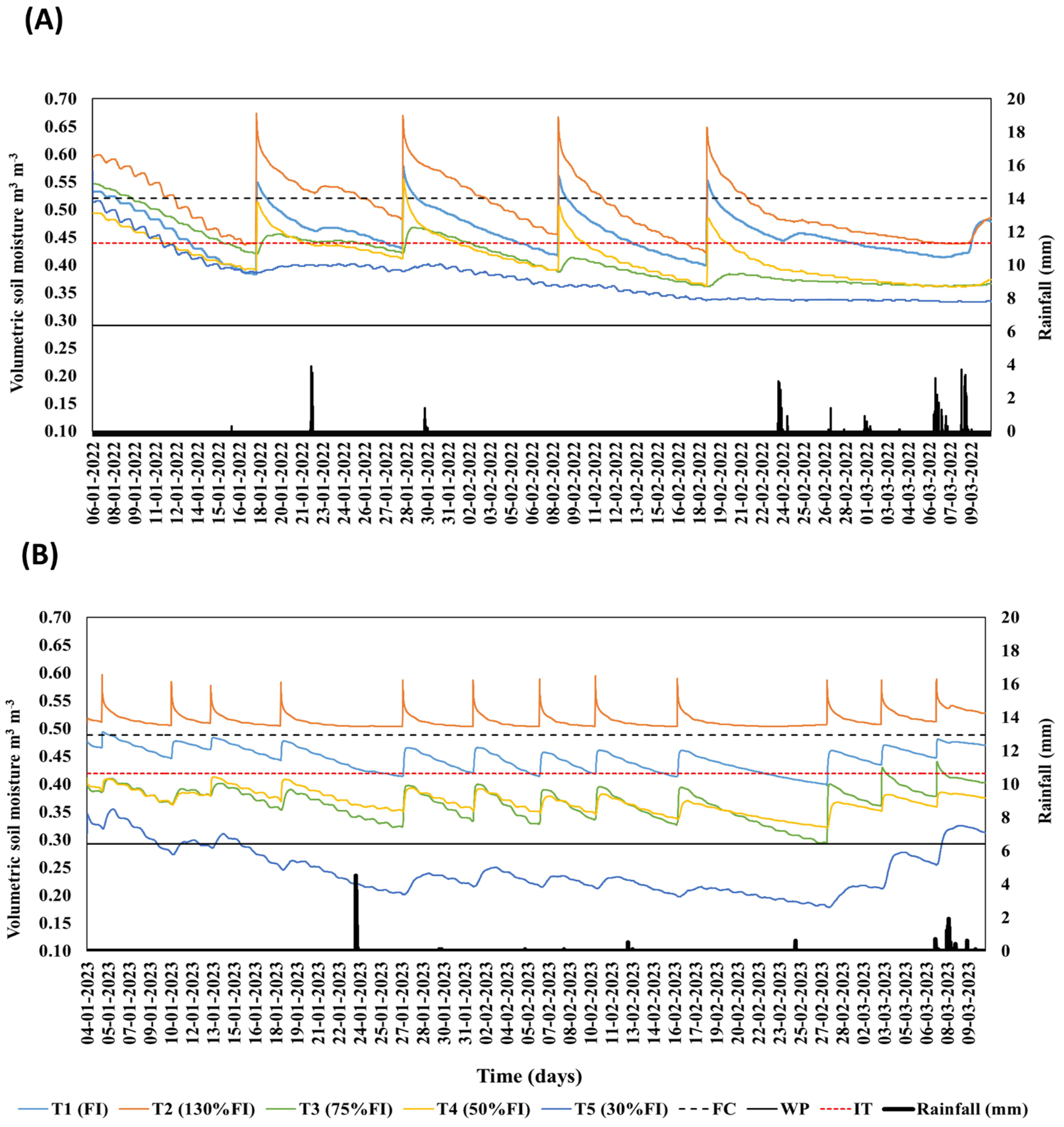
2.3. Soil Moisture
2.4. Chlorophyll a Fluorescence Parameter of PSII
2.5. Gas Exchange Measurements
2.6. Quantification of Photosynthetic Pigments
2.7. Yield Estimation
2.8. Experimental Design and Statistical Analysis
- Pn is the net photosynthesis rate (μmol CO2 m−2 s−1);
- gs is stomatal conductance (mol H2O m−2 s−1);
- E is transpiration rate (mmol H2O m−2 s−1);
- ΦPSII is the effective quantum yield of PSII;
- β₀ represents the intercept;
- β₁, β2, and β₃ are the regression coefficients estimating the contribution of each predictor to Pn.
3. Results
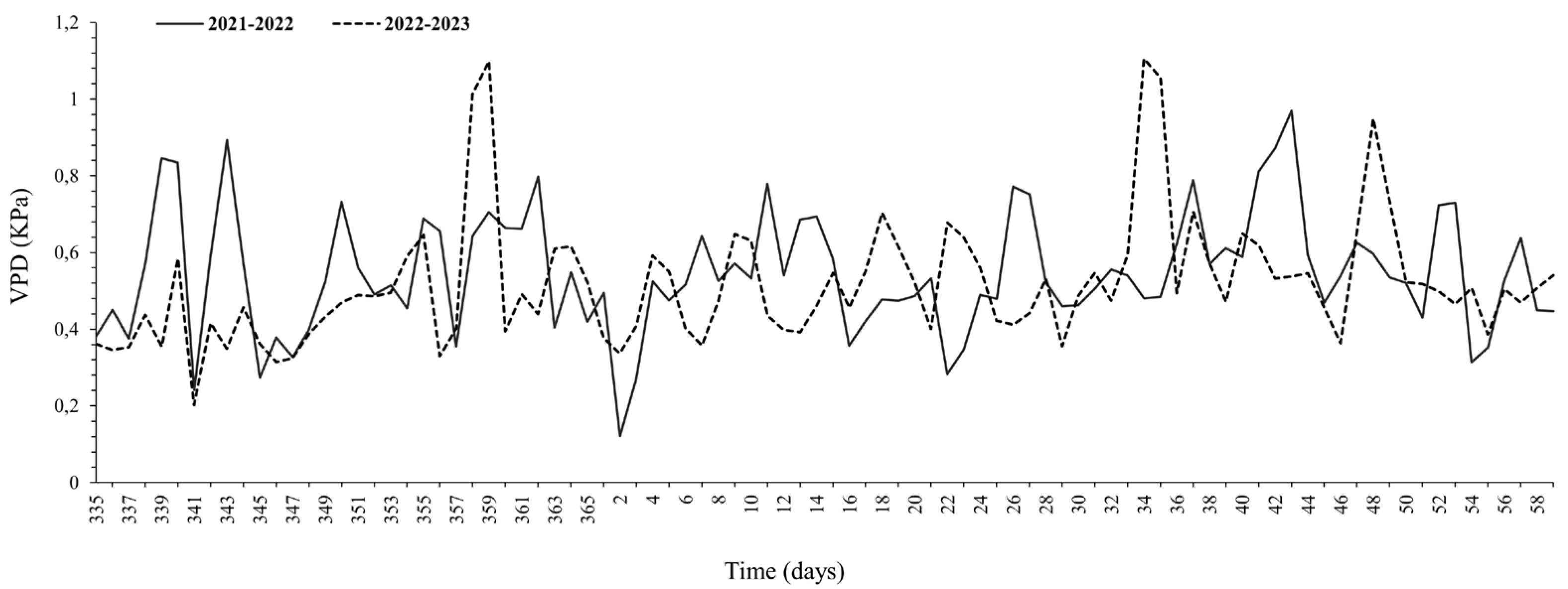
3.1. Meteorological Measurements and Soil Moisture Conditions
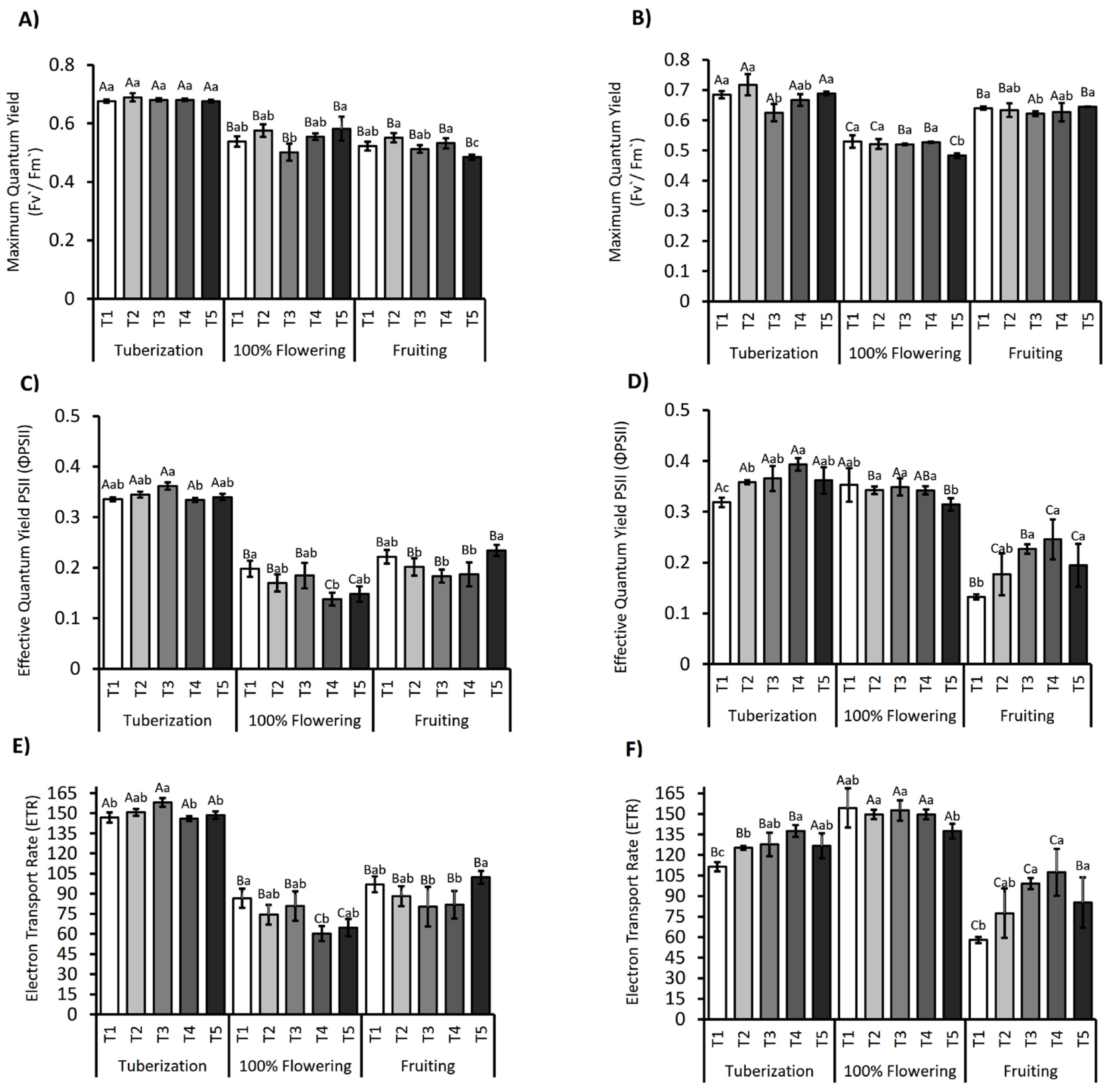
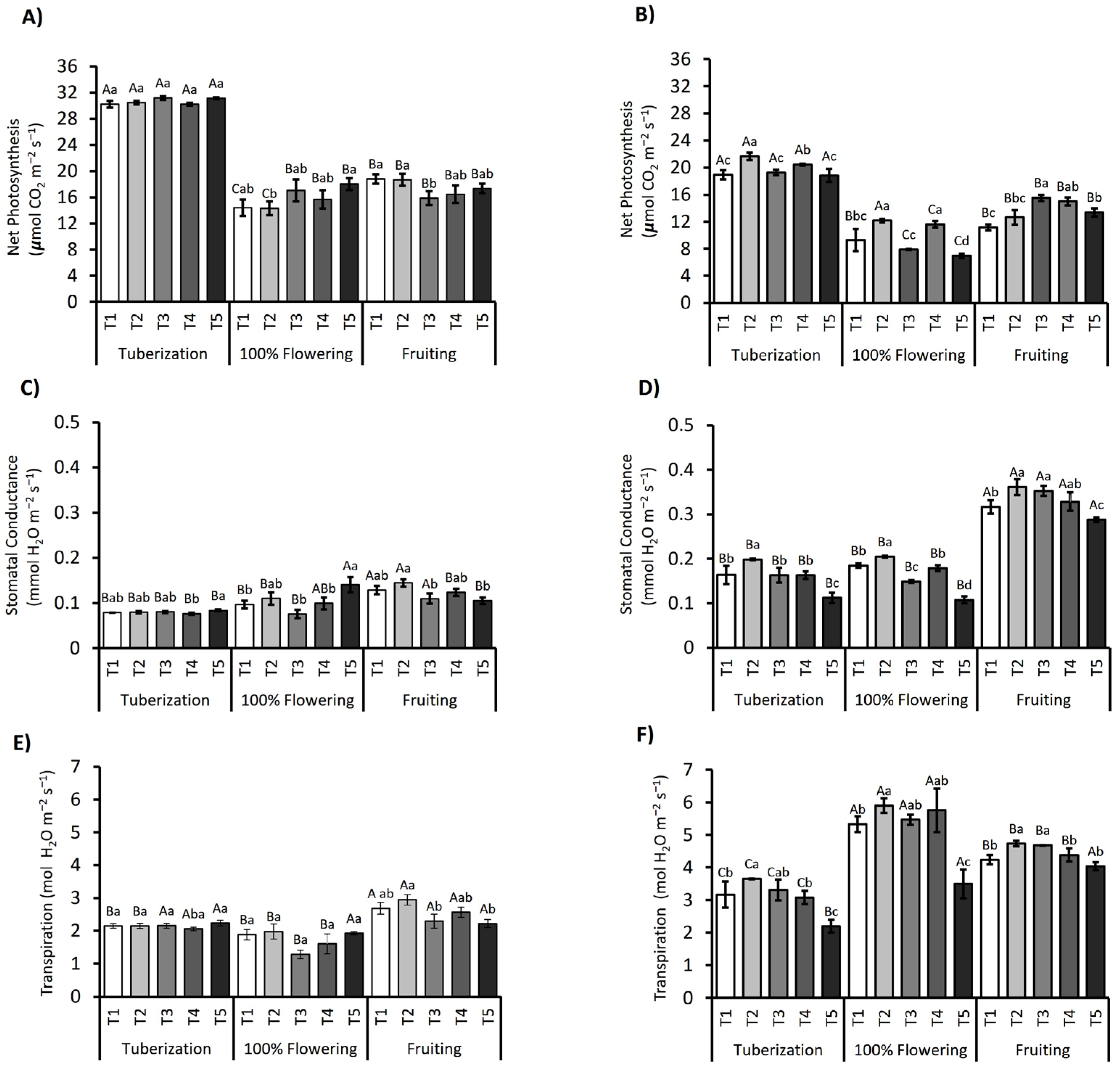
3.2. Photosynthetic Performance in S. tuberosum Subjected to Deficit Irrigation Strategies
3.3. Gas Exchange Measurement
3.4. Photosynthetic Pigments
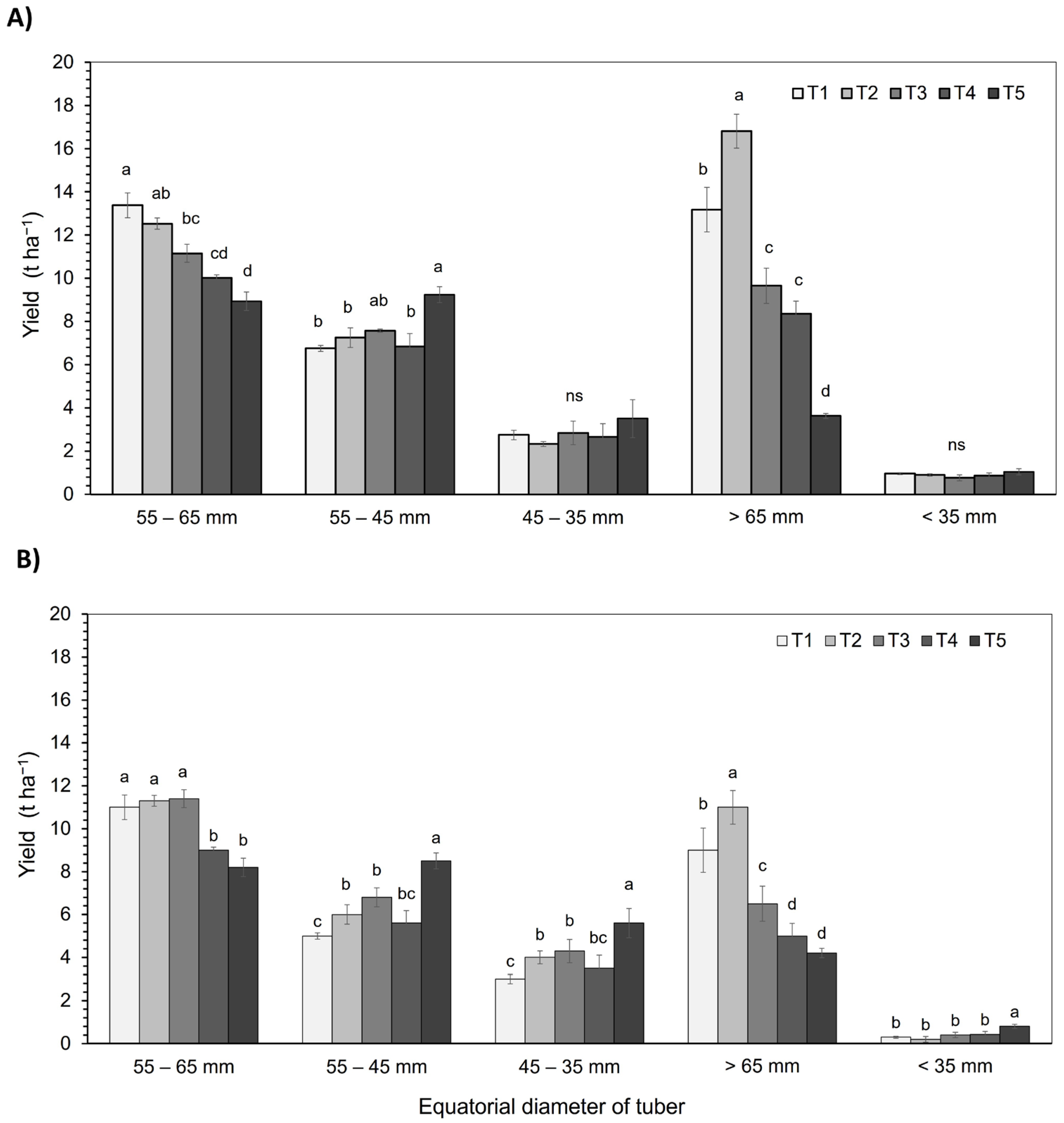
3.5. Yield
4. Discussion
4.1. Physiological Responses and Meteorological Conditions
4.2. Gas Exchange and Photosynthetic Pigments
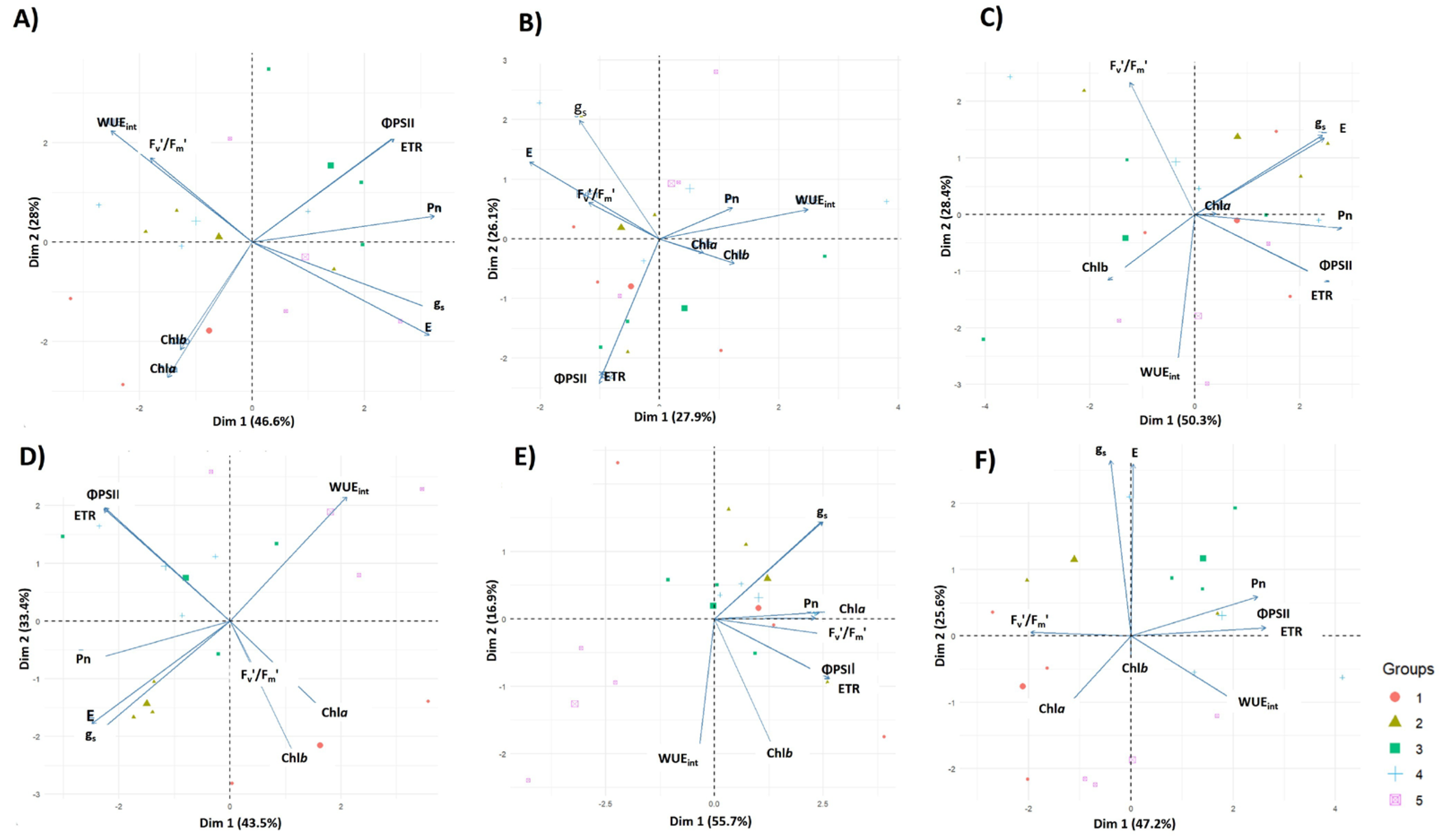
4.3. Principal Component Analysis (PCA)
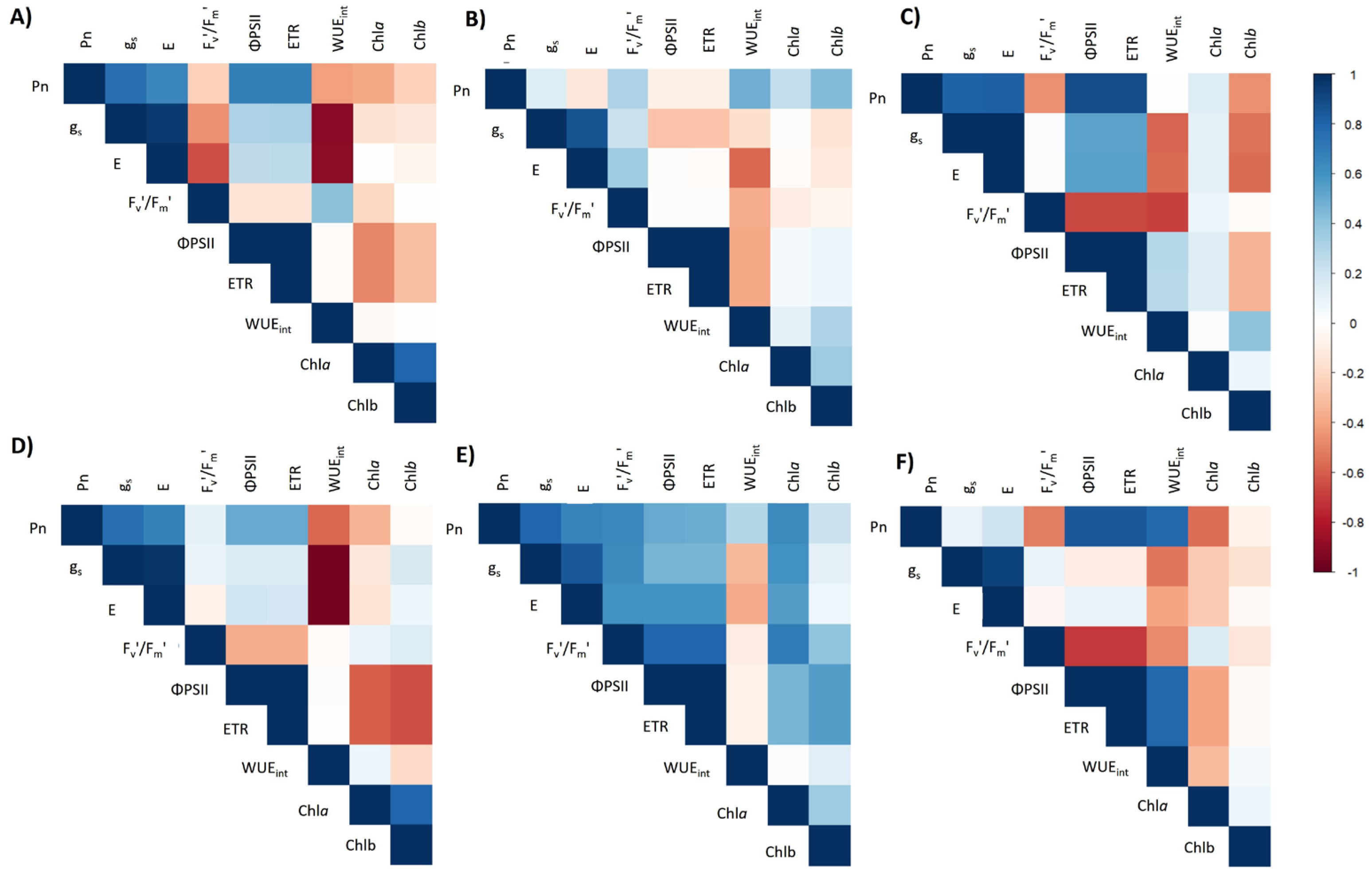
4.4. Correlation Matrix
4.5. Multiple Linear Regression Models
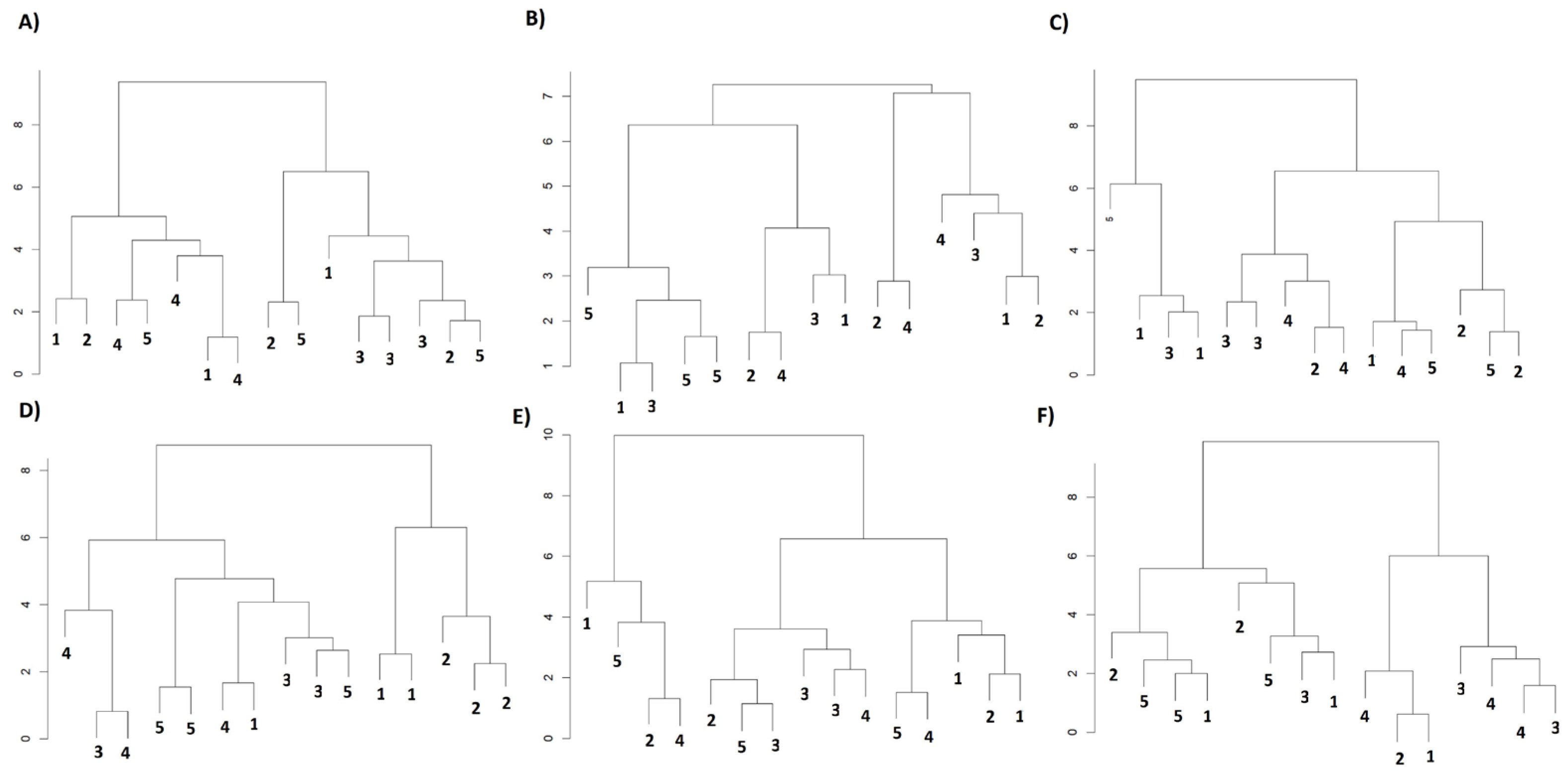
4.6. Hierarchical Clustering Analysis (HCA)
4.7. Yield
4.8. Seasonal Climate Variability and Transferability of the 75% ETc Threshold
5. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| Phenological Stage | T1 | T2 | T3 | T4 | T5 | Effective Rainfall |
|---|---|---|---|---|---|---|
| Season 2022/2022 | ||||||
| Pre-emergence | 20.0 | 26.0 | 15.0 | 10.0 | 6.0 | 0.0 |
| Emergence | 35.2 | 45.8 | 26.4 | 17.6 | 10.6 | 0.0 |
| Vegetative development | 28.6 | 37.2 | 21.5 | 14.3 | 8.6 | 0.0 |
| Tuber initiation | 19.2 | 25.0 | 14.4 | 9.6 | 5.8 | 26.1 |
| Flowering initiation | 45.8 | 59.5 | 34.3 | 22.9 | 13.7 | 26.1 |
| Flowering | 49.3 | 64.1 | 37.0 | 24.6 | 14.8 | 0.0 |
| Fruit formation | 53.1 | 69.0 | 39.8 | 26.6 | 15.9 | 11.3 |
| Fruitandseed | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Senescence | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total | 251.2 | 326.6 | 188.4 | 125.6 | 75.4 | 63.5 |
| Season 2022/2023 | ||||||
| Pre-emergence | 20.0 | 26.0 | 15.0 | 10.0 | 6.0 | 7.1 |
| Emergence | 32.0 | 41.6 | 24.0 | 16.0 | 9.6 | 0.0 |
| Vegetative development | 32.0 | 41.6 | 24.0 | 16.0 | 9.6 | 27.2 |
| Tuber initiation | 36.3 | 47.2 | 27.2 | 18.1 | 10.9 | 0.0 |
| Flowering initiation | 47.3 | 61.5 | 35.5 | 23.7 | 14.2 | 7.0 |
| Flowering | 55.2 | 71.8 | 41.4 | 27.6 | 16.6 | 0.0 |
| Fruit formation | 46.0 | 59.8 | 34.5 | 23.0 | 13.8 | 0.0 |
| Fruit & seed maturation | 13.0 | 16.9 | 9.8 | 6.5 | 3.9 | 0.0 |
| Senescence | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Total | 281.8 | 366.4 | 211.4 | 140.9 | 84.6 | 41.3 |
References
- FAOSTAT. Crops Database: Potato. Available online: https://www.fao.org/faostat (accessed on 10 May 2025).
- Oficina de Estudios y Políticas Agrarias (ODEPA). Boletín de la Papa, March 2024; Ministerio de Agricultura: Santiago, Chile, 2024.
- Food and Agriculture Organization of the United Nations (FAO); International Potato Center (CIP). Global Potato Facts & Figures; FAO–CIP: Rome, Italy, 2022. [Google Scholar]
- Monneveux, P.; Ramírez, D.A.; Pino, M.T. Drought tolerance in potato (Solanum tuberosum L.): Can we learn from research in cereals? Plant Sci. 2013, 205, 76–86. [Google Scholar] [CrossRef] [PubMed]
- van Loon, C.D. The effect of water stress on potato growth, development and yield. Am. Potato J. 1981, 58, 51–69. [Google Scholar] [CrossRef]
- Lahlou, O.; Ledent, J.F. Root mass and depth, stolons and roots formed on stolons in four cultivars of potato under water stress. Eur. J. Agron. 2005, 22, 159–173. [Google Scholar] [CrossRef]
- Nasir, M.W.; Toth, Z. Effect of drought stress on potato production: A review. Agronomy 2022, 12, 635. [Google Scholar] [CrossRef]
- Knox, J.W.; Weatherhead, E.K.; Bradley, R.I. Mapping the total volumetric irrigation water requirements in England and Wales. Agric. Water Manag. 1997, 33, 1–18. [Google Scholar] [CrossRef]
- Byrd, S.A.; Rowland, D.L.; Bennett, J.; Zotarelli, L.; Wright, D.; Alva, A.; Nordgaard, J. Reductions in a commercial potato irrigation schedule during tuber bulking in Florida: Physiological, yield and quality effects. J. Crop Improv. 2014, 28, 660–679. [Google Scholar] [CrossRef]
- Santibáñez, Q. Atlas Agroclimático de Chile. Estado Actual y Tendencias del Clima. Tomo IV: Regiones del Biobío y de La Araucanía; Universidad de Chile, Facultad de Ciencias Agronómicas: Santiago, Chile, 2017. [Google Scholar]
- Dirección General de Aguas (DGA). Informe Hídrico Nacional 2023: Diagnóstico y Proyecciones de la Disponibilidad de Recursos Hídricos en Chile; Ministerio de Obras Públicas: Santiago, Chile, 2023.
- Pourasadollahi, A.; Siosemardeh, A.; Hosseinpanahi, F.; Sohrabi, Y. Physiological and agromorphological response of potato to drought stress and hormone application. J. Plant Physiol. Breed. 2019, 9, 47–61. [Google Scholar] [CrossRef]
- Rudack, K.; Seddig, S.; Sprenger, H.; Köhl, K.; Uptmoor, R.; Ordon, F. Drought-stress-induced changes in starch yield and physiological traits in potato. J. Agron. Crop Sci. 2017, 203, 494–505. [Google Scholar] [CrossRef]
- Ierna, A.; Mauromicale, G. Ecophysiological and productive response of deficit-irrigated potatoes. Agronomy 2023, 13, 591. [Google Scholar] [CrossRef]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture—Not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Jadoski, S.O.; Suchoronczek, A.; dos Santos, J. Effect of water deficit on vegetative development, production and physiological disorders on ‘Agata’ potato tubers. Appl. Res. Agrotechnol. 2017, 10, 97–107. [Google Scholar]
- Díaz-Valencia, P.; Melgarejo, L.M.; Arcila, I.; Mosquera-Vásquez, T. Physiological, biochemical and yield-component responses of Solanum tuberosum L. Group Phureja genotypes to water deficit. Plants 2021, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kupriyanovich, Y.; Wagg, C.; Zheng, F.; Hann, S. Water deficit duration affects potato plant growth, yield and tuber quality. Agriculture 2023, 13, 2007. [Google Scholar] [CrossRef]
- Deblonde, P.M.K.; Ledent, J.F. Effects of moderate drought conditions on green-leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur. J. Agron. 2001, 14, 31–41. [Google Scholar] [CrossRef]
- Bijanzadeh, E.; Barati, V.; Emam, Y.; Pessarakli, M. Assessment of the crop water stress index and color quality of bur clover (Medicago polymorpha L.) under different irrigation regimes. Commun. Soil Sci. Plant Anal. 2019, 50, 2825–2835. [Google Scholar] [CrossRef]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.-L.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated deficit irrigation for crop production under drought stress: A review. Agron. Sustain. Dev. 2016, 36, 3. [Google Scholar] [CrossRef]
- Kalazich, J.; Uribe, M.; Santos, J.; López, H.; Acuña, I.; Orena, S.; Catalán, P.; Winkler, A. Puyehue-INIA: Variedad de Papa de Alto Rendimiento, Piel Roja, Pulpa Amarilla y Gran Calidad Para Consumo Fresco; Informativo INIA Remehue, No. 111; Instituto de Investigaciones Agropecuarias (INIA), Centro Regional de Investigación Remehue: Osorno, Chile, 2013; Available online: https://hdl.handle.net/20.500.14001/4765 (accessed on 10 May 2025).
- Centro de Información de Recursos Naturales (CIREN). Descripciones de Suelos, Materiales y Símbolos. Estudio Agrológico IX Región; Publicación 122; CIREN: Santiago, Chile, 2002. [Google Scholar]
- Hack, H.; Gall, H.; Klemke, T.; Klose, R.; Meier, U.; Stauss, R.; Witzenberger, A. Phänologische Entwicklungsstadien der Kartoffel (Solanum tuberosum L.) nach der erweiterten BBCH-Skala. Nachrbl. Dtsch. Pflanzenschutzd. 1993, 45, 11–19. [Google Scholar]
- Allen, R.; Pereira, L.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- López-Olivari, R.; Fuentes, S.; Poblete-Echeverría, C.; Quintulen-Ancapi, V.; Medina, L. Site-specific evaluation of canopy resistance models for estimating evapotranspiration over a drip-irrigated potato crop in southern Chile under water-limited conditions. Water 2022, 14, 2041. [Google Scholar] [CrossRef]
- Suleiman, A.A.; Tojo Soler, C.M.; Hoogenboom, J. Evaluation of FAO-56 crop-coefficient procedures for deficit-irrigation management of cotton in a humid climate. Agric. Water Manag. 2007, 91, 33–42. [Google Scholar] [CrossRef]
- Taghvaeian, S.; Chávez, J.L.; Bausch, W.C.; DeJonge, K.C.; Trout, T.J. Minimizing instrumentation requirements for estimating crop water stress index and transpiration of maize. Irrig. Sci. 2014, 32, 53–65. [Google Scholar] [CrossRef]
- López-Olivari, R.; Ortega-Klose, F. Response of red clover to deficit irrigation: Dry-matter yield, populations and irrigation-water-use efficiency in southern Chile. Irrig. Sci. 2021, 39, 173–189. [Google Scholar] [CrossRef]
- Logan, B.A.; Adams, W.W.; Demmig-Adams, B. Avoiding common pitfalls of chlorophyll-fluorescence analysis under field conditions. Funct. Plant Biol. 2007, 34, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon-isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Hay, R.K.M.; Walker, A.J. An Introduction to the Physiology of Crop Yield; Longman Scientific & Technical: Harlow, UK, 1989; p. 292. [Google Scholar]
- Steckel, J.R.; Gray, D. Drought tolerance in potatoes. J. Agric. Sci. 1979, 92, 375–381. [Google Scholar] [CrossRef]
- Wurr, D.C.E. Seed production and management. In The Potato Crop; Harris, P.M., Ed.; Chapman & Hall: London, UK, 1978; pp. 327–354. [Google Scholar] [CrossRef]
- Liu, F.; Jensen, C.R.; Shahanzari, A.; Andersen, M.N.; Jacobsen, S.E. ABA-regulated stomatal control and photosynthetic water-use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005, 168, 831–836. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, X.; Manevski, K.; Li, S.; Wei, Z.; Andersen, M.N.; Liu, F. Physiological and growth responses of potato (Solanum tuberosum L.) to air temperature and relative humidity under soil-water deficits. Plants 2022, 11, 1126. [Google Scholar] [CrossRef]
- Jefferies, R.A. Effects of drought on chlorophyll fluorescence in potato (Solanum tuberosum L.). II. Relations between plant growth and fluorescence. Potato Res. 1992, 35, 35–40. [Google Scholar] [CrossRef]
- Jefferies, R.A. Responses of potato genotypes to drought. I. Expansion of individual leaves and osmotic adjustment. Ann. Appl. Biol. 1993, 122, 93–104. [Google Scholar] [CrossRef]
- Jefferies, R.A.; MacKerron, D.K.L. Responses of potato genotypes to drought. II. Leaf-area index, growth and yield. Ann. Appl. Biol. 1993, 122, 105–112. [Google Scholar] [CrossRef]
- Fang, G.; Yang, S.; Ruan, B.; Ye, G.; He, M.; Su, W.; Zhou, Y.; Wang, J.; Yang, S. Research progress on physiological, biochemical and molecular mechanisms of potato in response to drought and high temperature. Horticulturae 2024, 10, 827. [Google Scholar] [CrossRef]
- Alvarez-Morezuelas, A.; Barandalla, L.; Ritter, E.; Ruiz de Galarreta, J.I. Genome-wide association study of agronomic and physiological traits related to drought tolerance in potato. Plants 2023, 12, 734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, X.; Xue, D.; Zhang, W.; Li, F.; Teng, A.; Zhang, C.; Lei, L.; Ba, Y. Dry-matter accumulation, water productivity and quality of potato in response to regulated deficit irrigation in a desert-oasis region. Plants 2024, 13, 1927. [Google Scholar] [CrossRef]
- García-Martínez, S.; López-Bonilla, L.; Ruiz-García, G. Deficit irrigation response and climate resilience of Mediterranean tomato landraces. Horticulturae 2024, 11, 74. [Google Scholar] [CrossRef]
- Tadele, S.; Tesfaye, A.; Dejene, T. Tomato yield and water-use efficiency as affected by nitrogen rate and irrigation regime. Sci. Rep. 2024, 14, 62884. [Google Scholar] [CrossRef]
- FAO. Crop Water Requirements of Potato: Guidelines for High Yields (120–150 Days); Irrigation and Drainage Paper 56; FAO: Rome, Italy, 2024. [Google Scholar]
- Hanson, B.R.; May, D.M. Crop evapotranspiration of processing tomato in the San Joaquin Valley of California, USA. Irrig. Sci. 2006, 24, 211–221. [Google Scholar] [CrossRef]
- Li, S.; Liu, F. Vapour-pressure deficit and endogenous ABA level modulate stomatal responses of tomato plants to soil-water deficit. Environ. Exp. Bot. 2022, 199, 104889. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll-fluorescence quenching during photoinhibition. In Photoinhibition; Kyle, D.J., Osmond, C.B., Arntzen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 137–152. [Google Scholar]
- Baker, N.R.; Bowyer, J.R. (Eds.) Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field; BIOS Scientific Publishers: Oxford, UK, 1994; 471p. [Google Scholar]
- Alves, P.L.D.C.; Magalhães, A.C.; Barja, P.R. The phenomenon of photoinhibition of photosynthesis and its importance in reforestation. Bot. Rev. 2002, 68, 193–208. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of plant responses to water stress and related genes: A review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Wang, S.; Hu, J.; Ren, B.; Liu, P.; Zhao, B.; Zhang, J. Effects of hydrogen-peroxide priming on yield, photosynthetic capacity and chlorophyll fluorescence of waterlogged summer maize. Front. Plant Sci. 2022, 13, 1042920. [Google Scholar] [CrossRef] [PubMed]
- Rolando, J.L.; Ramírez, D.A.; Yactayo, W.; Monneveux, P.; Quiroz, R. Leaf greenness as a drought-tolerance-related trait in potato (Solanum tuberosum L.). Environ. Exp. Bot. 2015, 110, 27–35. [Google Scholar] [CrossRef]
- Ghobadi, M.; Taherabadi, S.; Ghobadi, M.E.; Mohammadi, G.R.; Jalali-Honarmand, S. Antioxidant capacity, photosynthetic characteristics and water relations of sunflower (Helianthus annuus L.) cultivars in response to drought stress. Ind. Crops Prod. 2013, 50, 29–38. [Google Scholar] [CrossRef]
- Cho, K.S.; Han, E.H.; Kwak, S.S.; Cho, J.H.; Im, J.S.; Hong, S.Y.; Sohn, H.B.; Kim, Y.H.; Lee, S.W. Expressing the sweet-potato orange gene in transgenic potato improves drought tolerance and marketable tuber production. Comptes Rendus Biol. 2016, 339, 207–213. [Google Scholar] [CrossRef]
- Caliandro, R.; Nagel, K.A.; Kastenholz, B.; Bassi, R.; Li, Z.; Niyogi, K.K.; Pogson, B.J.; Schurr, U.; Matsubara, S. Effects of altered α- and β-branch carotenoid biosynthesis on photoprotection and whole-plant acclimation of Arabidopsis to photo-oxidative stress. Plant Cell Environ. 2013, 36, 438–453. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, L.; Ñústez, C.E.; Moreno, L.P. Drought stress affects physiological parameters but not tuber yield in three Andean potato (Solanum tuberosum L.) cultivars. Agron. Colomb. 2017, 35, 158–170. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef]
- Orsák, M.; Kotíková, Z.; Hnilička, F.; Lachman, J. Effect of long-term drought and waterlogging stress on photosynthetic pigments in potato. Plant Soil Environ. 2023, 69, 152–160. [Google Scholar] [CrossRef]
- Da Ros, L.; Elferjani, R.; Soolanayakanahally, R.; Kagale, S.; Pahari, S.; Kulkarni, M.; Wahab, J.; Bizimungu, B. Drought-induced regulatory cascades and their effects on the nutritional quality of developing potato tubers. Genes 2020, 11, 864. [Google Scholar] [CrossRef]
- Brown, C.R.; Haynes, K.G. Genetic variation for carotenoid content in cultivated potato. Am. J. Potato Res. 2007, 84, 365–372. [Google Scholar]
- Andre, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Alvarado, C.; Nomberto, G.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y.; Evers, D. Modification of the health-promoting value of potato tubers field-grown under drought stress: Emphasis on dietary antioxidant and glycoalkaloid contents in five Andean cultivars (Solanum tuberosum L.). J. Agric. Food Chem. 2009, 57, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Li, F.; Bai, Y.; Shen, Y.; Kang, S.; Zhang, Y.; Tong, L.; Sun, H. Deficit irrigation as an effective way to increase potato water use efficiency in Northern China: A meta-analysis. Agronomy 2024, 14, 1533. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Andersen, M.N.; Plauborg, F.; Jensen, C.R. Effects of partial root-zone drying and deficit irrigation on field-grown potato: Yield, tuber size distribution and water-use efficiency. Field Crops Res. 2010, 118, 14–23. [Google Scholar]
- Karam, F.; Sarkis, K.; Lahoud, R.; Masaad, R.; Rouphael, Y. Yield and water-use efficiency of drip-irrigated potato under different irrigation regimes in a semi-arid Mediterranean environment. Agric. Water Manag. 2014, 134, 126–136. [Google Scholar]
- Dalla Costa, L.; Delle Vedove, G.; Gianquinto, G.; Giovanardi, R.; Peressotti, A. Yield, water-use efficiency and nitrogen uptake in potato: Influence of drought stress. Potato Res. 1997, 40, 19–34. [Google Scholar] [CrossRef]
- Manna, T.; Nanda, M.K.; Sarkar, S.; Mukherjee, A.; Ray, M.; Alkeridis, L.A.; Sayed, S.; Gaber, A.; Hossain, A. Infrared-thermometry-based stress indices as indicators of yield performance and seasonal evapotranspiration in potato under different moisture and potassium regimes. Sci. Hortic. 2024, 330, 113086. [Google Scholar] [CrossRef]
- Svoboda, P.; Raimanová, I.; Duffková, R.; Fučík, P.; Kurešová, G.; Haberle, J. The effects of irrigation on root density profiles of potato, celery, and wheat. Agron. Res. 2020, 18, 567–578. [Google Scholar] [CrossRef]
- Evers, D.; Lefèvre, I.; Legay, S.; Lamoureux, D.; Hausman, J.-F.; Gutiérrez Rosales, R.O.; Tincopa Marca, L.R.; Hoffmann, L.; Bonierbale, M.; Schafleitner, R. Identification of drought-responsive compounds in potato through a combined transcriptomic and targeted metabolite approach. J. Exp. Bot. 2010, 61, 2327–2343. [Google Scholar] [CrossRef]
- Puértolas, J.; Ballester, C.; Elphinstone, E.D.; Dodd, I.C. Two potato (Solanum tuberosum) varieties differ in drought tolerance due to differences in root growth at depth. Funct. Plant Biol. 2014, 41, 1107–1118. [Google Scholar] [CrossRef]
- Dodd, I.C.; Theobald, J.C.; Bacon, M.A.; Davies, W.J. Alternation of wet and dry sides during partial root-zone drying irrigation alters root-to-shoot signalling of abscisic acid. Funct. Plant Biol. 2006, 33, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Fabeiro, C.M.; de Santa Olalla, F.M.; De Juan, J.A. Yield and size of deficit-irrigated potatoes. Agric. Water Manag. 2001, 48, 255–266. [Google Scholar] [CrossRef]
- Pawar, D.D.; Dingre, S.K. Yield and quality attributes of potato (Solanum tuberosum L.) under different irrigation methods and regimes. J. Nat. Resour. Conserv. Manag. 2020, 1, 151–156. [Google Scholar] [CrossRef]
- Djaman, K.; Irmak, S.; Koudahe, K.; Allen, S. Irrigation management in potato (Solanum tuberosum L.) production: A review. Sustainability 2021, 13, 1504. [Google Scholar] [CrossRef]
- Patel, J.C.; Patel, B.K. Response of potato to microsprinkler and furrow irrigation. J. Indian Potato Assoc. 2000, 27, 145–146. [Google Scholar]
- Mahmud, A.A. Improvement of Drought-Tolerant Potato Variety. Ph.D. Thesis, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh, 2012. [Google Scholar]
- Romero, A.P.; Alarcón, A.; Valbuena, R.I.; Galeano, C.H. Physiological assessment of water stress in potato using spectral information. Front. Plant Sci. 2017, 8, 1608. [Google Scholar] [CrossRef]
- Schittenhelm, S.; Sourell, H.; Löpmeier, F.J. Drought resistance of potato cultivars with contrasting canopy architecture. Eur. J. Agron. 2006, 24, 193–202. [Google Scholar] [CrossRef]
- Badr, M.A.; Abou Hussein, S.D.; El-Tohamy, W.A.; Gruda, N. Efficiency of subsurface drip irrigation for potato production under different dry-stress conditions. Gesunde Pflanz. 2010, 62, 63–70. [Google Scholar] [CrossRef]
- Yactayo, W.; Ramírez, D.A.; Gutiérrez, R.; Mares, V.; Posadas, A.; Quiroz, R. Effect of partial root-zone-drying irrigation timing on potato tuber yield and water-use efficiency. Agric. Water Manag. 2013, 123, 65–70. [Google Scholar] [CrossRef]
- Sprenger, H.; Kurowsky, C.; Horn, R.; Erban, A.; Seddig, S.; Rudack, K.; Fisher, A.; Walther, D.; Zuther, E.; Köhl, K.; et al. The drought response of potato reference cultivars with contrasting tolerance. Plant Cell Environ. 2016, 39, 2370–2389. [Google Scholar] [CrossRef]
- Al-Yahyai, R.; Al-Kharusi, L. Sub-optimal irrigation affects chemical quality attributes of dates during fruit development. Afr. J. Agric. Res. 2012, 7, 1498–1503. [Google Scholar] [CrossRef]
- Yang, W.; Kang, Y.; Feng, Z.; Gu, P.; Wen, H.; Liu, L.; Jia, Y. Sprinkler irrigation reduces nitrous-oxide emissions from an arid-region potato field: A two-year study. Atmosphere 2019, 10, 242. [Google Scholar] [CrossRef]
- Danielescu, S.; Zarei, A.; Mann, P.; Struik, P.C.; Jiang, Y.; MacKinnon, K.; Zilahi-Sebess, L. Crop water deficit and supplemental irrigation requirements for potato production in a temperate humid region (Prince Edward Island, Canada). Water 2022, 14, 2748. [Google Scholar] [CrossRef]
- Jiang, P.; Li, T.; Gao, G.; Wang, Y.; Sun, B.; Luo, Z.; Dong, R. Long-term potato response to different irrigation scheduling methods under arid conditions. Front. Agron. 2024, 6, 1426034. [Google Scholar]
- Qiao, X.; Lu, C.; Zhang, D.; Li, Y.; Sun, M.; Wang, J.; Zhang, J. Effects of deficit irrigation on potato yield and water productivity in Northeast China. PLOS Water 2024, 3, e0000266. [Google Scholar]
- Zhang, R.; Wei, W.; Wang, Q.; Li, X.; Zhou, W.; Yang, Y.; Liu, C.; Fang, Z. Shallow burial of drip irrigation tape improves water-use efficiency of potatoes in semiarid regions. Sci. Rep. 2025, 15, 89295. [Google Scholar]

| 2021/2022 Season | 2022/2023 Season | |||||
|---|---|---|---|---|---|---|
| Phenological State | Height | Irrigation | Effective Rainfall | Height | Irrigation | Effective Rainfall |
| (m) | (mm) | (mm) | (m) | (mm) | (mm) | |
| Pre-emergence | 0 | 20.0 | 0 | 0 | 20.0 | 7.1 |
| Emergence | 0.1 | 35.2 | 0 | 0.1 | 32.0 | 0 |
| Vegetative development | 0.21 | 28.6 | 0 | 0.22 | 32.0 | 27.2 |
| Tuber initiation | 0.45 | 19.2 | 26.1 | 0.4 | 36.3 | 0 |
| Flowering initiation | 0.67 | 45.8 | 26.1 | 0.5 | 47.3 | 7.0 |
| Flowering | 0.57 | 49.3 | 0 | 0.56 | 55.2 | 0 |
| Fruit formation | 0.4 | 53.1 | 11.32 | 0.5 | 46.0 | 0 |
| Fruit and seed maturation | 0.45 | 0 | 0 | 0.5 | 13 | 0 |
| Senescence | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 251.2 | 63.5 | 281.5 | 41.3 | ||
| Total water | 314.7 | 322.8 | ||||
| Treatment | Collection of Plant Material (2021/2022) | |||||
|---|---|---|---|---|---|---|
| Tuberization | Peak Flowering | Fruiting | ||||
| Average | Standard Error | Average | Standard Error | Average | Standard Error | |
| Chlorophyll a content (mg g−1 DW) | ||||||
| T1 | 0.64 ± 0.02 Aa | 0.55 ± 0.02 Bab | 0.44 ± 0.05 Cab | |||
| T2 | 0.55 ± 0.01 Ab | 0.55 ± 0.03 Aab | 0.45 ± 0.03 Ba | |||
| T3 | 0.52 ± 0.01 Ab | 0.54 ± 0.01 Aab | 0.35 ± 0.05 Bbc | |||
| T4 | 0.55 ± 0.00 Ab | 0.58 ± 0.02 Aa | 0.33 ± 0.07 Bc | |||
| T5 | 0.57 ± 0.02 Aab | 0.48 ± 0.01 Bb | 0.44 ± 0.02 Bab | |||
| Chlorophyll b content (mg g−1 DW) | ||||||
| T1 | 0.26 ± 0.00 Aa | 0.22 ± 0.01 ABa | 0.19 ± 0.02 Ba | |||
| T2 | 0.24 ± 0.01 Aab | 0.22 ± 0.01 ABa | 0.18 ± 0.01 Ba | |||
| T3 | 0.22 ± 0.01 Ab | 0.21 ± 0.01 Aa | 0.21 ± 0.05 Aa | |||
| T4 | 0.22 ± 0.01 Aab | 0.21 ± 0.01 Aa | 0.16 ± 0.01 Ba | |||
| T5 | 0.23 ± 0.00 Aab | 0.22 ± 0.01 Aa | 0.19 ± 0.00 Bab | |||
| Carotenoid content (mg g−1 DW) | ||||||
| T1 | 1.16 ± 0.04 Aa | 1.08 ± 0.07 Aba | 0.87 ± 0.05 Bab | |||
| T2 | 1.03 ± 0.03 Aa | 1.05 ± 0.07 Aab | 0.88 ± 0.03 Aa | |||
| T3 | 0.99 ± 0.03 Aa | 1.09 ± 0.04 Aa | 0.77 ± 0.05 Bab | |||
| T4 | 0.95 ± 0.10 Aa | 1.14 ± 0.05 ABa | 0.64 ± 0.07 Bb | |||
| T5 | 1.05 ± 0.01 Aa | 0.93 ± 0.06 Ab | 0.88 ± 0.02 Bab | |||
| Collection of plant material (2022/2023) | ||||||
| Chlorophyll a content (mg g−1 DW) | ||||||
| T1 | 0.65 ± 0.050 Ba | 0.70 ± 0.027 Aa | 0.60 ± 0.049 Ba | |||
| T2 | 0.60 ± 0.019 Bab | 0.65 ± 0.040 Aab | 0.55 ± 0.029 Ba | |||
| T3 | 0.62 ± 0.012 Ba | 0.68 ± 0.013 Aa | 0.58 ± 0.049 Ba | |||
| T4 | 0.58 ± 0.017 Ab | 0.60 ± 0.030 Abc | 0.52 ± 0.063 Aab | |||
| T5 | 0.50 ± 0.041 ABc | 0.55 ± 0.020 Ac | 0.45 ± 0.028 Bb | |||
| Chlorophyll b content (mg g−1 DW) | ||||||
| T1 | 0.30 ± 0.021 Ba | 0.35 ± 0.010 Aa | 0.28 ± 0.019 Ba | |||
| T2 | 0.28 ± 0.019 Abab | 0.32 ± 0.017 Ab | 0.25 ± 0.016 Bab | |||
| T3 | 0.30 ± 0.019 Aa | 0.33 ± 0.018 Aab | 0.27 ± 0.023 Aab | |||
| T4 | 0.27 ± 0.010 Bb | 0.30 ± 0.009 Ab | 0.24 ± 0.019 Bb | |||
| T5 | 0.22 ± 0.009 Bc | 0.25 ± 0.009 Ac | 0.20 ± 0.018 Bc | |||
| Carotenoid content (mg g−1 DW) | ||||||
| T1 | 1.20 ± 0.039 Ab | 1.30 ± 0.068 Abc | 1.15 ± 0.049 Ab | |||
| T2 | 1.15 ± 0.025 Ab | 1.20 ± 0.065 Ac | 1.20 ± 0.035 Ab | |||
| T3 | 1.25 ± 0.031 Bab | 1.40 ± 0.035 Ab | 1.20 ± 0.051 Bb | |||
| T4 | 1.30 ± 0.099 Bab | 1.45 ± 0.047 Aab | 1.35 ± 0.072 ABa | |||
| T5 | 1.30 ± 0.009 Ba | 1.50 ±0.063 Aa | 1.40 ± 0.018 Ba | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Sanhueza, R.; Tighe-Neira, R.; López-Olivari, R.; Inostroza-Blancheteau, C. Assessment of Different Irrigation Thresholds to Optimize the Water Use Efficiency and Yield of Potato (Solanum tuberosum L.) Under Field Conditions. Plants 2025, 14, 1734. https://doi.org/10.3390/plants14111734
Mora-Sanhueza R, Tighe-Neira R, López-Olivari R, Inostroza-Blancheteau C. Assessment of Different Irrigation Thresholds to Optimize the Water Use Efficiency and Yield of Potato (Solanum tuberosum L.) Under Field Conditions. Plants. 2025; 14(11):1734. https://doi.org/10.3390/plants14111734
Chicago/Turabian StyleMora-Sanhueza, Rodrigo, Ricardo Tighe-Neira, Rafael López-Olivari, and Claudio Inostroza-Blancheteau. 2025. "Assessment of Different Irrigation Thresholds to Optimize the Water Use Efficiency and Yield of Potato (Solanum tuberosum L.) Under Field Conditions" Plants 14, no. 11: 1734. https://doi.org/10.3390/plants14111734
APA StyleMora-Sanhueza, R., Tighe-Neira, R., López-Olivari, R., & Inostroza-Blancheteau, C. (2025). Assessment of Different Irrigation Thresholds to Optimize the Water Use Efficiency and Yield of Potato (Solanum tuberosum L.) Under Field Conditions. Plants, 14(11), 1734. https://doi.org/10.3390/plants14111734








