Enhanced Stress Tolerance in Rice Through Overexpression of a Chimeric Glycerol-3-Phosphate Dehydrogenase (OEGD)
Abstract
1. Introduction
2. Results
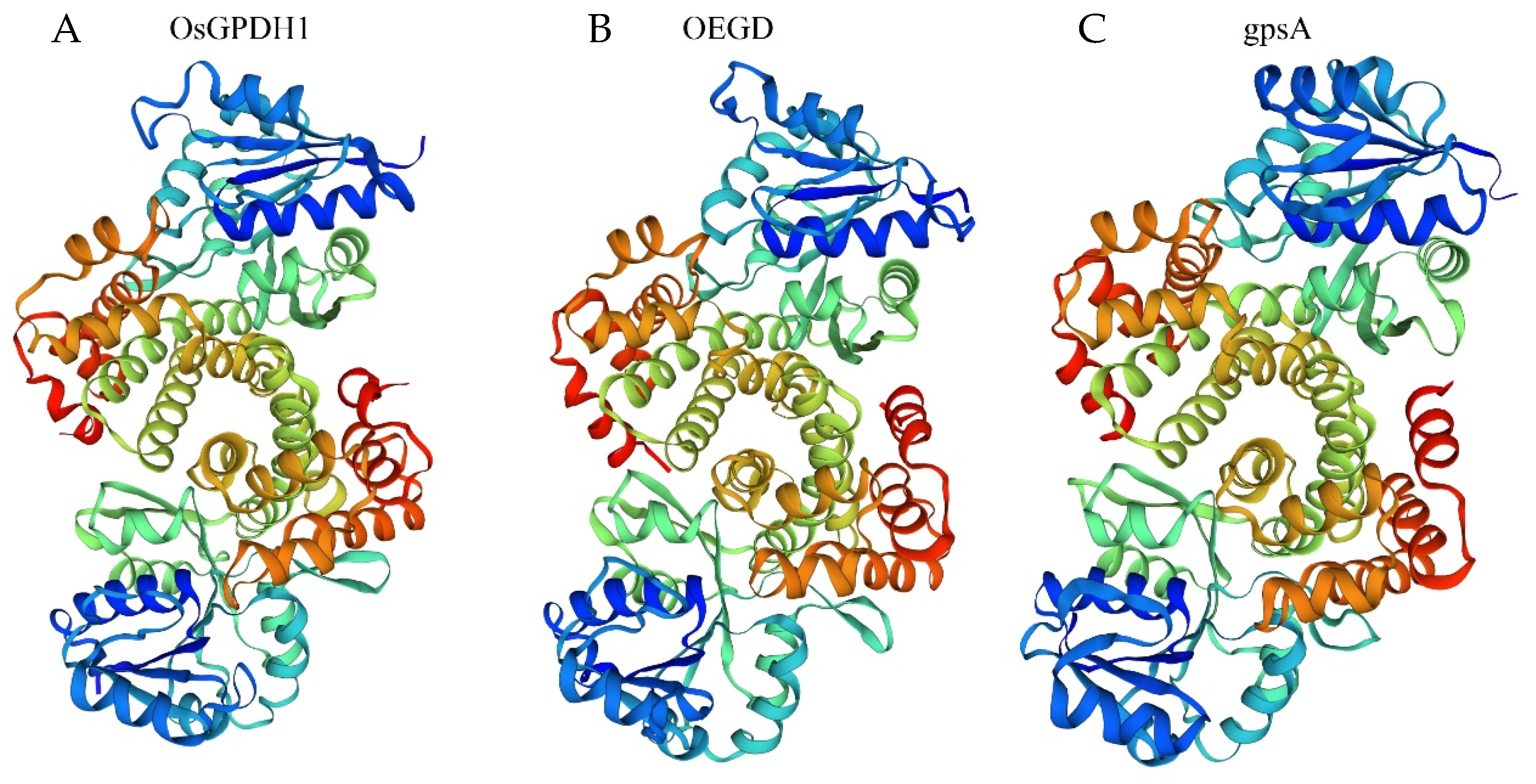
2.1. OEGD Design and Molecular Characterization
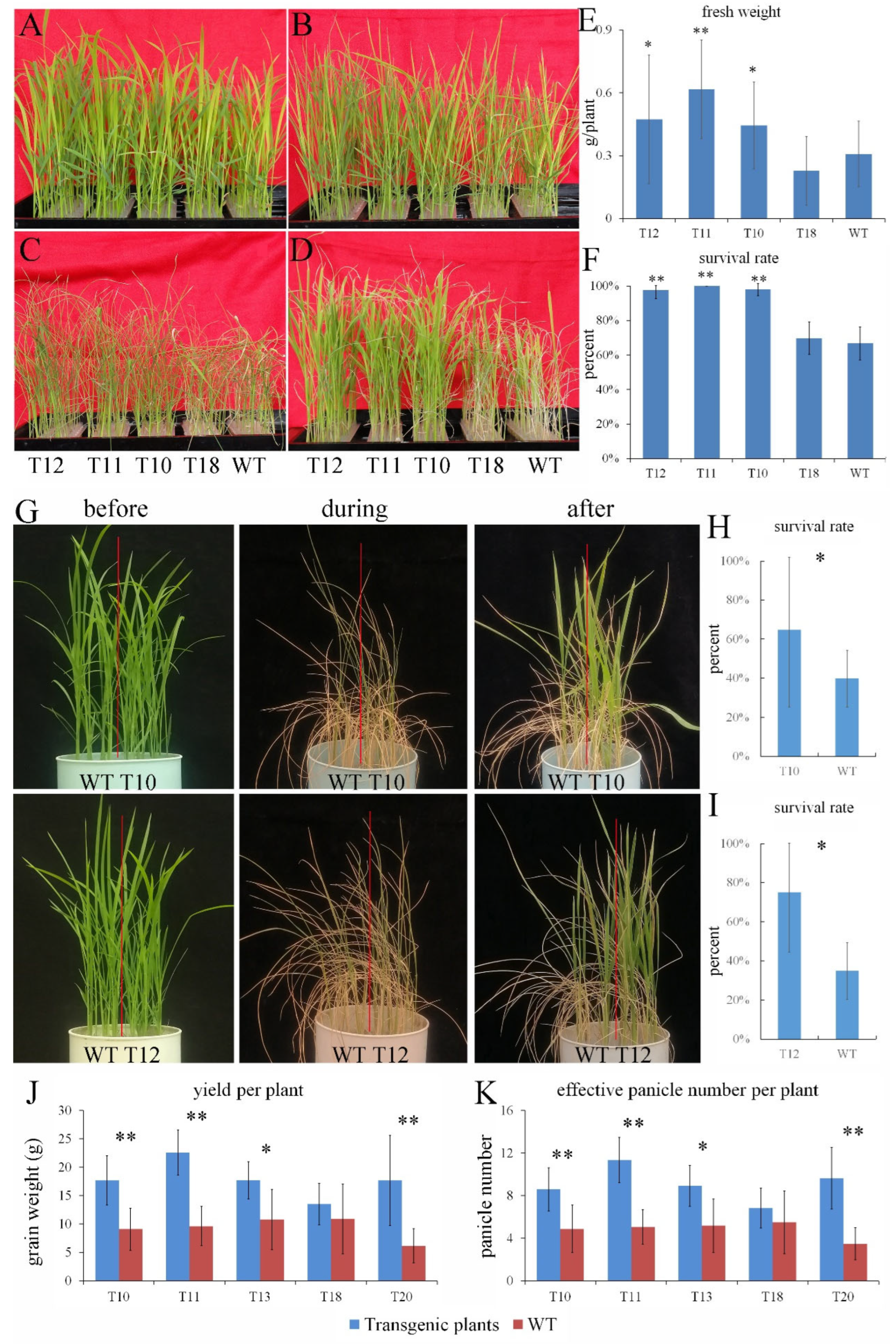
2.2. Transgenic Rice Plants of OEGD
2.3. Overexpression of OEGD Enhanced Tolerance to Drought Stress in Rice
2.4. Overexpression of OEGD Enhanced Tolerance to Low Phosphorus, Heat, and Cd2+ Stress in Rice
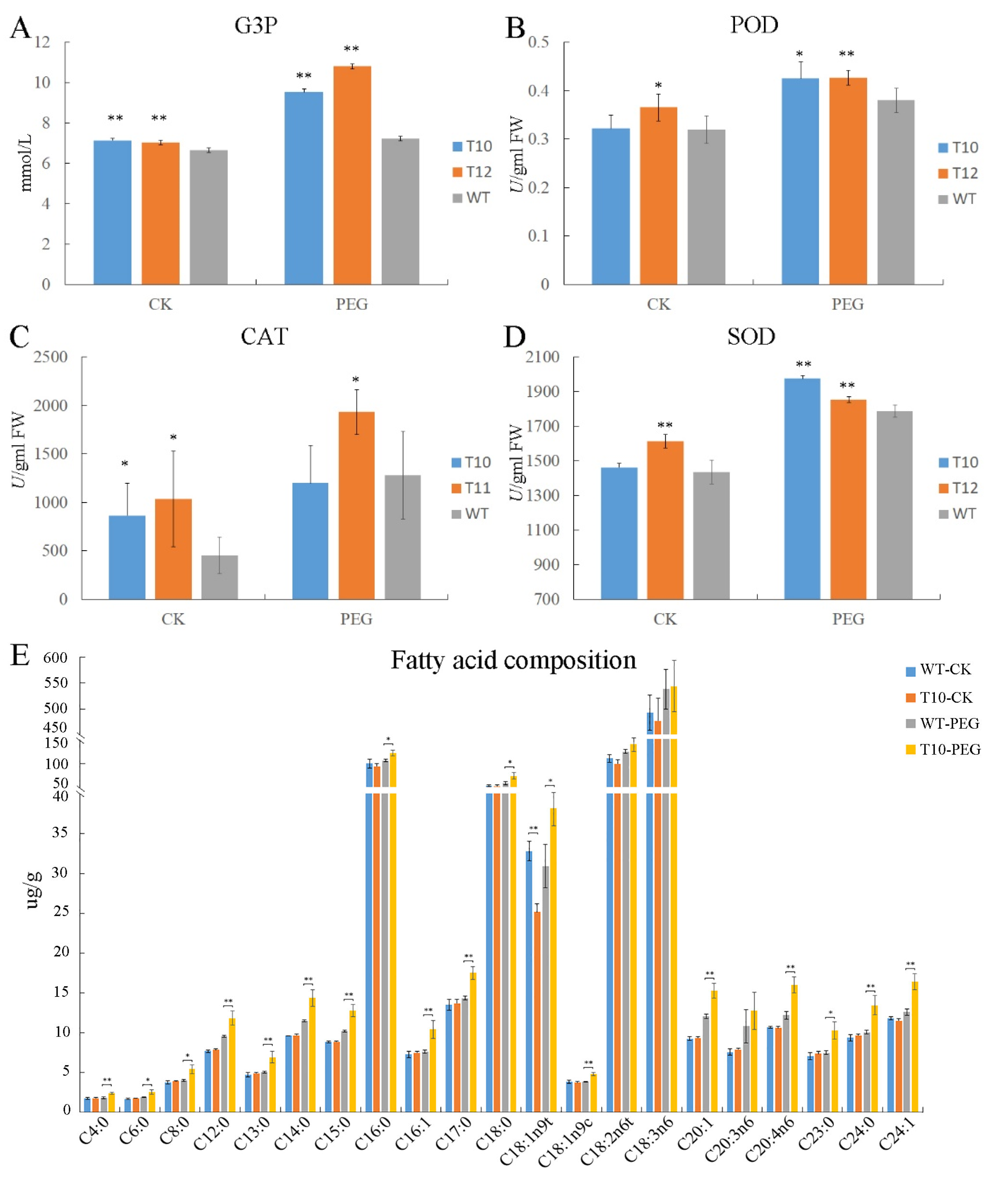
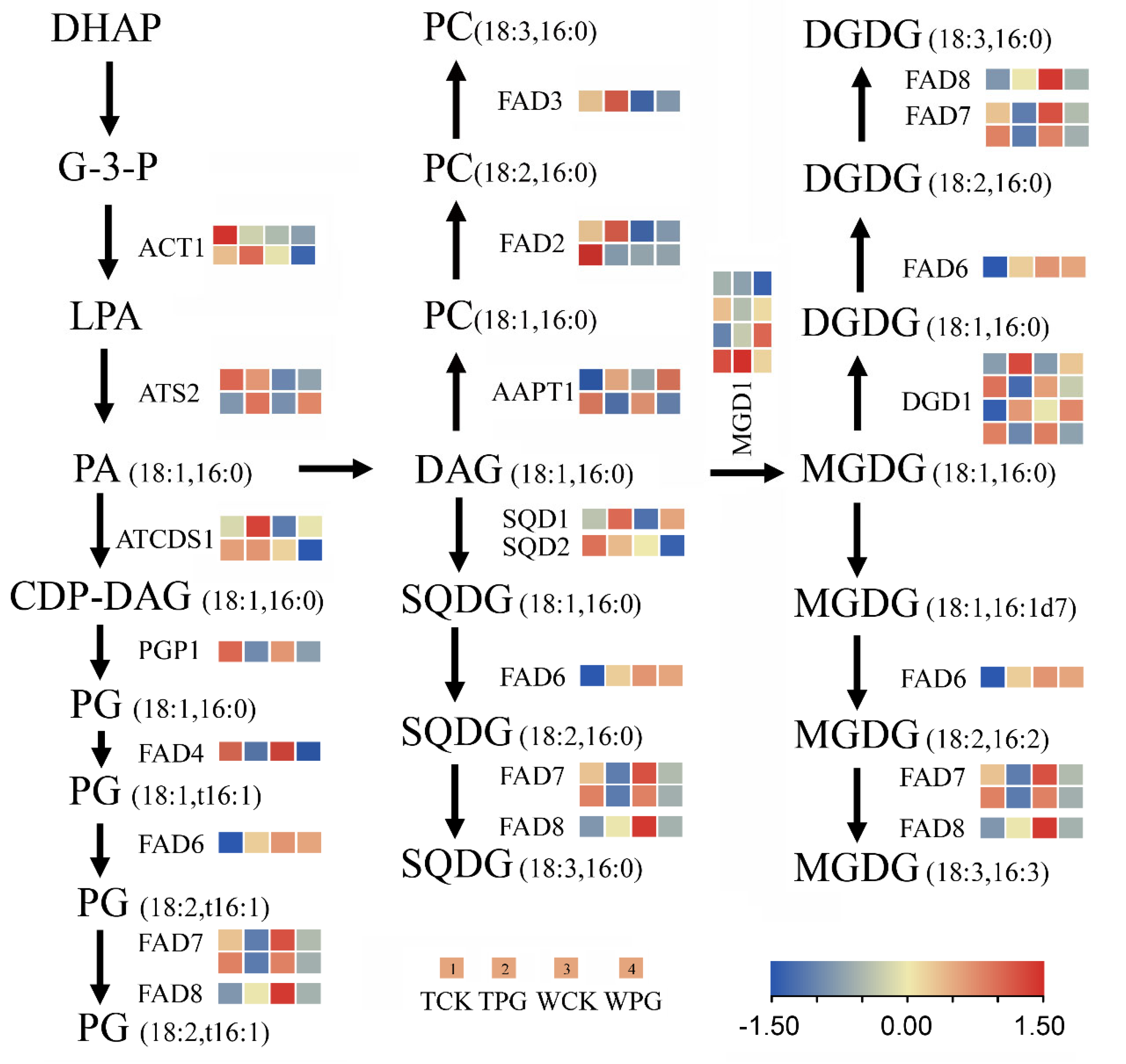
2.5. The OEGD Transgenic Plants Exhibited Altered Metabolite Accumulation Under Osmotic Stress
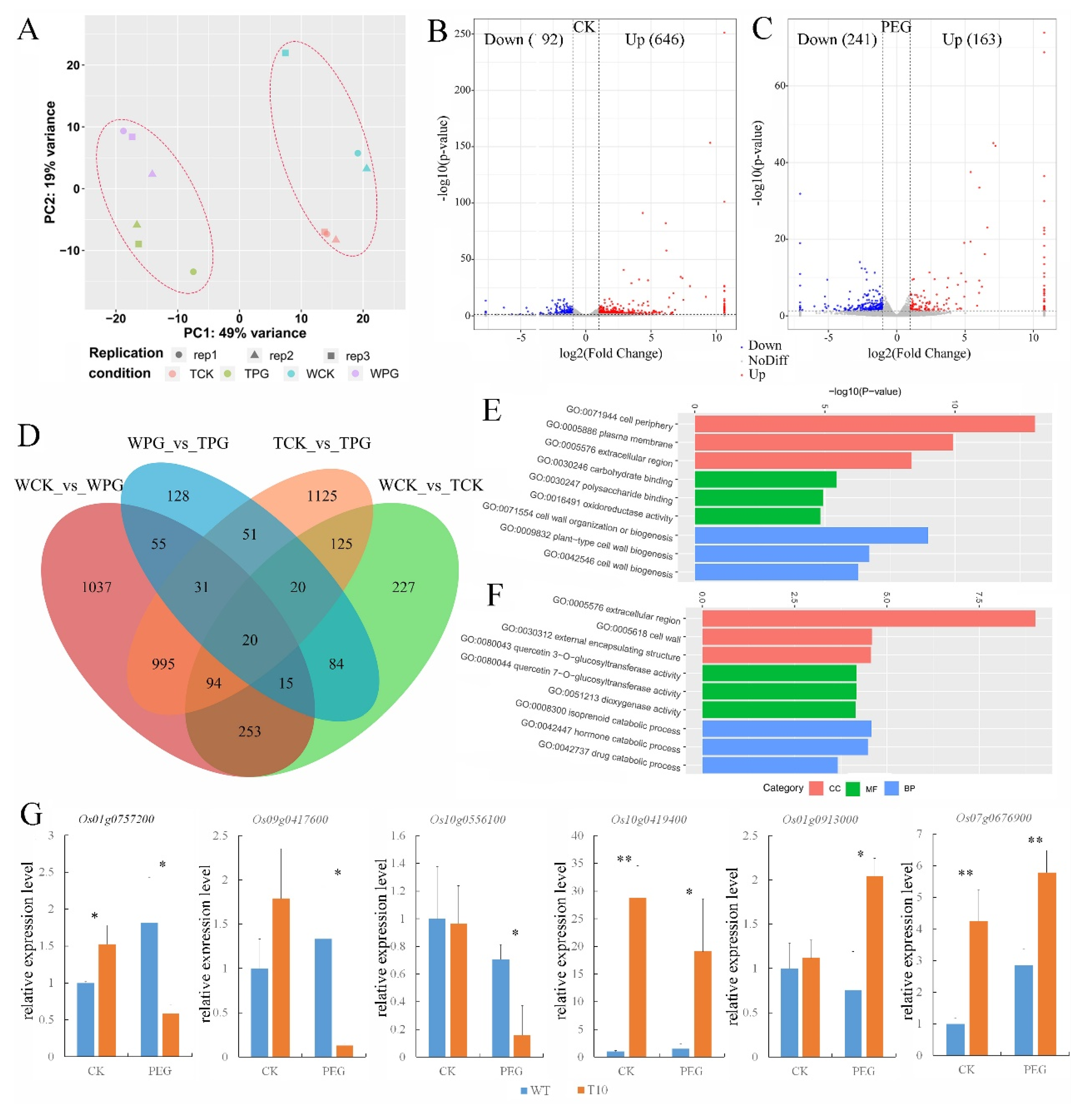
2.6. Transcriptomic Analysis of OEGD Transgenic Plants
3. Discussion
3.1. OEGD Is a Chimeric Glycerol-3-Phosphate Dehydrogenase Gene
3.2. OEGD Enhances Multi-Stress Tolerance Through G3P-Mediated Metabolic Reprogramming
3.3. Transcriptomic Insights into OEGD’s Mode of Action
3.4. OEGD Gene for Agricultural Relevance and Limitations
3.5. Conclusions
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Molecular Cloning and Transformation of Rice
4.3. Sourthern Blot and Reverse Transcription–Quantitative PCR (RT-qPCR) Analysis
4.4. Detection of G-3-P, POD, SOD, CAT, and Fatty Acid Component
4.5. RNA Sequencing
4.6. DNA and Protein Sequence Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef]
- Chanda, B.; Xia, Y.; Mandal, M.K.; Yu, K.; Sekine, K.T.; Gao, Q.M.; Selote, D.; Hu, Y.; Stromberg, A.; Navarre, D.; et al. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 2011, 43, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Wei, Y.; Dauk, M.; Tan, Y.; Taylor, D.C.; Selvaraj, G.; Zou, J. Involvement of a glycerol-3-phosphate dehydrogenase in modulating the NADH/NAD+ ratio provides evidence of a mitochondrial glycerol-3-phosphate shuttle in Arabidopsis. Plant Cell 2006, 18, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Haslam, R.P.; Sayanova, O.; Kim, H.J.; Cahoon, E.B.; Napier, J.A. Synthetic redesign of plant lipid metabolism. Plant J. 2016, 87, 76–86. [Google Scholar] [CrossRef]
- Singh, V.; Singh, P.K.; Siddiqui, A.; Singh, S.; Banday, Z.Z.; Nandi, A.K. Over-expression of Arabidopsis thaliana SFD1/GLY1, the gene encoding plastid localized glycerol-3-phosphate dehydrogenase, increases plastidic lipid content in transgenic rice plants. J. Plant Res. 2016, 129, 285–293. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Wang, J.; Zhou, Y. Glycerol affects root development through regulation of multiple pathways in Arabidopsis. PLoS ONE 2014, 9, e86269. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Liu, P.; Xing, H.; Li, C.; Wei, G.; Kang, Z. Glycerol-3-phosphate metabolism in wheat contributes to systemic acquired resistance against Puccinia striiformis f. sp. tritici. PLoS ONE 2013, 8, e81756. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shen, W.; Dauk, M.; Wang, F.; Selvaraj, G.; Zou, J. Targeted gene disruption of glycerol-3-phosphate dehydrogenase in Colletotrichum gloeosporioides reveals evidence that glycerol is a significant transferred nutrient from host plant to fungal pathogen. J. Biol. Chem. 2004, 279, 429–435. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, M.; He, L.; Li, X.; Wang, F.; Yan, B.; Wei, J.; Zhao, C.; Li, Z.; Xu, J. A cytosolic NAD+-dependent GPDH from maize (ZmGPDH1) is involved in conferring salt and osmotic stress tolerance. BMC Plant Biol. 2019, 19, 16. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Wang, F.; Zhao, X.; Gao, Y.; Zhao, C.; He, L.; Li, Z.; Xu, J. Glycerol-3-phosphate dehydrogenase (GPDH) gene family in Zea mays L.: Identification, subcellular localization, and transcriptional responses to abiotic stresses. PLoS ONE 2018, 13, e0200357. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yu, S.; Li, T.; Zhang, Y.; Chen, S.J.; Chen, C.; Li, J.; Hu, S.P. Cloning and Functional Identification of OsGPDH1 in Rice. J. Nucl. Agric. Sci. 2017, 5, 829–836. [Google Scholar] [CrossRef]
- Eastmond, P.J. Glycerol-insensitive Arabidopsis mutants: gli1 seedlings lack glycerol kinase, accumulate glycerol and are more resistant to abiotic stress. Plant J. 2004, 37, 617–625. [Google Scholar] [CrossRef]
- Pallapati, A.R.; Prasad, S.; Roy, I. Glycerol 3-phosphate dehydrogenase regulates heat shock response in Saccharomyces cerevisiae. BBA-Mol. Cell Res. 2022, 1869, 119238. [Google Scholar] [CrossRef] [PubMed]
- Pecinova, A.; Alan, L.; Brazdova, A.; Vrbacký, M.; Pecina, P.; Drahota, Z.; Houštěk, J.; Mráček, T. Role of Mitochondrial Glycerol-3-Phosphate Dehydrogenase in Metabolic Adaptations of Prostate Cancer. Cells 2020, 9, 1764. [Google Scholar] [CrossRef]
- He, Y.; Meng, X.; Fan, Q.; Sun, X.; Xu, Z.; Song, R. Cloning and characterization of two novel chloroplastic glycerol-3-phosphate dehydrogenases from Dunaliella viridis. Plant Mol. Biol. 2009, 71, 193–205. [Google Scholar] [CrossRef]
- Shen, W.; Wei, Y.; Dauk, M.; Zheng, Z.; Zou, J. Identification of a mitochondrial glycerol-3-phosphate dehydrogenase from Arabidopsis thaliana: Evidence for a mitochondrial glycerol-3-phosphate shuttle in plants. FEBS Lett. 2003, 536, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cui, H.; Wu, B.; Wang, W.; Yang, Q.; Zhang, Y.; Yang, S.; Zhao, Y.; Xu, D.; Liu, G.; et al. Genome-Wide Identification of Cotton (Gossypium spp.) Glycerol-3-Phosphate Dehydrogenase (GPDH) Family Members and the Role of GhGPDH5 in Response to Drought Stress. Plants 2022, 11, 592. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, P.; Cui, Y.; Liu, D.; Li, J.; Zhao, Y.; Yang, S.; Zhang, B.; Zhou, R.; Sun, M.; et al. Enhanced production of seed oil with improved fatty acid composition by overexpressing NAD+-dependent glycerol-3-phosphate dehydrogenase in soybean. J. Integr. Plant Biol. 2021, 63, 1036–1053. [Google Scholar] [CrossRef]
- Shen, W.; Li, J.Q.; Dauk, M.; Huang, Y.; Periappuram, C.; Wei, Y.; Zou, J. Metabolic and transcriptional responses of glycerolipid pathways to a perturbation of glycerol 3-phosphate metabolism in Arabidopsis. J. Biol. Chem. 2010, 285, 22957–22965. [Google Scholar] [CrossRef]
- Ou, X.; Ji, C.; Han, X.; Zhao, X.; Li, X.; Mao, Y.; Wong, L.-L.; Bartlam, M.; Rao, Z. Crystal structures of human glycerol 3-phosphate dehydrogenase 1 (GPD1). J. Mol. Biol. 2006, 357, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Liu, J.; Han, S.; Lin, M.; Guo, Z.; Chen, X. OsWRKY62 and OsWRKY76 Interact with Importin alpha1s for Negative Regulation of Defensive Responses in Rice Nucleus. Rice 2022, 15, 12. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, R.; Huang, K.; Huang, S.; Wang, H.; Wei, Z.; Li, Z.; Bian, M.; Jiang, W.; Wu, T.; et al. The OsWRKY63-OsWRKY76-OsDREB1B module regulates chilling tolerance in rice. Plant J. 2022, 112, 383–398. [Google Scholar] [CrossRef]
- Lo, S.; Yang, S.; Chen, K.; Hsing, Y.-I.; Zeevaart, J.A.; Chen, L.-J.; Yu, S.-M. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 2008, 20, 2603–2618. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Xiong, W.; Yin, C.; Xie, X.; Jin, Y.-J.; Zhang, S.; Yang, B.; Ye, G.; Chen, S.; Luan, W.-J. Overexpression of OsARD1 Improves Submergence, Drought, and Salt Tolerances of Seedling Through the Enhancement of Ethylene Synthesis in Rice. Front. Plant Sci. 2019, 10, 1088. [Google Scholar] [CrossRef]
- Raza, A.; Anas, M.; Bhardwaj, S.; Mir, R.A.; Charagh, S.; Elahi, M.; Zhang, X.; Mir, R.R.; Weckwerth, W.; Fernie, A.R.; et al. Harnessing metabolomics for enhanced crop drought tolerance. Crop J. 2025, 13, 311–327. [Google Scholar] [CrossRef]
- Gao, L.; Pan, L.; Shi, Y.; Zeng, R.; Li, M.; Li, Z.; Zhang, X.; Zhao, X.; Gong, X.; Huang, W.; et al. Genetic variation in a heat shock transcription factor modulates cold tolerance in maize. Mol. Plant 2024, 17, 1423–1438. [Google Scholar] [CrossRef]
- Moellering, E.R.; Benning, C. Galactoglycerolipid metabolism under stress: A time for remodeling. Trends Plant Sci. 2011, 16, 98–107. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Chen, M.; Yang, F.; Han, J.; Ma, X.; Li, T.; Liu, H.; Liang, B.; Yu, S. Enhanced Stress Tolerance in Rice Through Overexpression of a Chimeric Glycerol-3-Phosphate Dehydrogenase (OEGD). Plants 2025, 14, 1731. https://doi.org/10.3390/plants14111731
Wu J, Chen M, Yang F, Han J, Ma X, Li T, Liu H, Liang B, Yu S. Enhanced Stress Tolerance in Rice Through Overexpression of a Chimeric Glycerol-3-Phosphate Dehydrogenase (OEGD). Plants. 2025; 14(11):1731. https://doi.org/10.3390/plants14111731
Chicago/Turabian StyleWu, Jinhong, Meiyao Chen, Fangwen Yang, Jing Han, Xiaosong Ma, Tianfei Li, Hongyan Liu, Bin Liang, and Shunwu Yu. 2025. "Enhanced Stress Tolerance in Rice Through Overexpression of a Chimeric Glycerol-3-Phosphate Dehydrogenase (OEGD)" Plants 14, no. 11: 1731. https://doi.org/10.3390/plants14111731
APA StyleWu, J., Chen, M., Yang, F., Han, J., Ma, X., Li, T., Liu, H., Liang, B., & Yu, S. (2025). Enhanced Stress Tolerance in Rice Through Overexpression of a Chimeric Glycerol-3-Phosphate Dehydrogenase (OEGD). Plants, 14(11), 1731. https://doi.org/10.3390/plants14111731





