High-Quality Assembly of the Apple Fungal Pathogen Marssonina coronaria Genome and Functional Analysis of Candidate Effectors
Abstract
1. Introduction
2. Results
2.1. Marssonina coronaria Assembly and Candidate Effector Prediction
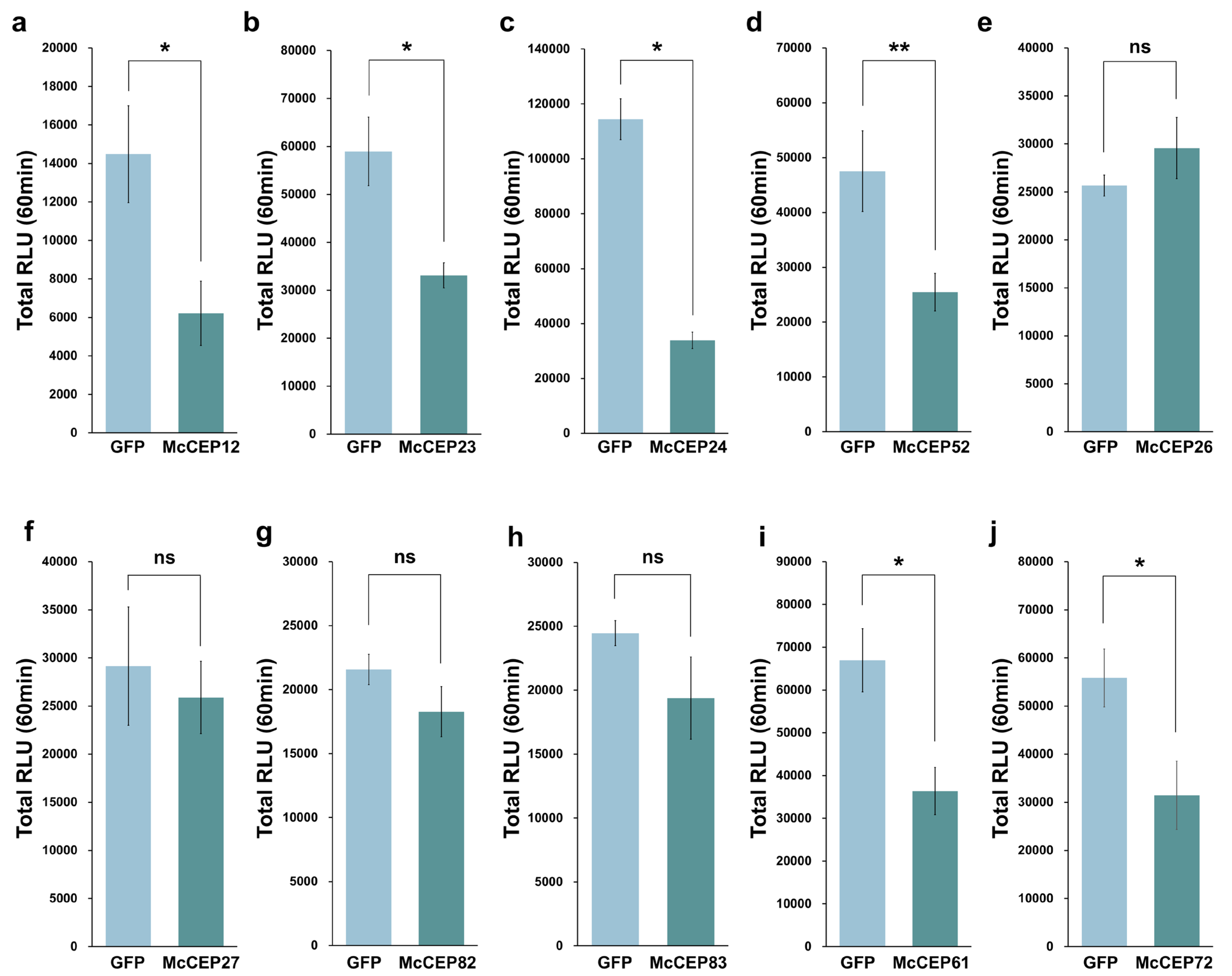
2.2. Screening of Effectors Suppressing BT-PCD
2.3. Screening of Effectors Inhibiting flg22-Induced Reactive Oxygen Species (ROS) Bursts
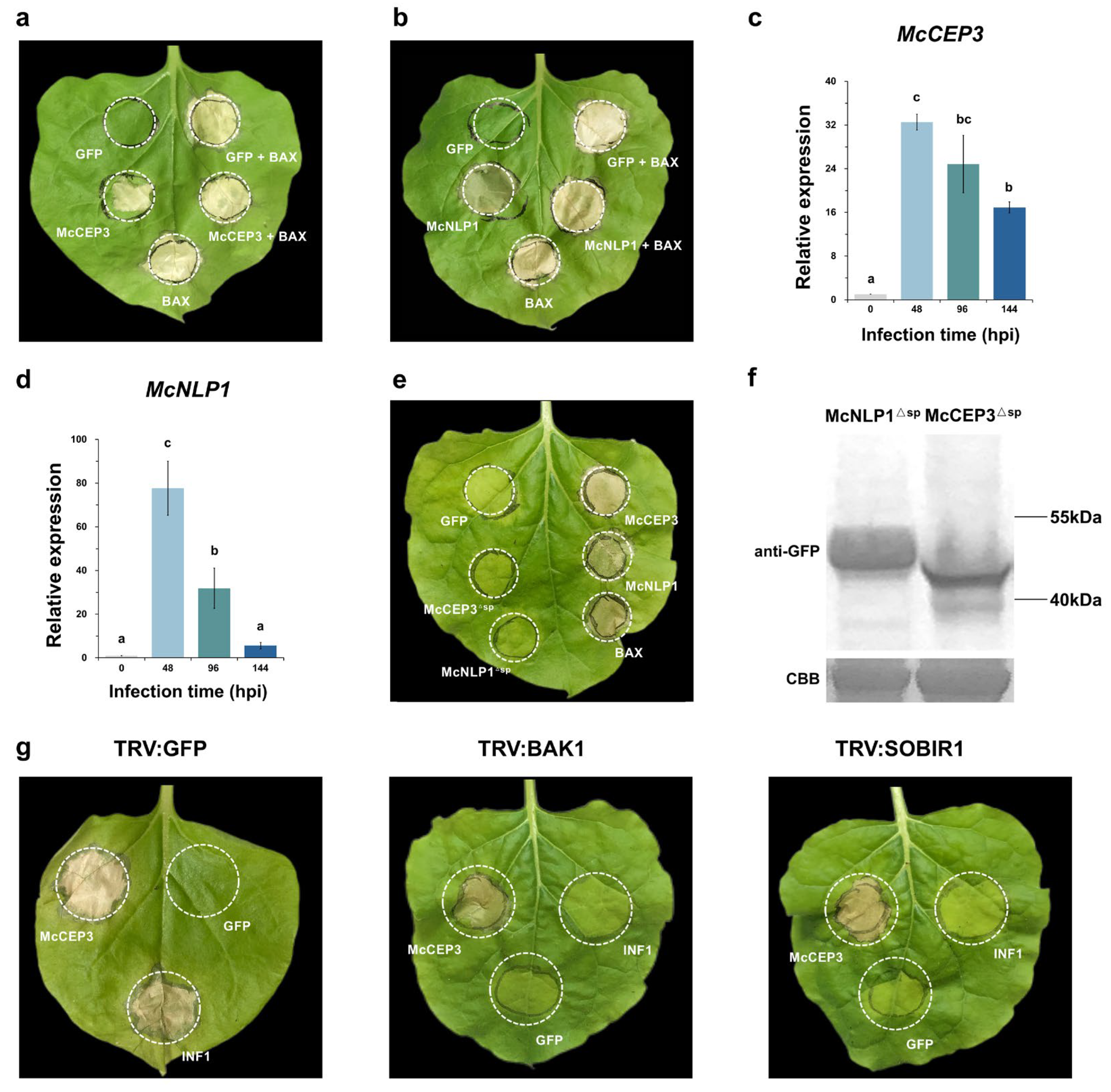
2.4. Effectors Inducing Cell Death in N. benthamiana
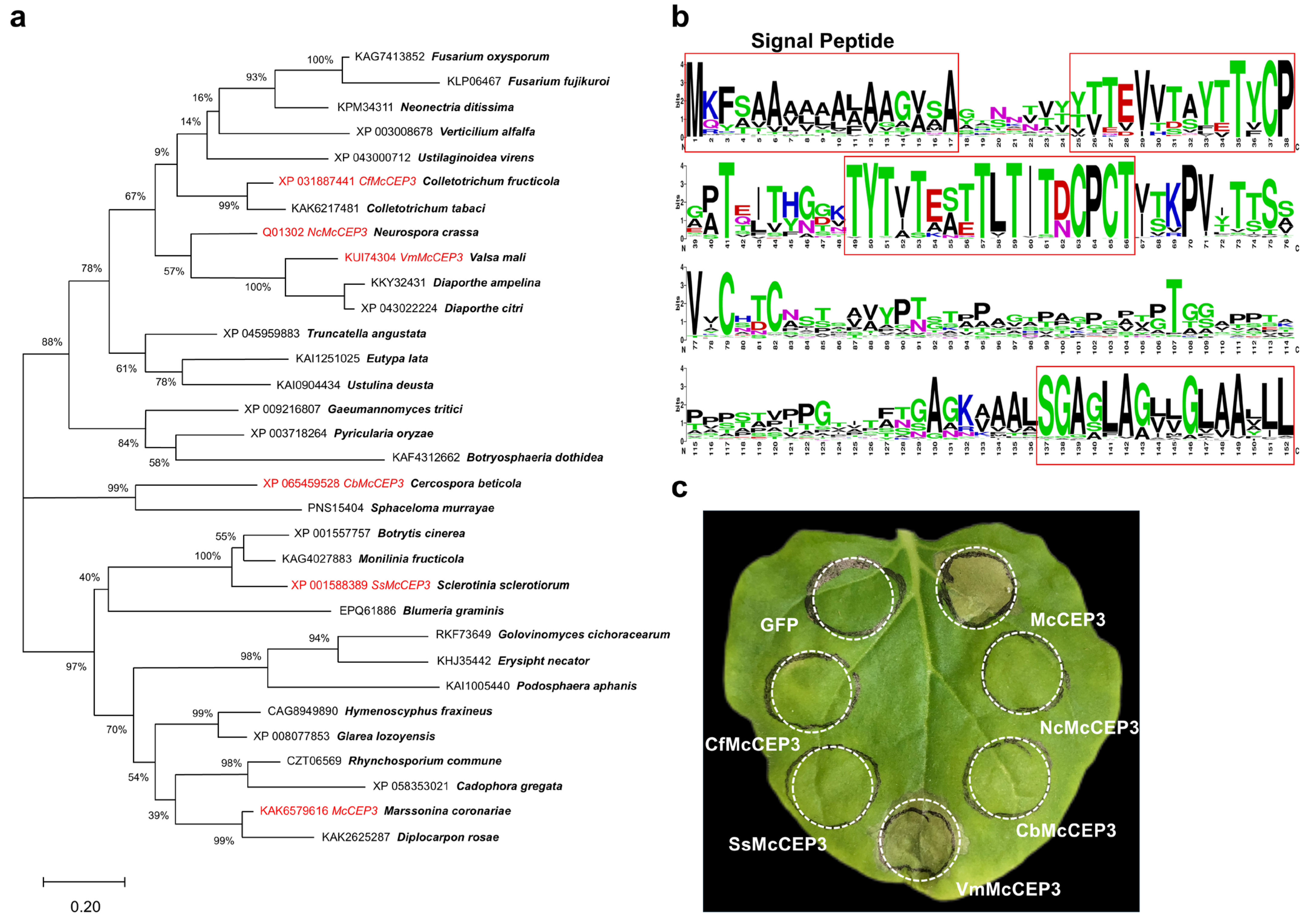
2.5. McCEP3 Represents a Conserved Class of Fungal Cell Death-Inducing Proteins
3. Discussion
3.1. Comparative Genomic Analysis of M. coronaria
3.2. Effector Identification and Functional Characterization
3.3. McCEP3 and the Mechanism of Cell Death Induction
4. Materials and Methods
4.1. Fungal and Plant Materials, and Pathogen Inoculation
4.2. Whole-Genome Sequencing and Annotation of YL1
4.3. RNA Extraction and Quantitative PCR (qPCR)
4.4. Candidate Effector Cloning and Plasmid Construction
4.5. Cell Death Induction and the BT-PCD Suppression Assay
4.6. Validation of SP Secretory Activities
4.7. Flg22-Induced ROS Suppression Assay
4.8. Protein Extraction and Western Blot Analysis
4.9. VIGS Assay
4.10. Phylogenetic Tree Construction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wohner, T.; Girichev, V.; Radatz, S.; Lauria-Baca, B.; Scheinpflug, H.; Hanke, M.V. Evaluation of Malus gene bank resources with German strains of Marssonina coronaria using a greenhouse-based screening method. Eur. J. Plant Pathol. 2019, 153, 743–757. [Google Scholar] [CrossRef]
- Wöhner, T.; Emeriewen, O.F. Apple blotch disease (Marssonina coronaria (Ellis & Davis) Davis)—Review and research prospects. Eur. J. Plant Pathol. 2018, 153, 657–669. [Google Scholar]
- Zhao, H.; Han, Q.; Wang, J.; Gao, X.; Xiao, C.-L.; Liu, J.; Huang, L. Cytology of infection of apple leaves by Diplocarpon mali. Eur. J. Plant Pathol. 2013, 136, 41–49. [Google Scholar] [CrossRef]
- Dou, D.; Zhou, J.M. Phytopathogen effectors subverting host immunity: Different foes, similar battleground. Cell Host Microbe 2012, 12, 484–495. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Irieda, H.; Inoue, Y.; Mori, M.; Yamada, K.; Oshikawa, Y.; Saitoh, H.; Uemura, A.; Terauchi, R.; Kitakura, S.; Kosaka, A.; et al. Conserved fungal effector suppresses PAMP-triggered immunity by targeting plant immune kinases. Proc. Natl. Acad. Sci. USA 2019, 116, 496–505. [Google Scholar] [CrossRef]
- Zheng, X.Z.; McLellan, H.; Fraiture, M.; Liu, X.Y.; Boevink, P.C.; Gilroy, E.M.; Chen, Y.; Kandel, K.; Sessa, G.; Birch, P.R.J.; et al. Functionally redundant RXLR effectors from Phytophthora infestans act at different steps to suppress early flg22-Triggered Immunity. PLoS Pathog. 2014, 10, e1004057. [Google Scholar] [CrossRef]
- Kleemann, J.; Rincon-Rivera, L.J.; Takahara, H.; Neumann, U.; Van Themaat, E.V.L.; van Der Does, H.C.; Hacquard, S.; Stüber, K.; Will, I.; Schmalenbach, W. Sequential delivery of host-induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum. PLoS Pathog. 2012, 8, e1002643. [Google Scholar] [CrossRef]
- Seidl, M.F.; Van den Ackerveken, G. Activity and phylogenetics of the broadly occurring family of microbial Nep1-like proteins. Annu. Rev. Phytopathol. 2019, 57, 367–386. [Google Scholar] [CrossRef]
- Saitoh, H.; Fujisawa, S.; Mitsuoka, C.; Ito, A.; Hirabuchi, A.; Ikeda, K.; Irieda, H.; Yoshino, K.; Yoshida, K.; Matsumura, H. Large-scale gene disruption in Magnaporthe oryzae identifies MC69, a secreted protein required for infection by monocot and dicot fungal pathogens. PloS Pathog. 2012, 8, e1002711. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, P.; Sharma, R.; Bhardwaj, L. The genus marssonina-its biology, pathology and management. Annu. Rev. Plant Pathol. 2005, 3, 271–292. [Google Scholar]
- Cheng, Q.; Yang, H.; Chen, J.; Zhao, L. Population Genomics Reveals Population Structure and Mating-Type Loci in Marssonina brunnea. J. Fungi 2022, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Rawandoozi, Z.J.; Young, E.L.; Yan, M.; Noyan, S.; Fu, Q.; Hochhaus, T.; Rawandoozi, M.Y.; Klein, P.E.; Byrne, D.H.; Riera-Lizarazu, O. QTL mapping and characterization of black spot disease resistance using two multi-parental diploid rose populations. Hortic. Res. 2022, 9, uhac183. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, Y.; Su, L.; Yang, S.; Yi, X.; Luo, L.; Yu, C.; Cheng, T.; Wang, J.; Zhang, Q. The Marssonina rosae effector MrSEP43 suppresses rose immunity by targeting the orphan protein RcBROG. J. Exp. Bot. 2024, 75, erae200. [Google Scholar] [CrossRef]
- Qian, Y.; Zheng, X.; Wang, X.; Yang, J.; Zheng, X.; Zeng, Q.; Li, J.; Zhuge, Q.; Xiong, Q. Systematic identification and functional characterization of the CFEM proteins in poplar fungus Marssonina brunnea. Front. Cell Infect. Microbiol. 2022, 12, 1045615. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, H.; Xu, B.; Zhu, S.; Hu, L.; Huang, M. Discovery of a novel small secreted protein family with conserved N-terminal IGY motif in Dikarya fungi. BMC Genom. 2014, 15, 1151. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, J.; Zhao, L. Draft genome sequence of Marssonina coronaria, causal agent of apple blotch, and comparisons with the Marssonina brunnea and Marssonina rosae genomes. PLoS ONE 2021, 16, e0246666. [Google Scholar] [CrossRef]
- Richter, S.; Kind, S.; Oberhänsli, T.W.; Schneider, M.; Nenasheva, N.; Hoff, K.; Keilwagen, J.; Yeon, I.-K.; Philion, V.; Moriya, S. Genome sequence of a European Diplocarpon coronariae strain and in silico structure of the mating-type locus. Front. Plant Sci. 2024, 15, 1437132. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, Q. Heterologous expression of Arabidopsis pattern recognition receptor RLP23 increases broad-spectrum resistance in poplar to fungal pathogens. Mol. Plant Pathol. 2023, 24, 80–86. [Google Scholar] [CrossRef]
- Böhm, H.; Albert, I.; Oome, S.; Raaymakers, T.M.; Van den Ackerveken, G.; Nürnberger, T. A conserved peptide pattern from a widespread microbial virulence factor triggers pattern-induced immunity in Arabidopsis. PLoS Pathog. 2014, 10, e1004491. [Google Scholar] [CrossRef]
- Xing, Q.; Cao, Y.; Peng, J.; Zhang, W.; Wu, J.; Zhou, Y.; Li, X.; Yan, J. A putative effector LtCSEP1 from Lasiodiplodia theobromae inhibits BAX-triggered cell death and suppresses immunity responses in Nicotiana benthamiana. Plants 2022, 11, 1462. [Google Scholar] [CrossRef] [PubMed]
- Rajput, N.A.; Zhang, M.; Ru, Y.; Liu, T.; Xu, J.; Liu, L.; Mafurah, J.J.; Dou, D. Phytophthora sojae effector PsCRN70 suppresses plant defenses in Nicotiana benthamiana. PLoS ONE 2014, 9, e98114. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lan, X.; Ye, W.; Liu, Y.; Song, S.; Lu, J. Plasmopara viticola effector PvRXLR159 suppresses immune responses in Nicotiana benthamiana. Plant Signal. Behav. 2019, 14, 1682220. [Google Scholar] [CrossRef]
- Lenarčič, T.; Albert, I.; Böhm, H.; Hodnik, V.; Pirc, K.; Zavec, A.B.; Podobnik, M.; Pahovnik, D.; Žagar, E.; Pruitt, R. Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 2017, 358, 1431–1434. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, F.; Xie, S.Q.; Zheng, Y.F.; Dai, Q.; Bray, T.; Wang, Y.X.; Xing, J.F.; Huang, Z.J.; Wang, D.P.; et al. Efficient assembly of nanopore reads via highly accurate and intact error correction. Nat. Commun. 2021, 12, 60. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Simao, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Brůna, T.; Hoff, K.J.; Lomsadze, A.; Stanke, M.; Borodovsky, M. BRAKER2: Automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genom. Bioinform. 2021, 3, lqaa108. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Jones, L.; Hamilton, A.J.; Voinnet, O.; Thomas, C.L.; Maule, A.J.; Baulcombe, D.C. RNA–DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 1999, 11, 2291–2301. [Google Scholar] [PubMed]
- Jacobs, K.A.; Collins-Racie, L.A.; Colbert, M.; Duckett, M.; Golden-Fleet, M.; Kelleher, K.; Kriz, R.; LaVallie, E.R.; Merberg, D.; Spaulding, V. A genetic selection for isolating cDNAs encoding secreted proteins. Gene 1997, 198, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-K.; Young, C.; Lee, M.; Oliva, R.; Bozkurt, T.O.; Cano, L.M.; Win, J.; Bos, J.I.; Liu, H.-Y.; van Damme, M. In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell 2009, 21, 2928–2947. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Macho, A.P. Analysis of PAMP-Triggered ROS Burst in Plant Immunity. In Plant Pattern Recognition Receptors; Springer: Berlin/Heidelberg, Germany, 2017; pp. 143–153. [Google Scholar]
- Liu, Y.; Schiff, M.; Marathe, R.; Dinesh-Kumar, S. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002, 30, 415–429. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]

| Genome Feature Annotation | Statistics |
|---|---|
| NCBI accession | PRJNA940293 |
| Genome size (bp) | 54,484,178.00 |
| Contigs | 23 |
| N50 contig (bp) | 4,071,588.00 |
| Maximum contig length (bp) | 2,368,877.30 |
| GC content (%) | 44.08 |
| Gene number | 8095 |
| mRNA average length (bp) | 2756.83 |
| CDS average length (bp) | 1495.95 |
| Transfer RNA number | 8275 |
| Ribosomal RNA number | 42,433 |
| Small nuclear RNA number | 3638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Fu, Y.; Zhong, L.; Cheng, Q. High-Quality Assembly of the Apple Fungal Pathogen Marssonina coronaria Genome and Functional Analysis of Candidate Effectors. Plants 2025, 14, 1638. https://doi.org/10.3390/plants14111638
Guo H, Fu Y, Zhong L, Cheng Q. High-Quality Assembly of the Apple Fungal Pathogen Marssonina coronaria Genome and Functional Analysis of Candidate Effectors. Plants. 2025; 14(11):1638. https://doi.org/10.3390/plants14111638
Chicago/Turabian StyleGuo, Huiting, Yicong Fu, Lichi Zhong, and Qiang Cheng. 2025. "High-Quality Assembly of the Apple Fungal Pathogen Marssonina coronaria Genome and Functional Analysis of Candidate Effectors" Plants 14, no. 11: 1638. https://doi.org/10.3390/plants14111638
APA StyleGuo, H., Fu, Y., Zhong, L., & Cheng, Q. (2025). High-Quality Assembly of the Apple Fungal Pathogen Marssonina coronaria Genome and Functional Analysis of Candidate Effectors. Plants, 14(11), 1638. https://doi.org/10.3390/plants14111638





