Scorzonera undulata: Traditional Applications, Phytochemical Analysis, and Biological and Pharmacological Attributes
Abstract
1. Introduction
2. Methodology
2.1. Inclusion and Exclusion Criteria
2.2. Rationale for Study Choices and Methods Used to Assess the Quality or Risk of Bias
3. Results and Discussion
3.1. Taxonomy
3.2. Geographic Distribution
3.3. Botanical Description
3.4. Ethnomedicinal Uses
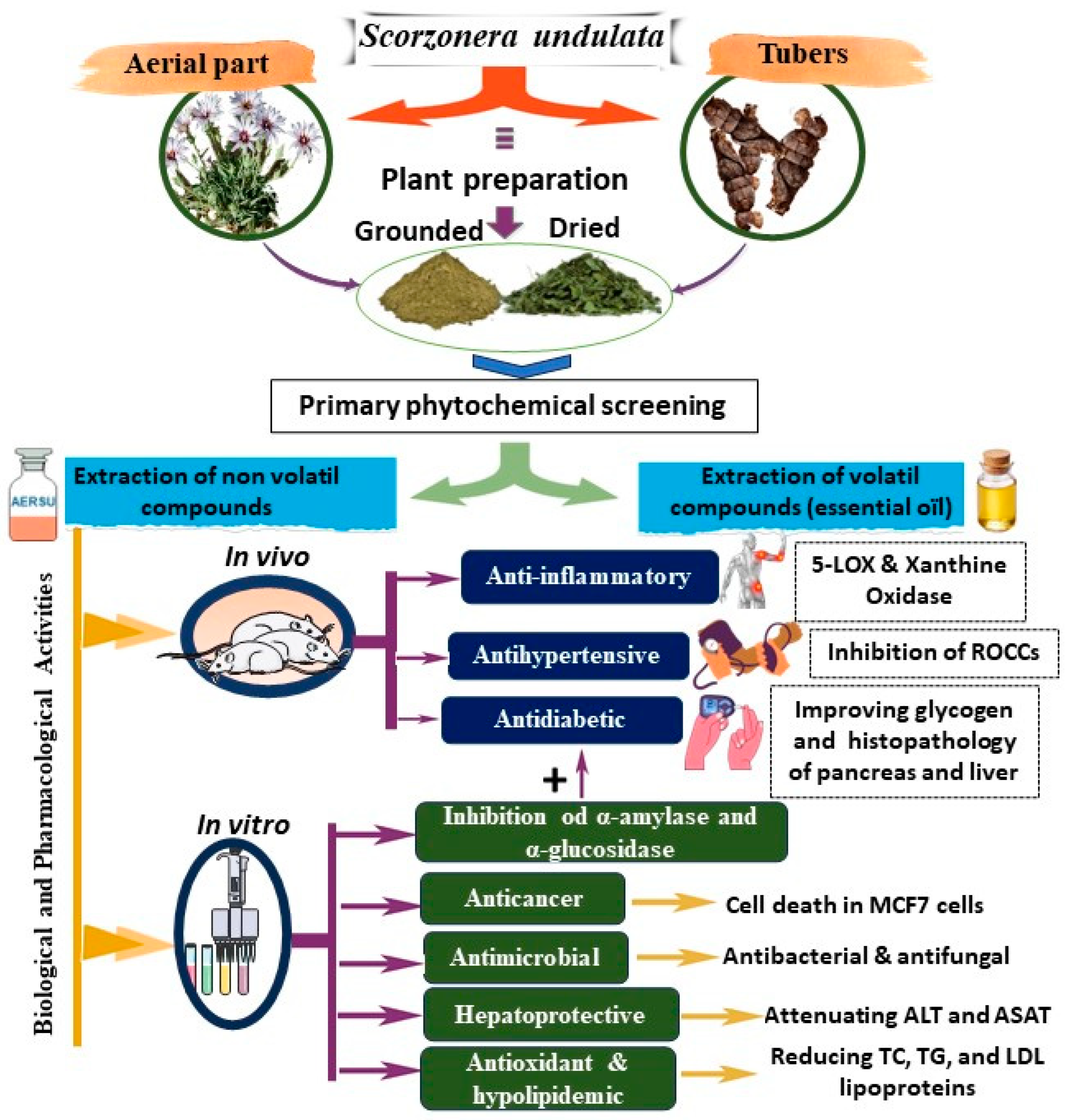
3.5. Phytochemical Properties of S. undulata
3.5.1. Nonvolatile Compounds
3.5.2. Volatile Compounds and Oils
3.6. Pharmacological and Biological Activities
3.6.1. Antidiabetic Activity
3.6.2. Anticancer Activity
3.6.3. Anti-Inflammatory Effects
3.6.4. Antimicrobial Activity
3.6.5. Hypolipidemic Activity
3.7. Toxicity
3.8. Antioxidant Abilities
3.9. Critical Assessment and Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gong, Y.; Shi, Z.-N.; Yu, J.; He, X.-F.; Meng, X.-H.; Wu, Q.-X.; Zhu, Y. The genus Scorzonera L. (Asteraceae): A comprehensive review on traditional uses, phytochemistry, pharmacology, toxicology, chemotaxonomy, and other applications. J. Ethnopharmacol. 2024, 320, 116787. [Google Scholar] [CrossRef] [PubMed]
- Mavrodiev, E.V.; Edwards, C.E.; Albach, D.C.; Gitzendanner, M.A.; Soltis, P.S.; Soltis, D.E. Phylogenetic relationships in subtribe Scorzonerinae (Asteraceae: Cichorioideae: Cichorieae) based on ITS sequence data. Taxon 2004, 53, 699–712. [Google Scholar] [CrossRef]
- Zahran, M.A.; Zahran, M. Afro-Asian Mediterranean Coastal Lands; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Özhatay, N.; Kültür, Ş. Check-list of additional taxa to the Supplement Flora of Turkey III. Turk. J. Bot. 2006, 30, 281–316. [Google Scholar]
- Grlić, L.; Vrščaj, D.; Tratnik, B. Samoniklo Jestivo Bilje; Prosvjeta: Zagred, Croatia, 1980. [Google Scholar]
- Bencheikh, N.; Elbouzidi, A.; Kharchoufa, L.; Ouassou, H.; Alami Merrouni, I.; Mechchate, H.; Es-Safi, I.; Hano, C.; Addi, M.; Bouhrim, M. Inventory of medicinal plants used traditionally to manage kidney diseases in North-Eastern Morocco: Ethnobotanical fieldwork and pharmacological evidence. Plants 2021, 10, 1966. [Google Scholar] [CrossRef]
- Barhoumi, T.; Abderraba, M. Ethnobotanical study of medicinal plant in Djerba Island, Tunisia. Arab. J. Med. Aromat. Plants 2019, 5, 67–97. [Google Scholar]
- Hotel, B.; Petra-Jordan, R. The 3rd International Symposium on Medicinal Plants, Their Cultivation and Aspects of Uses. Pharmacogn. Commun. 2012, 2, 84–86. [Google Scholar]
- Erden, Y.; Kırbağ, S.; Yılmaz, Ö. India Section B: Biological Sciences. Phytochemical composition and antioxidant activity of some Scorzonera species. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 271–276. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Efferth, T. Medicinal plants from near east for cancer therapy. Front Pharmacol. 2018, 9, 56. [Google Scholar] [CrossRef]
- Boussaada, O.; Saidana, D.; Chriaa, J.; Chraif, I.; Ammar; Mahjoub, M.A.; Mighri, Z.; Daami, M.; Helal, A.N. Chemical composition and antimicrobial activity of volatile components of Scorzonera undulata. J. Essent. Oil Res. 2008, 20, 358–362. [Google Scholar] [CrossRef]
- Rolnik, A.; Soluch, A.; Kowalska, I.; Olas, B. Antioxidant and hemostatic properties of preparations from Asteraceae family and their chemical composition–Comparative studies. Biomed. Pharmacother. 2021, 142, 111982. [Google Scholar] [CrossRef]
- Lendzion, K.; Gornowicz, A.; Bielawski, K.; Bielawska, A. Phytochemical composition and biological activities of Scorzonera species. Int. J. Mol. Sci. 2021, 22, 5128. [Google Scholar] [CrossRef] [PubMed]
- Hosni, H.A.; Shamso, E.M. Contribution to the Flora of Egypt: Taxonomic and Nomenclature changes. Taeckholmia 2022, 42, 12–26. [Google Scholar] [CrossRef]
- Faris, F.Z.; Oualidi, J.; Fennane, M.; Ibn Tattou, M.; Mathez, J.; Ouchbani, S.; Ouyahya, A.; Raymaud, C.; Salvo Tierra, Á.; Abidine, A.Z. Flore Pratique du Maroc; Institut Scientifique de Rabat: Rabat, Morocco, 1999. [Google Scholar]
- Amssayef, A.; Ajebli, M.; Eddouks, M. Study of the Antihypertensive Effect of Scorzonera undulata ssp. deliciosa in Albino Wistar Rats. Cardiovasc. Hematol. Agents Med. Chem. 2024, 22, 159–167. [Google Scholar] [CrossRef]
- Bowers, F.D. High elevation mosses of Costa Rica. J. Hattori Bot. Lab. 1970, 33, 7–35. [Google Scholar]
- Idoudi, S.; Othman, K.B.; Bouajila, J.; Tourrette, A.; Romdhane, M.; Elfalleh, W. Influence of extraction techniques and solvents on the antioxidant and biological potential of different parts of Scorzonera undulata. Life 2023, 13, 904. [Google Scholar] [CrossRef]
- Catullo, G.; Montmollin, B.d.; Radford, E.A. Zones Importantes Pour les Plantes en Méditerranée Méridionale et Orientale: Sites Prioritaires pour la Conservation. 2011. Available online: https://portals.iucn.org/library/sites/library/files/documents/2011-014-fr.pdf (accessed on 19 March 2025).
- Savo, V.; Caneva, G.; Camarda, I. Plants, History and Cultures in the Mediterranean Area; Academia: San Francisco, CA, USA, 2010. [Google Scholar]
- Eddouks, M.; Ajebli, M.; Hebi, M. Ethnopharmacological survey of medicinal plants used in Daraa-Tafilalet region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2017, 198, 516–530. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- Hassani, M.; Douiri, E.; Bammi, J.; Zidane, L.; Douira, A. Plantes médicinales de la Moyenne Moulouya (nord-est du Maroc). Ethnopharmacologia 2013, 50, 39. [Google Scholar]
- Abdelkader, H.B.; Salah, K.B.H.; Liouane, K.; Boussaada, O.; Gafsi, K.; Mahjoub, M.A.; Aouni, M.; Hellal, A.; Mighri, Z. Antimicrobial activity of Rhaponticum acaule and Scorzonera undulata growing wild in Tunisia. Afr. J. Microbiol. Res. 2010, 4, 1954–1958. [Google Scholar]
- Rebbas, K.; Bounar, R.; Gharzouli, R.; Ramdani, M.; Djellouli, Y.; Alatou, D. Plants of interest medicinal and ecological in the area of Ouanougha (M’sila, Algeria). Phytothérapie 2012, 10, 131–142. [Google Scholar] [CrossRef]
- Brahim, H.; Salah, A.; Bayet, C.; Laouer, H.; Dijoux-Franca, M. Evaluation of antioxidant activity, free radical scavenging and cuprac of two compounds isolated from Scorzonera undulata ssp. deliciosa. Adv. Environ. Biol. 2013, 7, 591–594. [Google Scholar]
- Kargol, H.S.; Elgadi, H.M.; Gadamsi, M.T.; Shubar, H.M.; Geroushi, A.M. Pharmacognostical, Antimicrobial and Laxative Study of Scorzonera undulata in Libya. Int. Res. J. Pharm. 2013, 4, 96–99. [Google Scholar] [CrossRef]
- Karous, O.; Ben Haj Jilani, I.; Ghrabi-Gammar, Z. Ethnobotanical study on plant used by semi-nomad descendants’ community in Ouled Dabbeb—Southern Tunisia. Plants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Baytop, T. Türkiye’de Bitkiler ile Tedavi: Geçmişte ve Bugün; Nobel Tıp Kitabevleri: Istanbuk, Turkey, 1999. [Google Scholar]
- Zidorn, C.; Ellmerer, E.P.; Sturm, S.; Stuppner, H. Tyrolobibenzyls E and F from Scorzonera humilis and distribution of caffeic acid derivatives, lignans and tyrolobibenzyls in European taxa of the subtribe Scorzonerinae (Lactuceae, Asteraceae). Phytochemistry 2003, 63, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tsevegsuren, N.; Edrada, R.; Lin, W.; Ebel, R.; Torre, C.; Ortlepp, S.; Wray, V.; Proksch, P. Biologically active natural products from Mongolian medicinal plants Scorzonera divaricata and Scorzonera pseudodivaricata. J. Nat. Prod. 2007, 70, 962–967. [Google Scholar] [CrossRef]
- Ajebli, M.; Amssayef, A.; Eddouks, M. Assessment of antihyperglycemic effect and acute toxicity of the aqueous Scorzonera undulata extract in rats. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 1130–1141. [Google Scholar] [CrossRef]
- Ajebli, M.; Amssayef, A.; Eddouks, M. Antihyperglycemic activity and safety assessment of the aqueous extract of aerial parts of Scorzonera undulata ssp deliciosa in rat. Cardiovasc. Haematol. Disord.-Drug Targets 2020, 20, 305–316. [Google Scholar] [CrossRef]
- Abdelkader, B.; Ouadah, N.; Kaabeche, M. Changements climatiques et paysages végétaux aux confins Saharo-Méditerranéens (Monts des Ksour, Algérie). Le J. Bot. 2016, 75, 69–79. [Google Scholar] [CrossRef]
- Ajebli, M.; Khan, H.; Eddouks, M. Natural alkaloids and diabetes mellitus: A review. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 111–130. [Google Scholar] [CrossRef]
- Guerine, L.; Hadjadj, K.; Soufan, W.; Belhadj, S.; Rihan, H.Z. Traditional use of Non-Timber Forest Products of Plant Origin from the Djebel Aissa National Park (Ksours Mountains, Algeria). Pak. J. Agric. Sci. 2024, 61, 1–11. [Google Scholar] [CrossRef]
- Bédoui, I.; Nasr, H.B.; Ksouda, K.; Ayadi, W.; Louati, N.; Chamkha, M.; Choura, S.; Gargouri, J.; Hammami, S.; Affes, H.; et al. Phytochemical Composition, Bioavailability and Pharmacokinetics of Scorzonera undulata Methanolic Extracts: Antioxidant, Anticancer, and Apoptotic Effects on MCF7 Cells. Pharmacogn. Mag. 2024, 20, 218–229. [Google Scholar] [CrossRef]
- Harkati, B.; Akkal, S.; Bayat, C.; Laouer, H.; Franca, M.D. Secondary metabolites from Scorzonera undulata ssp. deliciosa (Guss.) Maire (Asteracae) and their antioxidant activities. Rec. Nat. Prod. 2010, 4, 171. [Google Scholar]
- Benabdelaziz, I.; Haba, H.; Lavaud, C.; Benkhaled, M. Triterpenoids and flavonoid from Scorzonera undulata ssp. alexandrina. Int. J. Chem. Biol. Sci 2014, 5, 1–5. [Google Scholar]
- Brahim, H.; Salah, A.; Marie, G.D.F. Composition of the Volatile Components Extracted from the Roots of Scorzonera undulata ssp deliciosa (Guiss) Maire: From Algeria. Green Sustain. Chem. 2012, 2, 59–61. [Google Scholar]
- de Almeida, T.S.; Neto, J.J.L.; de Sousa, N.M.; Pessoa, I.P.; Vieira, L.R.; De Medeiros, J.L.; Boligon, A.A.; Hamers, A.R.; Farias, D.F.; Peijnenburg, A. Phenolic compounds of Triplaris gardneriana can protect cells against oxidative stress and restore oxidative balance. Biomed. Pharmacother. 2017, 93, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Mantravadi, P.K.; Kalesh, K.A.; Dobson, R.C.; Hudson, A.O.; Parthasarathy, A. The quest for novel antimicrobial compounds: Emerging trends in research, development, and technologies. Antibiotics 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Acıkara, Ö.B.; Ataman, U.; Zidorn, C.; Cvacka, J.; Kurtul, E.; Buděšínský, M.; Bednarova, L.; Sarıaltın, S.Y. Undescribed and known phenolic acid derivatives with significant antioxidant activity from the Scorzonera parviflora Jacq. roots. Fitoterapia 2025, 180, 106328. [Google Scholar] [CrossRef]
- Lendzion, K.; Gornowicz, A.; Strawa, J.W.; Bielawska, K.; Czarnomysy, R.; Popławska, B.; Bielawski, K.; Tomczyk, M.; Miltyk, W.; Bielawska, A. LC-PDA-MS and GC-MS Analysis of Scorzonera hispanica Seeds and Their Effects on Human Breast Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 11584. [Google Scholar] [CrossRef]
- Babalola, I.T.; Shode, F.O. Ubiquitous ursolic acid: A potential pentacyclic triterpene natural product. J. Pharmacogn. Phytochem. 2013, 2, 214–222. [Google Scholar]
- da Silva, K.A.S.; Paszcuk, A.F.; Passos, G.F.; Silva, E.S.; Bento, A.F.; Meotti, F.C.; Calixto, J.B. Activation of cannabinoid receptors by the pentacyclic triterpene α, β-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain 2011, 152, 1872–1887. [Google Scholar] [CrossRef]
- Ferrer, A.; Altabella, T.; Arró, M.; Boronat, A. Emerging roles for conjugated sterols in plants. Prog. Lipid Res. 2017, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kangsamaksin, T.; Chaithongyot, S.; Wootthichairangsan, C.; Hanchaina, R.; Tangshewinsirikul, C.; Svasti, J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS ONE 2017, 12, e0189628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, J.; Shao, L.; Guo, M. Current advances in acteoside biosynthesis pathway elucidation and biosynthesis. Fitoterapia 2020, 142, 104495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lomash, V.; Jatav, P.; Kumar, A.; Pant, S. Prenatal developmental toxicity study of n-heneicosane in Wistar rats. Toxicol. Ind. Health 2016, 32, 118–125. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Liu, F.; Sirisena, S.; Ng, K. Efficacy of flavonoids on biomarkers of type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 4916–4941. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Peng, L.; Zhang, Q.; Rong, X.; Luo, Y.; Li, J. Flavonoids: Potential therapeutic agents for cardiovascular disease. Heliyon 2024, 10, e32563. [Google Scholar] [CrossRef]
- Sudarshan, K.; Aidhen, I.S. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar] [CrossRef]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Cheung, J.; Beri, V.; Shiomi, K.; Rosenberry, T.L. Acetylcholinesterase complexes with the natural product inhibitors dihydrotanshinone I and territrem B: Binding site assignment from inhibitor competition and validation through crystal structure determination. J. Mol. Neurosci. 2014, 53, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Balappa Somappa, S. Synthetic and natural coumarins as potent anticonvulsant agents: A review with structure-activity relationship. J. Clin. Pharm. Ther. 2022, 47, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Skoreński, M.; Sieńczyk, M. The Fellowship of Privileged Scaffolds-One Structure to Inhibit Them All. Pharmaceuticals 2021, 14, 1164. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Ajebli, M.; Amssayef, A.; Eddouks, M. Hypolipidemic, Antioxidant and Cardioprotective Effects of the Aqueous Extract from Scorzanera Undulata Tubers in Streptozotocin-Induced Diabetic Rats. Cardiovasc. Hematol. Agents Med. Chem. 2021, 19, 17–23. [Google Scholar] [CrossRef]
- Ali Temiz, M. Antioxidant and antihyperglycemic activities of Scorzonera cinerea radical leaves in streptozocin-induced diabetic rats. Acta Pharm. 2021, 71, 603–617. [Google Scholar] [CrossRef]
- Şakul, A.A.; Kurtul, E.; Özbek, H.; Kırmızı, N.İ.; Bahtiyar, B.C.; Saltan, H.; Acıkara, Ö.B. Evaluation of antidiabetic activities of scorzonera species on alloxan induced diabetic mice. Clin. Exp. Health Sci. 2021, 11, 74–80. [Google Scholar] [CrossRef]
- El Hosry, L.; Al Ayash, S.; Matar Boumosleh, J.; Bou-Maroun, E. Chemical Composition, Antioxidant, and Anti-Diabetic Activities of Scorzonera phaeopappa Boiss. Stresses 2023, 3, 773–784. [Google Scholar] [CrossRef]
- Sonmez, N.I.K.; Acikara, O.B.; Sakul, A.A.; Bahtiyar, B.C.; Bardakci, H.; Barak, T.H.; Ozbek, H. HPTLC quantification, assessment of antioxidant potential and in vivo hypoglycemic activity of Scorzonera latifolia (Fisch. & C.A. Mey.) DC. and its major compounds. S. Afr. J. Bot. 2022, 150, 671–677. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Kulkarni, M.; Hardwick, J.M. Programmed cell death in unicellular versus multicellular organisms. Annu. Rev. Genet. 2023, 57, 435–459. [Google Scholar] [CrossRef]
- Singh, H.R.; Prakash, P.; Haidar, S. Addressing Challenges in Cell Lysis: Effective Strategies and Technologies. In Cytotoxicity—A Crucial Toxicity Test for In Vitro Experiments; Erkekoğlu, P., Ed.; IntechOpen: Rijeka, Croatia, 2025. [Google Scholar]
- Faucher, K.X.; Faucher, K.X. Metastability and Metastasis—A Deleuzian Approach to Information; SensePublishers: Rotterdam, The Netherlands, 2013; pp. 127–177. [Google Scholar]
- Ntanzi, N.; Khan, R.B.; Nxumalo, M.B.; Kumalo, H.M. Mechanisms of H2pmen-Induced cell death: Necroptosis and apoptosis in MDA cells, necrosis in MCF7 cells. Heliyon 2024, 10, e40654. [Google Scholar] [CrossRef]
- Sarimahmut, M. Investigation of the Dual Role of Scorzonera pygmaea: Cytotoxic Activity and Antioxidant Potential. J. Food Process. Preserv. 2023, 2023, 2663247. [Google Scholar] [CrossRef]
- Bahadır Acikara, Ö.; Hošek, J.; Babula, P.; Cvačka, J.; Budešínský, M.; Dračinský, M.; Saltan İşcan, G.; Kadlecová, D.; Ballová, L.; Šmejkal, K. Turkish Scorzonera species extracts attenuate cytokine secretion via inhibition of NF-κB activation, showing anti-inflammatory effect in vitro. Molecules 2015, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Sutar, I.; Bahadir Acikara, O.; Saltan Citoglu, G.; Keles, H.; Ergene, B.; Kupeli Akkol, E. In vivo and in vitro evaluation of the therapeutic potential of some Turkish Scorzonera species as wound healing agent. Curr. Pharm. Des. 2012, 18, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Bahadır-Acıkara, Ö.; Özbilgin, S.; Saltan-İşcan, G.; Dall’Acqua, S.; Rjašková, V.; Özgökçe, F.; Suchý, V.; Šmejkal, K. Phytochemical analysis of Podospermum and Scorzonera n-hexane extracts and the HPLC quantitation of triterpenes. Molecules 2018, 23, 1813. [Google Scholar] [CrossRef]
- Patil, P.; Soujanya, B.; KIRAN, K. A review on Lupeol: Superficial triterpenoid from horticulture crops. Internat. J. Chem. Stud 2018, 6, 3301–3305. [Google Scholar]
- Lucetti, D.L.; Lucetti, E.C.; Bandeira, M.A.M.; Veras, H.N.; Silva, A.H.; Leal, L.K.A.; Lopes, A.A.; Alves, V.C.; Silva, G.S.; Brito, G.A. Anti-inflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthus drasticus (Mart.) Plumel. J. Inflamm. 2010, 7, 60. [Google Scholar] [CrossRef]
- Feldmann, M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2002, 2, 364–371. [Google Scholar] [CrossRef]
- Johnston, W.F.; Salmon, M.; Su, G.; Lu, G.; Stone, M.L.; Zhao, Y.; Owens, G.K.; Upchurch, G.R., Jr.; Ailawadi, G. Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 294–304. [Google Scholar] [CrossRef]
- Dinarello, C.A. The interleukin-1 family: 10 years of discovery 1. FASEB J. 1994, 8, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.K.; Tansey, M.G. TNF signaling inhibition in the CNS: Implications for normal brain function and neurodegenerative disease. J. Neuroinflammation 2008, 5, 45. [Google Scholar] [CrossRef]
- Allan, S.M.; Tyrrell, P.J.; Rothwell, N.J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005, 5, 629–640. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Inflammasome activation and IL-1β; and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Ajaib, M.; Mati-ur-Rehman, A.; Khan, K.M.; Perveen, S.; Shah, S. Pulicaria undulata: A Potential Phytochemical, Antimicrobial and Antioxidant Source. J. Chem. Soc. Pak. 2015, 37, 559–566. [Google Scholar]
- Sarı, A.; Özbek, B.; Özgökçe, F. Antimicrobial activities of two Scorzonera species growing in Turkey. Asian J. Chem. 2009, 21, 4785–4788. [Google Scholar]
- Ugur, A.; Sarac, N.; Ceylan, O.; Duru, M.E.; Beyatli, Y. Chemical composition of endemic Scorzonera sandrasica and studies on the antimicrobial activity against multiresistant bacteria. J. Med. Food 2010, 13, 635–639. [Google Scholar] [CrossRef]
- Erden, Y.; Kırbağ, S. Chemical and biological activities of some Scorzonera species: An in vitro study. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 319–326. [Google Scholar] [CrossRef]
- Güçlü, G.; Eruygur, N.; Uçar, E.; Özbek, D.Ü.; Bal, H.; Akpolat, A.; Kahrizi, D. Biological Activity Evaluation of Scorzonera tomentosa L. Türk. Tarımsal Araştırmalar Derg. 2023, 10, 162–167. [Google Scholar] [CrossRef]
- Ak, G.; Gokhan, Z.; Stefano, D.A.; Irene, F.; Stefania, S.; Jasmina, G.; Marina, S.; Marija, N.; Annalisa, C.; Lucia, R.; et al. A new step on the chemical profiles and pharmacological effects of three Scorzonera species (S. hieraciifolia, S. hispanica and S. tomentosa). Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2023, 157, 119–128. [Google Scholar] [CrossRef]
- Angelini, P. Plant-derived antimicrobials and their crucial role in combating antimicrobial resistance. Antibiotics 2024, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and bacterial resistance—A short story of an endless arms race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Bhattacharjee, M.K. Antibiotics that inhibit nucleic acid synthesis. In Chemistry of Antibiotics and Related Drugs; Springer: Berlin/Heidelberg, Germany, 2022; pp. 125–148. [Google Scholar]
- Wei, E.; Sixi, Z.; Jinghui, Z.; Sitong, W.; and Wang, G. The evaluation of hepatoprotective effects of flavonoids from Scorzonera austriaca Wild against CCl4-induced acute liver injury in vitro and in vivo. Drug Chem. Toxicol. 2022, 45, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Bellassouad, K.; Feki, A.E.; Ayadi, H. Effect of extraction solvents on the biomolecules and antioxidant properties of Scorzonera undulata (Asteraceae): Application of factorial design optimization phenolic extraction. Acta Sci. Pol. Technol. Aliment. 2015, 14, 313–330. [Google Scholar]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; V. Anil Kumar, N.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I. Plant-derived bioactives and oxidative stress-related disorders: A key trend towards healthy aging and longevity promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Li, Y.; Liang, T.; Zhong, H.; Yang, L.; Xi, Y.; Zhang, J.; Ding, Y.; Wu, Q. Gut microbiota as an antioxidant system in centenarians associated with high antioxidant activities of gut-resident Lactobacillus. npj Biofilms Microbiomes 2022, 8, 102. [Google Scholar] [CrossRef]
- Pollier, J.; Moses, T.; Goossens, A. Combinatorial biochemistry in plants. In Combinatorial Biochemistry of Triterpene Saponins in Plants; Ghent University: Ghent, Belgium, 2011; Volume 3. [Google Scholar]
- Milella, L.; Bader, A.; De Tommasi, N.; Russo, D.; Braca, A. Antioxidant and free radical-scavenging activity of constituents from two Scorzonera species. Food Chem. 2014, 160, 298–304. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Menzel, A.; Samouda, H.; Dohet, F.; Loap, S.; Ellulu, M.S.; Bohn, T. Common and novel markers for measuring inflammation and oxidative stress ex vivo in research and clinical practice—Which to use regarding disease outcomes? Antioxidants 2021, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, C.; Li, W.; Adu-Frimpong, M.; Wang, Q.; Yu, J.; Xu, X. Preparation and characterization of Syringic acid–loaded TPGS liposome with enhanced oral bioavailability and in vivo antioxidant efficiency. AAPS PharmSciTech 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Ward, R.M.; Benjamin, D.; Barrett, J.S.; Allegaert, K.; Portman, R.; Davis, J.M.; Turner, M.A. Safety, dosing, and pharmaceutical quality for studies that evaluate medicinal products (including biological products) in neonates. Pediatr. Res. 2017, 81, 692–711. [Google Scholar] [CrossRef]
- Hobson, C.; Chan, A.N.; Wright, G.D. The antibiotic resistome: A guide for the discovery of natural products as antimicrobial agents. Chem. Rev. 2021, 121, 3464–3494. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Gupta, M.K.; Prakash, V.; Saxena, S. Endophytic fungi: A source of potential antifungal compounds. J. Fungi 2018, 4, 77. [Google Scholar] [CrossRef]
- North, B.; Kocher, H.M.; Sasieni, P. A new pragmatic design for dose escalation in phase 1 clinical trials using an adaptive continual reassessment method. BMC Cancer 2019, 19, 632. [Google Scholar] [CrossRef]
- Anderson, R.L.; DiMeglio, L.A.; Mander, A.P.; Dayan, C.M.; Linsley, P.S.; Herold, K.C.; Marinac, M.; Ahmed, S.T. Innovative Designs and Logistical Considerations for Expedited Clinical Development of Combination Disease-Modifying Treatments for Type 1 Diabetes. Diabetes Care 2022, 45, 2189–2201. [Google Scholar] [CrossRef]
- Gumbs, R.; Gray, C.L.; Böhm, M.; Burfield, I.J.; Couchman, O.R.; Faith, D.P.; Forest, F.; Hoffmann, M.; Isaac, N.J.; Jetz, W. The EDGE2 protocol: Advancing the prioritisation of Evolutionarily Distinct and Globally Endangered species for practical conservation action. PLoS Biol. 2023, 21, e3001991. [Google Scholar] [CrossRef]

| Species/ Subspecies | Vernacular Name | Geographical Area | Used Parts | Mode of Preparation | Application in Ethnomedicine | Ref |
|---|---|---|---|---|---|---|
| S. undulata Vahl | Tamtla/Guiz | Morocco | Flowers | Raw | Treating diabetes mellitus | [22] |
| S. undulata Vahl | Guiz | Morocco | Roots | Fresh | Used against thirst | [23] |
| S. undulata Vahl | Guiz | Tunisia | Tubers/roots | Ashes | Treating burns | [24] |
| S. undulata Vahl | Guiz | Tunisia | - | Decoction | Used as a purgative | [18] |
| S. undulata Vahl | Guiz | Algeria | Leaves Roots | Infusion - | Used as a diuretic, emollient, sudorific, and depurative | [25] |
| S. undulata subsp. deliciosa Maire | Guiz | Algeria | - | - | Treating snake bites | [26] |
| S. undulata subsp. deliciosa Maire | Elgiz | Libya | Leaves | - | Used as a laxative to treat constipation | [27] |
| S. undulata | Guiz | Tunisia | Leaves and roots | Raw sap (milk) drops in the eye | Against oral inflammation and digestive disorders; antidiarrheal effect and eye treatment | [7] |
| S. undulata | Guiz | Tunisia | Fleshy roots, flowers, and leaves | Fresh | Eaten at the site of collection, as a snack | [28] |
| Plant Organ | Extraction Method | Organic Solvent | Yield | Phytochemical Class | Identification Technique | Main Phytochemicals Found | Ref | |
|---|---|---|---|---|---|---|---|---|
| Volatile | Phenolic | |||||||
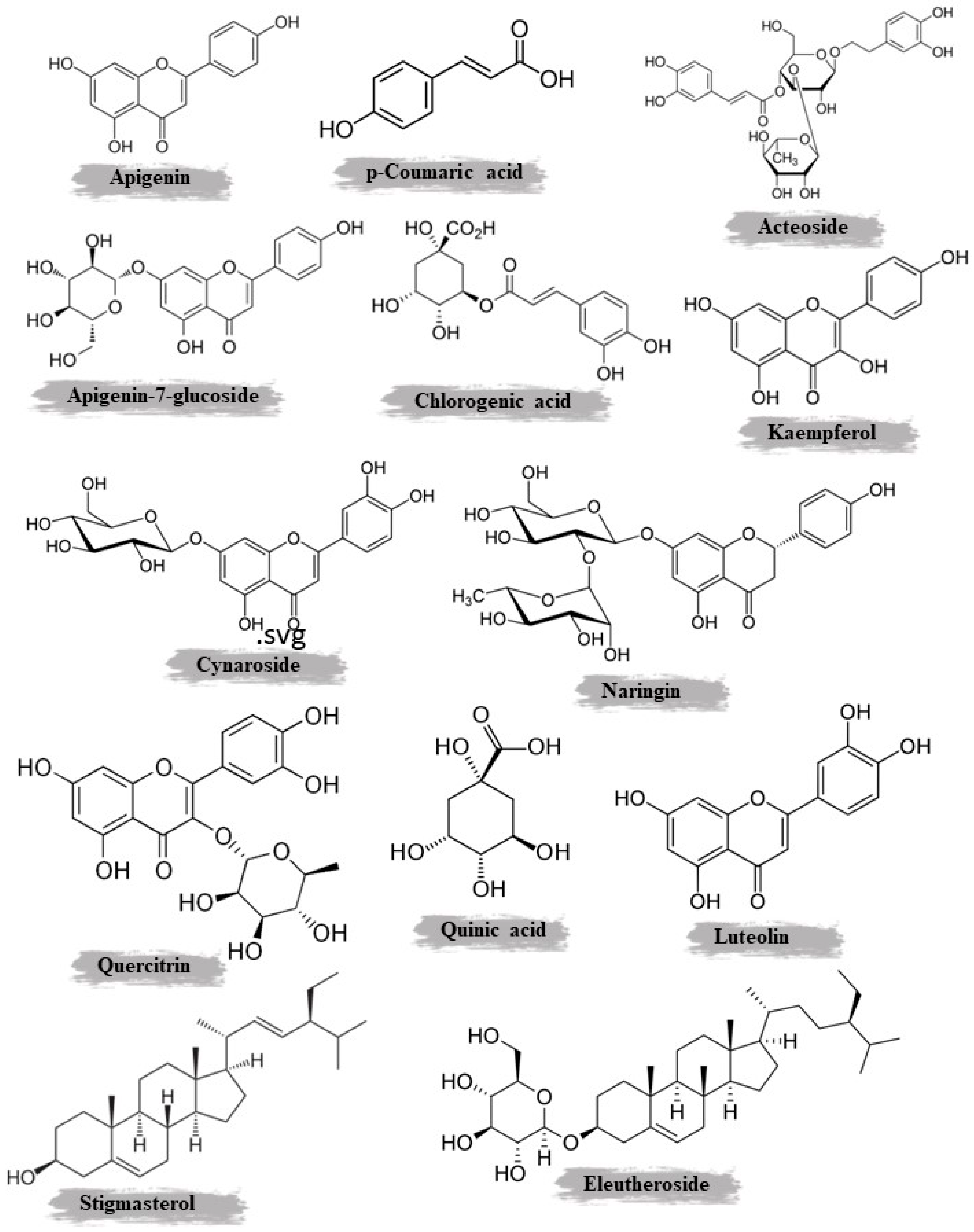
| Aerial parts | Maceration | Methanol | 0.78% | Polyphenols, flavonoids, and tannins | HPLC | Apigenin-7-glucoside, gallic acid, luteolin-7-glucoside and p-coumaric acid | [37] | |
| Roots | Maceration | Methanol | 0.86% | Polyphenols, flavonoids, and tannins | HPLC | Luteolin and chlorogenic acid | ||
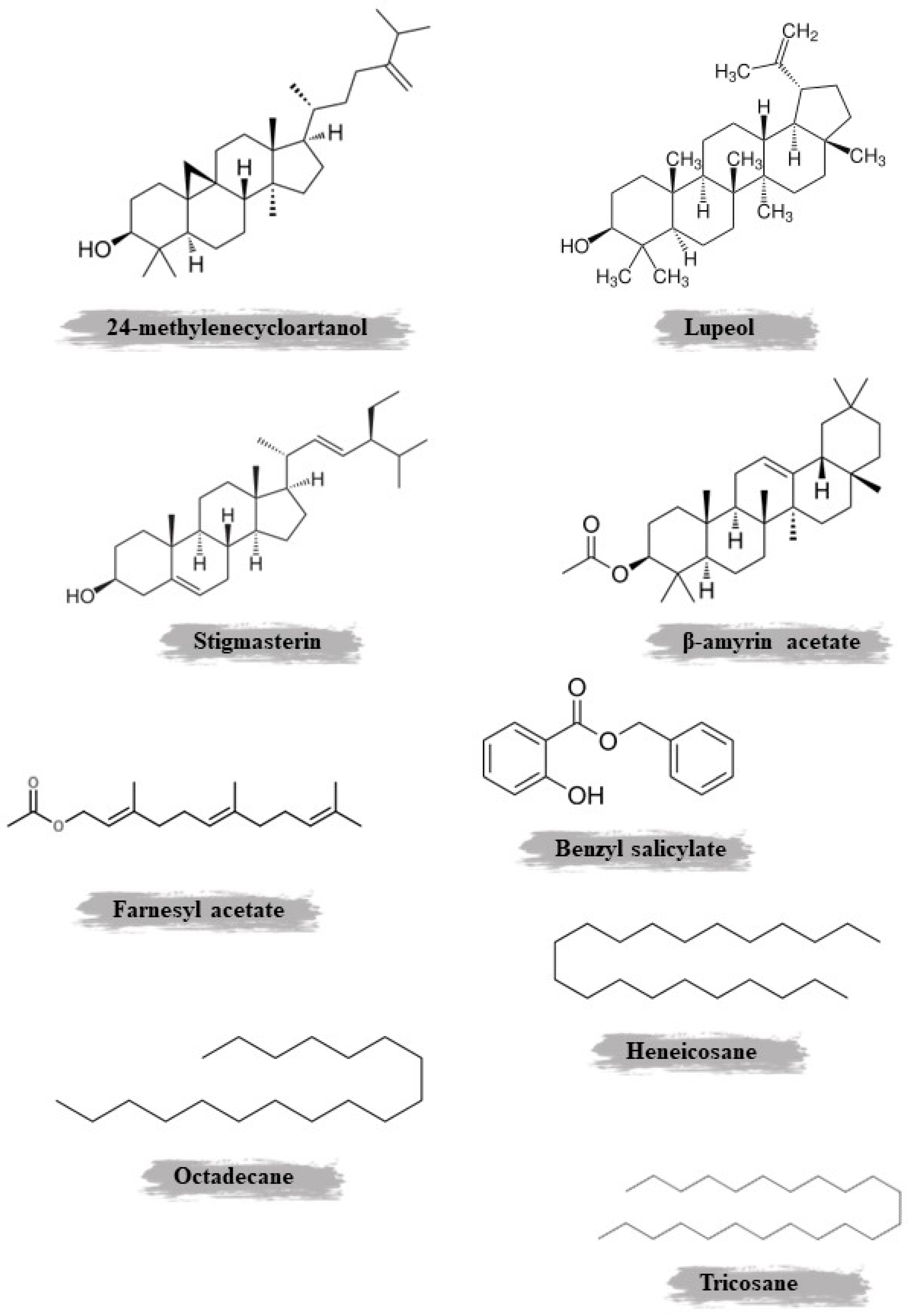
| Roots | Maceration | Chloroform | Polyphenols | MS, 1H, and 13C NMR, including COSY, HMQC, and HMBC | β-Amyrin acetate, methyl oleanate, stigmasterol, β-sitosterol, galangustin, coumarin-O-β-glycoside, and acteoside | [38] | ||
| Aerial parts | Steam distillation | Chloroformic extraction | Oils, hydrocarbons, terpenoids, aromatic compounds, fatty acids, and fatty acid esters | Gas chromatograph, mass spectrometer, and GC/MS | Octadecane, farnesyl acetate, benzyl salicylate, methyl hexadecanoate, heneicosane, methyl octadecanoate, methyl linolenate, tricosane | [11] | ||
| Aerial parts | Maceration | Petroleum ether and ethyl acetate | - | Triterpenoids | Flavonoid | Spectroscopic methods (1H-NMR, 13C-NMR, DEPT, HMBC, HSQC, COSY, NOESY), ESI-MS, and EI-MS |
| [39] |
| Roots | Maceration | Chloroform and methanol | - | Polyphenol and flavonoid | Column chromatography on Sephadex LH-20 and MPLC | Acteoside and galangustin | [26] | |
| Roots | Hydrodistillation using a Clevenger-type apparatus | - | - | Essential oil and fatty acids | Gas chromatography–mass spectrometry | Hexadecanoic acid, n-tetradecanoic acid, 9-octadecenoic acid, and 9-hexadecenoic acid | [40] | |
| Activity Type | Extract Source | Extract Method | Concentration | Positive Control/Reference | Inhibition Percentage (IP)/MIC (µg/mL) | Notes | Ref |
|---|---|---|---|---|---|---|---|
| Xanthine oxidase | Leaf | Ethanolic (maceration) | 50 mg/L | Allopurinol (1 µg/mL) | 0 ≤ IP ≤ 38.26 | Highest inhibition detected for leaf ethanolic extract due to luteolin-7-O-glucoside. | [18] |
| Xanthine oxidase | Tubers | Various | 50 mg/L | Allopurinol (1 µg/mL) | IP 0 | All tuber extracts were inactive, likely due to absence of flavonoids and hydroxycinnamic acids. | |
| Inflammatory (5-LOX) | Leaf | Ethanolic (ultrasound) | 50 mg/L | NDGA | IP 0–14.05 | Ethanolic leaf extract showed highest activity; aligns with low anti-inflammatory activity found in Lantara Camara essential oil. | |
| Anti-alpha glucosidase | Flower | Aqueous (ultrasound) | 50 mg/L | Acarbose (50 µg/mL) | IP 0–9.77 | Showed lower activity; first study, no previous comparisons. | |
| Anti-alpha amylase | Tuber | Aqueous (maceration) | 50 mg/L | Acarbose (50 µg/mL) | IP 0–31.34 | Showed some activity; first study, no previous comparisons. | |
| Anticancer | Aerial parts and tubers | Methanolic (maceration) | 4.22 ± 0.06 and 5.89 ± 0.08 mg/mL | - | 50% | Extracts from S. undulata caused cell death in MCF7 cells through a combination of cell lysis and apoptosis. | [37] |
| Antibacterial | Aerial part and root | Methanolic extracts | 25 mg/mL and 100 mg/mL | Phenol | - | The extract from the above-ground portion of the plant demonstrated antimicrobial effects against three standard bacterial strains: Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. | [27] |
| Antifungal | Aerial part and tubers | Methanolic extracts | 25 mg/mL and 100 mg/mL | - | - | Both extracts of S. undulate did not show any antifungal effect against the fungus Candida albicans. | |
| Laxative | Aerial part | Methanolic extracts | 200 and 400 mg/kg | Tween 80 | - | The laxative research was conducted solely on the above-ground portion of S. undulata. The method involved tracking the transit of a charcoal meal through the gastrointestinal system. The results showed a significant increase, dependent on the dose, in the percentage of the total length of the intestine affected. | |
| Antibacterial | Aerial parts and tubers | Butanolic, ethyl acetate, petroleum ether, and the product H2 | - | Gentamicin (10 µg) | MIC 500–2000 | The ethyl acetate extract from the aerial part of S. undulata exhibited greater activity compared to the petroleum extract. It displayed antibacterial effects against all tested bacterial strains except for E. coli. The tuber extract of S. undulata exhibited weaker antimicrobial activity when compared to the aerial extracts from the same plant. | [24] |
| Antifungal | Aerial parts and tubers | Butanolic, ethyl acetate, petroleum ether, and the product H2 | - | - | IP 0–64.24 | The petroleum ether and ethyl acetate extracts of the aerial parts showed potent inhibition against all tested fungi for antifungal activity. | |
| Antidiabetic | Aerial parts | Aqueous extract | 20 mg/kg | Glibenclamide (5 mg/kg) | - | The findings suggest that the hypoglycemic effect of the aqueous extract of S. undulata’s aerial parts may be attributed to the improvement of liver structure and function. Furthermore, the dosage used in the study was not found to be toxic. | [33] |
| Hepatoprotective | Aerial parts | Aqueous extract | 20 mg/kg | Glibenclamide (5 mg/kg) | - | Administering the water-based extract from the above-ground portion of S. undulata (SUA) orally over a period of 15 days resulted in elevated glycogen contents in the livers of diabetic rats. Additionally, it enhanced the histological architecture of the liver in diabetic rats treated with SUAP. This treatment also had a positive effect on certain biochemical markers, including ALT and ASAT. | |
| Antidiabetic | Tubers | Aqueous extract | 20 mg/kg | Glibenclamide (5 mg/kg) | - | The findings revealed that both single and repeated oral administration of the aqueous extract of S. undulata tubers (AERSU) at a dose of 20 mg/kg resulted in significant reductions in the blood glucose levels in both normal and streptozotocin (STZ)-induced diabetic rats. The extract was also found to inhibit the α-amylase activity. | [32] |
| Hepatoprotective | Tubers | Aqueous extract | 20 mg/kg | Glibenclamide (5 mg/kg) | - | Administering AERSU orally over a period of 15 days resulted in an enhancement of the histological architecture of the liver in diabetic rats treated with SUAP. This treatment also had a positive effect on the hepatic biochemical markers ALT and ASAT. | [32] |
| Antihypertensive | Aerial parts | Aqueous extract | 300 mg/kg | Furosemide (5 mg/kg) | - | AESU effectively reduced systolic, diastolic, and mean arterial blood pressure in hypertensive rats. The data analysis showed that AESU exerted its antihypertensive effect through its vasodilatory properties. The vasorelaxation ability of AESU could potentially be mediated by its interaction with receptor-operated calcium channels (ROCCs). | [16] |
| Hypolipidemic and cardioprotective | Tubers | Aqueous extract | 20 mg/kg | Glibenclamide (5 mg/kg) | - | The administration of AERSU led to significant enhancements in the weight of diabetic rats, along with reductions in plasma levels of total cholesterol, triglycerides, and LDL lipoprotein. Moreover, the extract positively influenced the atherogenic index (AI) and coronary risk index (CRI). | [61] |
| Extract Source | Type of Activity | Extraction Method | Solvent | Concentration | AA (%)/IC50 Value/E % (g/L) | Standard Control/Dose | Notes | Ref |
|---|---|---|---|---|---|---|---|---|
| Tubers | DPPH scavenging | Ultrasound | Aqueous | 50 µg/mL | 5.55% | VIT C (4 µg/mL) | Lower antioxidant activity regardless of extraction method or solvent. | [18] |
| Leaves | DPPH scavenging | Ultrasound | Ethanolic | 50 µg/mL | 25.06% | VIT C (4 µg/mL) | Highest antioxidant activity; positive correlation with TPC. | |
| Flowers | DPPH scavenging | Various | Various | 50 µg/mL | Varied | VIT C (4 µg/mL) | General pattern: aerial parts and ethanolic extracts showed higher antioxidant activity. | |
| Tubers | DPPH scavenging | Maceration | Various | 50 µg/mL | Lower | VIT C (4 µg/mL) | Lower activity explained by tubers being organ reserve; inulin presence noted. | |
| Leaves | DPPH scavenging | Maceration | Various | 50 µg/mL | Higher | VIT C (4 µg/mL) | Positive correlation with total polyphenolic content (TPC). | |
| Tubers | DPPH scavenging | Maceration | Chloroform and methanol | 0.16 mg | Trolox | Results demonstrated important radical scavenging activity. | [26] | |
| Tubers | CUPRAC | Maceration | Chloroform and methanol | 0.23 g/L | Trolox | Results demonstrated important radical scavenging activity. | ||
| Tubers | DPPH scavenging ABTS FRAP | Maceration | Methanol and n-hexane | 0.4 mg/mL | DPPH: 0.21 mg/mL FRAP: 0.31 Mm TE/g DW ABTS•+: 1.36 TE/g DW | BHT (0.4 mg/mL) and VIT C (0.4 mg/mL) | The methanolic fraction showed the highest scavenging activity against radicals, exhibiting effective IC50 values of 0.14 ± 0.02 mg/mL. Similarly, FRAP and ABTS•+ of methanol extract. | [95] |
| Tubers and aerial parts | DPPH and FRAP | Maceration | Methanol | 0.05−1 mg/mL |
| Ascorbic acid | The potential to scavenge DPPH and the total antioxidant activity of aerial parts were twofold greater than those of tubers. | [37] |
| Tubers | DPPH | Decoction | Aqueous | 31–500 μg/mL | 772.29 μg/mL | BHT 31–500 μg/mL | AERSU possesses potent antioxidant activity. | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajebli, M.; Amssayef, A.; Sabiri, M.; Radi, F.Z.; Bouhlali, E.D.T.; Eddouks, M. Scorzonera undulata: Traditional Applications, Phytochemical Analysis, and Biological and Pharmacological Attributes. Plants 2025, 14, 1606. https://doi.org/10.3390/plants14111606
Ajebli M, Amssayef A, Sabiri M, Radi FZ, Bouhlali EDT, Eddouks M. Scorzonera undulata: Traditional Applications, Phytochemical Analysis, and Biological and Pharmacological Attributes. Plants. 2025; 14(11):1606. https://doi.org/10.3390/plants14111606
Chicago/Turabian StyleAjebli, Mohammed, Ayoub Amssayef, Maryame Sabiri, Fatima Zahrae Radi, Eimad Dine Tariq Bouhlali, and Mohamed Eddouks. 2025. "Scorzonera undulata: Traditional Applications, Phytochemical Analysis, and Biological and Pharmacological Attributes" Plants 14, no. 11: 1606. https://doi.org/10.3390/plants14111606
APA StyleAjebli, M., Amssayef, A., Sabiri, M., Radi, F. Z., Bouhlali, E. D. T., & Eddouks, M. (2025). Scorzonera undulata: Traditional Applications, Phytochemical Analysis, and Biological and Pharmacological Attributes. Plants, 14(11), 1606. https://doi.org/10.3390/plants14111606







