Abstract
The Phalaenopsis genus, a horticulturally vital group within the Orchidaceae, dominates global floriculture markets through strategic cultivar innovation, scalable propagation, and data-driven cultivation. This review systematically examines the breeding, propagation, cultivation management, and potential applications of Phalaenopsis while providing insights into future research directions. The main contents include the following: Breeding innovations—This review outlines the taxonomy of the Phalaenopsis genus and highlights its intergeneric hybridization potential, which offers vast opportunities for developing novel horticultural varieties. By establishing clear breeding objectives, researchers employ diverse breeding strategies, including conventional crossbreeding and biotechnological approaches (e.g., mutation breeding, ploidy manipulation, genetic transformation, and CRISPR/Cas9 editing). Propagation and cultivation management—Analyses of Phalaenopsis tissue culture protocols covering explant selection, media optimization, and regeneration systems are summarized. Key factors for efficient cultivation are discussed, including temperature, light, water, nutrient management, cultivation medium selection, and integrated pest/disease management. Scientific environmental control ensures robust plant growth, synchronized flowering, and high-quality flower production. Emerging applications—Phalaenopsis exhibits promising applications in functional bioactive compound extraction (e.g., antioxidants and antimicrobial agents). This review summarizes current advancements in Phalaenopsis breeding, cultivation, and potential applications. Based on technological progress and market demands, future research directions are proposed to support the sustainable development of the Phalaenopsis industry.
1. Introduction
As one of the largest angiosperm families, the Orchidaceae comprises over 750 genera and 31,485 documented species [1,2,3]. Orchids are renowned for their extraordinary diversity and vibrant colors, captivating observers with their stunning array of hues and intricate inflorescences [4]. Their extended shelf life further enhances their appeal, solidifying their status as a key commodity in the floral industry [5,6]. The Phalaenopsis genus stands out as one of the most horticulturally significant groups within the Orchidaceae family, being widely regarded as the top ornamental orchid globally [7]. These plants are highly sought after as both cut and potted flowers, making them a major commodity in international trade [8,9].
Due to their low hybridization barriers and distinctive reproductive mechanisms [9], the Orchidaceae family exhibits a remarkable ability to hybridize. Since 2003, approximately 500 new species within this family have been identified each year [10,11]. Phalaenopsis is among the most extensively researched genera, with significant advancements in breeding studies [12,13]. Currently, it includes 210 species, comprising 104 accepted names, 96 synonyms, and 10 unverified classifications [1]. Additionally, the Royal Horticultural Society (RHS) International Orchid Register Database records over 39,000 artificially created Phalaenopsis hybrids [14].
Over 70% of orchid species thrive as epiphytes [15], while others are terrestrial and require support for growth. Most orchids are perennial plants lacking permanent woody structures and display two growth patterns: monopodial and sympodial [16]. Both species and hybrids of Phalaenopsis are epiphytic and monopodial orchids [9,17]. Their leaves are thick and fleshy, with efficient water and nutrient storage, and possess crassulacean acid metabolism (CAM), enabling them to adapt to a wide range of environments [18].
In the modern era, advancements in biotechnological interventions and cultivation techniques have significantly driven the growth of the orchid industry and its global trade [19]. The orchid industry not only generates sustainable outcomes but also establishes a high-value system. For instance, in 2020, the Phalaenopsis orchid market in the Netherlands was valued at EUR 422 million, with 117 million units sold [9]. The successful commercialization of Phalaenopsis orchids is closely tied to the development of new cultivars, efficient propagation systems, and intensive cultivation practices (Figure 1).
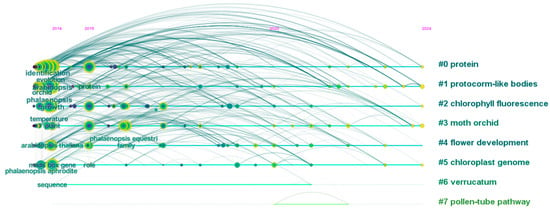
Figure 1.
A temporal evolution visualization of keyword frequencies in Phalaenopsis research (2014–2024). The co-occurrence network was generated from articles indexed in Web of Science Core Collection using “Phalaenopsis” as the search term (2014–2024 publications) and analyzed through CiteSpace software (version 6.2.R4).
The orchid industry still faces critical scientific challenges, including the molecular mechanisms underlying Phalaenopsis floral fragrance, pigmentation, and stress responses under adverse conditions, as well as technical bottlenecks such as difficulties in gene editing delivery, innovative flower color breeding, the development of high-quality cultivation protocols, and product commercialization. This article provides an overview of orchid breeding, with a specific focus on Phalaenopsis, covering its classification and breeding methods. It also explores current and future trends in the development of cultivars, cultivation practices, and potential application.
2. Orchid Breeding
Orchids are primarily found in the tropical regions stretching from Asia to Australia [16]. Morphologically and anatomically, the Orchidaceae family can be divided into 5 subfamilies: Apostasioideae (2 genera and 16 species), Cypripedioideae (5 genera and 130 species), Vanilloideae (15 genera and 180 species), Orchidoideae (208 genera and 3630 species), and Epidendroideae (over 500 genera and 20,000 species) [20,21,22]. Among these, Epidendroideae is the largest subfamily [23,24]. Orchids are among the most hybridized, captivating, and commercially significant ornamental plants in global floriculture [9], with the Phalaenopsis genus, along with its intergeneric and interspecific hybrids, dominating as key cut and potted flowers in international flower markets. Therefore, understanding the classification of the Phalaenopsis genus is crucial for Phalaenopsis breeding.
2.1. The Classification of the Phalaenopsis Genus
The Phalaenopsis genus, commonly known as the moth orchid, is part of the Vandaeae tribe within the Epidendroideae subfamily [17,25]. Its flowers are characterized by three outer sepals, two petals, and a distinct lip petal, with the carpel positioned centrally and the stigma and stamen being fused into a single column. Despite its widespread popularity, the taxonomy of Phalaenopsis remains unclear, and its classification has been a subject of uncertainty for nearly two centuries [7,25].
In 1753, Phalaenopsis was first named by Linnaeus and classified under Epidendrum as ‘Epidendrum amabile’ [26]. In 1814, Roxburgh reclassified it into Cymbidium and named it ‘Cymbidium amabilis’ [7]. The genus Phalaenopsis was formally established by Blume in 1825, who renamed it ‘Phalaenopsis amabilis’ [7,26]. In 1980, Sweet categorized 46 species within the Phalaenopsis genus into nine sections, Phalaenopsis, Proboscidioides, Aphyllae, Parishianae, Polychilos, Stauroglottis, Fuscatae, Amboinenses, and Zebrinae [27], a classification that became foundational for subsequent taxonomic studies. In 2001, Christenson, based on morphological characteristics, divided approximately 66 species into five subgenera: Proboscidioides, Aphyllae, Parishianae, Polychilos, and Phalaenopsis [27,28]. This system remains the most authoritative morphological classification and is widely used in horticultural applications. Moving forward, the application of molecular marker technology to orchid classification and phylogenetic analysis will likely lead to a re-evaluation and discussion of previous classification systems [29].
2.2. The Breeding of Phalaenopsis
In horticulture, the term ‘moth orchid’ extends beyond just Phalaenopsis species and their interspecific hybrids [28]. While, taxonomically, closely related species are more likely to produce hybrids, the focus in horticulture and commercial breeding is on creating new hybrid plants [28]. This means that the commercial classification of orchids must be distinguished from their botanical classification [30]. The existence of natural hybrids in Orchidaceae demonstrates that many genetic barriers between species or genera are not fully established as criteria for reproductive incompatibility [28]. Most commercial orchid flowers are derived from interspecific or intergeneric hybrids. Over 20 closely related genera can hybridize with Phalaenopsis, enabling the development of numerous new hybrid genera and species [28] (Figure 2). Consequently, the potential to create innovative horticultural cultivars is immense.

Figure 2.
A visualization of Phalaenopsis species (green circles) and Phalaenopsis hybrids (white circles) created using NRD Studio software (Version 3.3.1). These hybrids were derived from crossbreeding through interspecific and intergeneric (Table S1, data source: Ichihashi and Mii [28]).
2.2.1. Innovation in New Horticultural Cultivars
The breeding of new horticultural cultivars focuses on a variety of desirable traits, including flower color, size, shape, texture, longevity, and resistance to pathogens [28]. For example, blue flowers have always been highly sought after, with the development of blue Phalaenopsis orchids being a significant milestone. Mii successfully created the world’s first blue Phalaenopsis, representing a breakthrough in orchid breeding [31]. Additionally, there has been growing market demand for mini-sized Phalaenopsis orchids, which are ideal for use as table flowers. Several mini cultivars have been developed, such as ‘KS Little Gem’, ‘Queen Beer’, ‘Tony Pink’, ‘Vaviche’, and ‘Rorens’, with the petal lengths ranging from 24.9 to 33.8 mm and the widths from 15.9 to 22.6 mm [32]. Among these, the mini-sized P. ‘Sogo Vivien’ stands out for its abundance of beautiful pink flowers [33], while ‘Bravo Star’ is prized for its fragrant, miniature pink blooms [34].
In terms of petal texture, Phalaenopsis flowers exhibit two distinct natural textures: velvety and waxy [35]. Velvety petals are larger, thinner, and lighter, offering striking visual appeal. A notable example is P. Sogo Yukidian ‘V3’, a renowned cultivar with large, white velvety petals [36,37]. On the other hand, waxy petals are thicker, more durable, and longer-lasting, making them suitable for long-distance transport. ‘Frigdaas Oxford’ is a medium–small cultivar with a yellow ground color and red-purple patterns, featuring waxy petals. It is also known for its high heat tolerance and resistance to pathogens [35,38]. These advancements highlight the diverse and innovative breeding efforts in the development of Phalaenopsis cultivars.
2.2.2. Breeding Methods
Both conventional methods (e.g., selective breeding, hybridization) and biotechnological approaches, including haploid induction, polyploidization, genetic transformation, and CRISPR/Cas9 genome editing, are utilized to engineer flowers exhibiting diverse ornamental traits such as novel pigmentation, extended shelf life, and enhanced stress tolerance (Table 1). Despite technological advancements, conventional hybridization remains the dominant and most cost-effective strategy for generating new Phalaenopsis cultivars due to its high compatibility with interspecific crosses and predictable phenotypic outcomes [9,39].

Table 1.
Breeding methods for Phalaenopsis cultivars.
Hybridization Breeding
Traditional cross-hybridization remains the most widely used conventional breeding method in Phalaenopsis [64]. Hybrid breeding serves as the foundational approach for combining different genotypes, whether that be within the same cultivar, between different varieties, or even across different species and genera [42,65]. The selection of parent plants is critical and depends on the specific breeding objectives. For instance, most deep red large-flowered Phalaenopsis orchids trace their lineage to Doritaenosis hybrids. A notable example is the cultivar Dtps. Hinacity Glow, which was developed by crossing Dtps. Coral Gleam and P. Herbert Hager [66]. Similarly, yellow Phalaenopsis cultivars are predominantly derived from species in the subgenus Polychilos, such as P. amboinensis, P. fasciata, P. lueddemanniana, and P. venosa [67]. The renowned yellow cultivar P. Taipei Gold, for example, is a hybrid of P. Glays Read ‘Snow Queen’ and P. venosa [68].
Spotted Phalaenopsis orchids often inherit traits from P. lueddemanniana. A landmark in Phalaenopsis breeding is P. Paifang’s Queen, which was created by crossing P. Mount Kaala and P. lueddemanniana. Its exceptional individual, P. Paifang’s Queen ‘Brother’, has been extensively used as a parent to breed red-spotted Phalaenopsis varieties [69]. Other key parents for spotted types include P. Golden Sands, P. Stuartiana, P. Ho’s Francy Leopard, P. Golden Peoker, P. Sentra, and P. Super Stupid [68,69].
White-colored Phalaenopsis orchids are also highly popular. The primary native species contributing to white flowers are P. amabilis, P. aphrodite, and P. amabilis var. formosana [70,71]. Additionally, other outstanding parents for breeding white Phalaenopsis include P. Chieftain, P. Joseph Hampton, P. San Marino, and P. Barbara Kirch [66,69]. These examples highlight the strategic use of hybridization to achieve diverse and desirable traits in Phalaenopsis breeding.
The above guidelines pertain to parental selection for breeding Phalaenopsis cultivars with diverse floral pigmentation. Additionally, the following principles are typically applied: maternal parent, prioritizing plants exhibiting vigorous growth and stable phenotypic performance; paternal parent, selecting individuals producing abundant, viable pollen with high germination rates (>85%); and trait-directed selection, when specific genetic traits (e.g., striped tepals) require maternal inheritance dominance. The parent carrying these target characteristics is designated as the maternal line [28].
Mutagenesis Breeding
Mutagenesis breeding encompasses both naturally occurring mutations and artificially induced mutations through the application of specific chemical agents or physical mutagens [72]. Artificial methods are particularly effective in increasing the frequency of mutations in plants [46,72]. Among these, gamma irradiation is a widely used technique [73,74]. Studies have shown that lower doses of gamma irradiation (around 15 Gy) can induce early flowering in Phalaenopsis [49,75], while higher doses (above 40 Gy) tend to inhibit flowering [76]. For instance, the P. equestris irradiated with a dose of 40 Gy exhibited rapid growth and development within just ten days post treatment [77]. Additionally, gamma-irradiated protocorms of Phalaenopsis amabilis have been used to develop mutants with enhanced resistance to soft rot disease [47]. These findings highlight the potential of mutation breeding, particularly gamma irradiation, in improving Phalaenopsis traits such as flowering time, growth rate, and disease resistance.
Ploidy Breeding
Ploidy breeding is a pivotal technique in plant genetics and involves the manipulation of chromosome numbers through the loss or gain of chromosomes relative to the normal chromosome complement [78]. This approach encompasses both haploid and polyploid breeding strategies. Haploid breeding, typically utilizing another culture technology, facilitates the rapid development of pure diploid lines through chromosome doubling, thereby circumventing the extensive processes of separation, selection, and stabilization inherent in conventional hybrid breeding. This method significantly accelerates the breeding cycle [79]. Moreover, haploid mutations are expressed directly without allelic interference, rendering their callus tissues optimal for mutation breeding. Artificial selection at the cellular level of callus tissue proves to be markedly more efficient than selection at the individual level [80]. In addition, there have been other attempts to induce haploids; for instance, Kazumitsu et al. explored the induction of haploid moth orchids by cultivating pseudofertilized ovules [81]. Similarly, Teixeira da Silva et al. attempted in vitro organogenesis in various parts of immature and fully opened P. Gallant Beau ‘George Vazquez’ flowers to generate haploid organs [82]. Although these efforts were not successful, they underscored the potential for such regeneration techniques in haploid production.
Polyploid breeding, involving the doubling of chromosomes through natural or artificial induction, is a crucial method for introducing genetic variation [83]. Polyploid cultivars often exhibit superior vigor, growth, stem sturdiness, leaf and flower size, and resistance compared to their diploid counterparts [84]. The Phalaenopsis genus primarily consists of diploid wild species, with a basic chromosome number of 2n = 2x = 38 [85,86]. However, the chromosome numbers of cultivated varieties vary, including tetraploid, triploid, and aneuploid forms [87], with tetraploid cultivars being predominant in commercial markets [88]. The ploidy differences between wild species and commercial cultivars complicate the transfer of desirable genes. Additionally, significant variations in chromosome size among Phalaenopsis species often result in the infertility of interspecific hybrids [89].
Polyploid induction in Phalaenopsis is typically achieved through chemical agents such as colchicine, oryzalin, trifluralin, and nitrous oxide (N2O) [54,89,90]. Phalaenopsis amabilis is the most commonly used species for such inductions [85,91,92]. Other varieties have also been tested for in vitro polyploid induction, including P. amboinensis [92], P. equestris [93], P. bellina [94], P. aphrodite [95], and various hybrid orchids (Phalaenopsis spp. and Doritaenopsis sp.) [96]. Common induction materials include protocorms and protocorm-like bodies (PLBs) [94], with unreduced gametes [85] and buds [97] also being utilized.
In summary, through both haploid and polyploid strategies, ploidy breeding offers significant advancements in plant breeding by accelerating the development of new varieties and enhancing desirable traits. The application of these techniques in Phalaenopsis orchids highlights their potential in overcoming breeding challenges and improving commercial cultivars.
Genetic Transformation and CRISPR/Cas9 Genome Editing Technology
Genetic transformation is a powerful technique for introducing exogenous genes or DNA fragments into plant genomes, enabling the transfer of traits that are unattainable through conventional hybridization [9]. In Phalaenopsis, genetic transformation can be achieved through particle bombardment and Agrobacterium-mediated methods, both of which are recognized as efficient and reliable approaches [6,98].
Particle bombardment is a direct physical transformation process and has been effectively utilized in Phalaenopsis. For instance, Chew et al. established an efficient particle bombardment transformation system for P. bellina using PLBs as target tissues [57]. Similarly, Fan employed particle bombardment to generate P. equestris var. alba plants resistant to odontoglossum ringspot virus (ORSV) by overexpressing either sense or anti-sense strands of the ORSV coat protein gene [99]. Additionally, Anzai et al. successfully delivered marker genes encoding Escherichia coli β-glucuronidase and Aequorea victoria green fluorescent protein into Phalaenopsis cells using this method [100].
In contrast, the Agrobacterium-mediated transformation system is often preferred due to its simplicity [9]. Belarmino et al. successfully produced transgenic Phalaenopsis plantlets by transforming suspension culture cells using Agrobacterium tumefaciens strains LBA4404 (Ptok233) and EHA101 (Pig121Hm) [101]. Semiarti et al. developed a genetic transformation method for P. amabilis using protocorms as the target material, mediated by Agrobacterium tumefaciens [102]. They further improved the transformation efficiency by pre-culturing protocorms on a medium containing an extract from fully ripe tomato fruit [103]. In a subsequent study, they utilized pollen (pollinia and pollinaria) of P. amabilis as target materials, immersing the pollen in an overnight culture of Agrobacterium and self-pollinating the flowers with the inoculated pollen. This approach allowed for the selection of positive transformants from the next generation, bypassing the need for in vitro inoculation [104].
The CRISPR/Cas9 system is a groundbreaking genome editing tool and enables precise modifications of target genes through guided sequence recognition [105]. Nopitasari et al. developed a method for delivering T-DNA into P. amabilis protocorms using Agrobacterium tumefaciens EHA101 carrying a T-DNA construct with UBI::Cas9::U3::VAR2 in the pRGEB32 vector [61]. To refine molecular breeding methodologies using CRISPR/Cas9, Semiarti et al. sowed P. amabilis seeds on NP+ peptone media (2 g L−1) to obtain protocorms, which were then submerged in a culture of Agrobacterium tumefaciens containing a T-DNA construct of the pRGEB32 vector harboring sgRNA with the Phytoene desaturase (PDS3) sequence [63]. Xia et al. advanced the field by developing two multiplex genome editing tools for Phalaenopsis: the PTG-Cas9-HPG (polycistronic tRNA-gRNA) system and the RMC-Cpf1-HPG (ribozyme-based multi-crRNA) system [60]. Furthermore, the CRISPR/Cas9 system has been employed to accelerate flowering in P. amabilis by inactivating the Gibberellic acid insensitive (GAI) gene, a mutation that enhances the regulation of FLOWERING TIME (FT) genes in the flowering biosynthesis pathway [59].
In summary, genetic transformation and genome editing technologies, including particle bombardment, Agrobacterium-mediated transformation, and CRISPR/Cas9, have significantly advanced the breeding and genetic improvement of Phalaenopsis, offering precise and efficient methods for trait manipulation and accelerated breeding cycles. After successfully breeding a new Phalaenopsis variety, the next steps involve expanding the population of superior cultivars, providing optimal cultivation environments and management practices, and ultimately cultivating robust plants that produce high-quality blooms.
3. Phalaenopsis Orchid Propagation and Cultivation Practices
3.1. The Propagation of Phalaenopsis
In their natural state, Phalaenopsis orchids primarily propagate through seeds, and artificial aseptic sowing can yield a large number of sterile seedlings [28]. However, seed propagation fails to meet the demands of factory production in terms of cultivar consistency and quantity [106]. Additionally, seedlings propagated through vegetative methods often exhibit inconsistent characteristics [107]. Therefore, tissue culture has become the primary approach for mass-producing elite commercial Phalaenopsis cultivars [108].
A variety of explants can be used for Phalaenopsis tissue culture, including flower stalks [109,110,111], shoot tips [112,113,114], leaves [107,110,115], and roots [116,117]. Among these, flower stalks are the most commonly selected explants. The Murashige and Skoog (MS) culture medium is the most widely used for Phalaenopsis propagation.
For instance, Chen employed a root medium composed of 1/2 MS nutrients supplemented with 200 mL L−1 coconut liquid, 6.5 g L−1 agar, 10 g L−1 sucrose, 10 mg L−1 6-benzylaminopurine (BA), and 5.0 mg L−1 α-naphthaleneacetic acid (NAA) to cultivate P. Sogo Yukidian ‘V3’ [118]. This study also evaluated the effects of temperature and light irradiance on growth characteristics. Similarly, Minh utilized an MS culture medium enriched with BA, thidiazuron (TDZ), β-indol butyric acid (IBA), NAA, adenine, 6-furfurylaminopurine, 10 mg L−1 vitamin B1, 1 g L−1 peptone, 10% coconut water, 30 g L−1 sucrose, and 1 g L−1 activated carbon to investigate the large-scale propagation of Phalaenopsis orchids using bioreactor technology [115]. Furthermore, Barough et al. evaluated the effects of eight different culture media on the growth of in vitro mini plantlets of Phalaenopsis orchids. Among the eight culture media tested, the SM2 high-carbon formulation demonstrated the highest growth rate. Its balanced 20-20-20 NPK ratio mitigated the inhibitory effects of high nitrogen levels on seed germination while promoting leaf and root development to enhance regeneration efficiency. SM2’s replacement of synthetic vitamins (e.g., nicotinic acid, thiamine) with banana powder and other natural components reduced chemical mutagenesis risks, supporting genetic stability. When combined with the TIS-FA-Bio system, SM2 balanced the growth rate, regeneration capacity, and genetic stability while achieving a 72.5% cost reduction [119]. Their findings highlighted that the optimal culture medium for orchid growth is highly dependent on the specific cultivation system employed.
Tissue culture, particularly the use of flower stalks as explants and MS-based media, has become the cornerstone of Phalaenopsis propagation, enabling the production of consistent and high-quality cultivars for commercial purposes. Advances in culture media formulations and bioreactor technology further enhance the efficiency and scalability of this process.
3.2. Cultivation Practices of Phalaenopsis Orchid
3.2.1. The Biological Characteristics of Phalaenopsis
Phalaenopsis orchids exhibit unique biological traits that enable them to thrive in their natural environments. They possess short stems and thick, succulent leaves with stomata that open at night, an adaptation that minimizes water loss in water-limited conditions. This characteristic allows them to perform CAM photosynthesis, which enhances water use efficiency and maximizes CO2 uptake [120].
Native to subtropical and tropical regions, Phalaenopsis orchids are adapted to consistently warm temperatures [121]. Consequently, they are highly sensitive to low temperatures and susceptible to cold injury [122]. Temperature plays a critical role in their flowering process, with floral induction being inhibited when temperatures exceed 28 °C [123].
In their natural habitat, Phalaenopsis orchids grow epiphytically on trees, with their roots exposed to moist air [124]. This adaptation makes them vulnerable to both drought and waterlogging. Drought conditions cause leaves to become wrinkled and flaccid, while waterlogging damages the roots.
The leaves of Phalaenopsis orchids naturally droop, and the amount of light they receive is influenced by tree shade and the angle of light incidence. This light intensity is optimal for their growth [28]. Under typical temperature and low-light conditions, Phalaenopsis orchids can maintain a long flower display life [124].
The biological characteristics of Phalaenopsis orchids, including their CAM photosynthesis, temperature sensitivity, epiphytic growth habit, and light requirements, are key to their survival and ornamental value. Understanding these traits is essential for their successful cultivation and conservation.
3.2.2. Environmental Adaptability of Phalaenopsis Cultivars
The environmental adaptability of Phalaenopsis cultivars is a critical factor in their successful cultivation. For instance, cultivars bred in cooler regions often struggle to adapt to high-temperature summer conditions [28]. In areas with prolonged and intense summer heat, Phalaenopsis cultivation necessitates the use of cooling systems such as fans and water curtains, resulting in significant energy consumption [125]. Therefore, selecting cultivars with superior heat resistance is essential. P. Tongzhen, for example, exhibits the highest heat tolerance and is particularly suitable for cultivation in regions with hot summers, such as southern China [126].
Conversely, in low-temperature winter regions, heating systems are required to maintain optimal growing conditions, which not only increases costs but also leads to substantial energy consumption. Cold-tolerant cultivars, however, can thrive in relatively low temperatures without the need for excessive energy input [127]. Several Phalaenopsis cultivars are known for their strong low-temperature tolerance, including P. mannii, P. lobbi, P. Alishan, P. In the Mood for Love, P. Wedding Promenade, P. Love at First Sight, and P. Fule Star. These cultivars are well suited for cultivation in middle- to high-latitude regions [128,129].
Whether for heat tolerance in warm climates or cold tolerance in cooler regions, the selection of Phalaenopsis cultivars with appropriate environmental adaptability can significantly reduce energy consumption and operational costs, ensuring sustainable and efficient cultivation practices.
3.2.3. Environmental Factors of Phalaenopsis Cultivation
To obtain high-quality Phalaenopsis orchids, it is essential to provide a suitable environment and implement appropriate cultivation management practices. Below are key factors to consider (Table 2).

Table 2.
The cultivation and management of Phalaenopsis.
Temperature
Phalaenopsis orchids, like many tropical plants, are sensitive to cold temperatures. Prolonged exposure to temperatures below 15 °C can lead to physiological issues. Daems et al. investigated the effects of chilling temperatures on the photosynthetic performance of P. ‘Edessa’ and found that leaf photosynthesis was impaired after 24 h at 10 °C, with a significant decline in the performance index [130]. Similarly, Ma et al. studied P. amabilis ‘Green Bear’ and P. amabilis ‘Anna’ at 5 °C for 12, 24, and 48 h. They observed that chilling stress caused frozen spots on new leaves and the wilting of old leaves by the second day, with severe damage and leaf drop by the fourth day in P. amabilis ‘Anna’ [143]. Mu et al. suggested that spraying Phalaenopsis leaves with Sodium Nitroprusside solution could mitigate low-temperature stress [144].
For optimal growth and flowering, Phalaenopsis orchids are typically cultivated in greenhouses with controlled temperatures. High temperatures are maintained during the growth phase, while lower temperatures are necessary for inflorescence initiation [123,145]. Newton and Runkle emphasized that a daytime temperature of 26 °C or lower is crucial for inflorescence initiation, while exposure to temperatures above 29 °C for 8 or 12 h can inhibit flowering [146]. Jeong et al. found that high temperatures before the induction phase delayed flowering initiation and inflorescence development in P. Queen Beer ‘Mantefon’ [131]. Lee et al. demonstrated that intermittent high temperatures could prevent premature flowering by reducing the percentage of visible inflorescences and delaying their emergence while also decreasing soluble sugar content in leaves [123,147].
Temperature management is critical for the healthy growth and flowering of Phalaenopsis orchids. Both excessively low and high temperatures can disrupt physiological processes, emphasizing the need for precise temperature control in cultivation practices.
Water
Proper watering is essential for maintaining the health and vitality of Phalaenopsis orchids. When adequately watered, the relative water content of their leaves remains between 85 and 94%, and the roots exhibit a vibrant green color [134]. However, under drought stress, the roots take on a silvery hue, signaling water deficiency [124]. Tay et al. demonstrated that after 7 weeks of drought, P. cornu-cervi experienced a significant reduction in photosynthetic light utilization, with the relative water content in leaves dropping below 50% [134]. Ceusters et al. investigated the effects of drought stress on the photosynthetic performance of P. ‘Edessa’. Their study revealed that drought conditions led to increased thermal dissipation and the inactivation of partial PS‖ reaction centers, disrupting the electron flow from PS‖ to PS| [133]. Jeong and Oh examined the impact of 40 days of simulated shipping without watering on the flowering of P. Sogo Yukidian ‘V3’ [132]. Their findings indicated that drought-stressed plants experienced delayed flowering. To mitigate such stress, Gu proposed the application of chitosan spray on leaves, which was shown to alleviate drought stress in Phalaenopsis seedlings by enhancing the content of osmoregulatory substances [148].
Maintaining optimal watering practices is crucial for ensuring the photosynthetic efficiency, root health, and timely flowering of Phalaenopsis orchids. Drought stress not only impairs physiological functions but also delays developmental processes, underscoring the importance of consistent and adequate water management in cultivation.
Light
Light plays a pivotal role in photosynthesis and the overall health of Phalaenopsis orchids. However, excessive light exposure can lead to the formation of brown spots on the leaves, adversely affecting growth [135]. In computer-controlled greenhouses, the optimal light intensity for Phalaenopsis cultivation typically ranges between 200 and 300 µmol m−2 s−1 [149].
Ko et al. demonstrated that acclimating young tissue-cultured seedlings and 2.5′′ potted plants to blue light prior to transplantation significantly reduced photoinhibition, enhancing their adaptability to greenhouse conditions [137]. Magar et al. investigated the influence of light quality on Phalaenopsis flowering and discovered that red light markedly promoted flowering, increasing the number of florets per stem by 33.8% compared to blue light [136]. In a subsequent study, Magar et al. reaffirmed that red light was the most effective in stimulating flowering, whereas green light had the least impact. Far-red light exhibited effects similar to those of blue light [150].
Managing light intensity and quality is crucial for optimizing the growth and flowering of Phalaenopsis orchids. While excessive light can cause damage, appropriate light conditions, particularly the use of red light, may significantly enhance flowering performance.
Fertilization
While potted Phalaenopsis orchids can initially derive nutrients from the cultivation medium, essential fertility tends to leach away with each watering. Thus, maintaining a balanced and consistent supply of all necessary nutrients is critical for optimal growth and flowering [28]. Fertilization guidelines for Phalaenopsis vary, reflecting diverse approaches to nutrient management.
Poole and Seeley proposed an optimal fertilization regimen during the nutrient culture stage, consisting of nitrogen (N), potassium (K), and magnesium (Mg) at 100 ppm, 50–100 ppm, and 25 ppm, respectively [151]. Wang and Chang emphasized the importance of maintaining a steady supply of N throughout both the vegetative and reproductive phases to maximize growth and flowering potential. They also highlighted that Phalaenopsis orchids prefer nitrogen in the nitrate (NO3−) form, recommending NO3− as the primary N source [152].
In a recent study, Alves et al. explored the effects of calcium (Ca) fertilization on Phalaenopsis cultivation. Their results demonstrated that increasing Ca concentrations up to 6.25 mM significantly enhanced plant length, flower quality, and leaf texture. Additionally, higher Ca levels were correlated with increased N and phosphorus (P) contents in the substrate, further supporting plant health and development [153].
Effective fertilization practices for Phalaenopsis orchids require a balanced supply of essential nutrients, particularly nitrogen in the nitrate form, alongside potassium, magnesium, and calcium. Tailoring fertilization strategies to the specific growth stages and nutrient preferences of Phalaenopsis can significantly improve plant vigor, flower quality, and overall cultivation success.
Cultivation Medium
The cultivation medium is a critical component for orchid growth, providing anchorage, retaining moisture and nutrients, and ensuring proper root aeration. Commonly used media for orchid cultivation include tree bark, coconut husk chips, fir cocopeat, and sphagnum moss. Among these, sphagnum moss is the most widely used medium for Phalaenopsis orchids due to its superior performance.
Kaveriamma et al. compared the effects of coconut chips, cocopeat, and sphagnum moss on P. ‘Magic Kiss’. Their findings revealed that plants cultivated in sphagnum moss exhibited significantly better vegetative and floral characteristics compared to those grown in coconut husk chips or coconut husk bits. Specifically, Phalaenopsis orchids grown in sphagnum moss achieved greater height, with an average increase of 1.64 cm versus 0.21 cm in cocopeat and 0.40 cm in coconut husk chips. Moreover, inflorescence emergence occurred earliest in sphagnum moss (124.90 days) compared to 153.30 days in coconut husk chips and 155.90 days in cocopeat [141].
However, sphagnum moss is a natural resource and its extensive harvesting has led to increased market prices and concerns about depletion due to limited supply. This has spurred the development of alternative cultivation media. For instance, peanut shells have been explored as a viable substitute. Research by Hanik et al. demonstrated that peanut shell-based media performed comparably to fern-based media across key metrics, including leaf count, leaf width, root count, and average fresh weight [142]. This suggests that peanut shell mixed media can serve as a sustainable and effective alternative to traditional substrates.
Additionally, innovative approaches such as the use of artificial textile fibers have been investigated as cultivation substrates for Phalaenopsis orchids [154]. Furthermore, studies in closed plant factories have explored the cultivation of young Phalaenopsis plants without potting media, utilizing nutrient solutions and rhizosphere ventilation [155]. These alternative methods represent promising advancements in orchid cultivation, offering sustainable and efficient solutions for the future.
While sphagnum moss remains the preferred medium for Phalaenopsis cultivation due to its superior performance, the development of alternative substrates such as peanut shells and artificial fibers, as well as innovative soilless cultivation techniques, provides sustainable options to address resource limitations and environmental concerns.
Pests and Diseases
Phalaenopsis orchids are predominantly cultivated in greenhouses, where high humidity and elevated temperatures create favorable conditions for the proliferation of various pests and diseases. Kim et al. conducted a comprehensive two-year study on pest species affecting the above-ground parts of Phalaenopsis orchids across nine farms in South Korea. They identified a total of 10 insect species, with Tenuipalpus pacificus Baker and Frankliniella intonsa Trybom emerging as the primary pests impacting orchid health [156]. In Guangdong, China, Bradysia difformis Frey has been recognized as a significant pest, causing severe damage to seedlings in greenhouse environments [157]. Additionally, Dichromothrips corbetti, commonly known as Vanda thrips, has become a critical pest, posing substantial risks to the global commercial cultivation of Phalaenopsis orchids, particularly in large-scale production systems [158].
Among viral pathogens, Cymbidium mosaic virus (CymMV) and ORSV are the most widespread and economically significant, affecting Phalaenopsis orchids on a global scale [5]. Consequently, extensive research has been conducted on virus detection [159,160], virus–host interactions [161,162], and control measures [163,164]. Furthermore, the development of transgenic lines with dual resistance has been explored [165]. Recent studies have increasingly focused on the development of genetically modified orchids engineered for enhanced resistance to viral infections [11].
The cultivation of Phalaenopsis orchids in greenhouse environments is susceptible to a range of pests and diseases, including insect pests as well as viral pathogens like CymMV and ORSV. Ongoing research in virus detection, host interactions, control measures, and genetic engineering is crucial for mitigating these threats and ensuring the sustainable production of Phalaenopsis orchids.
The successful cultivation of Phalaenopsis orchids requires the careful management of temperature, water, light, fertilization, cultivation medium, and pests and diseases. Maintaining optimal conditions ensures healthy growth, timely flowering, and high-quality blooms.
4. The Potential Applications of Phalaenopsis
Beyond their widespread use as potted plants and cut flowers, Phalaenopsis orchids offer a range of promising applications, particularly in the fields of antioxidants, antimicrobials, and cosmetics. Among these, their antioxidant activity has been the most extensively studied (Table 3).

Table 3.
The antioxidant activity of Phalaenopsis.
Minh et al. identified that ethyl acetate extracts derived from the roots of hybrid Phalaenopsis spp. exhibited the highest level of antioxidant activity, suggesting that these root extracts could serve as an effective source of natural antioxidants [166,171]. Nguyen et al. further explored the antioxidant potential and secondary metabolite compounds in methanol extracts from four parts (roots, leaves, flower stalks, and flowers) of three differently colored orchids: white, yellow, and purple [167]. Their findings revealed that the leaf extract of the white orchid demonstrated the highest efficacy in the DPPH radical scavenging assay, while the flower extract of the white orchid exhibited the strongest reducing power.
Irimescu et al. investigated extracts derived from Phalaenopsis waste materials, such as dried leaves, stems, and roots [168]. Their results indicated that leaf and root extracts exhibited moderate to high antioxidant potential, with the leaf extract showing significant inhibitory activity against methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa. The stem extract also demonstrated inhibitory activity against Bacillus cereus. Yamada et al. proposed that Phalaenopsis orchid extract (Phex) inhibits the differentiation of melanocyte stem cells, suggesting its potential as a novel therapeutic agent for treating solar lentigines [172]. These findings highlight the potential of Phalaenopsis extracts in the pharmaceutical and cosmetic industries [173].
In addition to antioxidant activity, the antimicrobial properties of Phalaenopsis extracts have also been investigated. Studies have shown that extracts derived from PLBs of Phalaenopsis significantly inhibit the growth of Acidovorax citrulli, reduce bacterial attachment to watermelon seeds, and mitigate disease symptoms in infected watermelon seedlings. Researchers hypothesize that the reprogramming of cellular activity during the PLB regeneration process generates metabolites with potent antibacterial properties [169]. Tzou et al. demonstrated that mixed liposomes formed from monogalactosyldiacylglycerol (MGDG) glycolipids in Phalaenopsis leaves significantly enhance the suppression of Escherichia coli growth during both the growth and spike induction phases [174].
Chiu et al. examined the petals and sepals of nine moth orchid varieties with distinct colors and identified significant quantities of two C-glycosylated flavones, reaching up to 1.5% and 5.6% on a dry weight basis. These findings suggest that moth orchid flowers could serve as a valuable resource for the exploration of both known and yet-to-be-discovered applications [170].
Phalaenopsis orchids hold immense potential beyond their ornamental value, with applications in antioxidants, antimicrobials, and cosmetics. Their extracts demonstrate significant biological activities, paving the way for future innovations in pharmaceuticals, agriculture, and skincare industries.
5. Conclusions and Prospects
Currently, conventional breeding techniques remain the most widely used method for Phalaenopsis orchid breeding, yielding the majority of commercial varieties. Intergeneric hybridization also offers significant potential for creating novel orchid cultivars, expanding the diversity and unique characteristics of these plants [9]. Genetic transformation technology enables the development of varieties with desirable traits such as novel flower colors, unique flower shapes, and enhanced stress resistance, providing precise and effective methods to achieve specific breeding goals [175]. This technology is poised to become a powerful tool in Phalaenopsis orchid breeding, particularly for overcoming challenges such as breeding red orchids and developing pure true red Phalaenopsis varieties [176].
Phalaenopsis orchids are renowned for their distinctive fragrance and are highly prized in the ornamental flower market [177]. The harmonious blend of scent and visual beauty offers consumers a multi-sensory experience, while the fragrance itself evokes pleasant emotions, significantly enhancing the emotional allure of these orchids. Due to their unique qualities, fragrant Phalaenopsis orchids are particularly sought after in the premium floral market. With their distinctive sensory experience and emotional value, large-flowered and fragrant Phalaenopsis orchids are recognized as a key focus in Phalaenopsis breeding [178,179].
Resource-efficient Phalaenopsis orchid varieties also represent a crucial direction in breeding programs. In some regions where Phalaenopsis orchids are cultivated on a large-scale, year-round Phalaenopsis production incurs high energy costs due to strict temperature control requirements [180]. For instance, flower induction in summer demands nighttime temperatures of 18 °C and daytime temperatures of 25 °C [125,181], while winter cultivation requires nighttime temperatures no lower than 18 °C [182]. Therefore, breeding high-temperature flowering varieties capable of spike initiation and flowering at 20–25 °C alongside cold-tolerant varieties that withstand low temperatures of 15 °C during winter production can substantially reduce energy consumption. In natural conditions, Phalaenopsis orchids require approximately 3 years from seed germination to flowering [9]. Under tissue culture propagation, this timeline can be reduced to at least 18 months by extending photoperiods and implementing precise water and nutrient management. Breeding fast-growing Phalaenopsis varieties can further shorten cultivation cycles and reduce energy demands in production systems. Additionally, Phalaenopsis orchids often require staking during cultivation due to the gravitational stress exerted by their heavy multi-flowered inflorescences. Breeding programs targeting enhanced stem lignification and thus producing self-supporting flower spikes without artificial stabilization represent a strategic approach to streamline production costs in commercial orchid operations [183].
In Phalaenopsis cultivation, the development of a balanced nutrient formula specifically tailored for these orchids remains an urgent priority. The implementation of a showerhead-style downward irrigation system for fertilizer delivery effectively prevents the localized accumulation or deficiency of specific nutrients in the growing substrate. The integration of nanobubble technology with a balanced nutrient formulation can enhance absorption efficiency, thereby optimizing resource utilization and minimizing environmental pollution [184,185].
In terms of potential applications, Rizky et al. developed a micropropagation protocol utilizing natural compounds, enabling the cultivation of unique ‘orchid key holder’ prototypes in miniature glass vessels [186]. This innovation aims to advance agritourism through horticultural biotechnology. The growing global economy and expanding cultural prosperity have driven the increased consumer demand for cultural creative products, which are emerging as a pivotal economic force [187]. The development of Phalaenopsis-themed cultural creative products has garnered increasing attention as a niche market segment, synergizing botanical esthetics with cultural narratives to bridge horticultural innovation and consumer-driven value creation [188].
In conclusion, the future of Phalaenopsis breeding and cultivation lies in leveraging advanced technologies, optimizing resource efficiency, and exploring innovative applications (Figure 3). By integrating conventional breeding, genetic transformation, and sustainable cultivation practices, the Phalaenopsis industry can continue to thrive, meeting consumer demands while minimizing environmental impact.
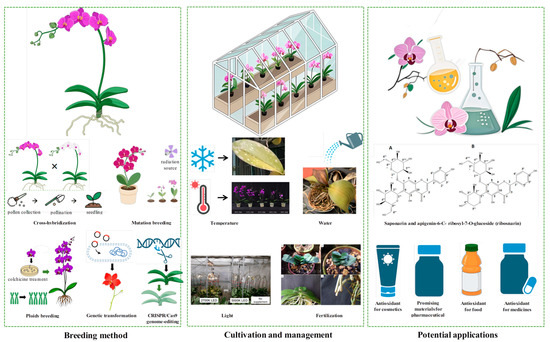
Figure 3.
Integrated framework of Phalaenopsis breeding, cultivation management, and potential applications. Icons were created using BioRender and AI-Doubao (https://www.doubao.com/chat/). All referenced images are credited below. In terms of ‘cultivation and management’, image data about ‘high temperature’ are sourced from Jeong et al. [131], image data about ‘light’ are sourced from Magar et al. [136], and image data about ‘fertilization’ are sourced from Novais et al. [140]. In terms of ‘potential applications’, image data about ‘Saponarin and apigenin-6-C-ribosyl-7-O-glucoside (ribosnarin)’ are sourced from Chiu et al. [170].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14111689/s1, Table S1: A comprehensive list of species names along with their respective parent.
Author Contributions
Conceptualization, writing—original draft preparation, C.H. and F.D.; investigation, Y.Q., Y.W. and J.Z.; writing—review and editing, X.L.; visualization, B.L. and L.Z.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong Key Research and Development Program of China, grant number 2023LZGCQY005; Shandong Provincial Natural Science Foundation of China (ZR2022MC099, ZR2024MC128); Ningxia Hui Autonomous Region Agricultural Science and Technology Independent Innovation Special Project-Science and Technology Innovation Guidance Project (NKYG-24-16); and East–West Science and Technology Collaboration Special Project (Tianjin-Gansu Cooperation and Shandong-Gansu Cooperation) 25CXNN010.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Binghai Li was employed by Dongying Shuangfu Fusheng Agricultural Development Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- The World Flora Online. Orchidaceae Family. 2024. Available online: https://www.worldfloraonline.org/search?query=+Orchidaceae&view=&limit=24&start=0&sort=&facet=taxon.taxonomic_status_s%3aAccepted&facet=taxon.taxon_rank_s%3aSPECIES (accessed on 24 October 2024).
- Wang, S.L.; An, H.R.; Tong, C.G.; Jang, S. Flowering and flowering genes: From model plants to orchids. Hortic. Environ. Biotechnol. 2021, 62, 135–148. [Google Scholar] [CrossRef]
- Tiwari, P.; Bose, S.K.; Gautam, A.; Chen, J.T. Emerging trends and insights into the cultivation strategies, ethnomedicinal uses, and socio-economic attributes of orchids. J. Hortic. Sci. Biotechnol. 2023, 98, 273–298. [Google Scholar] [CrossRef]
- Liang, C.Y.; Rengasamy, K.P.; Huang, L.M.; Hsu, C.C.; Jeng, M.F.; Chen, W.H.; Chen, H.H. Assessment of violet-blue color formation in Phalaenopsis orchids. BMC Plant Biol. 2020, 20, 212. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.W.; Lu, H.C.; Chan, M.T. Virus resistance in orchids. Plant Sci. 2014, 228, 26–38. [Google Scholar] [CrossRef]
- Wang, S.L.; Viswanath, K.K.; Tong, C.G.; An, H.R.; Jang, S.J.; Chen, F.C. Floral induction and flower development of orchids. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Wang, J.Y. Phylogenetic and Biogeographic Study of Phalaenopsis Blume. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Hsing, H.X.; Lin, Y.J.; Tong, C.G.; Li, M.J.; Chen, Y.J.; Ko, S.S. Efficient and heritable transformation of Phalaenopsis orchids. Bot. Stud. 2016, 57, 30. [Google Scholar] [CrossRef]
- Liyama, C.M.; Vilcherrez-Atoche, J.A.; Germanà, M.A.; Vendrame, W.A.; Cardoso, J.C. Breeding of ornamental orchids with focus on Phalaenopsis: Current approaches, tools, and challenges for this century. Heredity 2024, 132, 163–178. [Google Scholar]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Yusop, M.; Mohamed-Hussein, Z.; Ramzi, A.; Bunawan, H. Cymbidium mosaic virus infecting orchids: What, how, and what next. Iran. J. Biotechnol. 2022, 20, e3020. [Google Scholar]
- Vo, T.C.; Mun, J.H.; Yu, H.J.; Hwang, Y.J.; Chung, M.Y.; Kim, C.K.; Kim, H.Y.; Lim, K.B. Phenotypic analysis of parents and their reciprocal F1 hybrids in Phalaenopsis. Hortic. Environ. Biotechnol. 2015, 56, 612–617. [Google Scholar] [CrossRef]
- Yuan, S.C.; Bolaños-Villegas, P.; Chin, Y.T.; Chen, F.C. The breeding of Phalaenopsis hybrids. In The Orchid Genome; Chen, F.-C., Chin, S.-W., Eds.; Compendium of Plant Genomes; Springer: Cham, Switzerland, 2021; pp. 29–40. [Google Scholar]
- RHS. UK’s Leading Gardening Charity. Available online: https://www.rhs.org.uk/ (accessed on 24 October 2024).
- Hsu, C.C.; Chung, Y.N.; Chen, T.C.; Lee, Y.L.; Kuo, Y.T.; Tsai, W.C.; Hsiao, Y.Y.; Chen, Y.W.; Wu, W.L.; Chen, H.H. An overview of the Phalaenopsis orchid genome through BAC end sequence analysis. BMC Plant Biol. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.W.N.; Zhou, W.; Shen, L. Dissecting the function of MADS-Box transcription factors in orchid reproductive development. Front. Plant Sci. 2019, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.T.; Huang, K.L.; Shen, R.S.; Miyajima, I.; Hsu, S.T. Using cut-column pollination method to overcome crossing barriers in Phalaenopsis sunrise goldmour ‘KHM637’. J. Fac. Agric. Kyushu Univ. 2014, 59, 265–271. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zhao, X.W.; Li, Y.Y.; Ke, S.J.; Yin, W.L.; Lan, S.R.; Liu, Z.J. Advances and prospects of orchid research and industrialization. Hortic. Res. 2022, 9, uhac220. [Google Scholar] [CrossRef]
- Yuan, S.C.; Lekawatana, S.; Amore, T.D.; Chen, F.C.; Chin, S.W.; Vega, D.M.; Wang, Y.T. The Global Orchid Market. In The Orchid Genome; Chen, F.-C., Chin, S.-W., Eds.; Compendium of Plant Genomes; Springer: Cham, Switzerland, 2021; pp. 1–28. [Google Scholar]
- Tsai, W.C.; Dievart, A.; Hsu, C.C.; Hsiao, Y.Y.; Chiou, S.Y.; Huang, H.; Chen, H.H. Post genomics era for orchid research. Bot. Stud. 2017, 58, 61. [Google Scholar] [CrossRef]
- Lu, H.C.; Liu, Z.J.; Lan, S.R. Genome sequencing reveals the role of MADS-box gene families in the floral morphology evolution of orchids. Hortic. Plant J. 2019, 5, 247–254. [Google Scholar] [CrossRef]
- Song, C.; Wang, Y.; Manzoor, M.A.; Mao, D.; Wei, P.; Cao, Y.; Zhu, F. In-depth analysis of genomes and functional genomics of orchid using cutting-edge high-throughput sequencing. Front. Plant Sci. 2022, 13, 1018029. [Google Scholar] [CrossRef]
- Freudenstein, J.V.; Chase, M.W. Phylogenetic relationships in Epidendroideae (Orchidaceae), one of the great flowering plant radiations: Progressive specialization and diversification. Ann. Bot. 2015, 115, 665–681. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Y.; Huang, M.; Huang, W.; Liu, D.; Zhang, D.; Hu, H.; Downing, J.L.; Liu, Z.; Ma, H. Comprehensive phylogenetic analyses of Orchidaceae using nuclear genes and evolutionary insights into epiphytism. J. Integr. Plant Biol. 2023, 65, 1204–1225. [Google Scholar] [CrossRef]
- Goh, M.W.K.; Kumar, P.P.; Lim, S.H.; Tan, H.T.W. Random amplified polymorphic DNA analysis of the moth orchids, Phalaenopsis (Epidendroideae: Orchidaceae). Euphytica 2005, 141, 11–22. [Google Scholar] [CrossRef]
- Tsai, C.; Chou, C. Molecular phylogenetics of Phalaenopsis taxa: An updated review. Orchid Sci. Biotechnol. 2007, 1, 44–50. [Google Scholar]
- Tsai, C.C.; Huang, S.C.; Huang, P.L.; Chou, C.H. Phylogeny of the genus Phalaenopsis (Orchidaceae) with emphasis on the subgenus Phalaenopsis based on the sequences of the internal transcribed spacers 1 and 2 rDNA. J. Hortic. Sci. Biotechnol. 2003, 78, 879–887. [Google Scholar] [CrossRef]
- Ichihashi, S.; Mii, M. Practical Floral Horticulture Techniques: Cultivation and Production of Phalaenopsis Orchids; Seibundo Shinkosha Publishing Co., Ltd.: Tokyo, Japan, 2006. [Google Scholar]
- Li, M.; Gruss, O.; Liu, Z. Nomenclature changes in Phalaenopsis subgen. Hygrochilus (Orchidaceae; Epidendroideae; Vandeae) based on DNA evidence. Phytotaxa 2016, 275, 055–061. [Google Scholar]
- Cardoso, J.C.; Zanello, C.A.; Chen, J.T. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef]
- Mii, M. Ornamental plant breeding through interspecific hybridization, somatic hybridization and genetic transformation. Acta Hortic. 2012, 953, 43–54. [Google Scholar] [CrossRef]
- Vo, T.; Lee, H.; Deepo, D.; Hwang, Y.; Kim, H.; Lim, K. Comparisons of morphological and chromosomal characteristics of Phalaenopsis mini type cultivars. J. Agric. Life Sci. 2021, 55, 77–83. [Google Scholar] [CrossRef]
- Mursyanti, E.; Purwantoro, A.; Moeljopawiro, S.; Semiarti, E. Micropropagation of mini orchid hybrid Phalaenopsis “Sogo Vivien”. J. Trop. Biodivers. Biotechnol. 2016, 1, 45. [Google Scholar] [CrossRef]
- Been, C.; Hwang, J. Breeding of a fragrant, mini-multiflora Phalaenopsis hybrid orchid cultivar, ‘Brave Star’. Flower Res. J. 2019, 27, 211–215. [Google Scholar] [CrossRef]
- Xiao, W.; Li, Z.; Chen, H.; Lv, F. Visualization of micromorphology of petal epidermal features of waxy and velvety flowers in Phalaenopsis. ScienceAsia 2020, 46, 657–664. [Google Scholar] [CrossRef]
- Li, A.; Gong, Z.; Sun, J.; Zhang, Y.; Fang, Y.; Zhu, Z.; Liu, X. The correlation between leaf and flowering traits of Phalaenopsis Sogo Yukidian ‘V3’. Chin. Agric. Sci. Bull. 2018, 34, 75–80. [Google Scholar]
- Lu, J.; Su, J.; Cui, Y. Establishment of rapid propagation system with cluster shoots pathway of Phalaenopsis Sogo Yukidian ‘V3’. Molecular Plant Breeding. Available online: https://link.cnki.net/urlid/46.1068.S.20241115.1536.004 (accessed on 24 October 2024).
- Li, Z.; Xiao, W.; Chen, H.; You, Y.; Lv, F. Characteristics separation of F1 offspring from Phalaenopsis ‘Frigdaas Oxford’ × Phal. 316. Chin. J. Trop. Crops 2014, 35, 854–861. [Google Scholar]
- Yuan, S.; Chin, S.; Chen, F. Current trends of Phalaenopsis orchid breeding and study on pollen storage. Acta Hortic. 2015, 1078, 19–23. [Google Scholar] [CrossRef]
- Wu, R.; Tsai, Y.; Dai, T. Breeding of Yapara Tariflor Pink Fairy ‘Tainung No. 2-Pink Fairy’ by intergeneric hybridization. Hortscience 2025, 60, 301–302. [Google Scholar] [CrossRef]
- Badriah, D.S.; Pramanik, D.; Kartikaningrum, S.; Dewanti, M.; Mawaddah; Suryawati; Fibrianty, E.; Muharam, A.; Budiarto, K. Progeny evaluation from the crossing of novelty-type Phalaenopsis I Hsin Bee × Phalaenopsis pulcherrima var. champorensis 2024, 53, 249–265. [Google Scholar] [CrossRef]
- Devi, K.S.; Sanabam, R.; Singh, N.S.; Devi, E.J.; Devi, H.S. Intergeneric hybridization of two endangered orchids, Vanda stangeana and Phalaenopsis hygrochila, and molecular confirmation of hybridity using SSR and SCoT markers. S. Afr. J. Bot. 2023, 161, 140–150. [Google Scholar] [CrossRef]
- Wu, J.; Hsieh, T.; Tsao, C.; Chuang, K. Breeding of an indigo Phalaenopsis by intergeneric hybridization: Rhynchonopsis Tariflor Blue Kid ‘1030-4’. Hortscience 2022, 57, 489–490. [Google Scholar] [CrossRef]
- Lee, Y.; Tseng, Y.; Lee, Y.; Chung, M. Chromosome constitution and nuclear DNA content of Phalaenopsis hybrids. Sci. Hortic. 2020, 262, 109089. [Google Scholar] [CrossRef]
- Meng, Y.; Li, W.; Guan, Y.X.; Song, Z.H.; He, G.R.; Peng, D.H.; Ming, F. Mechanism underlying the rapid growth of Phalaenopsis equestris induced by 60Co-γ-ray irradiation. Mol. Genet. Genom. 2024, 299, 13. [Google Scholar] [CrossRef]
- Hartati, S.; Samanhudi; Sukaya; Aji, T.A. Diversity induction with gamma-ray irradiation on Phalaenopsis amboinensis var. common natural orchid generation M1V0. IOP Conf. Ser. Earth Environ. Sci. 2024, 1317, 012001. [Google Scholar] [CrossRef]
- Putri, H.A.; Purwito, A.; Sudarsono, S.; Sukma, D. Morphological, molecular and resistance responses to soft-rot disease variability among plantlets of Phalaenopsis amabilis regenerated from irradiated protocorms. Biodiversitas 2021, 22, 1077–1090. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, J.; Wang, Z.N.; Zheng, C. Effect of fast neutron radiation on the proliferation and differentiation of protocorm-like bodies and seedlings stem segments of Phalaenopsis. J. Nucl. Agric. Sci. 2014, 28, 0440–0455. [Google Scholar]
- Widiarsih, S.; Dwimahyani, I. Gamma irradiation application for mutation breeding in early flowering moth orchid (Phalaenopsis amabilis Bl.). J. Ilm. Apl. Isot. Dan Radiasi 2013, 9, 59–66. [Google Scholar]
- Wu, T.; Zhao, X.; Yang, S.; Yang, J.; Zhu, J.; Kou, Y.; Yu, X.; Ge, H.; Jia, R. Induction of 2n pollen with colchicine during microsporogenesis in Phalaenopsis. Breed. Sci. 2022, 72, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhu, J.; Yang, J.; Ge, H.; Yang, S.; Zhao, X.; Yu, X.; Jia, R. Polyploid induction of Phalaenopsis protocorms via colchicine treatment. J. Nucl. Agric. Sci. 2021, 35, 2463–2469. [Google Scholar]
- Mohammadi, M.; Kaviani, B.; Sedaghathoor, S. In vivo polyploidy induction of Phalaenopsis amabilis in a bubble bioreactor system using colchicine. Ornam. Hortic. 2021, 27, 204–212. [Google Scholar] [CrossRef]
- Putri, A.A.; Sukma, D.; Aziz, S.A.; Syukur, M. Protocorm growth medium composition before colchicine treatment to increase polyploidy on Phalaenopsis amabilis (L.) Blume. J. Agron. Indones. 2018, 46, 306–313. [Google Scholar] [CrossRef]
- Azmi, T.K.K.; Sukma, D.; Aziz, S.A.; Syukur, M. Polyploidy induction of moth orchid (Phalaenopsis amabilis (L.) Blume) by colchicine treatment on pollinated flowers. J. Agric. Sci. 2016, 11, 62–130. [Google Scholar] [CrossRef]
- Primasiwi, D.H.; Purwestri, Y.A.; Semiarti, E. Improving transient gene expression and agroinfiltration-based transformation effectiveness in Indonesian orchid Phalaenopsis amabilis (L.) Blume. Indones. J. Biotechnol. 2024, 29, 111–120. [Google Scholar] [CrossRef]
- Hsieh, K.T.; Liu, S.H.; Wang, I.W.; Chen, L.J. Phalaenopsis orchid miniaturization by overexpression of OsGA2ox6, a rice GA2-oxidase gene. Bot. Stud. 2020, 61, 10. [Google Scholar]
- Chew, Y.; Abdullah, W.; Kok, A.; Abdullah, J.; Mahmood, M.; Lai, K. Development of an efficient particle bombardment transformation system for the endemic orchid, Phalaenopsis bellina. Sains Malays. 2019, 48, 1867–1877. [Google Scholar] [CrossRef]
- Mursyanti, E.; Purwantoro, A.; Moeljopawiro, S.; Semiarti, E. Induction of somatic embryogenesis through overexpression of ATRKD4 Genes in Phalaenopsis “Sogo Vivien”. Indones. J. Biotechnol. 2015, 20, 42–53. [Google Scholar] [CrossRef]
- Suputri, N.P.A.E.O.; Prasojo, I.S.; Prabowo, L.A.T.; Purwestri, Y.A.; Purnomo; Semiarti, E. Identification of early flowering mutant gene in Phalaenopsis amabilis (L.) Blume for sgRNA construction in CRISPR/Cas9 genome editing system. Braz. J. Biol. 2024, 84, e268133. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Zhang, D.; Xu, X.; Li, G.; Yang, Y.; Chen, Z.; Wang, X.; Zhang, G.; Sun, H.; Gu, Y. Protoplast technology enables the identification of efficient multiplex genome editing tools in Phalaenopsis. Plant Sci. 2022, 322, 111368. [Google Scholar] [CrossRef] [PubMed]
- Nopitasari, S.; Setiawati, Y.; Lawrie, M.D.; Purwantoro, A.; Widada, J.; Sasongko, A.B.; Yoshioka, Y.; Matsumoto, S.; Ninomiya, K.; Asano, Y.; et al. Development of an Agrobacterium-delivered CRISPR/Cas9 for Phalaenopsis amabilis (L.) Blume genome editing system. AIP Conf. Proc. 2020, 2260, 1–10. [Google Scholar]
- Tong, C.; Wu, F.; Yuan, Y.; Chen, Y.; Lin, C. High-efficiency CRISPR/Cas-based editing of Phalaenopsis orchid MADS genes. Plant Biotechnol. J. 2020, 18, 889–891. [Google Scholar] [CrossRef]
- Semiarti, E.; Nopitasari, S.; Setiawati, Y.; Lawrie, M.D.; Purwantoro, A.; Widada, J.; Ninomiya, K.; Asano, Y.; Matsumoto, S.; Yoshioka, Y. Application of CRISPR/Cas9 genome editing system for molecular breeding of orchids. Indones. J. Biotechnol. 2020, 25, 61–68. [Google Scholar] [CrossRef]
- Zhu, G. Progress in germplasm resources and crossbreeding of Phalaenopsis. Guangdong Agric. Sci. 2015, 2015, 31–38. [Google Scholar]
- Tsai, C.; Chiang, Y.; Huang, S.; Liu, W.; Chou, C. Intergeneric hybridization, embryo rescue and molecular detection for intergeneric hybrids between Ascocenda and Phalaenopsis. Acta Hortic. 2009, 829, 413–416. [Google Scholar] [CrossRef]
- Ding, P.; Guo, W.; Sun, J.; Zhang, J.; Liu, X. Research advance on cross breeding of Phalaenopsis spp. J. Anhui Agric. Sci. 2014, 42, 1954–1956. [Google Scholar]
- Lee, Y.; Chung, M. Chromosome analysis of Phalaenopsis yellow cultivars. In The Orchid Genome; Chen, F.-C., Chin, S.-W., Eds.; Compendium of Plant Genomes; Springer: Cham, Switzerland, 2021; pp. 67–72. [Google Scholar]
- Xu, S.; Zhang, T.; Liao, F.; Lian, F. A review on studies of Phalaenopsis germplasm resources and breeding. Chinese Hortic. Abstract 2010, 2010, 27–30. [Google Scholar]
- Zhu, G.F. Common parents for hybrid breeding of Phalaenopsis orchids. China Flowers Hortic. 2002, 2002, 24–25. [Google Scholar]
- Lu, S.C. Chinese and Exotic Orchids; Jindun Press: Beijing, China, 1994; pp. 97–104. [Google Scholar]
- Zhang, G.; Zhao, Y.; Liu, X.; Wang, R.; Jiang, S.; Yang, S. Research progress of Phalaenopsis breeding technology in China. Guizhou Agric. Sci. 2020, 48, 86–92. [Google Scholar]
- Melsen, K.; van de Wouw, M.; Contreras Ryan, N. Mutation breeding in ornamentals. Hortscience 2021, 56, 1154–1165. [Google Scholar] [CrossRef]
- Magdalita, P.M.; Alangelico, O.; San Pascual, R.L. Villareal. Evaluation of plant and flower characteristics of selected 15-Gy irradiated Phalaenopsis aphrodite. Mindanao J. Sci. Technol. 2022, 20, S1. [Google Scholar] [CrossRef]
- Farid, N.; Ulinnuha, Z.; Dinuriah, I. Evaluation of flower diversity of selected Phalaenopsis orchids mutant irradiated by gamma ray. Int. J. Agric. Biol. 2024, 31, 277–284. [Google Scholar]
- Magdalita, P.M.; Pascual, A.S.; Villareal, R. Characterization and flowering behavior of eleven philippine native Phalaenopsis species and gamma irradiation effects on Phalaenopsis aphrodite. Philipp. J. Sci. 2019, 149, 1–10. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, R.; Yang, S.; Feng, J.; Zhao, Y.; Wang, S.; Wang, J. Effect of 60Co-γrays on growth of Phalaenopsis. Shanxi J. Agric. Sci. 2024, 70, 12–18. [Google Scholar]
- Li, W. Germplasm Innovation and Mechanism Exploration of Phalaenopsis equestris in 60Co-γ-Ray Radiation. Master’s Thesis, Shanghai Normal University, Shanghai, China, 2022. [Google Scholar]
- Li, Y.M.; Yin, L.J.; He, X.Y.; Hu, C.L.; Wu, R.H.; Long, Q.; Xiao, S.X.; Yuan, D.Y. Ploidy and fruit trait variation in oil-tea Camellia: Implications for ploidy breeding. J. Integr. Agric. 2024, 23, 2662–2673. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, J.; Zhao, H.; Zong, M.; Li, M.; Gong, G.; Wang, J.; Zhang, J.; Ren, Y.; Zhang, H.; et al. Production of double haploid watermelon via maternal haploid induction. Plant Biotechnol. J. 2023, 21, 1308–1310. [Google Scholar] [CrossRef]
- Devaux, P.; Kilian, A.; Kleinhofs, A. Anther culture and Hordeum bulbosum-derived barley doubled haploids mutations and methylation. Mol. Genet. Genom. 1993, 241, 674–679. [Google Scholar] [CrossRef]
- Kazumitsu, M.; Mii, M.; Ken, T.; Hisashi, K. Research on haploid breeding in Orchidaceae plants 1 Induction of moth orchid haploids by pseudofertilized ovule culture. Horicultural Res. 2012, 11, 241. [Google Scholar]
- Silva, J.T.; Giang, D.T.T. Unsuccessful in vitro regeneration from Phalaenopsis (Orchidaceae) flowers. All-Results J. Biol. 2014, 5, 18–22. [Google Scholar]
- Marasek-Ciołakowska, A.; Xie, S.; Arens, P.; Van Tuyl, J.M. Ploidy manipulation and introgression breeding in Darwin hybrid tulips. Euphytica 2014, 198, 389–400. [Google Scholar] [CrossRef]
- Van Tuyl, J.M.; Lim, K.B.; Ramanna, M.S. Interspecific hybridization and introgression. In Breeding for Ornamentals: Classical and Molecular Approaches; Vainstein, A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherland, 2002; pp. 85–103. [Google Scholar]
- Wongprichachan, P.; Huang, K.L.; Hsu, S.T.; Chou, Y.M.; Liu, T.Y.; Okubo, H. Induction of polyploid Phalaenopsis amabilis by N2O treatment. J. Fac. Agric. Kyushu Univ. 2013, 58, 33–36. [Google Scholar]
- Bolaños -Villegas, P.; Chen, F.C. Advances and perspectives for polyploidy breeding in orchids. Plants 2022, 11, 1421. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, G.; Ye, Q.; Chen, H. Cytological observations on chromosome numbers in 50 hybrid cultivars and species of Phalaenopsis. Chin. J. Trop. Crops 2013, 34, 1871–1876. [Google Scholar]
- Zhuang, D.; Qu, Y.; Xu, D.; Li, J.; Chen, Z. Analysis on chromosome number and morphology of varieties in Phalaenopsis. Acta Hortic. Sin. 2007, 34, 1257–1262. [Google Scholar]
- Chen, W.H.; Tang, C.Y. Genome Size Variation in Species of the Genus Phalaenopsis Blume (Orchidaceae) and Its Application in Variety Improvement; Orchid Biotechnology Ⅱ; World Scientific: Singapore, 2017; p. 2. [Google Scholar]
- Wu, T.; Jia, R.; Yang, S.; Zhao, X.; Yu, X.; Guo, Y.; Ge, H. Research advances and prospects on Phalaenopsis polyploid breeding. Acta Hortic. Sin. 2022, 49, 448–462. [Google Scholar]
- Wongprichachan, P.; Shen, T.; Huang, K.; Okubo, H. Meiotic behavior, capsule setting and seed germination of diploid and polyploid Phalaenopsis amabilis. J. Fac. Agric. Kyushu Univ. 2012, 57, 405–409. [Google Scholar] [CrossRef]
- Rahayu, E.M.D.; Sukma, D.; Syukur, M.; Rawati. Induksi Poliploidi Phalaenopsis amabilis (L.) Blume dan Phalaenopsis amboinensis J. J. Smith dengan Kolkisin dalam Kultur In Vitro. J. Agron. Indones. 2016, 43, 219. [Google Scholar] [CrossRef]
- Griesbach, R.J. Colchicine-induced polyploidy in Phalaenopsis orchids. Plant Cell Tissue Organ Cult. 1981, 1, 103–107. [Google Scholar] [CrossRef]
- Miguel, T.P.; Leonhardt, K.W. In vitro polyploid induction of orchids using oryzalin. Sci. Hortic. 2011, 130, 314–319. [Google Scholar] [CrossRef]
- Chen, W.H.; Tang, C.Y. A Protocol for the Induction of Polyploids in Phalaenopsis orchids by In Vitro Method Without Using Anti-Microtubule Agents. Orchid Propagation: From Laboratories to Greenhouses-Methods and Protocols; Humana Press: New York, NY, USA, 2018. [Google Scholar]
- Hartati, S.; Samanhudi; Cahyono, O.; Wibowo, A.; Herviana, A. The Chromosome of Phalaenopsis spp. and Doritaenopsis sp. Hybrid Induced by Colchicine. IOP Conf. Ser. Earth Environ. Sci. 2023, 1133, 012064. [Google Scholar] [CrossRef]
- Liu, Y. Distinct Cross Incompatibility and Induction of Alloployploid in Phalaenopsis. Master’s Dissertation, South China Agricultural University, Guangzhou, China, 2012. [Google Scholar]
- Cao, Z. Study on Agrobacterium-Mediated Transgenic Technology of Orchid. Master’s Dissertation, South China Agricultural University, Guangzhou, China, 2000. [Google Scholar]
- Fan, S. Genetic engineering of ORSV-resistant Phalaenopsis. In Proceedings of the 4th International Conference on Biomedical Engineering and Informatics, Shanghai, China, 15–17 October 2011; pp. 1432–1435. [Google Scholar]
- Anzai, H.; Ishii, Y.; Shichinohe, M.; Nojiri, C.; Morikawa, H.; Tanaka, M. Transformation of Phalaenopsis by particle bombardment. Plant Tissue Cult. Lett. 1996, 13, 265–272. [Google Scholar] [CrossRef]
- Belarmino, M.M.; Mii, M. Agrobacterium-mediated genetic transformation of a Phalaenopsis orchid. Plant Cell Rep. 2000, 19, 435–442. [Google Scholar] [CrossRef]
- Semiarti, E.; Indrianto, A.; Purwantoro, A.; Isminingsih, S.; Suseno, N.; Ishikawa, T.; Yoshioka, Y.; Machida, Y.; Machida, C. Agrobacterium-mediated transformation of the wild orchid species Phalaenopsis amabilis. Plant Biotechnol. 2007, 24, 265–272. [Google Scholar] [CrossRef]
- Semiarti, E.; Indrianto, A.; Purwantoro, Y.H.; Martiwi, I.N.A.; Feroniasanti, Y.M.L.; Nadifah, F.; Mercuriana, I.S.; Dwiyani, R.; Iwakawa, H.; Yoshioka, Y.; et al. High-frequency genetic transformation of Phalaenopsis amabilis orchid using tomato extract-enriched medium for the pre-culture of protocorms. J. Hortic. Sci. Biotechnol. 2010, 85, 205–210. [Google Scholar] [CrossRef]
- Semiarti, E.; Purwantoro, A.; Mercuriani, I.S.; Anggriasari, A.M.; Jang, S.; Suhandono, S.; Machida, Y.; Machida, C. In planta transformation method for T-DNA transfer in orchids. AIP Conf. Proc. 2014, 1589, 303–307. [Google Scholar]
- Li, R. CRISPR/Cas9-Mediated Gene Editing of Fd and FNR in Oncidium. Master’s Dissertation, Fujian Agriculture and Forestry University, Fuzhou, China, 2019. [Google Scholar]
- Zhang, H.; He, D.; Li, X.; Dun, B.; Wu, D.; Huang, G. The establishment of rapid propagation system of ‘RED SUN’ Phalaenopsis aphrodite. Sustainability 2022, 14, 15305. [Google Scholar] [CrossRef]
- Zahara, M. A review: Micropropagation of Phalaenopsis sp. from leaf and flower stalk explants. J. Nat. 2017, 17, 91–95. [Google Scholar] [CrossRef]
- Roh, H.; Lee, S.; Lee, Y.; Baek, S.; Kim, J. Recent trends in tissue culture and genetic transformation of Phalaenopsis. J. Plant Biotechnol 2012, 39, 225–234. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Induction of embryogenic callus and cell suspension culture from shoot tips excised from flower stalk buds of Phalaenopsis (Orchidaceae). In Vitro Cellular Developmental Biol.-Plant 2001, 37, 457–461. [Google Scholar] [CrossRef]
- Ghahremani, R.; Daylami, S.D.; Mirmasoumi, M.; Askari, N. Refining a protocol for somatic embryogenesis and plant regeneration of Phalaenopsis amabilis cv. Jinan from mature tissues. Turk. J. Agric. For. 2021, 45, 356–364. [Google Scholar] [CrossRef]
- Sarmah, D.; Mohapatra, P.P.; Seleiman, M.F.; Mandal, T.K.; Mandal, N.; Pramanik, K.; Jena, C.; Sow, S.; Alhammad, B.A.; Ali, N.; et al. Efficient regeneration of in vitro derived plants and genetic fidelity assessment of Phalaenopsis orchid. Front. Sustain. Food Syst. 2024, 8, 1359486. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, Z. Study on key technique of rapid propagation of Phalaenopsis amabilis. South. Hortic. 2011, 22, 3–5. [Google Scholar]
- Winarto, B.; Atmini, K.D.; Badriah, D.S.; Wegadara, M. In vitro embryogenesis derived from shoot tips in mass propagation of two selected-clones of Phalaenopsis. Not. Sci. Biol. 2016, 8, 317–325. [Google Scholar] [CrossRef]
- Preetha, L.; Shylaraj, K.S.; Rohini, P.C. An improved method for rapid propagation of Phalaenopsis hybrids via culture of longitudinally bisected shoot tips. J. Trop. Agric. 2017, 55, 45–51. [Google Scholar]
- Minh, T.V. Industrial propagation of Phalaenopsis sp. by bioreactor technique. Int. J. Res. Innov. Appl. Sci. 2023, 8, 29–39. [Google Scholar] [CrossRef]
- Li, J.; Liao, J.; Ke, L.; Cai, P. Tissue culture of the root segment of Phalaenopsis. Brief Commun. Plant Tissue Cult. 2000, 36, 37. [Google Scholar]
- Yang, H.; Huang, S.; Bao, Z.; Xin, J.; Wu, Z.; Huang, J. Tissue culture with Phalaenopsis root tip. J. For. Eng. 2009, 23, 120–123. [Google Scholar]
- Chen, C. Application of growth models to evaluate the microenvironmental conditions using tissue culture plantlets of Phalaenopsis Sogo Yukidian ‘V3’. Sci. Hortic. 2015, 191, 25–30. [Google Scholar] [CrossRef]
- Barough, A.M.; Daylami, S.D.; Fadavi, A.; Aliniaeifard, S.; Vahdati, K. Enhancing photosynthetic efficiency in Phalaenopsis amabilis through bioreactor innovations. BMC Plant Biol. 2024, 24, 1166. [Google Scholar]
- Ko, S.S. Phalaenopsis aphrodite (moth orchid): Functional genomics and biotechnology. J. Plant Biotechnol. Microbiol. 2020, 3, 28–33. [Google Scholar] [CrossRef]
- Christenson, E.A. Phalaenopsis: A monograph; International Phalaenopsis Allicance, Timber Press, Inc.: Portland, OR, USA, 2001; 330p. [Google Scholar]
- Yuan, X.; Liang, F.; Jiang, S.; Wan, M.; Ma, J.; Zhang, X.; Cui, B. Differential protein expression in Phalaenopsis under low temperature. Appl. Biochem. Biotechnol. 2014, 175, 909–924. [Google Scholar] [CrossRef]
- Lee, H.B.; Lee, J.H.; Jeong, S.J.; An, S.K.; Kang, B.C.; Kim, K.S. Intermittent high temperature reduces leaf sugar content and inhibits inflorescence initiation in Phalaenopsis hybrid. Environ. Exp. Botany 2021, 189, 104562. [Google Scholar] [CrossRef]
- South, K.A.; Thomas, P.A.; Iersel, M.W.; Young, C.; Jones, M.L. Ice cube irrigation of potted Phalaenopsis orchids in bark media does not decrease display life. Hortscience 2017, 52, 1271–1277. [Google Scholar] [CrossRef]
- Wen, Y.; Li, L.; Yu, X. Study on the production mode for flower forcing of Phalaenopsis via plant factory. J. Beijing Univ. Agric. 2017, 32, 68–72. [Google Scholar]
- Xiao, W.; Li, Z.; Chen, H.; Lv, F. Comparative measurement and evaluation of heat tolerance of different phalaenopsis varieties. Chin. J. Trop. Crops 2018, 38, 43–48. [Google Scholar]
- Wang, S.; Yang, S.; Jiang, S.; Zhang, G.; Wang, R.; Wang, J. Research progress of response to low temperature stress in Phalaenopsis. North. Hortic. 2023, 2023, 124–131. [Google Scholar]
- Feng, X.; Kong, Y.; Sun, Y.; Jiang, N.; Fang, Y.; Li, Y.; Niu, X. Cold tolerance of different Phalaenopsis cultivars: An evaluation. Chin. Agric. Sci. Bull. 2022, 38, 59–67. [Google Scholar]
- Xie, Z.; Liu, G.; Lu, Z.; Huang, X.; Qin, Q.; Luo, Q. Evaluation of cold resistance in 22 Phalaenopsis varieties under natural low temperature. North. Hotric. 2024, 2024, 58–65. [Google Scholar]
- Daems, S.; Ceusters, N.; Valcke, R.; Ceusters, J. Effects of chilling on the photosynthetic performance of the CAM orchid Phalaenopsis. Front. Plant Sci. 2022, 13, 981581. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Lee, H.B.; An, S.K.; Kim, K.S. High temperature stress prior to induction phase delays flowering initiation and inflorescence development in Phalaenopsis queen beer ‘Mantefon’. Sci. Hortic. 2020, 263, 109092. [Google Scholar] [CrossRef]
- Jeong, J.H.; Oh, W. Drought and darkness during long-term simulated shipping delay post-shipping flowering of Phalaenopsis Sogo Yukidian ‘V3’. Horticulture 2021, 7, 483. [Google Scholar] [CrossRef]
- Ceusters, N.; Valcke, R.; Frans, M.; Claes, J.E.; Ende, W.V.; Ceusters, J. Performance index and PSII connectivity under drought and contrasting light regimes in the CAM orchid Phalaenopsis. Front. Plant Sci. 2019, 10, 1012. [Google Scholar] [CrossRef]
- Tay, S.; He, J.; Yam, T.W. CAM plasticity in epiphytic tropical orhid species responding to environmental stress. Bot. Stud. 2019, 60, 7. [Google Scholar] [CrossRef]
- Mubarok, S.; Yulianty, V.; Farida, F. Vegetative growth response of Phalaenopsis sp. hybrids (Moon Orchid) in response to light intensity and fertilizer concentration. Ornam. Hortic. 2024, 30, e242694. [Google Scholar] [CrossRef]
- Magar, Y.G.; Noguchi, A.; Furufuji, S.; Kato, H.; Amaki, W. Effects of light quality during supplemental lighting on Phalaenopsis flowering. Acta Hortic. 2019, 1262, 75–80. [Google Scholar] [CrossRef]
- Ko, S.S.; Jhong, C.M.; Shih, M.C. Blue light acclimation reduces the photoinhibition of Phalaenopsis aphrodite (Moth Orchid). Int. J. Mol. Sci. 2020, 21, 6167. [Google Scholar] [CrossRef]
- Ha, B.Y.; Kim, H.R.; Kim, D.H.; Woo, J.W.; Jo, Y.J.; Kwon, S. Growth effects of the application of new controlled-release fertilizers on Phalaenopsis spp. Appl. Biol. Chem. 2018, 61, 625–633. [Google Scholar] [CrossRef]
- Lin, J.A.; Susilo, H.; Lei, J.Y.; Chang, Y.C.A. Effects of fertilizer nitrogen shortly before forcing through flowering on carbon-nitrogen composition and flowering of Phalaenopsis. Sci. Hortic. 2019, 252, 61–70. [Google Scholar] [CrossRef]
- Novais, S.V.; Novais, R.F.; Alvarez, V.H.H.; Villani, E.M.D.; Zenero, M.D.O. Phosphorus-Zinc interaction and iron and manganese uptake in the growth and nutrition of Phalaenopsis (Orchidaceae). Rev. Bras. De Cienc. Do Solo 2016, 40, e0160054. [Google Scholar] [CrossRef][Green Version]
- Kaveriamma, M.M.; Rajeevan, P.K.; Girija, D.; Nandini, K. Sphagnum moss as growing medium in Phalaenopsis Orchid. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2118–2123. [Google Scholar] [CrossRef]
- Hanik, N.R.; Harsono, S.; Nugroho, A.A. Selection of peanut skin as a growing medium for moon orchid (Phalaenopsis amabilis). J. Biol. Trop. 2020, 20, 237–244. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Q.; Zhang, Q.; Qian, R.; Liu, H.; Zhang, X. Physiological response of cold stress on 2 cultivars of Phalaenopsis amabilis. J. Southwest For. Univ. 2021, 41, 72–78. [Google Scholar]
- Mu, X.; Liu, L.; Meng, P.; Jin, K. Physiological mechanism of exogenous nitric oxide on alleviating low temperature stress of Phalaenopsis spp. Acta Bot. Boreali-Occident. Sin. 2015, 35, 978–984. [Google Scholar]
- Chen, J.; Chen, C. The effect of temperature on the inflorescence formation model for Phalaenopsis. Plants 2024, 13, 1280. [Google Scholar] [CrossRef]
- Newton, L.; Runkle, E.S. High-temperature inhibition of flowering of Phalaenopsis and Doritaenopsis orchids. Hortscience 2009, 44, 1271–1276. [Google Scholar] [CrossRef]
- Lee, H.B.; An, S.K.; Kim, K.S. Inhibition of premature flowering by intermittent high temperature treatment to young Phalaenopsis plants. Hortic. Environ. Biotechnol. 2015, 56, 618–628. [Google Scholar] [CrossRef]
- Gu, L. Effects of exogenous chitosan on physiological characteristics of Phalaenopsis seedlings under drought stress. Southwest China J. Agric. Sci. 2011, 24, 90–93. [Google Scholar]
- Lee, N. Phalaenopsis orchid light requirements. Horttechnology 2000, 10, 430. [Google Scholar] [CrossRef]
- Magar, Y.G.; Noguchi, A.; Furufuji, S. Effects of far-red light irradiation and its timing on the flowering in Phalaenopsis amabilis. Acta Hortic. 2023, 1377, 405–410. [Google Scholar] [CrossRef]
- Poole, H.A.; Seeley, J.G. Nitrogen, potassium and magnesium nutrition of three orchid genera1. J. Am. Soc. Hortic. Sci. 1978, 103, 485–488. [Google Scholar] [CrossRef]
- Wang, Y.T.; Chang, Y.C.A. Effects of nitrogen and the various forms of nitrogen on Phalaenopsis orchid-A Review. HortTechnology 2017, 27, 144–149. [Google Scholar] [CrossRef]
- Alves, G.A.C.; Hoshino, R.T.; Tejo, D.P.; Pedro, S.; Takane, R.J.; Faria, R.T. Calcium fertilization on the Phalaenopsis ssp. cultivation (Orchidaceae). J. Plant Nutr. 2024, 47, 3860–3867. [Google Scholar] [CrossRef]
- Chang, K.H.; Dai, T.; Huang, S.C.; Tsao, C.Y.; Tsai, W.T.; Wang, F.N.; Chang, A.H.; How, F.W. Application of artifical textile fiber as growing medium for Phalaenopsis cultivation. In Proceedings of the Korean Society for Horticultural Science, 2006 Abstracts 27th International Horticultural Congress & Exhibition, Seoul, Republic of Korea, 13–19 August 2006; pp. 62–63. [Google Scholar]
- Min, S.Y.; Oh, W. Effects of nutrient solution application methods and rhizospheric ventilation on vegetative growth of young moth orchids without a potting medium in a closed-type plant factory. J. People Plants Environ. 2020, 23, 545–554. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, M.; Kang, T.J.; Yang, C.Y.; Kim, H.H.; Yoon, J.B. The status of pest occurrence on Phalaenopsis orchid in Korea. Korean J. Appl. Entomol. 2015, 54, 345–349. [Google Scholar] [CrossRef]
- Han, Q.X.; Cheng, D.; Luo, J.; Zhou, C.Z.; Lin, Q.S.; Xiang, M.M. First report of Bradysia difformis (Diptera: Sciaridae) damage to Phalaenopsis orchid in China. J. Asia-Pac. Entomol. 2015, 18, 77–81. [Google Scholar] [CrossRef]
- Masarovic, R.; Stefánik, M.; Zvaríková, M.; Sigmund, J.; Fedor, P. First record of a new alien economically important Thrips Dichromothrips corbetti (Priesner, 1936) (Thysanoptera: Thripidae) in Slovakia—Short Communication. Plant Prot. Sci. 2017, 53, 177–180. [Google Scholar] [CrossRef]
- Sun, A.; Wang, L.; Zhang, Y.; Yang, X.; Wu, Y.; Yan, D.; Li, W.; Wu, X. Establishment of a triplex TaqMan quantitative real-time PCR assay for simultaneous detection of Cymbidium mosaic virus, Odontoglossum ringspot virus and Cymbidium ringspot virus. Front. Microbiol. 2023, 14, 1129259. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.J.; Cho, I.S.; Jeong, R.D. Identification of viruses infecting Phalaenopsis orchids using nanopore sequencing and development of an RT-RPA-CRISPR/Cas12a for rapid visual detection of Nerine Latent Virus. Int. J. Mol. Sci. 2024, 25, 2666. [Google Scholar] [CrossRef] [PubMed]
- Pai, H.; Jean, W.; Lee, Y.; Chang, Y.; Lin, N. Genome-wide analysis of small RNAs from Odontoglossum ringspot virus and Cymbidium mosaic virus synergistically infecting Phalaenopsis. Mol. Plant Pathol. 2019, 21, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.Y.; Hu, C.C.; Huang, Y.W.; Lee, C.W.; Luo, M.J.; Tu, C.W.; Lee, C.; Lin, N.S.; Hsu, Y.H. Argonaute 5 family proteins play crucial roles in the defence against Cymbidium mosaic virus and Odontoglossum ringspot virus in Phalaenopsis aphrodite subsp. formosana. Mol. Plant Pathol. 2021, 22, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Mahfut, M. Identification and efforts to control infection Odontoglossum ringspot virus (ORSV) on Orchid. Int. J. Eng. Sci. Inf. Technol. 2021, 1, 25–29. [Google Scholar] [CrossRef]
- Campol, J.R.; Naing, A.H.; Aung, H.M.; Cho, S.B.; Kang, H.; Chung, M.Y.; Kim, C.K. Production of genetically stable and Odontoglossum ringspot virus-free Cymbidium orchid ‘New True’ plants via meristem-derived protocorm-like body (PLB) subcultures. Plant Methods 2024, 20, 145. [Google Scholar] [CrossRef]
- Chen, T.; Pai, H.; Hou, L.; Lee, S.; Lin, T.; Chang, C.; Hsu, F.; Hsu, Y.; Lin, N. Dual resistance of transgenic plants against Cymbidium mosaic virus and Odontoglossum ringspot virus. Sci. Rep. 2019, 9, 10230. [Google Scholar] [CrossRef]
- Minh, T.; Tuyen, P.; Khang, D.; Quan, N.; Ha, P.; Quan, N.; Andriana, Y.; Fan, X.; Van, T.; Khanh, T.; et al. Potential use of plant waste from the moth orchid (Phalaenopsis Sogo Yukidian “V3”) as an antioxidant source. Foods 2017, 6, 85. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Lin, K.H.; Huang, M.Y.; Yang, C.M.; Shih, T.H.; Hsiung, T.C.; Lin, Y.C.; Tsao, F.C. Antioxidant activities of the methanol extracts of various parts of Phalaenopsis orchids with white, yellow, and purple flowers. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 457–465. [Google Scholar] [CrossRef]
- Irimescu, L.S.; Olivares, C.G.; Preda, C.I.; Digu, C.F.; Lu, G.; Blan, D.; Matei, F. Characterisation of the antimicrobial and antioxidant profile of Phalaenopsis orchid wastes. AgroLife Sci. J. 2021, 10, 101–108. [Google Scholar] [CrossRef]
- Ho, B.L.; Chen, J.C.; Huang, T.P.; Fang, S.C. Protocorm-like-body extract of Phalaenopsis aphrodite combats watermelon fruit blotch disease. Front. Plant Sci. 2022, 13, 1054586. [Google Scholar] [CrossRef]
- Chiu, P.C.; Li, Y.J.; Lo, C.Y.; Lin, S.M.; Huang, C.; Chiou, R.Y.Y. Properties characterization and identification of saponarin and ribosnarin (apigenin-6-C-ribosyl -7-O-glucoside) extracted abundantly from the white-color moth orchid flowers (Phalaenopsis Hybrids). Food Nutr. J. 2024, 8, 314. [Google Scholar]
- Minh, N.; Khang, D.; Tuyen, P.; Minh, L.; Anh, L.; Quan, N.; Ha, P.; Quan, N.; Toan, N.; Elzaawely, A.; et al. Phenolic compounds and antioxidant activity of Phalaenopsis orchid hybrids. Antioxidants 2016, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Hasegawa, S.; Inoue, Y.; Kunita, M.; Ohsumi, K.; Sakaida, T.; Yashiro, Y.; Nakata, S. Inhibitory effect of Phalaenopsis orchid extract on WNT1-induced immature melanocyte precursor differentiation in a novel in vitro solar lentigo model. Biosci. Biotechnol. Biochem. 2016, 80, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Hung, H.Y.; Yang, M.L.; Chen, H.H.; Kuo, P.C.; Wu, T.S. Chemical constituents from Phalaenopsis hybrids and their bioactivities. Nat. Prod. Commun. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Tzou, D.; Lee, C.; Wu, Y.; Tang, Y.; Cheng, C. Glycolipids of monogalactosyldiacylglycerol in Phalaenopsis leaves during growth and spike induction periods form mixed liposomes that enhance the inhibition of Escherichia coli growth. J. Chin. Chem. Soc. 2023, 71, 232–239. [Google Scholar] [CrossRef]
- Giovannini, A.; Laurà, M.; Nesi, B.; Savona, M.; Cardi, T. Genes and genome editing tools for breeding desirable phenotypes in ornamentals. Plant Cell Rep. 2021, 40, 461–478. [Google Scholar] [CrossRef]
- Hsu, S.T.; Chuang, H.T.; Shen, T.M. Breeding barriers in red Phalaenopsis orchids. Acta Hortic. 2010, 2010, 145–152. [Google Scholar] [CrossRef]
- An, H.R.; Kwon, O.K.; Lee, S.Y.; Park, P.H.; Park, P.M.; Choi, I.; Lee, H.; Yoo, J.H. Breeding of yellow small-type Phalaenopsis ‘Yellow Scent’ with fragrance. Hortic. Sci. Technol. 2019, 37, 304–310. [Google Scholar] [CrossRef]
- Chen, J.M.; Zhu, X.Y.; Zheng, R.Y.; Tong, Y.; Peng, Y.K.; Xie, K.; Su, Q.L.; Huang, R.L.; Zhan, S.Y.; Shen, M.L.; et al. Orchestrating of native Phalaenopsis flower scents lighted the way through artificial selective breeding partiality in the current resource utilization. Ind. Crops Prod. 2024, 217, 118850. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Ming, F.; Liu, M.; Wang, X.; Zhang, Y. Correlation between floral color attributes and volatile components among 10 fragrant Phalaenopsis cultivars. Phyton-Int. J. Exp. Bot. 2025, 94, 379–391. [Google Scholar] [CrossRef]
- Roberta, P.; Stefania, D.P. Effects of plant size, temperature, and light intensity on flowering of Phalaenopsis hybrids in mediterranean greenhouses. Sci. World J. 2014, 2014, 420807. [Google Scholar]
- Su, W.R.; Chen, W.S.; Koshioka, M.; Mander, L.N.; Hung, L.S.; Chen, W.H.; Fu, Y.M.; Huang, K.L. Changes in gibberellin levels in the flowering shoot of Phalaenopsis hybrida under high temperature conditions when flower development is blocked. Plant Physiol. Biochem. 2001, 39, 45–50. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, L.; Wang, Y.; Li, J.; Luo, P.; Xin, J.; Cui, Y. Genome-wide identification of DREB transcription factor family and functional analysis of PaDREB1D associated with low-temperature stress in Phalaenopsis aphrodite. Horticulturae 2024, 10, 933. [Google Scholar] [CrossRef]
- Pramanik, D.; Spaans, M.; Kranenburg, T.; Bogarín, D.; Heijungs, R.; Lens, F.; Smets, E.; Gravendeel, B. Inflorescence lignification of natural species and horticultural hybrids of Phalaenopsis orchids. Sci. Hortic. 2022, 295, 110845. [Google Scholar] [CrossRef]
- Puspa, A.; Rahayu, T.; Jayanti, G.E. Analysis of nanobubbles (NBs) technology and foliar fertilization on the growth of Phalaenopsis sp. orchid. Berk. Sainstek 2024, 12, 37. [Google Scholar] [CrossRef]
- Syafitri, F.I.; Rahayu, T.; Jayanti, G.E. Potential of ecoenzymes and N2 nanobubbles on the growth of Phalaenopsis sp. orchid at the acclimatization stage. J. Ilmu Dasar 2024, 25, 41. [Google Scholar] [CrossRef]
- Rizky, W.H.; Nuraini, A.; Gunawan, R.H. Micropropagation of Phalaenopsis orchid by natural substances for unique-formed “orchid key-holder” on small bottle in order to agritourism development. Sci. Works—Univ. Agron. Sci. Vet. Med. Buchar. Ser. B Hortic. 2011, 2011, 236–241. [Google Scholar]
- Zhao, J.; Ishak, S.M.M.; Yahaya, M.F. Design factors influencing consumer acceptance in cultural and creative products: An integrated review. Int. J. Acad. Res. Bus. Soc. Sci. 2024, 14, 1405–1420. [Google Scholar]
- Chow, W.; Shieh, M.D. A study of the cultural and creative product design of Phalaenopsis in Taiwan. J. Interdiscip. Math. 2018, 21, 389–395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).