A Fragment Insertion of AgDFR Results in a White Flower Phenotype in Arundina graminifolia (Orchidaceae)
Abstract
1. Introduction
2. Results
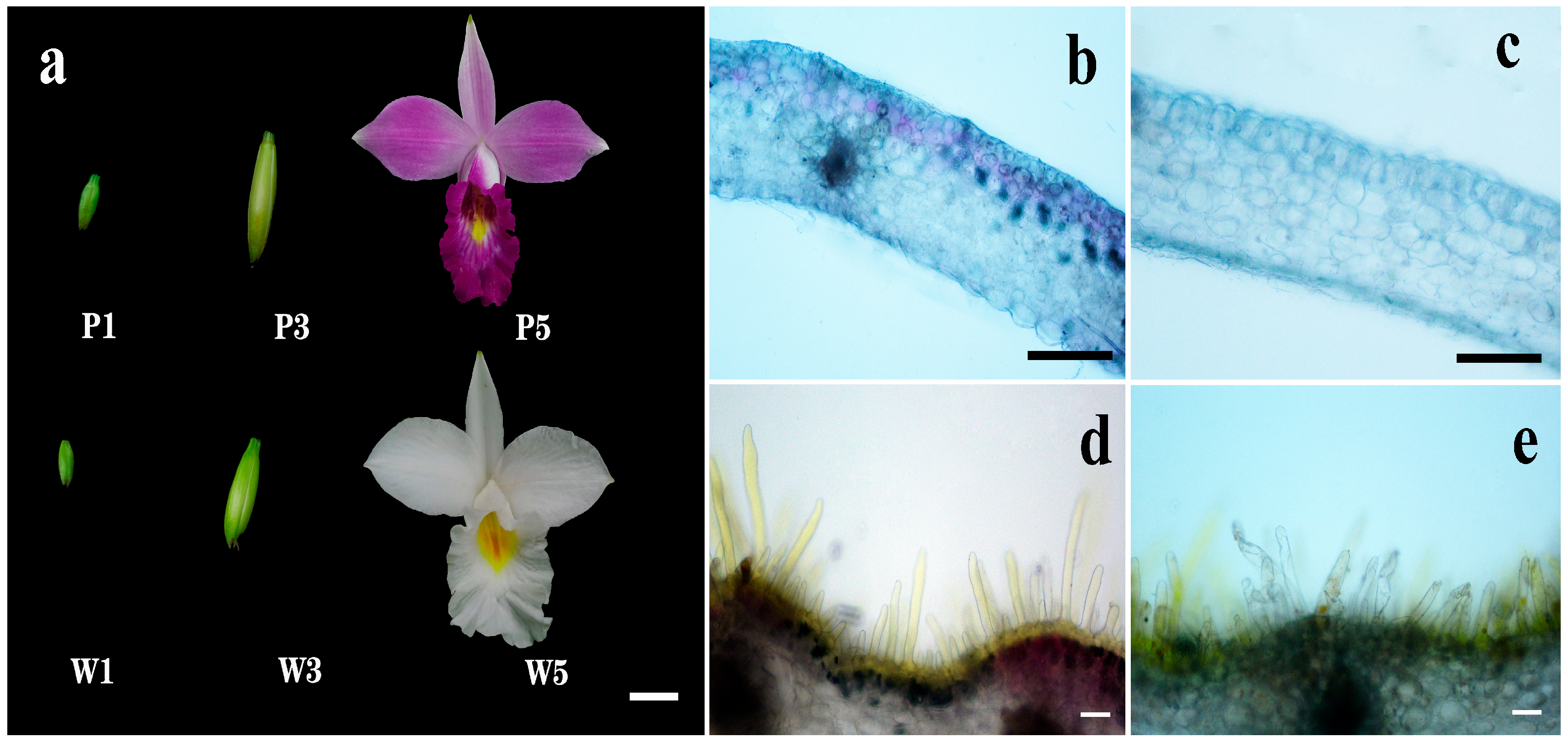
2.1. Flower Phenotypes and Spatial Localization of Pigments
2.2. Overview of RNA Sequencing
2.3. Identification of Differentially Expressed Genes (DEGs) in Anthocyanin Biosynthesis
2.4. Validation of the Expression of Anthocyanin-Related Genes
2.5. Isolation of AgDFR Gene and Phylogenetic Analysis
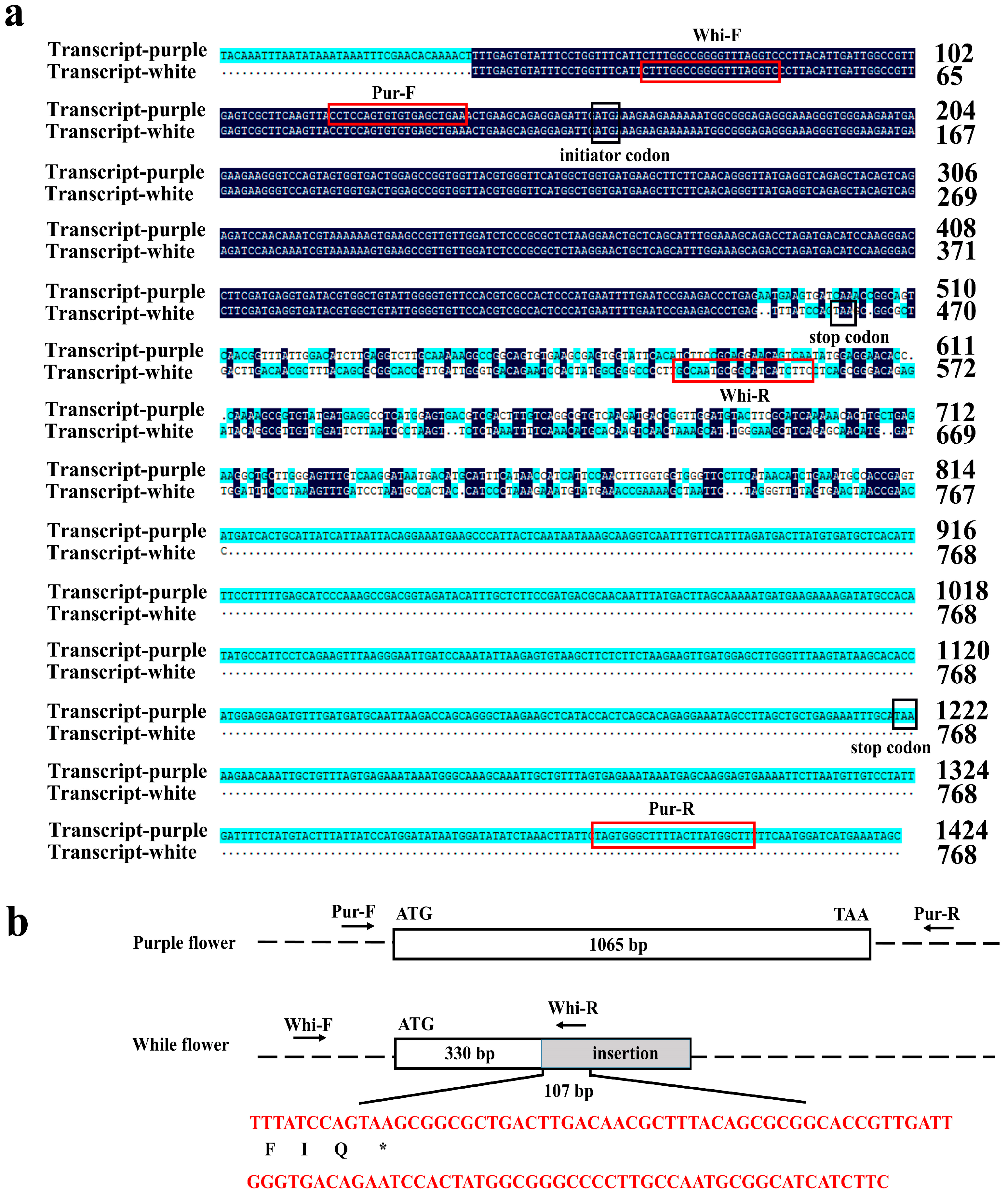
2.6. Cloning of the AgDFR Gene Promoter
2.7. Molecular Marker Identification and Proposed Model of White Flowers
3. Discussion
4. Materials and Methods
4.1. Isolation of the AgDFR Promoter
4.2. Tissue Section Observation
4.3. RNA Extraction, Library Preparation and Sequencing
4.4. De Novo Transcriptome Assembly Annotation
4.5. Functional Analysis of Differentially Expressed Genes
4.6. Cloning of AgDFR
4.7. Promoter Cloning
4.8. Multiple Sequence Alignment and Phylogenetic Analysis
4.9. Transcription Profiling by RT-qPCR
4.10. Molecular Marker Identification
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABP | Anthocyanin biosynthesis pathway |
| DEGs | Differentially expressed genes |
| CHS | Chalcone synthase |
| CHI | Chalcone isomerase |
| F3H | Flavanone 3-hydroxylase |
| F3′H | Flavonoid 3′-hydroxylase |
| F3′5′H | Flavonoid 3′,5′-hydroxylase |
| DFR | Dihydroflavonol 4-reductase |
| ANS | Anthocyanidin synthase |
| UFGT | Anthocyanidin 3-O-glucosyltransferase |
| PAL | Phenylalanine ammonia-lyase |
| 4CL | 4-coumarate-CoA ligase |
| C4H | Cinnamic acid 4-hydroxylase |
| FLS | Flavonol synthase |
| MYB | v-myb avian myeloblastosis viral oncogene homolog |
| bHLH | Basic helix-loop-helix |
| HY5 | Elongated hypocotyl 5 |
| ORF | Open reading frame |
References
- Li, J.; Wang, Z.H. Integrative metabolomic and transcriptome analysis reveal the differential mechanisms of spot color in the lips of Dendrobium chrysotoxum. J. Plant Biol. 2023, 66, 359–371. [Google Scholar]
- Meng, X.; Li, G.; Gu, L.; Sun, Y.; Li, Z.; Liu, J.; Wu, X.; Dong, T.; Zhu, M. Comparative metabolomic and transcriptome analysis reveal distinct flavonoid biosynthesis regulation between petals of white and purple Phalaenopsis amabilis. J. Plant Growth Regul. 2020, 39, 823–840. [Google Scholar]
- Li, B.J.; Zheng, B.Q.; Wang, J.Y.; Tsai, W.C.; Lu, H.C.; Zou, L.H.; Wan, X.; Zhang, D.; Qiao, H.; Liu, Z. New insight into the molecular mechanism of color differentiation among floral segments in orchids. Commun. Biol. 2020, 3, 89. [Google Scholar]
- Zhang, Y.; Zhou, T.; Dai, Z.; Dai, X.; Li, W.; Cao, M.; Li, C.; Tsai, W.C.; Wu, X.; Zhai, J.; et al. Comparative transcriptomics provides insight into floral color polymorphism in a Pleione limprichtii orchid population. Int. J. Mol. Sci. 2019, 21, 247. [Google Scholar]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-mediated regulation of anthocyanin biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar]
- Hsu, C.C.; Chen, Y.Y.; Tsai, W.C.; Chen, W.H.; Chen, H.H. Three R2R3-MYB transcription factors regulate distinct floral pigmentation patterning in Phalaenopsis spp. Plant Physiol. 2015, 168, 175–191. [Google Scholar] [CrossRef]
- Fu, Z.Z.; Shang, H.Q.; Jiang, H.; Gao, J.; Dong, X.Y.; Wang, H.J.; Li, Y.; Wang, L.; Zhang, J.; Shu, Q.; et al. Systematic identification of the light-quality responding anthocyanin synthesis-related transcripts in Petunia petals. Hortic. Plant J. 2020, 6, 428–438. [Google Scholar] [CrossRef]
- Li, C.; Qiu, J.; Ding, L.; Huang, M.; Huang, S.; Yang, G.; Yin, J. Anthocyanin biosynthesis regulation of DhMYB2 and DhbHLH1 in Dendrobium hybrids petals. Plant Physiol. Biochem. 2017, 112, 335–345. [Google Scholar] [CrossRef]
- Kaur, H.; Sena, S.; Jha, P.; Lekhak, M.M.; Singh, S.K.; Goutam, U.; Arencibia, A.D.; Kumar, V. Arundina graminifolia (D.Don) Hochr. (Orchidaceae): A review of its medicinal importance, phytochemistry and pharmacology activities. S. Afr. J. Bot. 2022, 150, 956–964. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Du, Y.; Su, P.; Qiu, Y.; Ning, J.; Liu, M. Phytochemistry and pharmacological activities of Arundina graminifolia (D.Don) Hochr. and other common Orchidaceae medicinal plants. J. Ethnopharmacol. 2021, 276, 114143. [Google Scholar] [CrossRef]
- Auberon, F.; Olatunji, O.J.; Krisa, S.; Antheaume, C.; Herbette, G.; Bonté, F.; Mérillon, J.M.; Lobstein, A. Two new stilbenoids from the aerial parts of Arundina graminifolia (Orchidaceae). Molecules 2016, 21, 1430. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Gao, J.; Wei, Y.; Lu, C.; Zhu, G.; Yang, F. The transcriptome profiling of flavonoids and bibenzyls reveals medicinal importance of rare orchid Arundina graminifolia. Front. Plant Sci. 2022, 13, 923000. [Google Scholar] [CrossRef]
- Trunschke, J.; Lunau, K.; Pyke, G.H.; Ren, Z.X.; Wang, H. Flower color evolution and the evidence of pollinator-mediated selection. Front. Plant Sci. 2021, 12, 617851. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ohnishi, N.; Morikawa, N.; Ishigami, A.; Otake, S.; Rabah, I.O.; Sakata, Y.; Hashimoto, F. A 94-bp deletion of anthocyanidin synthase gene in acyanic flower lines of lisianthus [Eustoma grandiflorum (Raf.) Shinn.]. J. Jpn. Soc. Hortic. Sci. 2011, 80, 434–442. [Google Scholar] [CrossRef]
- Lin, C.; Xing, P.; Jin, H.; Zhou, C.; Li, X.; Song, Z. Loss of anthocyanidin synthase gene is associated with white flowers of Salvia miltiorrhiza Bge. f. alba, a natural variant of S. miltiorrhiza. Planta 2022, 256, 15. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Guan, R.; Sun, X.; Zhang, C.; Shan, C.; Liu, M.; Cui, N.; Wang, P.; Lin, H. Integrated metabolome and transcriptome analyses of anthocyanin biosynthesis reveal key candidate genes involved in color variation of Scutellaria baicalensis flowers. BMC Plant Biol. 2023, 23, 643. [Google Scholar] [CrossRef]
- Qiu, Y.; Cai, C.; Mo, X.; Zhao, X.; Wu, L.; Liu, F.; Li, R.; Liu, C.; Chen, J.; Tian, M. Transcriptome and metabolome analysis reveals the effect of flavonoids on flower color variation in Dendrobium nobile Lindl. Front. Plant Sci. 2023, 14, 1220507. [Google Scholar] [CrossRef]
- Zhou, Z.; Ying, Z.; Wu, Z.; Yang, Y.; Fu, S.; Xu, W.; Yao, L.; Zeng, A.; Huang, J.; Lan, S.; et al. Anthocyanin genes involved in the flower coloration mechanisms of Cymbidium kanran. Front. Plant Sci. 2021, 12, 737815. [Google Scholar] [CrossRef]
- Gates, D.J.; Olson, B.J.S.C.; Clemente, T.E.; Smith, S.D. A novel R3 MYB transcriptional repressor associated with the loss of floral pigmentation in Iochroma. New Phytol. 2018, 217, 1346–1356. [Google Scholar] [CrossRef]
- Lim, S.H.; Park, B.; Kim, D.H.; Park, S.; Yang, J.H.; Jung, J.A.; Lee, J.; Lee, J.Y. Cloning and functional characterization of Dihydroflavonol 4-reductase gene involved in anthocyanin biosynthesis of Chrysanthemum. Int. J. Mol. Sci. 2020, 21, 7960. [Google Scholar] [CrossRef]
- Davies, K.M.; Schwinn, K.E.; Deroles, S.C.; Manson, D.G.; Lewis, D.H.; Bloor, S.J.; Bradley, J.M. Enhancing anthocyanin production by altering competition for substrate between flavonol synthase and dihydroflavonol 4-reductase. Euphytica 2003, 131, 259–268. [Google Scholar] [CrossRef]
- Luo, P.; Ning, G.; Wang, Z.; Shen, Y.; Jin, H.; Li, P.; Huang, S.; Zhao, J.; Bao, M. Disequilibrium of flavonol synthase and dihydroflavonol-4-reductase expression associated tightly to white vs. red color flower formation in plants. Front Plant Sci. 2016, 6, 1257. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.R.; Berg, J.J.; Blinka, S.; Rausher, M.D.; Baum, D.A. Precise spatio-temporal regulation of the anthocyanin biosynthetic pathway leads to petal spot formation in Clarkia gracilis (Onagraceae). New Phytol. 2013, 197, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Luo, S.; Ma, W.; Li, N.; Zhang, W.; Tikunov, Y.; Xuan, S.; Zhao, J.; Wang, Y.; et al. Discovery of a DFR gene that controls anthocyanin accumulation in the spiny Solanum group: Roles of a natural promoter variant and alternative splicing. Plant J. 2022, 111, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.C.; Liu, K.W.; Chen, L.J.; He, Y.; Xu, Q.; Bian, C.; et al. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chung, O.; Kim, J.; Bolser, D.; Kim, H.M.; Jun, J.H.; Choi, J.P.; Jang, H.D.; Cho, Y.S.; Bhak, J.; Kwak, M. A chromosome-scale genome assembly and annotation of the spring orchid (Cymbidium goeringii). Mol. Ecol. Resour. 2022, 22, 1168–1177. [Google Scholar] [CrossRef]
- Mudalige, R.G.; Kuehnle, A.R.; Amore, T.D. Pigment distribution and epidermal cell shape in Dendrobium species. HortScience 2003, 38, 573–577. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wei, Y.; Jin, J.; Gao, J.; Xie, Q.; Yang, F.; Zhu, G. A Fragment Insertion of AgDFR Results in a White Flower Phenotype in Arundina graminifolia (Orchidaceae). Plants 2025, 14, 1680. https://doi.org/10.3390/plants14111680
Li J, Wei Y, Jin J, Gao J, Xie Q, Yang F, Zhu G. A Fragment Insertion of AgDFR Results in a White Flower Phenotype in Arundina graminifolia (Orchidaceae). Plants. 2025; 14(11):1680. https://doi.org/10.3390/plants14111680
Chicago/Turabian StyleLi, Jie, Yonglu Wei, Jianpeng Jin, Jie Gao, Qi Xie, Fengxi Yang, and Genfa Zhu. 2025. "A Fragment Insertion of AgDFR Results in a White Flower Phenotype in Arundina graminifolia (Orchidaceae)" Plants 14, no. 11: 1680. https://doi.org/10.3390/plants14111680
APA StyleLi, J., Wei, Y., Jin, J., Gao, J., Xie, Q., Yang, F., & Zhu, G. (2025). A Fragment Insertion of AgDFR Results in a White Flower Phenotype in Arundina graminifolia (Orchidaceae). Plants, 14(11), 1680. https://doi.org/10.3390/plants14111680







