1. Introduction
Brazil is the world’s leading producer of sugarcane (
Saccharum spp.), setting a record in the 2023/2024 harvest by processing 713.2 million tons [
1]. Sugarcane is one of the most important crops globally, with diverse uses, including food production (liquid, refined, crystal, and organic demerara sugars), beverages, biofuels (as a feedstock for ethanol), renewable energy through ethanol and biogas, and biomaterial production using bacterial cellulose [
2,
3].
Several factors can influence sugarcane production, with nutrient management and nutrient assimilation rates playing critical roles. Medium-textured soils with low organic matter content are common in tropical conditions, offering highly favorable physical properties for agricultural production. However, they present chemical limitations [
4], which can be mitigated through effective management and fertilization strategies.
The nutritional requirements of sugarcane vary according to the expected yield and crop cycle. The crop’s developmental stage directly influences the highest nutrient uptake around 200 days after planting. In the soil/nutrient dynamic, various reactions can affect nutrient availability and plant absorption, directly influencing the amount of mineral fertilization required [
5].
Among the most demanded nutrients by sugarcane is nitrogen (N), a vital element for metabolic processes and an essential component of amino acids, proteins, and nucleic acids, contributing directly to chlorophyll biosynthesis [
6]. Urea [CO(NH
2)
2] is the most used source to meet plant N requirements due to its industrial advantages, such as high N concentration per unit mass (45–46%) and lower production costs compared to other N sources [
7]. Once applied to the soil, urea is hydrolyzed by the enzyme urease, producing ammonia (NH
3-N), which is rapidly lost to the atmosphere as gas [
8,
9].
Mineral fertilization can be employed to meet the crop’s N demand; however, this increases production costs. Moreover, the long-term intensive use of these inputs can lead to adverse environmental impacts, such as soil acidification and contamination of water resources by chemical compounds present in fertilizers, among other effects [
5]. In sugarcane cultivation, fertilizers make up 20–30% of the production costs, with a rather variable range of fertilization recommendations, both for plant cane and ratoon crops. In Brazil, more than 15% of the fertilizers are used for this crop [
10,
11]. Several strategies have been used to increase fertilization efficiency. The most frequently used strategy has been the application of enhanced-efficiency fertilizers [
12] One approach to reducing reliance on mineral N fertilizers is the use of plant growth-promoting bacteria (PGPB), which has shown promising results in agricultural production systems [
13].
PGPB have the potential to stimulate plant growth through biological N-fixation. They contribute directly to greater root system development, the synthesis of phytohormones, and phosphate solubilization and may also enhance plant resistance to both biotic and abiotic stresses [
14].
The N-fixing ability of these bacteria grants them diazotrophic capabilities, making their use in agriculture a highly sustainable alternative. This reduces the need for mineral fertilizers and, consequently, dependence on non-renewable industrial processes [
15,
16].
Azospirillum is a well-studied genus of bacteria due to its ability to fix atmospheric N (N
2) and produce phytohormones that enhance plant development. Scudeletti et al. [
17], when evaluating doses of
Azospirillum brasilense in sugarcane, observed an increase in sugar production, energy output, and yield when the inoculant was applied during the sprouting and tillering stages. Additionally, Ferreira et al. [
18] highlighted the potential for improving raw material quality, which can translate into strategic gains in final sugarcane productivity.
The objective of this study was to evaluate the application of Azospirillum brasilense in the planting furrow of sugarcane, combined with reduced N fertilization, focusing on the plant’s nutritional status, nutrient extraction from the aerial parts, stalk productivity and technological quality, and total fresh and dry biomass production.
3. Discussion
A significant difference was observed in total fresh and dry matter production (Area 1). In the total aboveground fresh mass production, the application of 0.6 L ha
−1 of the inoculant solution resulted in the highest yield, reaching 216.33 Mg ha
−1. These results are consistent with those found in the literature, which highlights the use of PGPB-based inoculants as an effective practice to increase productivity in crops such as sugarcane [
19].
The fresh mass production in treatment T5 was 34% higher than in treatment T2.
Azospirillum can stimulate plant growth through mechanisms such as biological N-fixation, phytohormone production, and phosphate solubilization [
20,
21]. For total aboveground dry mass production, the highest yield was observed in T1. These results are like those reported by Fukami et al. [
22], who noted that excessive doses of inoculants can have antagonistic effects due to an imbalance in soil microbiota or the increase in inhibitory substances, leading to negative impacts on production. Stalk productivity in both experimental areas exceeded the regional average, which, in the 2022/2023 season, was 80.47 Mg ha
−1 [
1]. Sugarcane plants tend to exhibit the highest productivity in the first harvest, and it tends to decline in subsequent seasons [
23].
Table 2 presents the results of the technological analyses of sugarcane in two experimental areas. The technological parameters (
Table 2) did not show a response to the treatments. These findings are supported by Scudeletti et al. [
17], who, when evaluating the application of
Azospirillum brasilense in plant cane and first ratoon, did not observe a significant increase in the crop’s technological parameters. However, the data related to the technological parameters are in line with those recommended by [
24].
The responses observed for P in both areas can be attributed to
Azospirillum’s ability to promote root growth, which increases the soil exploration area and consequently enhances phosphorus uptake, a nutrient that is relatively immobile in the soil [
25]. Additionally,
Azospirillum can release organic acids and phosphatases that solubilize soil P, making it more available to the plant [
26]. Some authors report that
Azospirillum may act synergistically with phosphate fertilizers, enhancing P use efficiency, which is crucial for crops such as sugarcane, with high P demand [
21].
In Area 1, the concentration of Cu in the leaves observed in T1 was significantly higher than in all other treatments, while in Area 2, T1 was statistically like T2 and T3. The behavior observed for Cu can be explained by the possible saturation of absorption sites or changes in Cu availability in the soil due to microbial activity induced by
Azospirillum brasilense. This microorganism can influence Cu availability to a lesser extent than mineral N, directly impacting the absorption of various micronutrients [
27]. The decrease in Cu concentration at higher doses may indicate that an increase in the microbial population could have led to Cu immobilization in the soil or competition between microorganisms and plants for the element, reducing its availability to plants [
25]. Moreover, Cu is an essential micronutrient for many enzymatic functions, but its absorption can be limited by the presence of high levels of P or other antagonistic elements [
28].
Fe showed a significant difference in both areas studied. In Area 1, treatments T2, T3, T4, and T5 had higher concentrations compared to T1 and T6, whereas in Area 2, the highest concentrations were observed in treatments T1, T3, and T5, which differed statistically from the other treatments. It was noted that T6 had lower responses compared to the other treatments in both areas, indicating that high doses of the inoculant may reduce the concentration of this element in sugarcane leaves. This pattern may be related to the excessive production of siderophores by
Azospirillum, which, when overproduced, can limit the nutrient’s availability to plants [
20].
Mn responded significantly to the inoculant application in both areas. In Area 1, there were no differences between treatments, while in Area 2, treatments T1 and T3 were statistically different from the others. For Zn, the only significant response was observed in T5 in Area 1.
The foliar concentrations of Fe and Zn were within the adequate range for sugarcane, which is Fe (40.0–250.0 mg kg
−1) and Zn (10.0–50.0 mg kg
−1). For Mn, the concentrations exceeded the adequate range, which is 10.0–50.0 mg kg
−1, while Cu was below the adequate range in all cases (6.0–15.0 mg kg
−1) [
29].
Table 4 shows the average extraction of macro- and micronutrients from the aboveground part of sugarcane in two experimental areas. The extraction of elements N, P, K, Ca, Mg, S, Cu, Fe, Mn, and Zn in Area 1 responded to the combined application of microorganisms and half of the mineral N fertilization. The same response was observed in Area 2, except for N and Cu (
Table 4).
For N, no significant difference was observed between treatments. However, T5 significantly contributed to the extraction of P, K, Ca, and Mg, with this treatment showing higher averages than T1 in both areas for P extraction. This demonstrates the potential of
Azospirillum to enhance fertilizer use efficiency. This combination suggests that
Azospirillum can improve P availability and absorption, possibly by promoting root growth, better soil exploration, and increased solubilization [
30,
31]. Research by Hungria et al. [
21] indicated that the use of
Azospirillum in crops such as corn and sugarcane can improve the efficiency of N and P extraction, reducing fertilizer costs and environmental impacts.
In Area 1, the highest extraction of K, Ca, and Mg occurred with the application of T5, which differed significantly from the other treatments according to the Scott–Knott test. In Area 2, no significant differences were observed among treatments for K, but for Ca and Mg, the highest extractions were in T1, T3, T5, and T6 (
Table 4). The greater extraction of K in Area 1 may be related to
Azospirillum brasilense’s ability to promote root growth and increase the absorption surface, which is particularly important for K uptake. K is a mobile nutrient in the soil and is essential for various physiological functions, including osmotic regulation and enzymatic activation [
32]. No significant differences were observed among treatments for S extraction in either area.
Although the observed responses for Ca, Mg, and S are consistent with the trends in concentrations, the microorganism’s ability to sustain these extraction levels suggests that the inoculant could be used as a tool to stabilize plant nutrition, even in scenarios where soil nutrient availability is limiting [
33].
The extraction of all micronutrients analyzed (Cu, Fe, Mn, and Zn) in Area 1 responded significantly to the application of the inoculant solution, whereas in Area 2, only Cu showed no significant response. In Area 1, the extraction of Cu and Zn showed similar behaviors, with T5 being the only treatment to respond equally to T1 (control). In Area 2, for Zn extraction, T4 was the only treatment that responded differently and inferiorly to the others.
For Mn extraction in Area 1, treatment T5 was statistically superior to all other treatments, while in Area 2, T3 responded statistically the same as T1, with these two treatments showing the highest extractions. In terms of Fe extraction, no significant differences were found among treatments in Area 1; however, in Area 2, the lowest extractions were observed in T2 and T4. The results for Fe in both areas suggest that although
Azospirillum can enhance Fe extraction, its effectiveness may be limited compared to traditional mineral fertilizers. This limitation may be related to how Fe is available in the soil and the plant’s ability to translocate it after absorption [
27].
The results for Fe and Zn are particularly relevant, as these micronutrients play crucial roles in biochemical and physiological processes, such as photosynthesis and chlorophyll synthesis, which are vital for the healthy growth of sugarcane [
34].
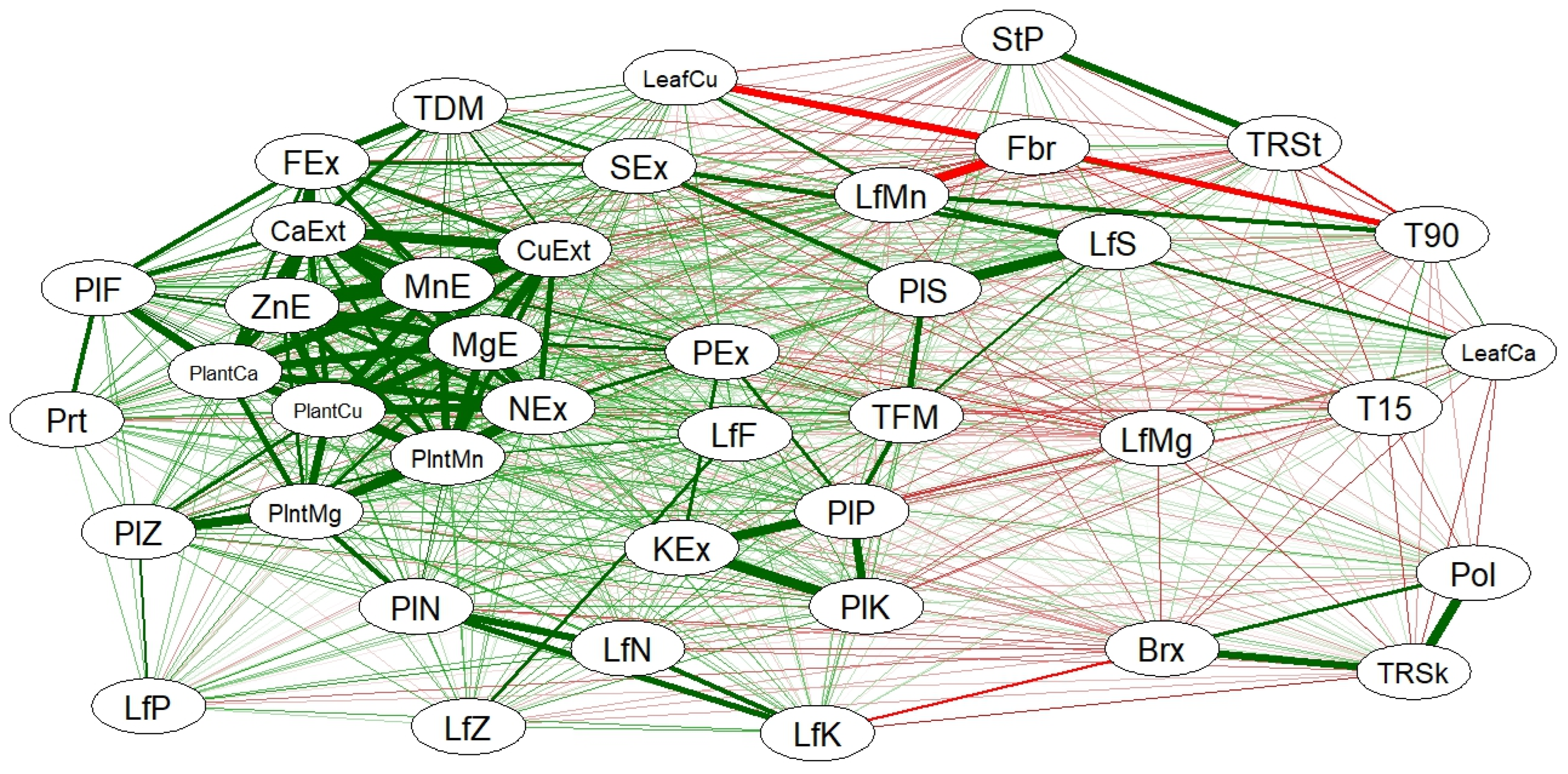
Stalk yield and TRS t ha
−1 were also strongly linked, with a positive correlation of 0.86, indicating that the amount of biomass is directly related to TRS t ha
−1 (
Figure 2). This synergy can be maximized through proper nutrient management and agricultural practices that promote plant development [
35].
Negative relationships were observed for cane fiber (Fbr), which negatively impacted the accumulation of Cu (CuF) and Mn (MnF) in the leaves, with correlation values of −0.96 and −0.91, respectively, and for stalk tillering at 90 days (P90), with a correlation of −0.90. In both cases, these are strong negative correlations (
Figure 2). This suggests that an increase in fiber content may be associated with a reduced availability or absorption of these micronutrients, which could affect plant development. However, excessive fiber is a limiting factor for the quality of raw materials for industrial purposes [
36].
Regarding N, the relationship between N in the leaves (NF) and K in the leaves (KF) was strongly correlated (0.88). The extraction of N (ExN) was also strongly associated with the extraction of various micronutrients (
Figure 2), highlighting a beneficial relationship consistent with nitrogen’s role in promoting the absorption and mobilization of other essential nutrients.
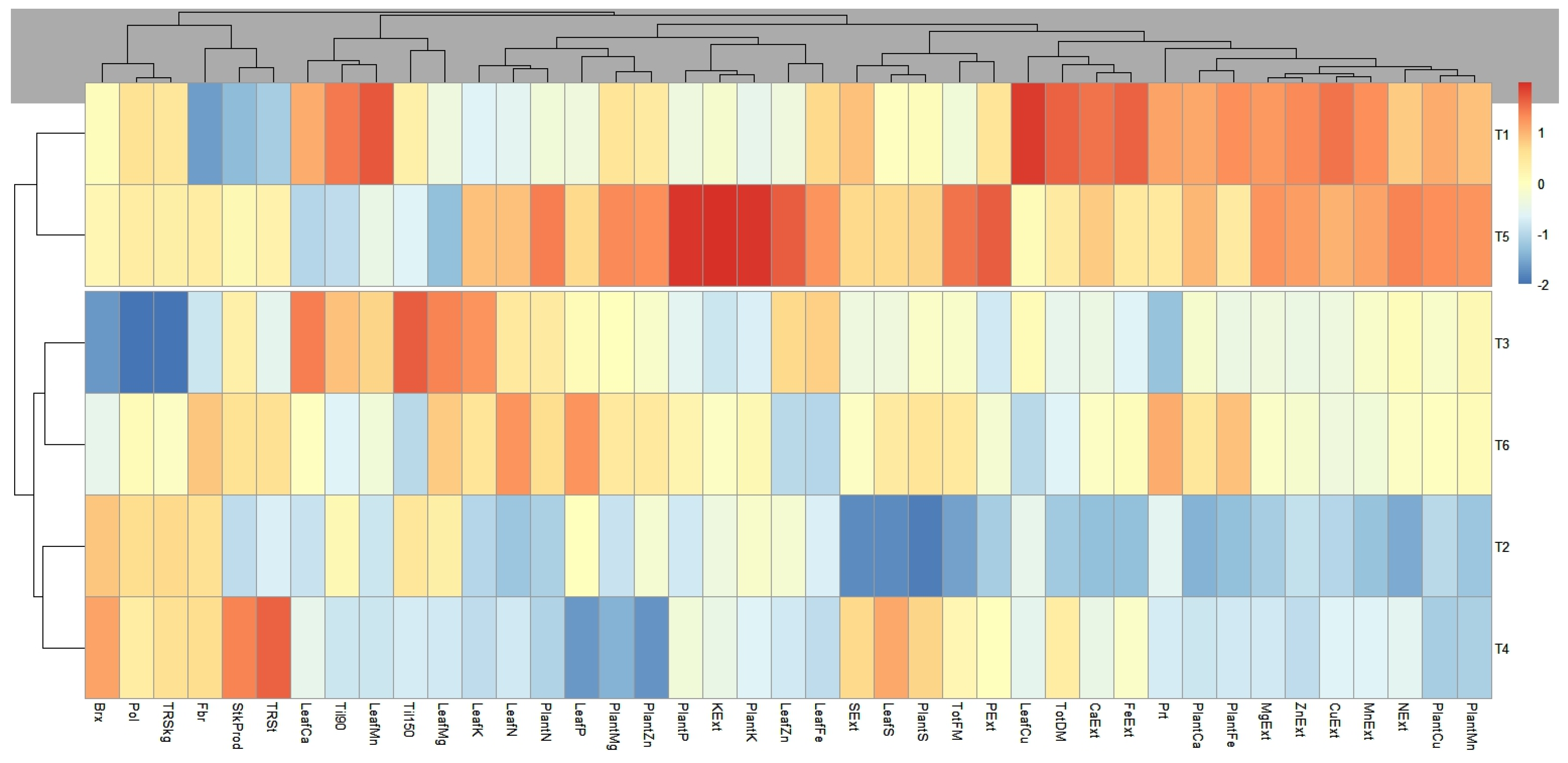
The resulting dendrogram, presented in
Figure 3, illustrates the hierarchical structure of the clusters. The observations were grouped into two clusters, defined according to the cut-off point established by the analysis. This cut-off point was determined based on the visual inspection of the dendrogram and the analysis of the consistency of the groupings. The treatments T2 and T4 exhibited the most consistent patterns, with a separate grouping for T5, T1, and T3.
Notably, the proximity between T1 and T5 formed a superior cluster compared to the others. This result suggests that the application of the inoculant creates conditions very similar to those achieved with conventional mineral fertilizers, indicating that inoculants may be as effective as fertilizers.
5. Conclusions
The application of 0.6 L ha−1 of the solution containing Azospirillum brasilense at the planting furrow of sugarcane, combined with 50% of the nitrogen dose, increased the total fresh biomass production in Area 1. However, the total dry mass, stalk productivity, tillering, and technological parameters of the crop were not affected by the presence of the bacteria.
The nutrient concentrations in the foliar diagnosis did not differ with the application of Azospirillum and full or half doses of nitrogen fertilization.
In nutrient extraction, the treatment with 0.6 L ha−1 of the inoculant solution, combined with 50% of the nitrogen dose, consistently ranked among the treatments with the highest averages of P, K, Ca, Mg, S, Fe, Mn, and Zn in both experimental areas.
According to the dendrogram, the control treatment and T5—the application of 0.6 L ha−1 of the inoculant solution and a half dose of mineral nitrogen—displayed a superior clustering compared to the others, with a similar coloration, indicating very comparable conditions between the two treatments.