The Overexpression of ORR3 Negatively Regulates the Growth of Young Rice Roots by Reducing the Cell Size and the Number in the Root Meristematic Zone
Abstract
1. Introduction
2. Result
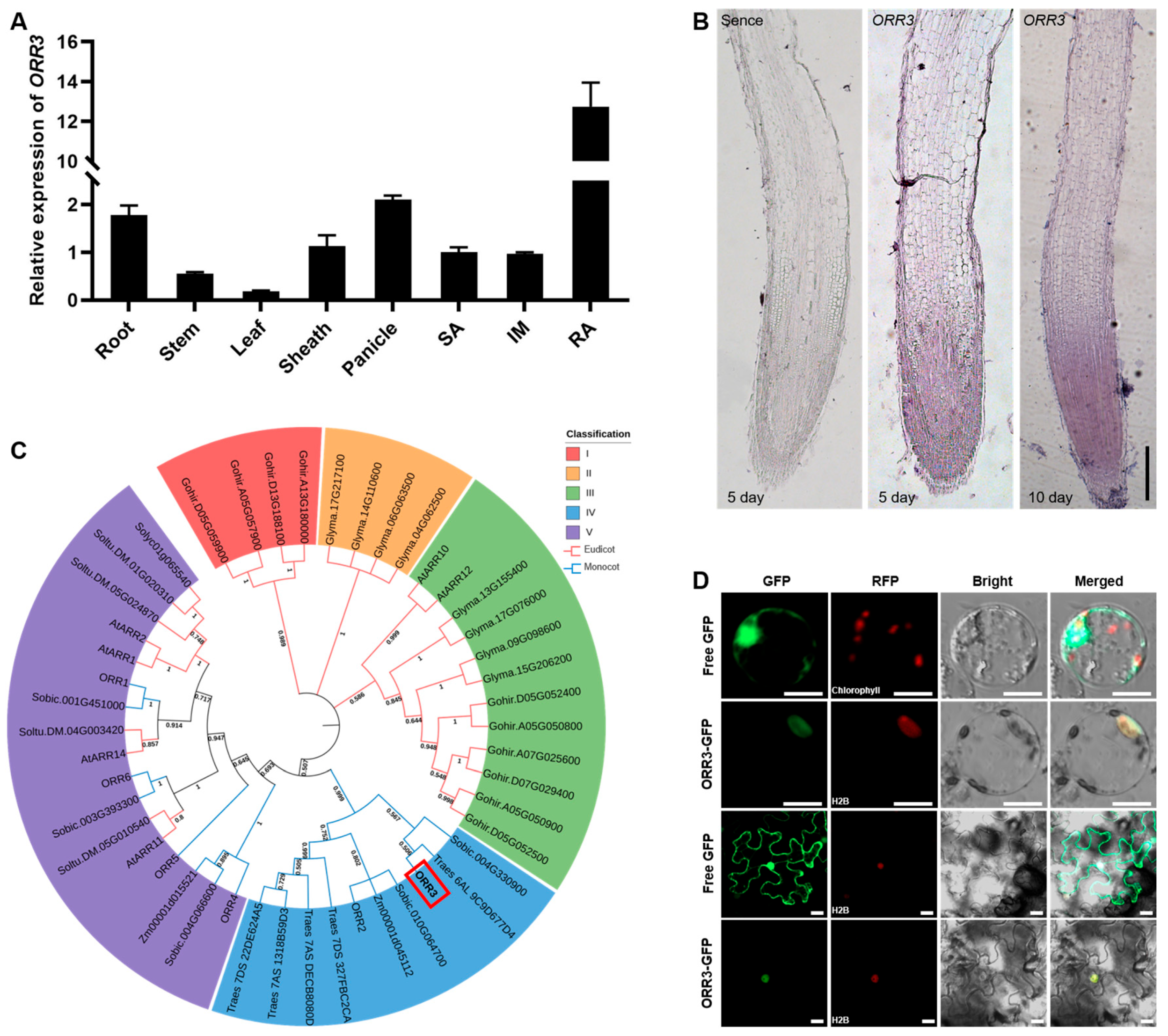
2.1. Expression Pattern and Homology Analysis of ORR3, and Subcellular Localization of ORR3 Protein
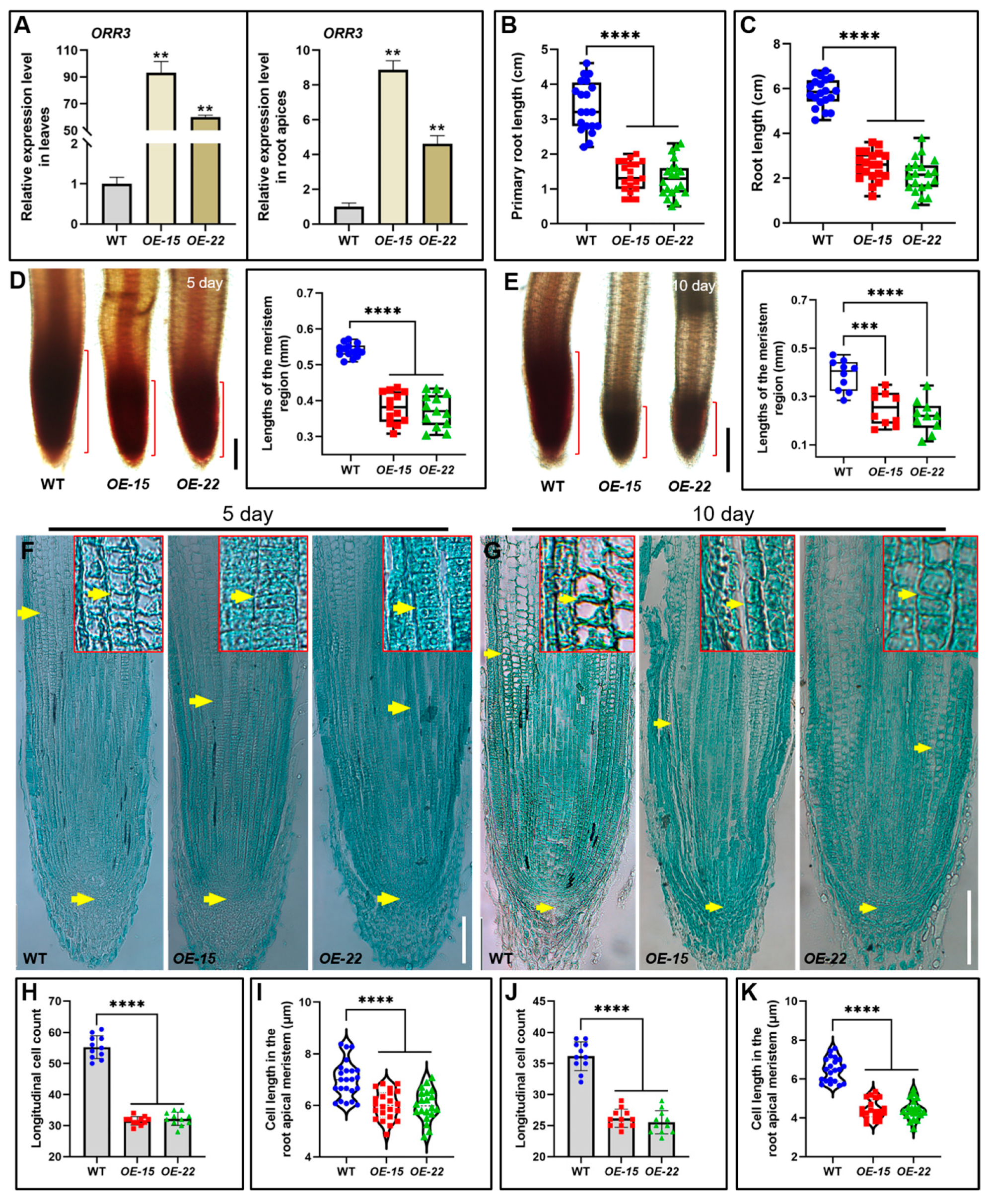
2.2. The Overexpression of ORR3 Leads to a Reduction in Both the Number and Size of Cells in the Root Meristematic Zone of Rice Seedlings
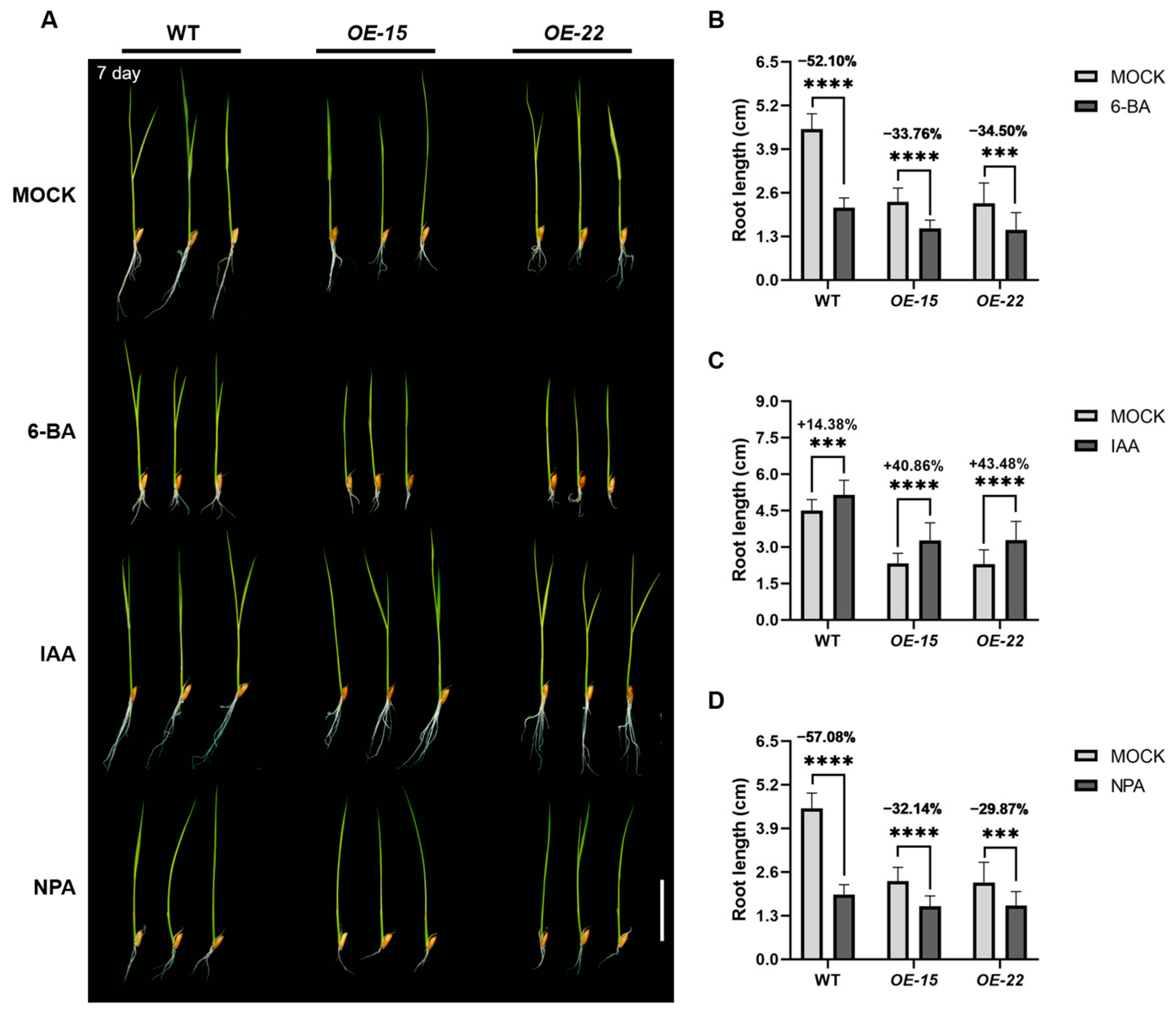
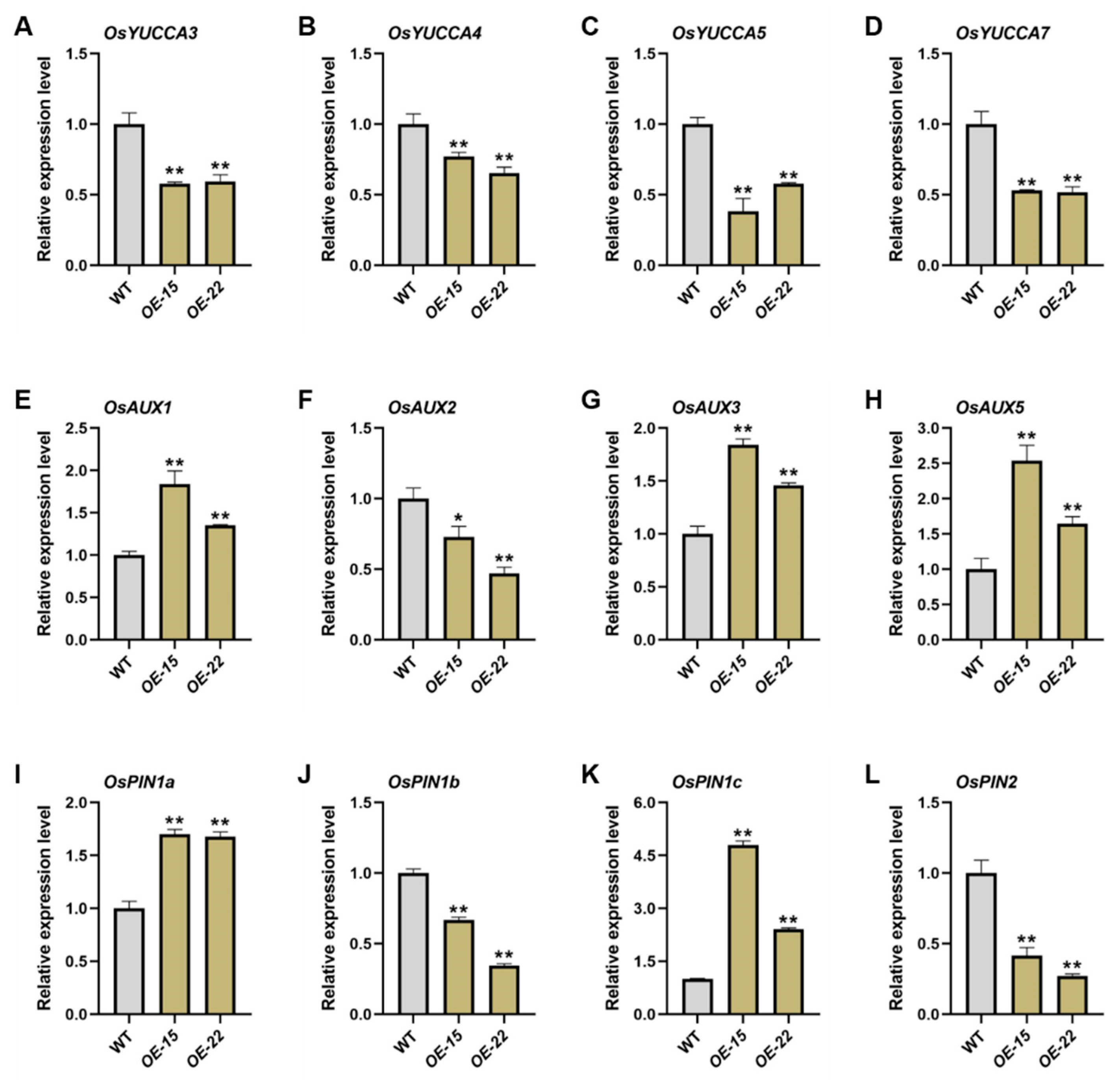
2.3. ORR3 Inhibits Root Tip Cell Division by Affecting the Auxin Pathway
2.4. The Overexpression of ORR3 Impacts Both Hormone Metabolism and Cell Wall Metabolism in the Root Tip
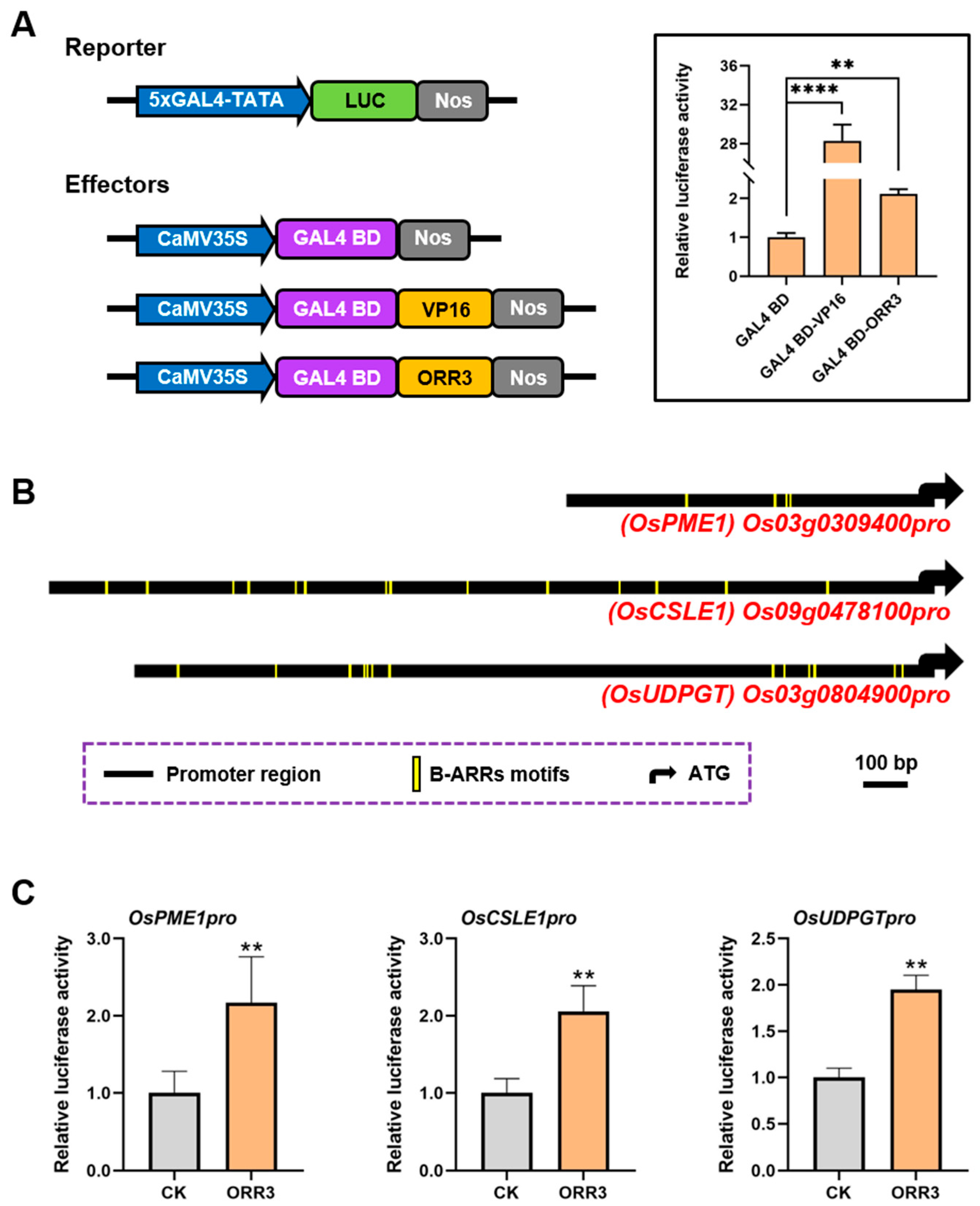
2.5. ORR3 Functions as a Transcriptional Activator That Regulates the Transcription of Cell Wall Metabolism-Related Genes
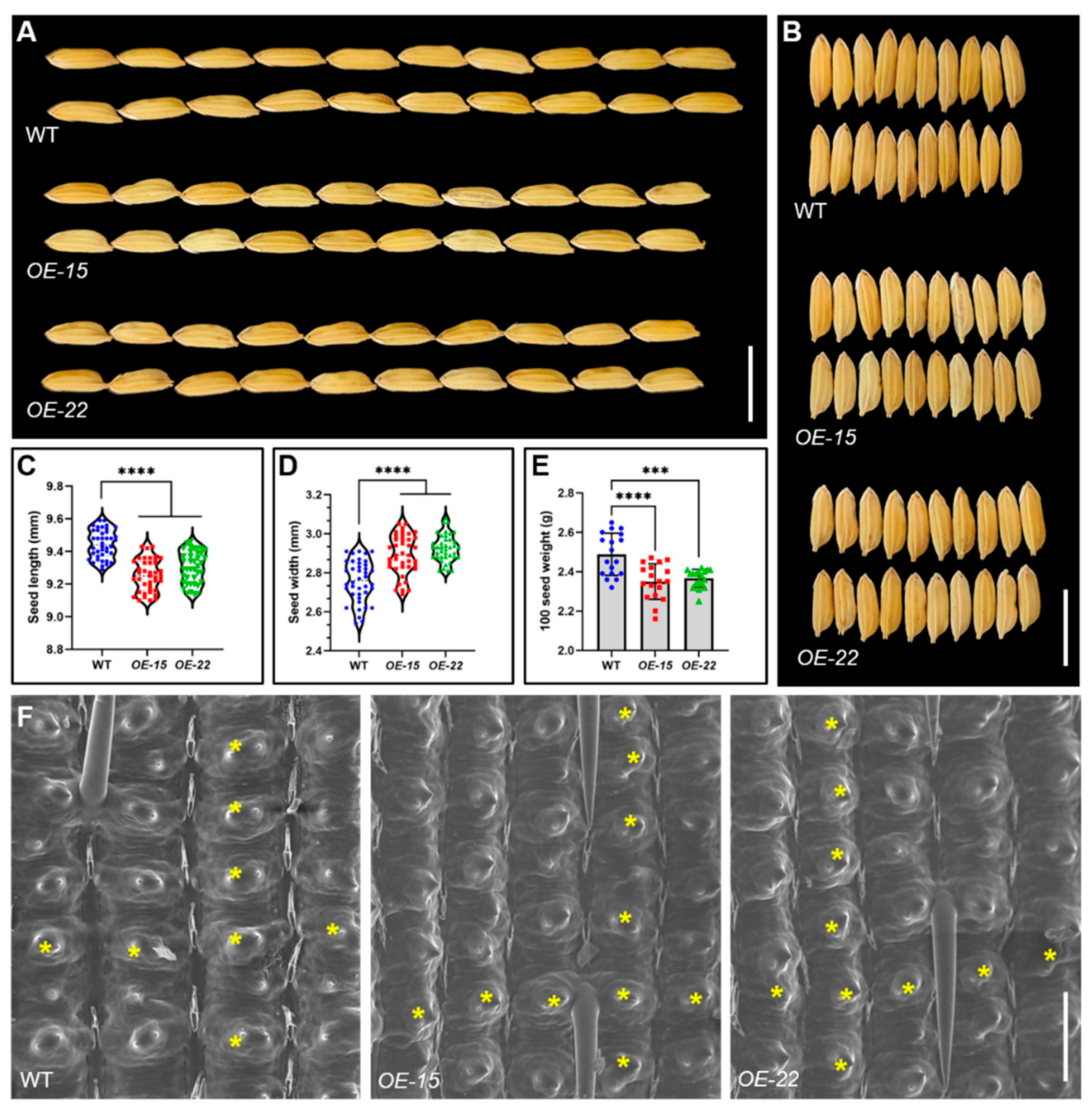
2.6. The Overexpression of ORR3 Affects Rice Grain Development
3. Discussion
3.1. Overexpression of ORR3 Negatively Regulates the Development of Young Rice Roots
3.2. ORR3 May Regulate the Number of Longitudinal Cells in the Root Meristematic Zone by Modulating Auxin Levels or Signaling
3.3. ORR3 May Regulate the Length of Root Meristematic Zone Cells by Affecting Cell Wall Metabolism
4. Materials and Methods
4.1. Construction of Plant Materials and Phenotypic Observation
4.2. RNA Isolation and qRT-PCR Analysis
4.3. In Situ Hybridization
4.4. Subcellular Localization
4.5. Phylogenetic Analysis
4.6. Microscopy Analysis
4.7. Transcriptome Analysis
4.8. Hormone Treatments
4.9. Analysis of Transcriptional Activity and Transient Expression Assay
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Accession Numbers
References
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges and perspective. Plant J. 2022, 110, 23–42. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Chen, X.; Wang, F.; Peng, P.; Zhou, Y.; Miao, Y.; Zhang, Y.; Gao, Y.; Qi, Y.; et al. Rice OsDOF15 contributes to ethylene-inhibited primary root elongation under salt stress. New Phytol. 2019, 223, 798–813. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Tan, M.; Deng, Q.; Wang, Y.; Zhang, T.; Hu, X.; Ye, M.; Lian, X.; Zhou, D.X.; Zhao, Y. Transcription factors WOX11 and LBD16 function with histone demethylase JMJ706 to control crown root development in rice. Plant Cell 2024, 36, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Feldman, L.J. Regulation of root apical meristem development. Annu. Rev. Cell Dev. Biol. 2005, 21, 485–509. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Jia, Y.; Niu, T.; Yu, Q.; Ding, Z. The key players of the primary root growth and development also function in lateral roots in Arabidopsis. Plant Cell Rep. 2014, 33, 745–753. [Google Scholar] [CrossRef]
- Qin, H.; Pandey, B.K.; Li, Y.; Huang, G.; Wang, J.; Quan, R.; Zhou, J.; Zhou, Y.; Miao, Y.; Zhang, D.; et al. Orchestration of ethylene and gibberellin signals determines primary root elongation in rice. Plant Cell 2022, 34, 1273–1288. [Google Scholar] [CrossRef]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What makes adventitious roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef]
- Cui, H.; Levesque, M.P.; Vernoux, T.; Jung, J.W.; Paquette, A.J.; Gallagher, K.L.; Wang, J.Y.; Blilou, I.; Scheres, B.; Benfey, P.N. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 2007, 316, 421–425. [Google Scholar] [CrossRef]
- Chen, R.; Xu, N.; Yu, B.; Wu, Q.; Li, X.; Wang, G.; Huang, J. The WUSCHEL-related homeobox transcription factor OsWOX4 controls the primary root elongation by activating OsAUX1 in rice. Plant Sci. 2020, 298, 110575. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Meng, B.; Miao, L.; Fan, Y. Genome-Wide Identification and Evolutionary and Expression Analyses of the Cyclin B Gene Family in Brassica napus. Plants 2024, 13, 1709. [Google Scholar] [CrossRef]
- Jung, J.K.; McCouch, S. Getting to the roots of it: Genetic and hormonal control of root architecture. Front. Plant Sci. 2013, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Kuderová, A.; Urbánková, I.; Válková, M.; Malbeck, J.; Brzobohaty, B.; Némethová, D.; Hejátko, J. Effects of conditional IPT-dependent cytokinin overproduction on root architecture of Arabidopsis seedlings. Plant Cell Physiol. 2008, 49, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, M.S.; Zhou, H.; Cao, Y.; Liu, B.; Xia, Y. The effect of humic acid on endogenous hormone levels and antioxidant enzyme activity during in vitro rooting of evergreen azalea. Sci. Hortic. 2018, 227, 234–243. [Google Scholar] [CrossRef]
- Fu, X.; Harberd, N.P. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 2003, 421, 740–743. [Google Scholar] [CrossRef]
- Uragami, T.; Kiba, T.; Kojima, M.; Takebayashi, Y.; Tozawa, Y.; Hayashi, Y.; Kinoshita, T.; Sakakibara, H. The cytokinin efflux transporter ABCC4 participates in Arabidopsis root system development. Plant Physiol. 2024, 197, kiae628. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, N.; Chen, H.; Wang, G.; Huang, J. OsMADS25 regulates root system development via auxin signalling in rice. Plant J. 2018, 95, 1004–1022. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Mao, X.; Li, L.; Chang, X.; Zhang, X.; Jing, R. TaARF4 genes are linked to root growth and plant height in wheat. Ann. Bot. 2019, 124, 903–915. [Google Scholar] [CrossRef]
- Wang, Z.; Hua, J.; Yin, Y.; Gu, C.; Yu, C.; Shi, Q.; Guo, J.; Xuan, L.; Yu, F. An Integrated Transcriptome and Proteome Analysis Reveals Putative Regulators of Adventitious Root Formation in Taxodium ‘Zhongshanshan’. Int. J. Mol. Sci. 2019, 20, 1225. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Feng, C.; Wei, Y.; Peng, X.; Guo, X.; Guo, X.; Zhai, Z.; Li, J.; Shen, X.; et al. Overexpression of MsGH3.5 inhibits shoot and root development through the auxin and cytokinin pathways in apple plants. Plant J. 2020, 103, 166–183. [Google Scholar] [CrossRef]
- Meng, F.; Xiang, D.; Zhu, J.; Li, Y.; Mao, C. Molecular Mechanisms of Root Development in Rice. Rice 2019, 12, 1. [Google Scholar] [CrossRef]
- Wang, X.; Cnops, G.; Vanderhaeghen, R.; De Block, S.; Van Montagu, M.; Van Lijsebettens, M. AtCSLD3, a cellu lose synthase-like gene important for root hair growth in Arabidopsis. Plant Physiol. 2001, 126, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Xu, L.; Wu, Y.R.; Chen, X.A.; Liu, Y.; Zhu, S.H.; Ding, W.N.; Wu, P.; Yi, K.K. OsGLU3, a putative mem brane-bound endo-1,4-beta-glucanase, is required for root cell elongation and division in rice (Oryza sativa L.). Mol. Plant 2012, 59, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Inukai, Y.; Sakamoto, T.; Morinaka, Y.; Miwa, M.; Kojima, M.; Tanimoto, E.; Yamamoto, H.; Sato, K.; Katayama, Y.; Matsuoka, M.; et al. ROOT GROWTH INHIBITING, a Rice Endo-1,4-β-d-Glucanase, Regulates Cell Wall Loosening and is Essential for Root Elongation. J. Plant Growth Regul. 2012, 31, 373–381. [Google Scholar] [CrossRef]
- Aoi, Y.; Hira, H.; Hayakawa, Y.; Liu, H.; Fukui, K.; Dai, X.; Tanaka, K.; Hayashi, K.I.; Zhao, Y.; Kasahara, H. UDP-glucosyltransferase UGT84B1 regulates the levels of indole-3-acetic acid and phenylacetic acid in Arabidopsis. Biochem. Biophys. Res. Commun. 2020, 532, 244–250. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Li, P.; Wang, T.; Zheng, C.; Hou, B. An Arabidopsis Cytokinin-Modifying Glycosyltransferase UGT76C2 Improves Drought and Salt Tolerance in Rice. Front. Plant Sci. 2020, 11, 560696. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, Q.; Xie, Z.; Yu, B.; Zeng, R.; Min, Q.; Huang, J. OsFPFL4 is Involved in the Root and Flower Development by Affecting Auxin Levels and ROS Accumulation in Rice (Oryza sativa). Rice 2020, 13, 2. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Peaucelle, A.; Wightman, R.; Höfte, H. The Control of Growth Symmetry Breaking in the Arabidopsis Hypocotyl. Curr. Biol. 2015, 25, 1746–1752. [Google Scholar] [CrossRef]
- Haas, K.T.; Wightman, R.; Meyerowitz, E.M.; Peaucelle, A. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 2020, 367, 1003–1007. [Google Scholar] [CrossRef]
- Aryal, B.; Jonsson, K.; Baral, A.; Sancho-Andres, G.; Routier-Kierzkowska, A.L.; Kierzkowski, D.; Bhalerao, R.P. Interplay between cell wall and auxin mediates the control of differential cell elongation during apical hook development. Curr. Biol. 2020, 30, 1733–1739. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Deng, S.; Jin, C.; Shang, Z.; Chang, L.; Wang, J.; Han, X.; Wang, A.; Jin, D.; Wang, Y.; et al. Origin and evolution of auxin-mediated acid growth. Proc. Natl. Acad. Sci. USA 2024, 121, e2412493121. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Kurata, N. Identification and characterization of cytokinin-signalling gene families in rice. Gene 2006, 382, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 2001, 413, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.M.; Li, J.X.; Zhang, T.Q.; Xu, Z.G.; Ma, M.L.; Zhang, P.; Wang, J.W. The structure of B-ARR reveals the molecular basis of transcriptional activation by cytokinin. Proc. Natl. Acad. Sci. USA 2024, 121, e2319335121. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, N.; Zhang, T.; Zhang, Q.; Du, D.; Chen, X.; Lu, X.; Zhang, Y.; Zhu, M.; Liu, M.; et al. SHORT-ROOT 1 is critical to cell division and tracheary element development in rice roots. Plant J. 2021, 105, 1179–1191. [Google Scholar] [CrossRef]
- Dello Ioio, R.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A Genetic Framework for the Control of Cell Division and Differentiation in the Root Meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef]
- Sadowski, I.; Ma, J.; Triezenberg, S.; Ptashne, M. GAL4-VP16 is an unusually potent transcriptional activator. Nature 1988, 335, 563–564. [Google Scholar] [CrossRef]
- Lally, D.; Ingmire, P.; Tong, H.Y.; He, Z.H. Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell 2001, 13, 1317–1331. [Google Scholar]
- Yu, H.; Hu, M.; Hu, Z.; Liu, F.; Yu, H.; Yang, Q.; Gao, H.; Xu, C.; Wang, M.; Zhang, G.; et al. Insights into pectin dominated enhancements for elimination of toxic Cd and dye coupled with ethanol production in desirable lignocelluloses. Carbohydr. Polym. 2022, 286, 119298. [Google Scholar] [CrossRef]
- Xie, M.; Chen, H.; Huang, L.; O’Neil, R.C.; Shokhirev, M.N.; Ecker, J.R. A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nat. Commun. 2018, 9, 1604. [Google Scholar]
- Chun, Y.; Fang, J.; Savelieva, E.M.; Lomin, S.N.; Shang, J.; Sun, Y.; Zhao, J.; Kumar, A.; Yuan, S.; Yao, X.; et al. The cytokinin receptor OHK4/OsHK4 regulates inflorescence architecture in rice via an IDEAL PLANT ARCHITECTURE1/WEALTHY FARMER’S PANICLE-mediated positive feedback circuit. Plant Cell 2023, 36, 40–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, 39, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ren, L.; Chen, X.; Yu, H.; Liu, C.; Shen, Y.; Shi, W.; Tang, D.; Du, G.; Li, Y.; et al. The OsRR24/LEPTO1 Type-B Response Regulator is Essential for the Organization of Leptotene Chromosomes in Rice Meiosis. Plant Cell 2018, 30, 3024–3037. [Google Scholar] [CrossRef] [PubMed]
- Worthen, J.M.; Yamburenko, M.V.; Lim, J.; Nimchuk, Z.L.; Kieber, J.J.; Schaller, G.E. Type-B response regulators of rice play key roles in growth, development and cytokinin signaling. Development 2019, 146, dev174870. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gong, C.; Wu, J.; Yang, L.; Zhou, L.; Wu, B.; Gao, L.; Ling, F.; You, A.; Li, C.; et al. Improvement of Rice Agronomic Traits by Editing Type-B Response Regulators. Int. J. Mol. Sci. 2022, 23, 14165. [Google Scholar] [CrossRef]
- Cho, L.H.; Yoon, J.; Tun, W.; Baek, G.; Peng, X.; Hong, W.J.; Mori, I.C.; Hojo, Y.; Matsuura, T.; Kim, S.R.; et al. Cytokinin increases vegetative growth period by suppressing florigen expression in rice and maize. Plant J. 2022, 110, 1619–1635. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Xu, J.; Qi, J.; Liu, X.; Guo, L.; Zhang, H. Research on the Mechanisms of Phytohormone Signaling in Regulating Root Development. Plants 2024, 13, 3051. [Google Scholar] [CrossRef]
- Sakai, H.; Honma, T.; Aoyama, T.; Sato, S.; Kato, T.; Tabata, S.; Oka, A. Oka, ARR1, a transcription factor for genes immediately responsive to cytokinins. Science 2001, 294, 1519–1521. [Google Scholar] [CrossRef]
- Mandal, D.; Datta, S.; Raveendar, G.; Mondal, P.K.; Nag Chaudhuri, R. RAV1 mediates cytokinin signaling for regulating primary root growth in Arabidopsis. Plant J. 2023, 113, 106–126. [Google Scholar] [CrossRef]
- Mason, M.G.; Mathews, D.E.; Argyros, D.A.; Maxwell, B.B.; Kieber, J.J.; Alonso, J.M.; Ecker, J.R.; Schaller, G.E. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 2005, 17, 3007–3018. [Google Scholar] [CrossRef]
- Yokoyama, A.; Yamashino, T.; Amano, Y.; Tajima, Y.; Imamura, A.; Sakakibara, H.; Mizuno, T. Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol. 2007, 48, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Argyros, R.D.; Mathews, D.E.; Chiang, Y.H.; Palmer, C.M.; Thibault, D.M.; Etheridge, N.; Argyros, D.A.; Mason, M.G.; Kieber, J.J.; Schaller, G.E. Type B Response Regulators of Arabidopsis Play Key Roles in Cytokinin Signaling and Plant Development. Plant Cell 2008, 20, 2102–2116. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Guo, W.; Hu, X.; Liu, M.; Xu, X.; Hu, F.; Lan, Y.; Lv, C.; Fang, Y.; Liu, M.; et al. Static magnetic field regulates Arabidopsis root growth via auxin signaling. Sci. Rep. 2019, 9, 14384. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Ni, H.; Zuo, W.; Shi, H.; Liao, W.; Liu, H.; Chen, J.; Bai, Y.; Yue, H.; et al. Systematic characterization of plant-associated bacteria that can degrade indole-3-acetic acid. PLoS Biol. 2024, 22, e3002921. [Google Scholar] [CrossRef]
- Shang, E.; Tu, Q.; Yu, Z.; Ding, Z. Cell wall dynamic changes and signaling during plant lateral root development. J. Integr. Plant Biol. 2025, 67, 632–648. [Google Scholar] [CrossRef]
- Zhou, H.L.; He, S.J.; Cao, Y.R.; Chen, T.; Du, B.X.; Chu, C.C.; Zhang, J.S.; Chen, S.Y. OsGLU1, a putative mem brane-bound endo-1,4-beta-glucanase from rice, affects plant internode elongation. Plant Mol. Biol. 2006, 60, 137–151. [Google Scholar] [CrossRef]
- Hirose, T.; Scofied, G.N.; Terao, T. An expression anal ysis profile for the entire sucrose synthase gene family in rice. Plant Sci. 2008, 174, 534–543. [Google Scholar] [CrossRef]
- Kanneganti, V.; Gupta, A.K. Isolation and Expression analysis of OsPME1, encoding for a putative Pectin Methyl Esterase from Oryza sativa (subsp. indica). Physiol. Mol. Biol. Plants 2009, 15, 123–131. [Google Scholar] [CrossRef][Green Version]
- Yan, Y.; Qi, B.W.; Mo, T.; Wang, X.H.; Wang, J.; Shi, S.P.; Liu, X.; Tu, P.F. Research Progress of Rhamnosyltransferase. Chin. J. Org. Chem. 2018, 38, 2281–2295. [Google Scholar] [CrossRef]
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J.; et al. Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol. Plant 2021, 14, 1714–1732. [Google Scholar] [CrossRef]
- Tu, B.; Zhang, T.; Wang, Y.; Hu, L.; Li, J.; Zheng, L.; Zhou, Y.; Li, J.; Xue, F.; Zhu, X.; et al. Membrane-associated xylanase-like protein OsXYN1 is required for normal cell wall deposition and plant development in rice. J. Exp. Bot. 2020, 71, 4797–4811. [Google Scholar] [CrossRef] [PubMed]
- Tundo, S.; Mandalà, G.; Sella, L.; Favaron, F.; Bedre, R.; Kalunke, R.M. Xylanase Inhibitors: Defense Players in Plant Immunity with Implications in Agro-Industrial Processing. Int. J. Mol. Sci. 2022, 23, 14994. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Lee, C.; Cui, D.; Phillips, D.R.; Adams, E.R.; Jeong, H.Y.; Jung, K.H.; Ye, Z.H. Identification of xylan arabinosyl 2-O-xylosyltransferases catalyzing the addition of 2-O-xylosyl residue onto arabinosyl side chains of xylan in grass species. Plant J. 2022, 112, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Juge, N. Plant protein inhibitors of cell wall degrading enzymes. Trends Plant Sci. 2006, 11, 359–367. [Google Scholar] [CrossRef]
- Pinzón-Latorre, D.; Deyholos, M.K. Characterization and transcript profiling of the pectin methylesterase (PME) and pectin methylesterase inhibitor (PMEI) gene families in flax (Linum usitatissimum). BMC Genom. 2013, 14, 742. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zeng, Z.H.; Yan, J.Y.; Fan, W.; Bian, H.W.; Zhu, M.Y.; Yang, J.L.; Zheng, S.J. Association of specific pectin methylesterases with Al-induced root elongation inhibition in rice. Physiol. Plant. 2013, 148, 502–511. [Google Scholar] [CrossRef]
- Koshani, R.; Pitcher, M.L.; Yu, J.; Mahajan, C.L.; Kim, S.H.; Sheikhi, A. Plant Cell Wall-Like Soft Materials: Micro- and Nanoengineering, Properties, and Applications. Nano-Micro Lett. 2025, 17, 103. [Google Scholar] [CrossRef]
- Vega-Sánchez, M.E.; Verhertbruggen, Y.; Christensen, U.; Chen, X.; Sharma, V.; Varanasi, P.; Jobling, S.A.; Talbot, M.; White, R.G.; Joo, M.; et al. Loss of Cellulose synthase-like F6 function affects mixed-linkage glucan deposition, cell wall mechanical properties, and defense responses in vegetative tissues of rice. Plant Physiol. 2012, 159, 56–69. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Ma, L.; Sang, X.; Ling, Y.; Wang, Y.; Yu, P.; Zhuang, H.; Huang, J.; Wang, N.; et al. LATERAL FLORET 1 induced the three-florets spikelet in rice. Proc. Natl. Acad. Sci. USA 2017, 114, 9984–9989. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef]
- Wang, J.R.; Hu, H.; Wang, G.H.; Li, J.; Chen, J.Y.; Wu, P. Expression of PIN genes in rice (Oryza sativa L.): Tissue specificity and regulation by hormones. Mol. Plant 2009, 2, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.S.; Figueiredo, D.D.; Cordeiro, A.M.; Almeida, D.M.; Lourenço, T.; Abreu, I.A.; Sebastián, A.; Fernandes, L.; Contreras-Moreira, B.; Oliveira, M.M.; et al. OsRMC, a negative regulator of salt stress response in rice, is regulated by two AP2/ERF transcription factors. Plant Mol. Biol. 2013, 82, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ohkubo, M.; Hatakeyama, H.; Ohashi, K.; Yoshizawa, R.; Kojima, S.; Hayakawa, T.; Yamaya, T.; Mae, T.; Makino, A. Increased Rubisco content in transgenic rice transformed with the ‘sense’ rbcS gene. Plant Cell Physiol. 2007, 48, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, L.; Zhou, H.; Liu, X.; Li, W.; Min, Y.; Yan, Y.; Ji, J.; Zhang, H.; Zhao, X. Mutation in OsFWL7 Affects Cadmium and Micronutrient Metal Accumulation in Rice. Int. J. Mol. Sci. 2021, 22, 12583. [Google Scholar] [CrossRef]
- Zaka, A.; Grande, G.; Coronejo, T.; Quibod, I.L.; Chen, C.W.; Chang, S.J.; Szurek, B.; Arif, M.; Cruz, C.V.; Oliva, R. Natural variations in the promoter of OsSWEET13 and OsSWEET14 expand the range of resistance against Xanthomonas oryzae pv. oryzae. PLoS ONE 2018, 13, e0203711. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Xiao, J.; Wang, S. Comprehensive analysis of VQ motif-containing gene expression in rice defense responses to three pathogens. Plant Cell Rep. 2014, 33, 1493–1505. [Google Scholar] [CrossRef]
- Feng, P.; Shi, J.; Zhang, T.; Zhong, Y.; Zhang, L.; Yu, G.; Zhang, T.; Zhu, X.; Xing, Y.; Yin, W.; et al. Zebra leaf 15, a receptor-like protein kinase involved in moderate low temperature signaling pathway in rice. Rice 2019, 12, 83. [Google Scholar] [CrossRef]
- You, J.; Xiao, W.; Zhou, Y.; Shen, W.; Ye, L.; Yu, P.; Yu, G.; Duan, Q.; Zhang, X.; He, Z.; et al. The APC/CTAD1-WIDE LEAF 1-NARROW LEAF 1 pathway controls leaf width in rice. Plant Cell 2022, 34, 4313–4328. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Yin, W.; Wei, G.; Xu, H.; Ma, L.; Tian, W.; Yang, G.; Li, Y.; Wu, R.; et al. Full-length EFOP3 and EFOP4 proteins are essential for pollen intine development in Arabidopsis thaliana. Plant J. 2023, 115, 37–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Wei, G.; Tian, W.; Ling, Y.; Wang, N.; Zhang, T.; Sang, X.; Zhu, X.; He, G.; et al. The WOX9-WUS modules are indispensable for the maintenance of stem cell homeostasis in Arabidopsis thaliana. Plant J. 2024, 120, 910–927. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, X.; Dai, Y.; Li, Y.; Ban, Y.; Tian, W.; Zhang, X.; Feng, X.; Zhang, X.; Jia, L.; et al. Transcription factor OsNF-YC1 regulates grain size by coordinating the transcriptional activation of OsMADS1 in Oryza sativa L. Plant J. 2024, 119, 1465–1480. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Florea, L.; Song, L.; Salzberg, S.L. Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues. F1000Research 2013, 2, 188. [Google Scholar] [CrossRef]
- Li, Y.F.; Zeng, X.Q.; Li, Y.; Wang, L.; Zhuang, H.; Wang, Y.; Tang, J.; Wang, H.L.; Xiong, M.; Yang, F.Y.; et al. MULTI-FLORET SPIKELET 2, a MYB Transcription Factor, Determines Spikelet Meristem Fate and Floral Organ Identity in Rice. Plant Physiol. 2020, 184, 988–1003. [Google Scholar] [CrossRef]
- Huang, H.; Wang, H.L.; Zhang, T.; Zeng, X.Q.; Chen, H.; Wang, Z.W.; Zhang, J.; Zheng, H.; Tang, J.; Ling, Y.H.; et al. NONSTOP GLUMES1 Encodes a C2H2 Zinc Finger Protein That Regulates Spikelet Development in Rice. Plant Cell 2020, 32, 392–413. [Google Scholar]
- Zhang, Q.; Wu, R.; Hong, T.; Wang, D.; Li, Q.; Wu, J.; Zhang, H.; Zhou, K.; Yang, H.; Zhang, T.; et al. Natural variation in the promoter of qRBG1/OsBZR5 underlies enhanced rice yield. Nat. Commun. 2024, 15, 8565. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Yu, W.; Chen, X.; Yun, H.; Wang, T.; Wang, N.; Zhang, T.; He, G. The Overexpression of ORR3 Negatively Regulates the Growth of Young Rice Roots by Reducing the Cell Size and the Number in the Root Meristematic Zone. Plants 2025, 14, 1627. https://doi.org/10.3390/plants14111627
Wei G, Yu W, Chen X, Yun H, Wang T, Wang N, Zhang T, He G. The Overexpression of ORR3 Negatively Regulates the Growth of Young Rice Roots by Reducing the Cell Size and the Number in the Root Meristematic Zone. Plants. 2025; 14(11):1627. https://doi.org/10.3390/plants14111627
Chicago/Turabian StyleWei, Gang, Wenjing Yu, Xinlong Chen, Han Yun, Tongming Wang, Nan Wang, Ting Zhang, and Guanghua He. 2025. "The Overexpression of ORR3 Negatively Regulates the Growth of Young Rice Roots by Reducing the Cell Size and the Number in the Root Meristematic Zone" Plants 14, no. 11: 1627. https://doi.org/10.3390/plants14111627
APA StyleWei, G., Yu, W., Chen, X., Yun, H., Wang, T., Wang, N., Zhang, T., & He, G. (2025). The Overexpression of ORR3 Negatively Regulates the Growth of Young Rice Roots by Reducing the Cell Size and the Number in the Root Meristematic Zone. Plants, 14(11), 1627. https://doi.org/10.3390/plants14111627






