Endocarp Morphology of Premna (Lamiaceae) in Thailand and Its Taxonomic Significance
Abstract
1. Introduction
2. Results
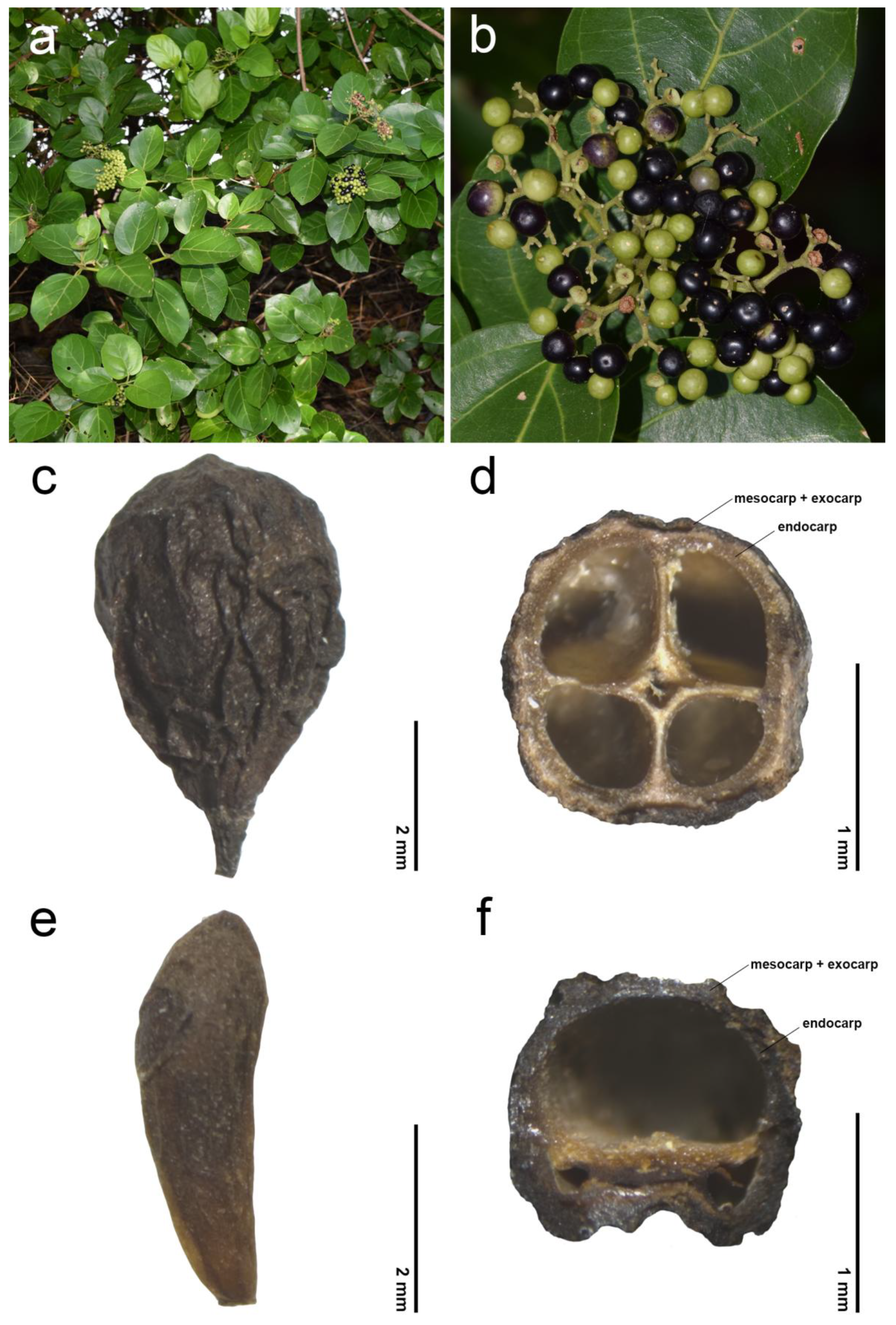
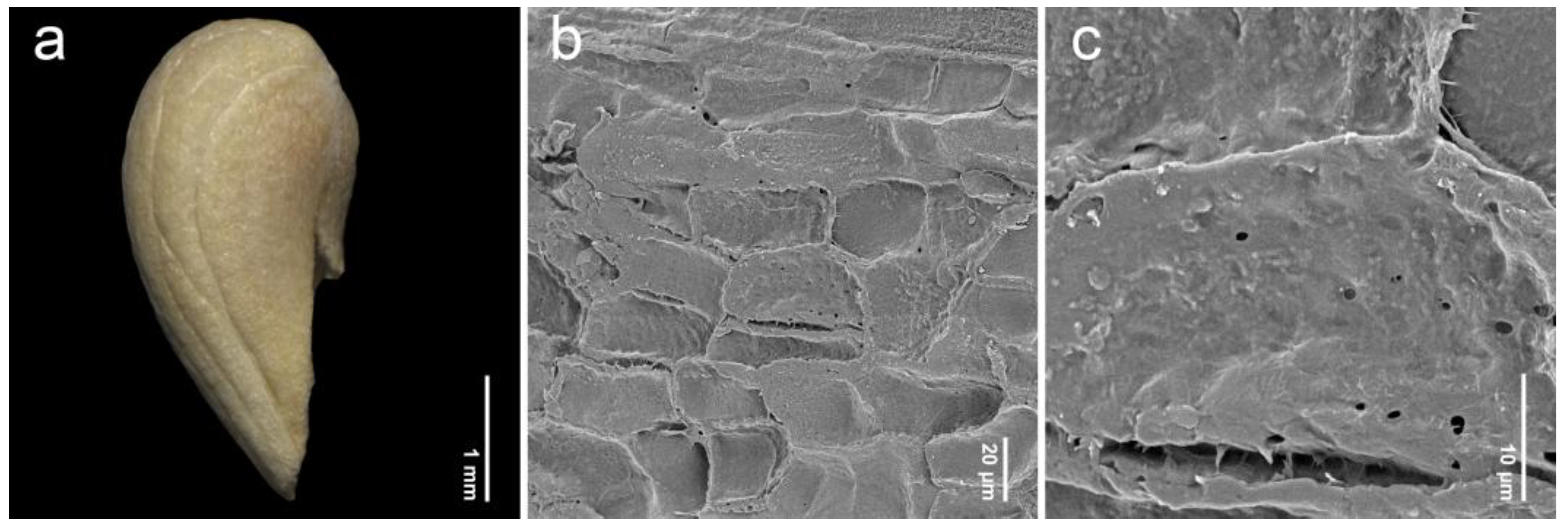
2.1. General Information of Fruit Morphology
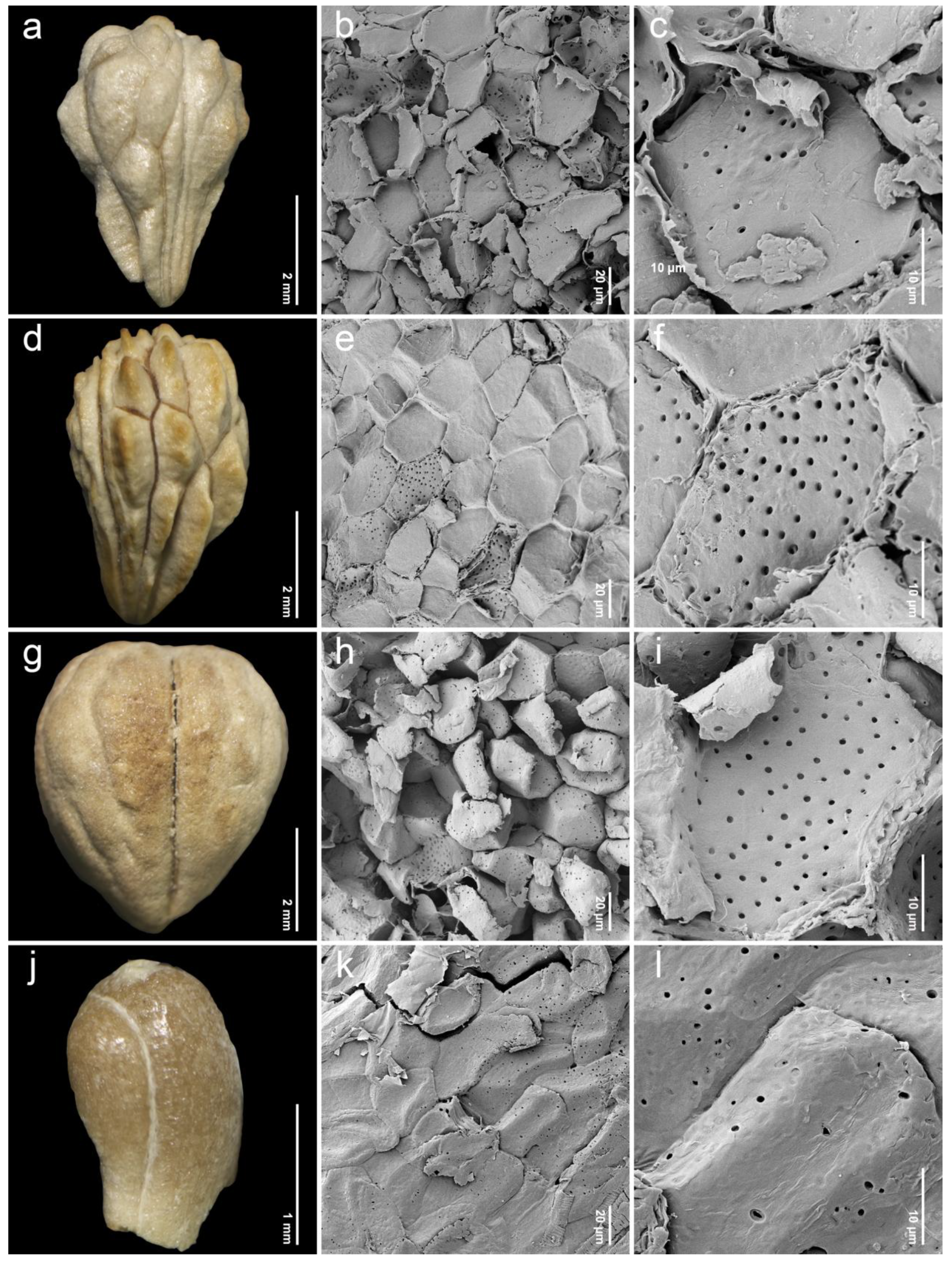
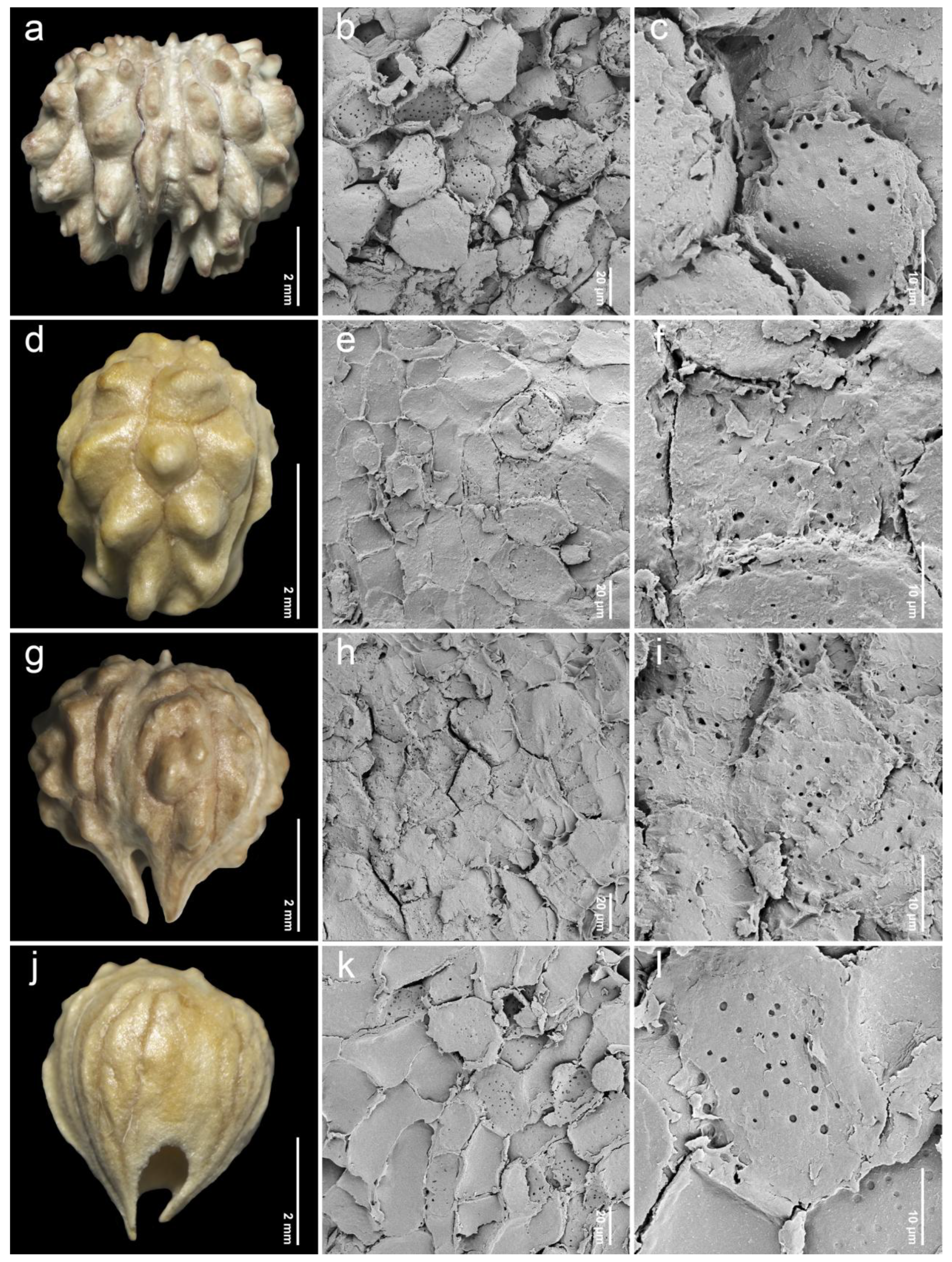
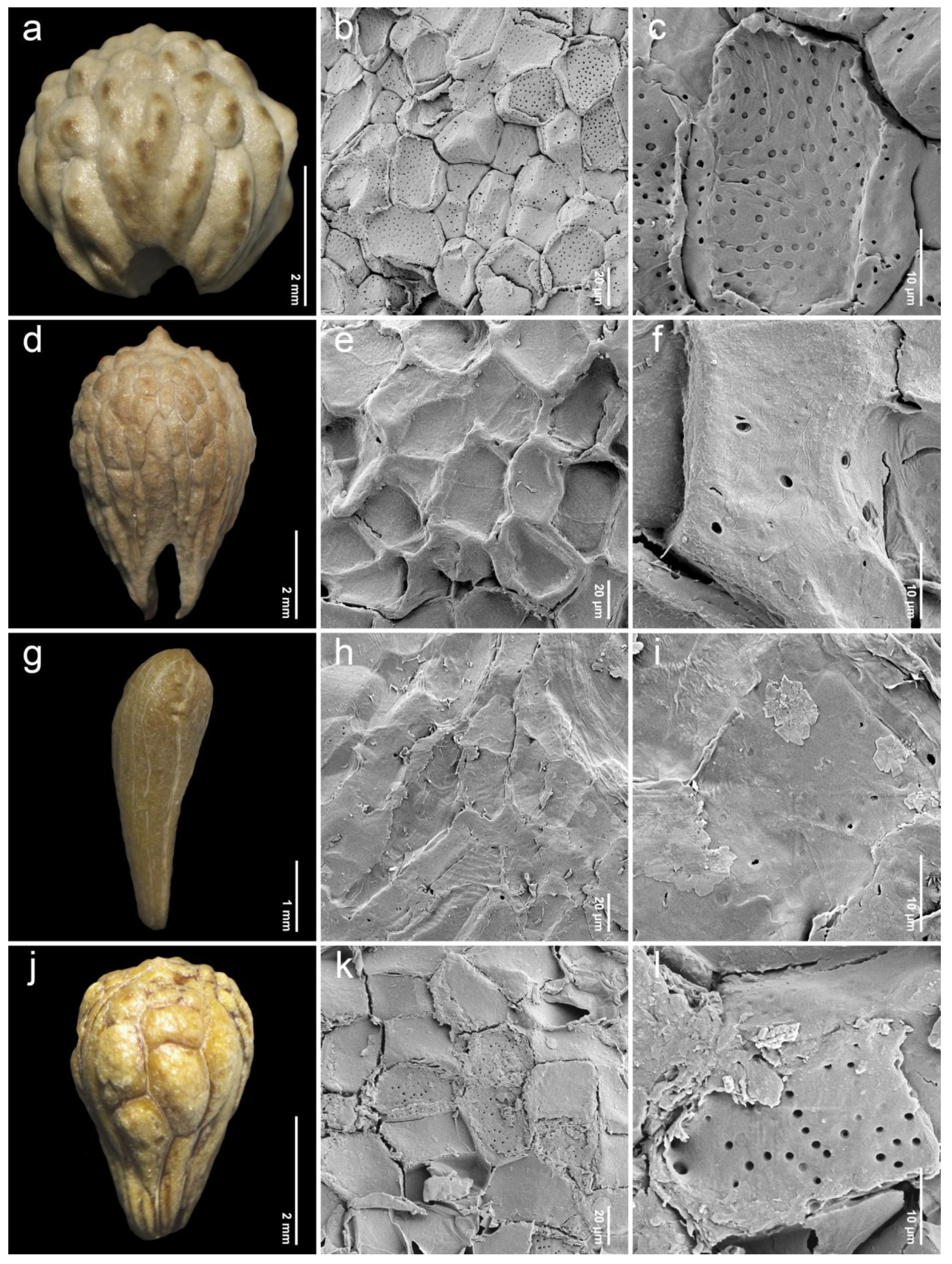
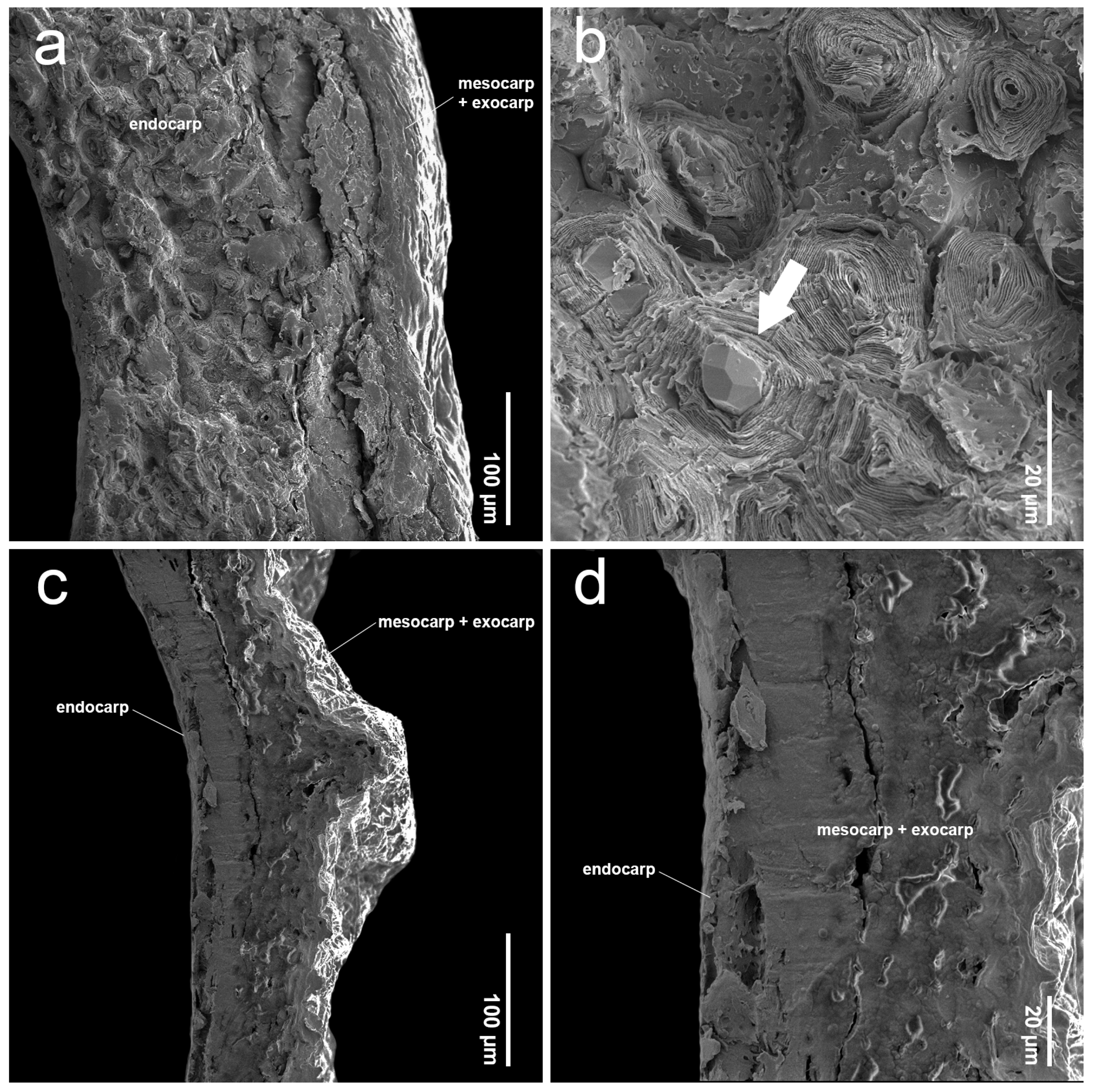
2.2. Endocarp Morphology and Micromorphology
2.3. Phenetic Analyses
3. Discussion
3.1. Fruit Diversity of the Genus Premna in Thailand
3.2. Comparative Study of Endocarp Morphology and Micromorphology
3.3. Taxonomic Significance
4. Materials and Methods
4.1. Sample Collection and Herbarium Specimen Preparation
4.2. Assessment of Fruit Morphological Characters
4.3. Examination of Endocarp Morphology and Micromorphology
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munir, A.A. A taxonomic revision of the genus Premna L. (Verbenaceae) in Australia. J. Adel. Bot. Gard. 1984, 7, 1–43. [Google Scholar]
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; de Kok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae. In The Families and Genera of Vascular Plants: Flowering Plants, Dicotyledons; Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 7, pp. 167–275. [Google Scholar]
- Li, B.; Cantino, P.D.; Olmstead, R.G.; Bramley, G.L.; Xiang, C.L.; Ma, Z.H.; Tan, Y.H.; Zhang, D.X. A large-scale chloroplast phylogeny of the Lamiaceae sheds new light on its subfamilial classification. Sci. Rep. 2016, 6, 34343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chen, Y.P.; Salmaki, Y.; Drew, B.T.; Wilson, T.C.; Scheen, A.C.; Celep, F.; Brauchler, C.; Bendiksby, M.; Wang, Q.; et al. An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biol. 2021, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Linnaeus, C. Mantissa Plantarum 2; Impensis Direct; Laurentius Salvius: Stockholm, Sweeden, 1771. [Google Scholar]
- Cantino, P.D.; Harley, R.M.; Wagstaff, S.J. Genera of Labiatae: Status and classification. In Advances in Labiate Science; Harley, R.M., Reynolds, T., Eds.; Royal Botanic Gardens, Kew: London, UK, 1992; pp. 511–522. [Google Scholar]
- Hai, D.V.; Min, D.Z.; Khang, N.S.; Tan, Y.H.; Thoa, P.T.K.; Bramley, G.L.C.; de Kok, R.P.J.; Li, B. Premna vietnamensis (Lamiaceae, Premnoideae), a distinct new species from the Central Highlands of Vietnam. PLoS ONE 2018, 13, e0195811. [Google Scholar] [CrossRef]
- Leeratiwong, C.; Chantaranothai, P.; Paton, A.J. A synopsis of the genus Premna L. (Lamiaceae) in Thailand. Trop. Nat. Hist. 2009, 9, 113–142. [Google Scholar] [CrossRef]
- De Kok, R. The genus Premna L. (Lamiaceae) in the Flora Malesiana area. Kew Bull. 2013, 68, 55–84. [Google Scholar] [CrossRef]
- De Kok, R. Premna. In Flora Malesiana: Series 1: Spermatophyta; Bramley, G.L.C., Ed.; National Parks Board Singapore: Singapore, 2019; pp. 314–335. [Google Scholar]
- Fletcher, H.R. Contributions to the Flora of Siam. Additamentum XLIX. Bull. Misc. Inf. Kew 1938, 1938, 199–209. [Google Scholar]
- Chen, S.L. Premna. In Flora of China; Wu, C.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1994; Volume 17, pp. 16–27. [Google Scholar]
- Demissew, S.; Harley, M.M. Trichome, seed surface and pollen characters in Stachys, Lamioideae (Labiatae) in Tropical Africa. In Advances in Labiatae Science; Harley, R.M., Reynolds, T., Eds.; Royal Botanic Gardens, Kew: London, UK, 1992; pp. 149–166. [Google Scholar]
- Krawczyk, K.; Głowacka, K. Nutlet micromorphology and its taxonomic utility in Lamium L. (Lamiaceae). Plant Syst. Evol. 2015, 301, 1863–1874. [Google Scholar] [CrossRef]
- Ryding, O. Pericarp structure and phylogeny of Lamiaceae subfamily Pogostemonoideae. Nord. J. Bot. 1994, 14, 59–63. [Google Scholar] [CrossRef]
- Walsingham, L.J. Patraeovitex. In Flora Malesiana: Series 1: Spermatophyta; Bramley, G.L.C., Ed.; National Parks Board Singapore: Singapore, 2019; pp. 270–279. [Google Scholar]
- Bongcheewin, B.; Paton, A. A taxonomic revision of Glossocarya (Lamiaceae: Ajugoideae) in Thailand. Blumea 2023, 68, 52–62. [Google Scholar] [CrossRef]
- Budantsev, A.L.; Lobova, T.A. Fruit morphology, anatomy and taxonomy of tribe Nepeteae (Labiatae). Edinb. J. Bot. 1997, 54, 183–216. [Google Scholar] [CrossRef]
- Ryding, O. Pericarp structure and phylogeny within Lamiaceae subfamily Nepetoideae tribe Ocimeae. Nord. J. Bot. 1992, 12, 273–298. [Google Scholar] [CrossRef]
- Moon, H.-K.; Hong, S.-P.; Smets, E.; Huysmans, S. Micromorphology and character evolution of nutlets in Tribe Mentheae (Nepetoideae, Lamiaceae). Syst. Bot. 2009, 34, 760–776. [Google Scholar] [CrossRef]
- Jeon, Y.C.; Jang, T.S.; Hong, S.P. Nutlet morphology in the tribe Elsholtzieae (Lamiaceae). Nord. J. Bot. 2020, 38, NJB12423. [Google Scholar] [CrossRef]
- Ryding, O. Pericarp structure and phylogeny of tribe Mentheae (Lamiaceae). Plant Syst. Evol. 2010, 285, 165–175. [Google Scholar] [CrossRef]
- Ryding, O. Pericarp structure in the subtribe Melittidinae (Lamiaceae-Lamoideae) and its systematic implications. Bot. Jahrb. Syst. 1994, 115, 547–555. [Google Scholar]
- Oran, S.A. Ultrastructure of nutlet surface of the genus Salvia L. in Jordan and the neighbouring countries. Dirasat Nat. Eng. Sci. 1996, 23, 393–408. [Google Scholar]
- Salmaki, Y.; Zarre, S.; Ryding, O.; Lindqvist, C.; Brauchler, C.; Heubl, G.; Barber, J.; Bendiksby, M. Molecular phylogeny of tribe Stachydeae (Lamiaceae subfamily Lamioideae). Mol. Phylogenetics Evol. 2013, 69, 535–551. [Google Scholar] [CrossRef]
- Dinç, M.; Pinar, N.M.; Dugu, S.; Yildirimli, S. Micromorphological studies of Lallemantia L. (Lamiaceae) species growing in Turkey. Acta Biol. Cracoviensia Ser. Bot. 2009, 51, 45–54. [Google Scholar]
- Kaya, A.; Satil, F.; Gogel, F. Nutlet surface micromorphology of Turkish Satureja (Lamiaceae). Biologia 2009, 64, 902–907. [Google Scholar] [CrossRef][Green Version]
- Özkan, M.; Aktas, K.; Özdemir, C.; Guerin, G.R. Nutlet morphology and its taxonomic utility in Salvia (Lamiaceae: Mentheae) from Turkey. Acta Bot. Croat. 2009, 68, 105–115. [Google Scholar]
- Kahraman, A.; Celep, F.; Doğan, M.; Guerin, G.R.; Bagherpour, S. Mericarp morphology and its systematic implications for the genus Salvia L. section Hymenosphace Benth. (Lamiaceae) in Turkey. Plant Syst. Evol. 2011, 292, 33–39. [Google Scholar] [CrossRef]
- Abdel Khalik, K.N. A systematic revision of the genus Plectranthus L. (Lamiaceae) in Saudi Arabia based on morphological, palynological, and micromorphological characters of trichomes. Am. J. Plant Sci. 2016, 7, 1429–1444. [Google Scholar] [CrossRef]
- Kasem, W.T. Pollen grains and seed-morphology as related to biochemical patterns in five species of genus Ocimum L. (Lamiaceae Juss.) of Saudi Arabia. J. Phytol. 2017, 8, 42–48. [Google Scholar] [CrossRef]
- Eyvazadeh Khosroshahi, E.; Salmaki, Y. Nutlet micromorphology and its systematic implications in Phlomoides Moench (Lamiaceae). Nova Biol. Reper. 2018, 5, 82–94. [Google Scholar] [CrossRef]
- Sudarmono. Seed micromorphology of Orthosiphon spp. and its relatives (Lamiaceae). Int. J. Adv. Res. Biol. Sci. 2020, 14, 215–219. [Google Scholar]
- Cosmo, N.L.; Gogosz, A.M.; Nogueira, A.C.; Bona, C.; Kuniyoshi, Y.S. Morfologia do fruto, da semente e morfo-anatomia da plântula de Vitex megapotamica (Spreng.) Moldenke (Lamiaceae). Acta Bot. Bras. 2009, 23, 389–397. [Google Scholar] [CrossRef]
- Hassan, S.A.; Thobaiti, A.T., II. Pericarp anatomical study of some Lamiaceae nutlets in Saudi Arabia and its taxonomic significance. Pak. J. Bot. 2014, 45, 1643–1651. [Google Scholar]
- Ryding, O. Pericarp structure in Monarda (Lamiaceae). Bot. Jahrb. Syst. 2009, 127, 453–458. [Google Scholar] [CrossRef]
- Ru, J.; Liu, M.; Wang, C.; Cheng, X.-Y.; Liang, L. Morphological and anatomical features of Lamiaceae fruits in northeasten China and notes on taxonomic value. Acta Prataculturae Sin. 2015, 24, 190–205. [Google Scholar]
- Srisombat, P.; Chitchak, N.; Rattanakrajang, P.; Stewart, A.B.; Traiperm, P. The Argyreia collinsiae species complex (Convolvulaceae): Phenetic analysis and geographic distribution reveal subspecies new to science. PeerJ 2024, 12, e18294. [Google Scholar] [CrossRef] [PubMed]
- Chitchak, N.; Traiperm, P.; Staples, G.; Rattanakrajang, P.; Sumanon, P. Species delimitation of some Argyreia (Convolvulaceae) using phenetic analyses: Insights from leaf anatomical data reveal a new species. Botany 2018, 96, 217–233. [Google Scholar] [CrossRef]
- Traiperm, P.; Chow, J.; Nopun, P.; Staples, G.; Swangpol, S.C. Identification among morphologically similar Argyreia (Convolvulaceae) based on leaf anatomy and phenetic analyses. Bot. Stud. 2017, 58, 25. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.K.; Hong, S.P. Nutlet morphology and anatomy of the genus Lycopus (Lamiaceae: Mentheae). J. Plant Res. 2006, 119, 633–644. [Google Scholar] [CrossRef]
- Buyukkartal, H.N.; Kahraman, A.; Colgecen, H.; Dogan, M.; Karabacak, E. Mericarp micromorphology and anatomy of Salvia hedgeana Donmez, S. huberi Hedge and S. rosifolia Sm. (section Salvia Hedge, Lamiaceae). Acta Bot. Croat. 2011, 70, 65–80. [Google Scholar] [CrossRef]
- Ryding, O. The distribution and evolution of myxocarpy in Lamiaceae. In Advances in Labiate Science; Harley, R.M., Reynolds, T., Eds.; Royal Botanic Gardens, Kew: London, UK, 1992; pp. 85–97. [Google Scholar]
- Ryding, O. Pericarp structure and phylogeny of the Lamiaceae-Verbenaceae-complex. Plant Syst. Evol. 1995, 198, 101–141. [Google Scholar] [CrossRef]
- Ryding, O. Myxocarpy in the Nepetoideae (Lamiaceae) with Notes on Myxodiaspory in General. Syst. Geogr. Plants 2001, 71, 503–514. [Google Scholar] [CrossRef]
- Hedge, I.C. Observations on the mucilage of Salvia fruits. Notes R. Bot. Gard. Edinb. 1970, 30, 79–95. [Google Scholar]
- Paton, A. The adaptive significance of calyx and nutlet morphology in Scutellaria. In Advances in Labiate Science; Harley, R.M., Reynolds, T., Eds.; Royal Botanic Gardens, Kew: London, UK, 1992; pp. 203–210. [Google Scholar]
- Pak, J.; Park, J.; Whang, S.S. Systematic implications of fruit wall anatomy and surface sculpturing of Microseris (Asteraceae, Lactuceae) and relatives. Int. J. Plant Sci. 2001, 162, 209–220. [Google Scholar] [CrossRef]
- Fahn, A. Plant Anatomy, 4th ed.; Pergamon: Oxford, UK, 1990. [Google Scholar]
- Mack, A.L. An advantage of large seed size: Tolerating rather than succumbing to seed predators. Biotropica 1998, 30, 604–608. [Google Scholar] [CrossRef]
- Rehling, F.; Jaroszewicz, B.; Braasch, L.V.; Albrecht, J.; Jordano, P.; Schlautmann, J.; Farwig, N.; Schabo, D.G. Within-species trait variation can lead to size limitations in seed dispersal of small-fruited plants. Front. Ecol. Evol. 2021, 9, 698885. [Google Scholar] [CrossRef]
- Choat, B.; Cobb, A.R.; Jansen, S. Structure and function of bordered pits: New discoveries and impacts on whole-plant hydraulic function. New Phytol. 2008, 177, 608–626. [Google Scholar] [CrossRef] [PubMed]
- Come, D. Les Obstacles à la Germination des Graines. Monographies de Physiologie Végétale 6; Masson et Cie: Paris, France, 1970. [Google Scholar]
- Thomas, R.J. Bordered pit aspiration in angiosperms. Wood Fiber 1972, 3, 236–237. [Google Scholar]
- Carlquist, S. Comparative Wood Anatomy: Systematic, Ecological, and Evolutionary Aspects of Dicotyledon Wood; Springer: New York, NY, USA, 2001. [Google Scholar]
- Sebaa, H.S.; Kaid Harche, M. Anatomical structure and ultrastructure of the endocarp cell walls of Argania spinosa (L.) Skeels (Sapotaceae). Micron 2014, 67, 100–106. [Google Scholar] [CrossRef]
- Denis, L.; Coelho, V.; Vear, F. Pericarp structure and hullability in sunflower inbred lines and hybrids. Agronomie 1994, 14, 453–461. [Google Scholar] [CrossRef]
- Heo, K. Fruit wall anatomy of Ocotea (Lauraceae). Korean J. Plant Res. 1996, 9, 298–304. [Google Scholar]
- Wu, Y.; Sun, X.R.; Peng, C.Y.; Shen, Y.B.; Visscher, A.M.; Pritchard, H.W.; Wang, M.Z.; Deng, Z.Y. Cryo-attenuated properties of Tilia miqueliana pericarps and seeds. Front. Plant Sci. 2023, 14, 1228069. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Fromm, J.; Rockel, B.; Lautner, S.; Windeisen, E.; Wanner, G. Lignin distribution in wood cell walls determined by TEM and backscattered SEM techniques. J. Struct. Biol. 2003, 143, 77–84. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989. [Google Scholar]
- Jocković, J.; Rajčević, N.; Terzić, S.; Zorić, L.; Jocković, M.; Miladinović, D.; Luković, J. Pericarp features of wild perennial Helianthus L. species as a potential source for improvement of technical and technological properties of cultivated sunflower. Ind. Crops Prod. 2020, 144, 112030. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Korth, K.L.; Doege, S.J.; Park, S.H.; Goggin, F.L.; Wang, Q.; Gomez, S.K.; Liu, G.; Jia, L.; Nakata, P.A. Medicago truncatula mutants demonstrate the role of plant calcium oxalate crystals as an effective defense against chewing insects. Plant Physiol. 2006, 141, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, A.; Buyukkartal, H.N.; Dogan, M. Pericarp ultrastructure of Salvia section Hemisphace (Mentheae; Nepetoideae; Lamiaceae). Commagene J. Biol. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Adu, O.T.; Naidoo, Y.; Adu, T.S.; Sivaram, V.; Dewir, Y.H.; Rihan, H. Micromorphology and histology of the secretory apparatus of Diospyros villosa (L.) de Winter leaves and stem bark. Plants 2022, 11, 2498. [Google Scholar] [CrossRef]
- Ostroumova, T.; Zakharova, E. The study of crystals in the fruits of some Apiaceae species using energy-dispersive spectroscopy. Int. J. Plant Biol. 2023, 14, 347–360. [Google Scholar] [CrossRef]
- Leeratiwong, C.; Chantaranothai, P.; Paton, A.J. Taxonomic notes on the genus Premna L. (Lamiaceae) in Thailand. Thai For. Bull. Bot. Bangk. 2016, 44, 122–124. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. Available online: http://sweetgum.nybg.org/ih/ (accessed on 6 February 2025).
- Leeratiwong, C.; Chantaranothai, P.; Paton, A. Three New Records of Premna L. (Lamiaceae) for Thailand. Nat. Hist. J. Chulalongkorn Univ. 2008, 8, 7–18. [Google Scholar] [CrossRef]
- Yang, S.Z.; Chen, P.H. Endocarps of menispermaceous plants in Taiwan. Bot. Stud. 2016, 57, 14. [Google Scholar] [CrossRef]
- Xiang, C.L.; Funamoto, T.; Evangelista, E.V.; Zhang, Q.; Peng, H. Pollen morphology of the East Asiatic genus Chelonopsis (Lamioideae: Lamiaceae) and allied genera, with reference to taxonomic implications and potential pollination ecology. Plant Biosyst. 2013, 147, 620–628. [Google Scholar] [CrossRef]
- Bai, R.Z.; Zhao, F.; Drew, B.T.; Xu, G.; Cai, J.; Shen, S.K.; Xiang, C.L. Seed morphology of Hypericum (Hypericaceae) in China and its taxonomic significance. Microsc. Res. Tech. 2023, 86, 1496–1509. [Google Scholar] [CrossRef]
- Barthlott, W. Epidermal and seed surface characters of plants: Systematic applicability and some evolutionary aspects. Nord. J. Bot. 1981, 1, 345–355. [Google Scholar] [CrossRef]
- Salmaki, Y.; Zarre, S.; Jamzad, Z. Nutlet micromorphology and its systematic implication in Stachys L. (Lamiaceae) in Iran. Feddes Repert. 2008, 119, 607–621. [Google Scholar] [CrossRef]
- Beentje, H. The Kew Plant Glossary: An Illustrated Dictionary of Plant Terms, 2nd ed.; Kew Publishing: Richmond, UK; London, UK, 2016. [Google Scholar]
- Crang, R.; Lyons-Sobaski, S.; Wise, R. Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants; Springer Nature: Cham, Switzerland, 2018. [Google Scholar]
- RCoreTeam. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 10 January 2025).
- Pagès, J. Analyse Factorielle de Donnees Mixtes. Rev. Stat. Appl. 2004, 4, 93–111. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: AnRPackage for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses (R Package Version 1.0.7); CRAN—Contributed Packages: Vienna, Austria, 2020. [Google Scholar]
- Kassambara, A. Ggpubr: ‘Ggplot2’ Based Publication Ready Plots, R package version 0.6.0; CRAN: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]



| Species | Shape | Length (mm) Min–Max (Average ± SD) | Width (mm) Min–Max (Average ± SD) | Length/Width Min–Max (Average ± SD) | Sculptured Cell Shape of Endocarp | Protrusion Type | Voucher Specimens |
|---|---|---|---|---|---|---|---|
| Fruit type I | |||||||
| 1. Premna annulata H.R.Fletcher (n = 20, Figure 3a–c) | narrowly obovoid | 4.23–5.40 (4.83 ± 0.32) | 3.24–4.39 (3.66 ± 0.32) | 1.09–1.56 (1.33 ± 0.13) | P | I | Leeratiwong 16-841 (PSU) Smitinand 2874 (BKF, K) Maxwell 84-236 (BKF) |
| 2. Premna cordifolia Roxb. (n = 20, Figure 3d–f) | narrowly obovoid | 5.32–6.65 (5.92 ± 0.42) | 4.25–5.66 (4.91 ± 0.40) | 1.08–1.40 (1.21 ± 0.08) | P | II | Leeratiwong 18-1484 (PSU) |
| 3. Premna fulva Craib (n = 20, Figure 3g–i) | broadly obovoid | 4.29–5.38 (5.04 ± 0.31) | 4.04–5.49 (4.76 ± 0.42) | 0.92–1.20 (1.06 ± 0.08) | P | III | Geesink 6843 (K) Leeratiwong 08-356 (PSU) |
| 4. Premna herbacea Roxb. (n = 20, Figure 4a–c) | narrowly obovoid | 3.20–4.19 (3.71 ± 0.27) | 2.46–3.49 (2.95 ± 0.31) | 1.01–1.46 (1.27 ± 0.13) | IT, P | I | Geesink et al. 7062 (K) Kostermans 281 (K) Leeratiwong 05-240 (PSU) Suddee et al. 871 (K) |
| 5. Premna mollissima Roth (n = 20, Figure 4g–i) | narrowly obovoid | 4.18–5.05 (4.68 ± 0.23) | 3.28–4.97 (3.99 ± 0.51) | 0.94–1.42 (1.19 ± 0.14) | P | I | Kostermans 93 (K) Leeratiwong 05-222 (PSU) |
| 6. Premna nana Collett & Hemsl. (n = 20, Figure 4j–l) | narrowly obovoid | 3.11–3.93 (3.55 ± 0.23) | 2.60–3.77 (3.03 ± 0.32) | 0.98–1.42 (1.18 ± 0.11) | IT, P | I | Leeratiwong 04-2 (KKU) Leeratiwong 05-217 (PSU, KKU) Puudjaa et al. 1908 (BKF) |
| 7. Premna odorata Blanco (n = 21, Figure 5a–c) | broadly obovoid | 4.94–7.48 (5.79 ± 0.72) | 4.65–7.71 (5.90 ± 0.72) | 0.88–1.17 (0.98 ± 0.07) | P | II | Geesink & Santisuk 5434 (K) Kerr 15383 (K) Leeratiwong 05-228 (PSU) Leeratiwong 17-1466 (PSU) |
| 8. Premna paniculata H.R.Fletcher (n = 20, Figure 5d–f) | narrowly obovoid | 3.22–4.20 (3.70 ± 0.27) | 2.33–3.82 (3.23 ± 0.48) | 0.98–1.57 (1.17 ± 0.19) | IT, P | II | Kerr 20536 (K) Leeratiwong 16-545 (PSU) |
| 9. Premna pubescens Blume (n = 34, Figure 5g–i) | broadly obovoid | 3.40–5.80 (4.53 ± 0.66) | 3.27–6.17 (4.57 ± 0.73) | 0.88–1.12 (1.00 ± 0.06) | P | I | Leeratiwong 05-229 (BKF) Leeratiwong 05-233 (PSU) Phonsena et al. 5926 (BKF) Pooma et al. 1814 (K) Pooma et al. 7077 (K) Winit 1405 (BKF) |
| 10. Premna punctulata C.B.Clarke (n = 9, Figure 5j–l) | narrowly obovoid | 4.37–5.70 (5.09 ± 0.42) | 3.79–5.69 (4.49 ± 0.68) | 0.94–1.30 (1.15 ± 0.11) | IT, P | I | Leeratiwong 18-1487 (PSU) Leeratiwong 22-2202 (PSU) |
| 11. Premna rabakensis Moldenke (n = 27, Figure 6a–c) | broadly obovoid | 3.08–4.75 (3.93 ± 0.57) | 2.96–4.25 (3.55 ± 0.39) | 0.95–1.28 (1.10 ± 0.08) | IT, P | I | Leeratiwong 04-16 (PSU) Geesing & Phengkhlai 6151 (K) Put 4011 (K) |
| 12. Premna repens H.R.Fletcher (n = 6, Figure 6d–f) | narrowly obovoid | 4.12–5.83 (5.02 ± 0.73) | 3.45–4.68 (4.17 ± 0.54) | 1.01–1.31 (1.21 ± 0.12) | IT, P | I | Leeratiwong 04-46 (PSU) Leeratiwong 04-155 (PSU |
| 13. Premna serrata H.R.Fletcher (n = 20, Figure 6j–l) | narrowly obovoid | 2.38–4.03 (3.39 ± 0.44) | 2.27–3.26 (2.70 ± 0.28) | 1.00–1.48 (1.26 ± 0.12) | OP | I | Leeratiwong 05-243 (PSU) Pooma et al. 5345 (K) Suddee & Puudjaa 1103 (K) Tagawa et al. T-9920 (K) |
| 14. Premna serratifolia L. (n = 22, Figure 7a–c) | narrowly obovoid | 3.33–4.89 (4.22 ± 0.35) | 2.58–2.99 (2.84 ± 0.11) | 1.12–1.75 (1.49 ± 0.12) | IT, P | I | Satthaphorn 235 (PSU) |
| 15. Premna stenobotrys Merr. (n = 20, Figure 7g–i) | narrowly obovoid | 4.58–5.62 (5.05 ± 0.35) | 3.31–5.20 (4.03 ± 0.49) | 1.06–1.40 (1.26 ± 0.10) | IT, P | I | Larsen & Larsen 33855 (K) Leeratiwong 05-269 (KKU) Nielsen et al. 1926 (BKF, K) Phonsena et al. 4661 (BKF) |
| 16. Premna tomentosa Willd. (n = 25, Figure 7j–l) | narrowly obovoid | 4.02–4.92 (4.51 ± 0.25) | 3.14–4.85 (3.97 ± 0.43) | 0.97–1.32 (1.14 ± 0.10) | IT, P | I | Kerr 13117 (K) Leeratiwong 04-54 (PSU) Parnell et al. 95-296 (K) Prapat 64 (K) Winit 414 (K) |
| Fruit type II | |||||||
| 17. Premna garrettii H.R.Fletcher (n = 20, Figure 3j–l) | clavoid | 2.89–3.38 (3.15 ± 0.17) | 1.21–1.77 (1.44 ± 0.14) | 1.82–2.54 (2.21 ± 0.20) | OIT, OP | III | Leeratiwong 05-242 (KKU, PSU) |
| 18. Premna interrupta Wall. ex Schauer (n = 20, Figure 4d–f) | clavoid | 1.52–2.49 (2.11 ± 0.27) | 0.98–1.37 (1.13 ± 0.12) | 1.48–2.31 (1.88 ± 0.27) | IT, P | III | Kerr 5379 (K) |
| 19. Premna scandens Roxb. (n = 30, Figure 6g–i) | clavoid | 3.37–4.47 (4.04 ± 0.31) | 1.03–1.87 (1.40 ± 0.22) | 1.90–3.81 (2.94 ± 0.48) | OIT, OP | III | Put 79 (K) Chantaranothai 329 (KKU) |
| 20. Premna siamensis H.R.Fletcher (n = 10, Figure 7d–f) | clavoid | 2.04–2.63 (2.42 ± 0.21) | 1.12–1.34 (1.23 ± 0.07) | 1.63–2.20 (1.98 ± 0.20) | IT, P | III | Sutheesorn 2441 (BK) |
| 21. Premna trichostoma Miq. (n = 20, Figure 8a–c) | clavoid | 2.99–3.95 (3.39 ± 0.26) | 1.32–1.86 (1.52 ± 0.14) | 1.90–2.60 (2.25 ± 0.24) | IT, P | III | Leeratiwong 05-237 (PSU) Leeratiwong 06-335 (PSU) Vidal & Niyomdham 6351 (K) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satthaphorn, J.; Paton, A.J.; Sutthinon, P.; Leeratiwong, C. Endocarp Morphology of Premna (Lamiaceae) in Thailand and Its Taxonomic Significance. Plants 2025, 14, 1706. https://doi.org/10.3390/plants14111706
Satthaphorn J, Paton AJ, Sutthinon P, Leeratiwong C. Endocarp Morphology of Premna (Lamiaceae) in Thailand and Its Taxonomic Significance. Plants. 2025; 14(11):1706. https://doi.org/10.3390/plants14111706
Chicago/Turabian StyleSatthaphorn, Jiratthi, Alan J. Paton, Pornsawan Sutthinon, and Charan Leeratiwong. 2025. "Endocarp Morphology of Premna (Lamiaceae) in Thailand and Its Taxonomic Significance" Plants 14, no. 11: 1706. https://doi.org/10.3390/plants14111706
APA StyleSatthaphorn, J., Paton, A. J., Sutthinon, P., & Leeratiwong, C. (2025). Endocarp Morphology of Premna (Lamiaceae) in Thailand and Its Taxonomic Significance. Plants, 14(11), 1706. https://doi.org/10.3390/plants14111706





