Height and Light-Obtaining Ability of Leymus chinensis Increased After a Decade of Warming in the Typical Steppe of Inner Mongolia, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Measurements and Analysis
2.2.1. Plant Height
2.2.2. Resource Utilization Efficiency
2.2.3. Morphological Characteristics
2.2.4. Leaf Anatomical Structure
2.2.5. Data Analysis
3. Results
3.1. Height of L. chinensis
3.2. Photosynthetic Capacity of L. chinensis
3.3. L. chinensis: Ability to Obtain Light
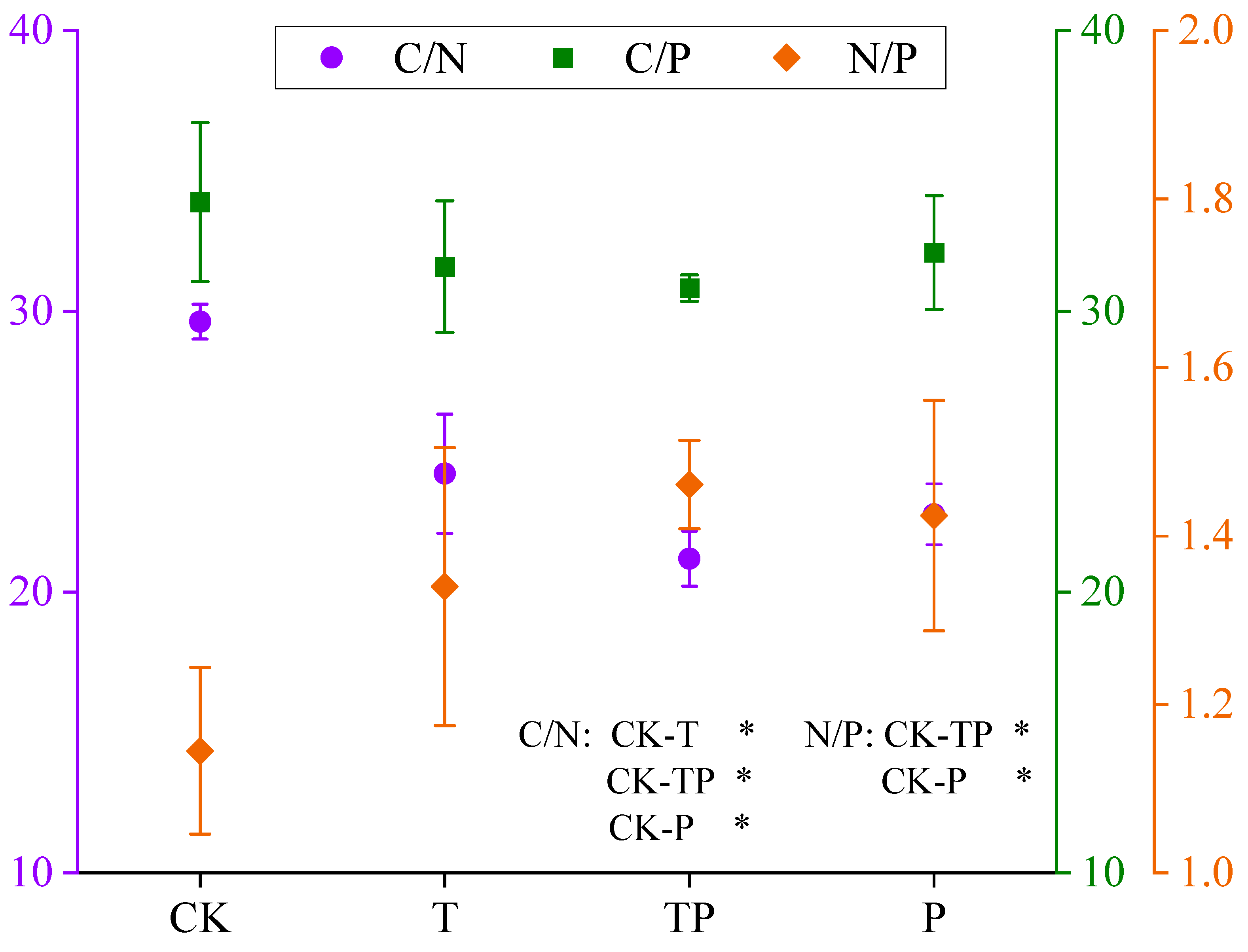
3.4. Resource Utilization Efficiency of Leymus Chinensis
3.5. Leaf Anatomical Structure of L. chinensis
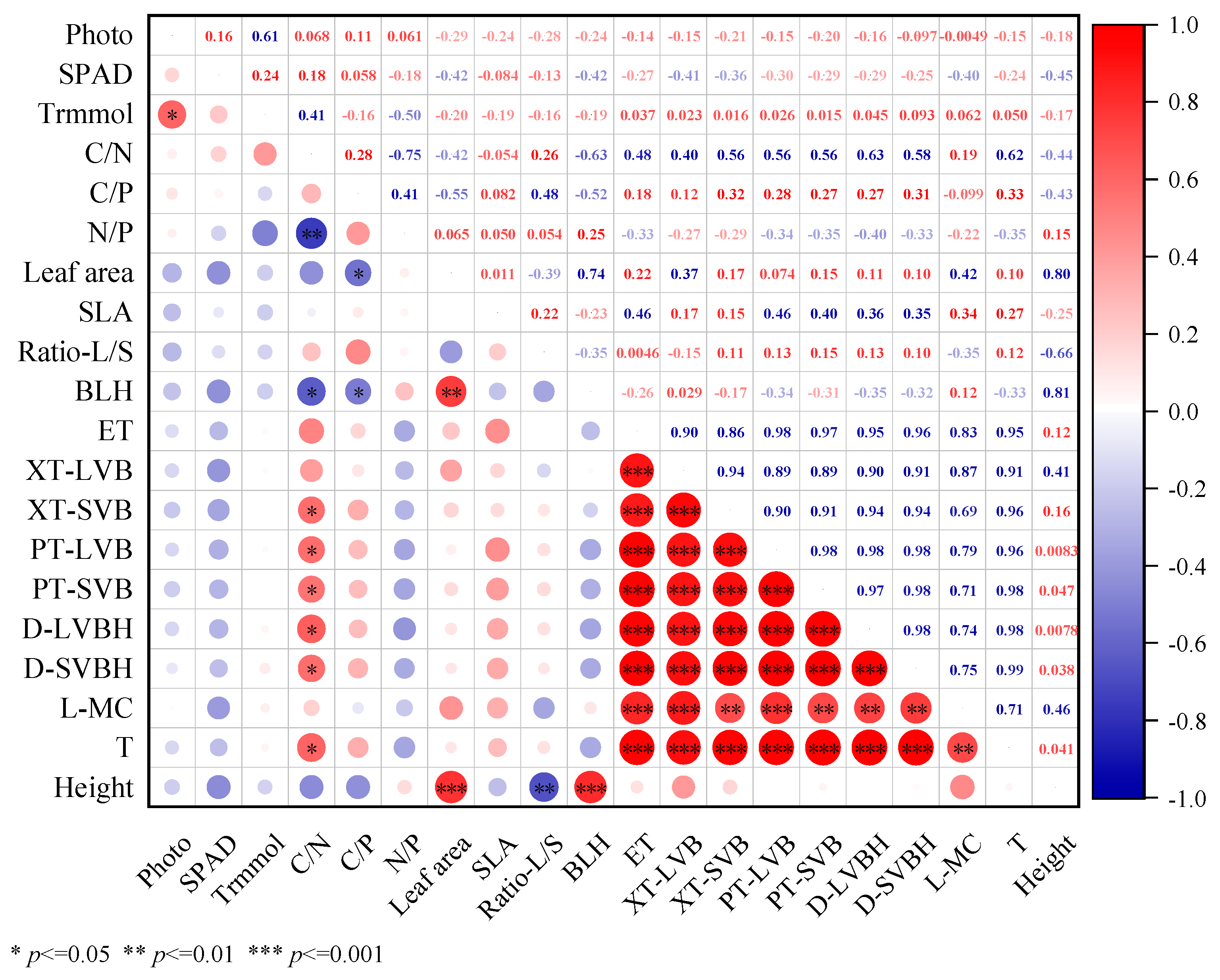
3.6. Relationship Between Photosynthetic Efficiency, Ability to Obtain Light, Resource Utilization Efficiency, Leaf Anatomical Structure, and Plant Height
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y.Q.; Zhou, X.X.; Sherry, R.A. Terrestrial carbon-cycle feedback to climate warming. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 683–712. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K.; Collins, S.L. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 2009, 90, 3279–3289. [Google Scholar] [CrossRef]
- Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Normand, S.; Rüger, N.; Beck, P.S.A.; Blach-Overgaard, A.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Plant functional trait change across a warming tundra biome. Nature 2018, 562, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, C.M.; Branfireun, B.A.; McLaughlin, J.W.; Lindo, Z. Climate change drives a shift in peatland ecosystem plant community: Implications for ecosystem function and stability. Glob. Chang. Biol. 2015, 21, 388–395. [Google Scholar] [CrossRef]
- Riibak, K.; Bennett, J.A.; Kook, E.; Reier, U.; Tamme, R.; Bueno, G.; Pärtel, M. Drivers of plant community completeness differ at regional and landscape scales. Agric. Ecosyst. Environ. 2020, 301, 107004. [Google Scholar] [CrossRef]
- Avolio, M.L.; Forrestel, E.J.; Chang, C.C.; Pierre, K.; Burghardt, K.T.; Smith, M.D. Demystifying dominant species. New Phytol. 2019, 223, 1106–1126. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. (Contribution of Working Group I to the Fifth Assessment. Report of the Intergovernmental Panel on Climate Change); Cambridge University Press: Cambridge, UK, 2022; p. 1535. [Google Scholar]
- Pauli, H.; Gottfried, M.; Reiter, K.; Klettner, C.; Grabherr, G. Signals of range expansions and contractions of vascular plants in the high Alps: Observations (1994-2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob. Chang. Biol. 2006, 13, 147–156. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; Rasmussen, R.M.; Parsons, D.B. The changing character of precipitation. Bull. Am. Meteorol. Soc. 2010, 84, 1205–1217. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K. Dominant species maintain ecosystem function with non-random species loss. Ecol. Lett. 2003, 6, 509–517. [Google Scholar] [CrossRef]
- Houseman, G.R.; Mittelbach, G.G.; Reynolds, E.L.; Gross, T.L. Perturbations alter community convergence, divergence, and formation of multiple community states. Ecology 2008, 89, 2172–2180. [Google Scholar] [CrossRef]
- Craine, J.M.; Nippert, J.B.; Towne, E.G.; Tucker, S.; Kembel, S.W.; Skibbe, A.; McLauchlan, K.K. Functional consequences of climate change-induced plant species loss in a tallgrass prairie. Oecologia 2011, 165, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.F.; Han, X.G.; Wu, J.G.; Chen, Z.Z.; Li, L.H. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 2004, 431, 181–184. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Qing, H.; Yang, L.; Zhao, L.Q. Non-destructive aboveground biomass estimation of Leymus chinensis individual across large scale. Ecol. Indic. 2021, 131, 108212. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Wang, Z.; Wu, X.; Li, X.; Hu, J.; Shi, H.; Guo, F.; Zhang, Y.; Hou, X.; et al. Pathways of Leymus chinensis Individual aboveground biomass decline in natural semiarid grassland induced by overgrazing: A study at the plant functional trait scale. PLoS ONE 2015, 10, e0124443. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Richards, C.L.; Holsinger, K.E.; Fox, G.A.; Zhang, Z.; Zhou, C. Genetic structure in patchy populations of a candidate foundation plant: A case study of Leymus chinensis using genetic and clonal diversity. Am. J. Bot. 2021, 108, 2371–2387. [Google Scholar] [CrossRef]
- Meng, B.; Li, J.; Yao, Y.; Nippert, J.B.; Williams, D.G.; Chai, H.; Collins, S.; Sun, W. Soil N enrichment mediates carbon allocation through respiration in a dominant grass during drought. Funct. Ecol. 2022, 36, 1204–1215. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wang, S.; Wu, X.; Wang, Y.; Wang, X.; Li, J.; Liang, Z.; Luo, T.; Yu, Y.; et al. Phenotypic plasticity couples with transcriptomic flexibility in Leymus chinensis under diverse edaphic conditions. Environ. Exp. Bot. 2022, 197, 104838. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Lv, X. Responses of plant biomass, photosynthesis and lipid peroxidation to warming and precipitation change in two dominant species (Stipa grandis and Leymus chinensis) from north China grasslands. Ecol. Evol. 2016, 6, 1871. [Google Scholar] [CrossRef]
- Jakob, T.; Schreiber, U.; Kirchesch, V.; Langner, U.; Wilhelm, C. Estimation of chlorophyll content and daily primary production of the major algal groups by means of multiwavelength-excitation PAM chlorophyll fluorometry: Performance and methodological limits. Photosynth. Res. 2005, 83, 343–361. [Google Scholar] [CrossRef]
- Rosbakh, S.; Ro¨mermann, C.; Poschlod, P. Specific leaf area correlates with temperature: New evidence of trait variation at the population, species and community levels. Alp. Bot. 2015, 125, 79–86. [Google Scholar] [CrossRef]
- Deleglise, C.; Meisser, M.; Mosimann, E.; Spiegelberger, T.; Signarbieux, C.; Jeangros, B.; Buttler, A. Drought-induced shifts in plants traits, yields and nutritive value under realistic grazing and mowing managements in a mountain grassland. Agric. Ecosyst. Environ. 2015, 213, 94–104. [Google Scholar] [CrossRef]
- Forrestel, E.J.; Donoghue, M.J.; Edwards, E.J.; Jetz, W.; Du Toit, J.C.O.; Smith, M.D. Different clades and traits yield similar grassland functional responses. Proc. Natl. Acad. Sci. USA 2017, 114, 705–710. [Google Scholar] [CrossRef]
- Gao, R.R.; Yang, X.J.; Liu, G.F.; Huang, Z.Y.; Walck, J.L. Effects of rainfall pattern on the growth and fecundity of a dominant dune annual in a semi-arid ecosystem. Plant Soil 2015, 389, 335–347. [Google Scholar] [CrossRef]
- Sarangi, D.; Irmak, S.; Lindquist, J.L.; Knezevic, S.Z.; Jhala, A.J. Effect of water stress on the growth and fecundity of common waterhemp (Amaranthus rudis). Weed Sci. 2016, 64, 42–52. [Google Scholar] [CrossRef]
- Baghalian, K.; Abdoshah, S.; Khalighi-Sigaroodi, F.; Paknejad, F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.). Plant Physiol. Biochem. 2011, 49, 201–207. [Google Scholar] [CrossRef]
- Ullah, U.; Ashraf, M.; Shahzad, S.; Siddiqui, A.; Piracha, M.; Suleman, M. Growth behavior of tomato (Solanum lycopersicum L.) under drought stress in the presence of silicon and plant growth promoting rhizobacteria. Soil Environ. 2016, 35, 65–75. [Google Scholar]
- Ma, F.; Na, X.; Xu, T. Drought responses of three closely related Caragana species: Implication for their vicarious distribution. Ecol. Evol. 2016, 6, 2763–2773. [Google Scholar] [CrossRef]
- Shi, Z.; Sherry, R.; Xu, X.; Hararuk, O.; Souza, L.; Jiang, L.F.; Xia, J.Y.; Liang, J.Y.; Luo, Y.Q. Evidence for long-term shift in plant community composition under experimental climate warming. J. Ecol. 2015, 103, 1131–1140. [Google Scholar] [CrossRef]
- Wen, J.; Qin, R.M.; Zhang, S.Y.; Yang, X.Y.; Xu, M.H. Effects of long-term warming on the aboveground biomass and species diversity in an alpine meadow on the Qinghai-Tibetan Plateau of China. J. Arid. Land 2020, 12, 252–266. [Google Scholar] [CrossRef]
- Wan, Z.Q.; Ganjurjav, H.; Gu, R.; Hu, G.Z.; Gornish, E.; Chun, X.; Zhou, H.J.; Gao, Q.Z. Changes in plant species dominance maintain community biomass production under warming and precipitation addition in temperate steppe in Inner Mongolia, China. Agric. For. Meteorol. 2023, 341, 109671. [Google Scholar] [CrossRef]
- Zhai, Z.W.; Gong, J.R.; Luo, Q.P.; Pan, Y.; Baoyin, T.; Xu, S.; Liu, M.; Yang, L.L. Effects of nitrogen addition on photosynthetic characteristics of Leymus chinensis in the temperate grassland of Nei Mongol, China. Chin. J. Plant Ecol. 2017, 41, 196–208. [Google Scholar] [CrossRef][Green Version]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits, prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.X.; Luo, T.X.; Wu, B. Optimal balance of water use efficiency and leaf construction cost with a link to the drought threshold of the desert steppe ecotone in northern China. Ann. Bot. 2016, 118, mcw127. [Google Scholar] [CrossRef]
- Naudts, K.; Van Den Berge, J.; Janssens, I.A.; Nijs, I.; Ceulemans, R. Does an extreme drought event alter the response of grassland communities to a changing climate? Environ. Exp. Bot. 2011, 70, 151–157. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Klanderud, K.; Yang, L. Responses in leaf functional traits and resource allocation of a dominant alpine sedge (Kobresia pygmaea) to climate warming in the Qinghai-Tibetan Plateau permafrost region. Plant Soil 2011, 349, 377–387. [Google Scholar] [CrossRef]
- Xu, Z.F.; Hu, T.X.; Wang, K.Y.; Zhang, Y.B.; Xian, J.R. Short-term responses of phenology, shoot growth and leaf traits of four alpine shrubs in a timberline ecotone to simulated global warming, Eastern Tibetan Plateau, China. Plant Species Biol. 2009, 24, 27–34. [Google Scholar] [CrossRef]
- Day, T.A.; Ruhland, C.T.; Xiong, F.S. Warming increases aboveground plant biomass and C stocks in vascular-plant-dominated Antarctic tundra. Glob. Chang. Biol. 2008, 14, 1827–1843. [Google Scholar] [CrossRef]
- Weih, M. Evidence for increased sensitivity to nutrient and water stress in a fast-growing hybrid willow compared with a natural willow clone. Tree Physiol. 2001, 21, 1141–1148. [Google Scholar] [CrossRef]
- Hudson, J.M.G.; Henry, G.H.R.; Cornwell, W.K. Taller and larger: Shifts in Arctic tundra leaf traits after 16 years of experimental warming. Glob. Chang. Biol. 2011, 17, 1013–1021. [Google Scholar] [CrossRef]
- Wang, C.S.; Wang, S.P. A review of research on responses of leaf traits to climate change. Chin. J. Plant Ecol. 2015, 39, 206–216. [Google Scholar] [CrossRef]
- Golodets, C.; Sternberg, M.; Kigel, J. A community-level test of the leaf-height-seed ecology strategy scheme in relation to grazing conditions. J. Veg. Sci. 2009, 20, 392–402. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C: N: P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Aerts, R.; Callaghan, T.V.; Dorrepaal, E.; Van Logtestijn, R.S.P.; Cornelissen, J.H.C. Seasonal climate manipulations result in species-specific changes in leaf nutrient levels and isotopic composition in a sub-arctic bog. Funct. Ecol. 2009, 23, 680–688. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, S.B.; Guo, R.; Guo, J.X. Warming and nitrogen addition alter photosynthetic pigments, sugars and nutrients in a temperate meadow ecosystem. PLoS ONE 2016, 11, e0155375. [Google Scholar] [CrossRef]
- Habermann, E.; Contin, D.R.; Afonso, L.F.; Barosela, J.R.; de Pinho Costa, K.A.; Viciedo, D.O.; Groppo, M.; Martinez, C.A. Future warming will change the chemical composition and leaf blade structure of tropical C3 and C4 forage species depending on soil moisture levels. Sci. Total Environ. 2022, 821, 153342. [Google Scholar] [CrossRef]
- Sandel, B.; Low, R. Intraspecific trait variation, functional turnover and trait differences among native and exotic grasses along a precipitation gradient. J. Veg. Sci. 2019, 30, 633–643. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Xu, M.; Shen, R.C.; Qiu, S. Effects of artificial warming on the structural, physiological, and biochemical changes of maize (Zea mays L.) leaves in northern China. Acta Physiol. Plant. 2013, 35, 2891–2904. [Google Scholar] [CrossRef]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Yang, H.; Auerswald, K.; Bai, Y.F.; Han, X.G. Complementarity in water sources among dominant species in typical steppe ecosystems of Inner Mongolia, China. Plant Soil 2011, 340, 303–313. [Google Scholar] [CrossRef]
- Sasaki, T.; Lauenroth, W.K. Dominant species, rather than diversity, regulates temporal stability of plant communities. Oecologia 2011, 166, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Shi, B.; Zhong, S.; Chai, H.; Sun, W. Drought sensitivity of aboveground productivity in Leymus chinensis meadow steppe depends on drought timing. Oecologia 2019, 191, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Song, M.H.; Zong, N.; Jiang, J.; Shi, P.L.; Zhang, X.Z.; Gao, J.Q.; Zhou, H.K.; Li, Y.K.; Loreau, M. Nutrient induced shifts of dominant species reduce ecosystem stability via increases in species synchrony and population variability. Sci. Total Environ. 2019, 692, 441–449. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef]
- Olsen, S.L.; Tpper, J.P.; Skarpaas, O.; Vandvik, V.; Klanderud, K. From facilitation to competition: Temperature-driven shift in dominant plant interactions affects population dynamics in seminatural grasslands. Glob. Chang. Biol. 2016, 22, 1915–1926. [Google Scholar] [CrossRef]
- Partzsch, M. Warming differently affects the inter- and intraspecific interactions among semi-dry grassland species. Perspect. Plant Ecol. Evol. Syst. 2019, 40, 125481. [Google Scholar] [CrossRef]
- Li, Z.; Lin, J.; Zhang, T.; Zhang, N.; Mu, C.; Wang, J. Effects of summer nocturnal warming on biomass production of Leymus chinensis in the Songnen grassland of China: From bud bank and photosynthetic compensation. J. Agron. Crop Sci. 2014, 200, 66–76. [Google Scholar] [CrossRef]
- Denton, E.M.; Dietrich, J.D.; Smith, M.D.; Knapp, A.K. Drought timing differentially affects above- and belowground productivity in a mesic grassland. Plant Ecol. 2017, 218, 317–328. [Google Scholar] [CrossRef]
- Yang, Z.L.; Zhang, Q.; Su, F.L.; Zhang, C.H.; Pu, Z.C.; Xia, J.Y.; Wan, S.Q.; Jiang, L. Daytime warming lowers community temporal stability by reducing the abundance of dominant, stable species. Glob. Chang. Biol. 2016, 23, 154–163. [Google Scholar] [CrossRef]
- Evans, S.E.; Byrne, K.M.; Lauenroth, W.K.; Burke, I.C. Defining the limit to resistance in a drought-tolerant grassland: Long-term severe drought significantly reduces the dominant species and increases ruderals. J. Ecol. 2011, 99, 1500–1507. [Google Scholar] [CrossRef]
- Chen, L.P.; Zhao, N.X.; Zhang, L.H.; Gao, Y.B. Responses of two dominant plant species to drought stress and defoliation in the Inner Mongolia steppe of China. Plant Ecol. 2013, 214, 221–229. [Google Scholar] [CrossRef]



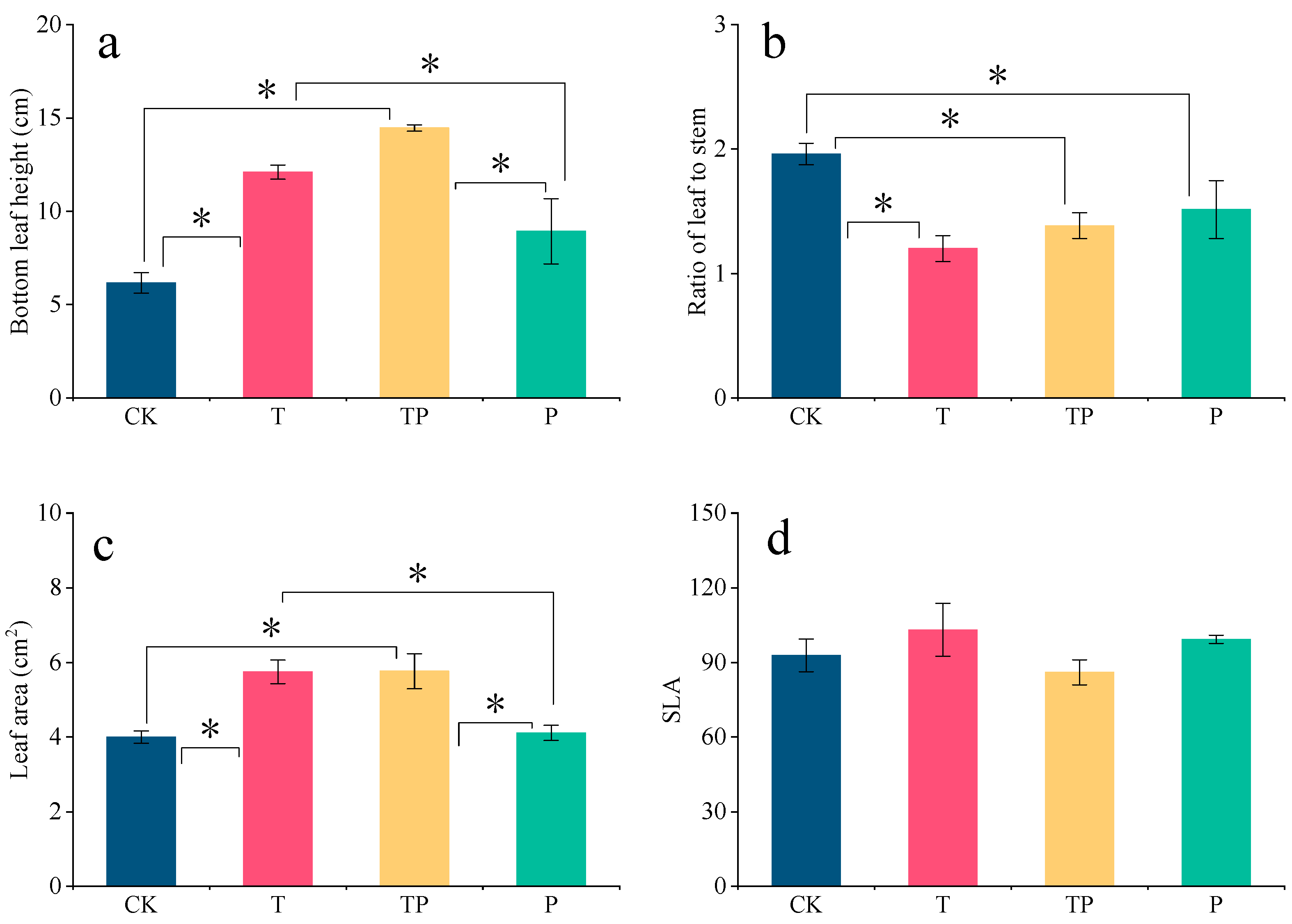


| CK | T | TP | P | |
|---|---|---|---|---|
| Epidermal thickness (µm) | 20.88 ± 0.68 a | 22.34 ± 0.71 a | 12.34 ± 0.28 b | 12.17 ± 0.31 b |
| Xylem thickness of large vascular bundle (µm) | 75.60 ± 5.22 a | 85.57 ± 7.56 a | 50.17 ± 2.03 b | 35.24 ± 1.97 c |
| Xylem thickness of small vascular bundle (µm) | 34.75 ± 1.55 a | 32.74 ± 1.89 a | 20.22 ± 0.91 b | 13.82 ± 0.83 c |
| Phloem thickness of large vascular bundle (µm) | 75.64 ± 1.68 a | 74.61 ± 2.36 a | 42.66 ± 0.45 b | 44.57 ± 0.91 b |
| Phloem thickness of small vascular bundle (µm) | 60.56 ± 1.24 a | 56.39 ± 1.86 a | 36.45 ± 0.67 b | 33.25 ± 0.94 c |
| Diameter of large vascular bundle sheath (µm) | 27.50 ± 1.05 a | 25.57 ± 0.92 a | 12.06 ± 0.37 b | 11.18 ± 0.24 b |
| Diameter of small vascular bundle sheath (µm) | 16.97 ± 0.60 a | 15.85 ± 0.48 a | 8.29 ± 0.23 b | 7.71 ± 0.18 b |
| Length of motor cell (µm) | 59.12 ± 1.40 b | 90.33 ± 2.44 a | 47.07 ± 1.13 c | 47.96 ± 1.35 c |
| Leaf thickness (µm) | 339.86 ± 17.71 a | 316.05 ± 12.02 a | 199.34 ± 6.62 b | 177.59 ± 5.35 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Z.; Gu, R.; Liang, Y.; Chun, X.; Zhou, H.; Zhang, W. Height and Light-Obtaining Ability of Leymus chinensis Increased After a Decade of Warming in the Typical Steppe of Inner Mongolia, China. Plants 2025, 14, 1702. https://doi.org/10.3390/plants14111702
Wan Z, Gu R, Liang Y, Chun X, Zhou H, Zhang W. Height and Light-Obtaining Ability of Leymus chinensis Increased After a Decade of Warming in the Typical Steppe of Inner Mongolia, China. Plants. 2025; 14(11):1702. https://doi.org/10.3390/plants14111702
Chicago/Turabian StyleWan, Zhiqiang, Rui Gu, Yan Liang, Xi Chun, Haijun Zhou, and Weiqing Zhang. 2025. "Height and Light-Obtaining Ability of Leymus chinensis Increased After a Decade of Warming in the Typical Steppe of Inner Mongolia, China" Plants 14, no. 11: 1702. https://doi.org/10.3390/plants14111702
APA StyleWan, Z., Gu, R., Liang, Y., Chun, X., Zhou, H., & Zhang, W. (2025). Height and Light-Obtaining Ability of Leymus chinensis Increased After a Decade of Warming in the Typical Steppe of Inner Mongolia, China. Plants, 14(11), 1702. https://doi.org/10.3390/plants14111702





