IAA-Mediated Haustorium Formation in Phelipanche aegyptiaca: Transcriptional Insights and Anti-Parasitic Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. 5-Ethynyl-2′-Deoxyuridine (EdU) Staining
2.3. RNA Sequencing
2.4. Identification and Functional Enrichment Analysis of DEGs
2.5. qRT-PCR
2.6. Greenhouse Experiment
2.7. Statistical Analysis
3. Results
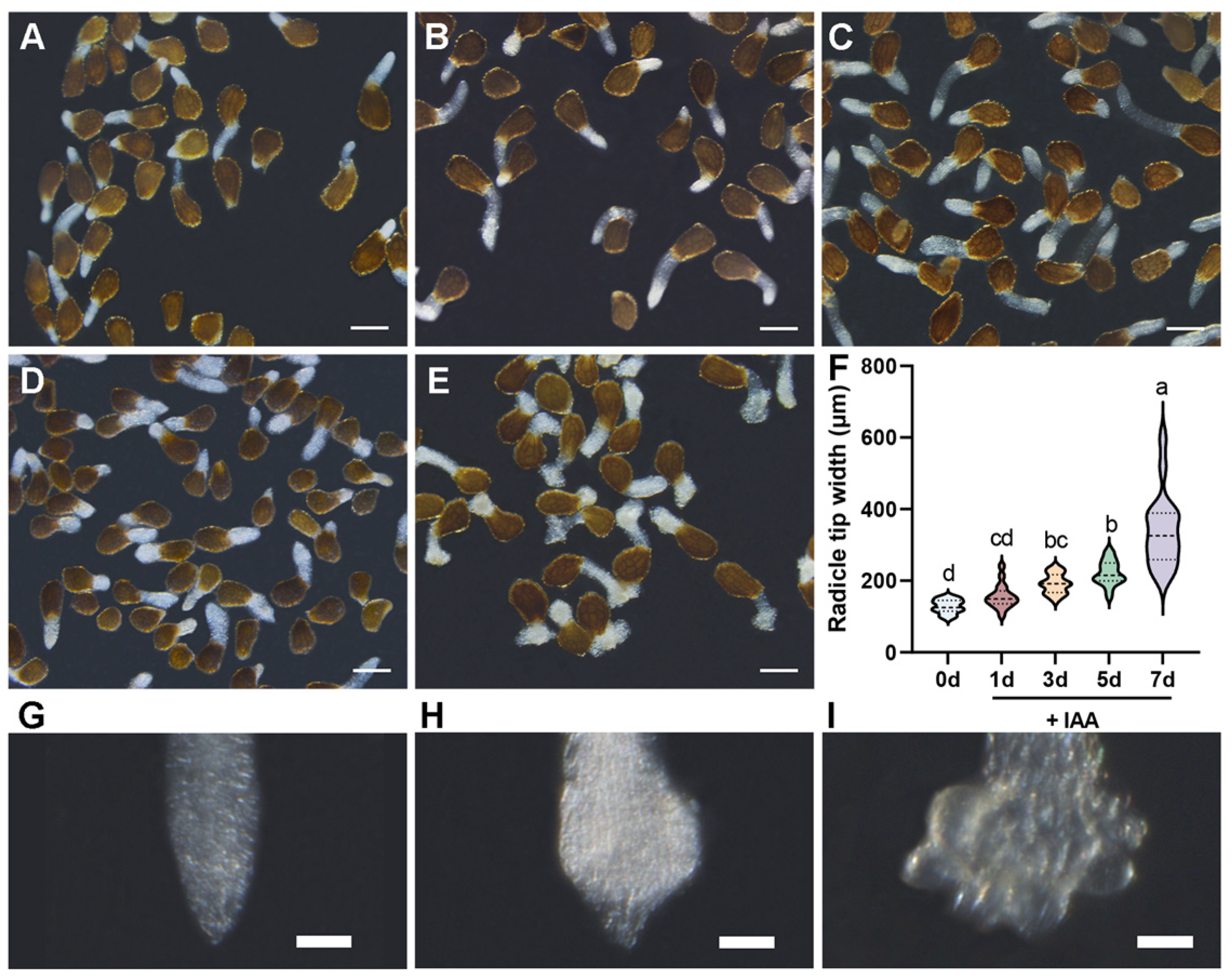
3.1. Temporal Dynamics of Haustorium Development in P. aegyptiaca Induced by IAA
3.2. Spatiotemporal Dynamics of P. aegyptiaca Radicles at Different Developmental Stages
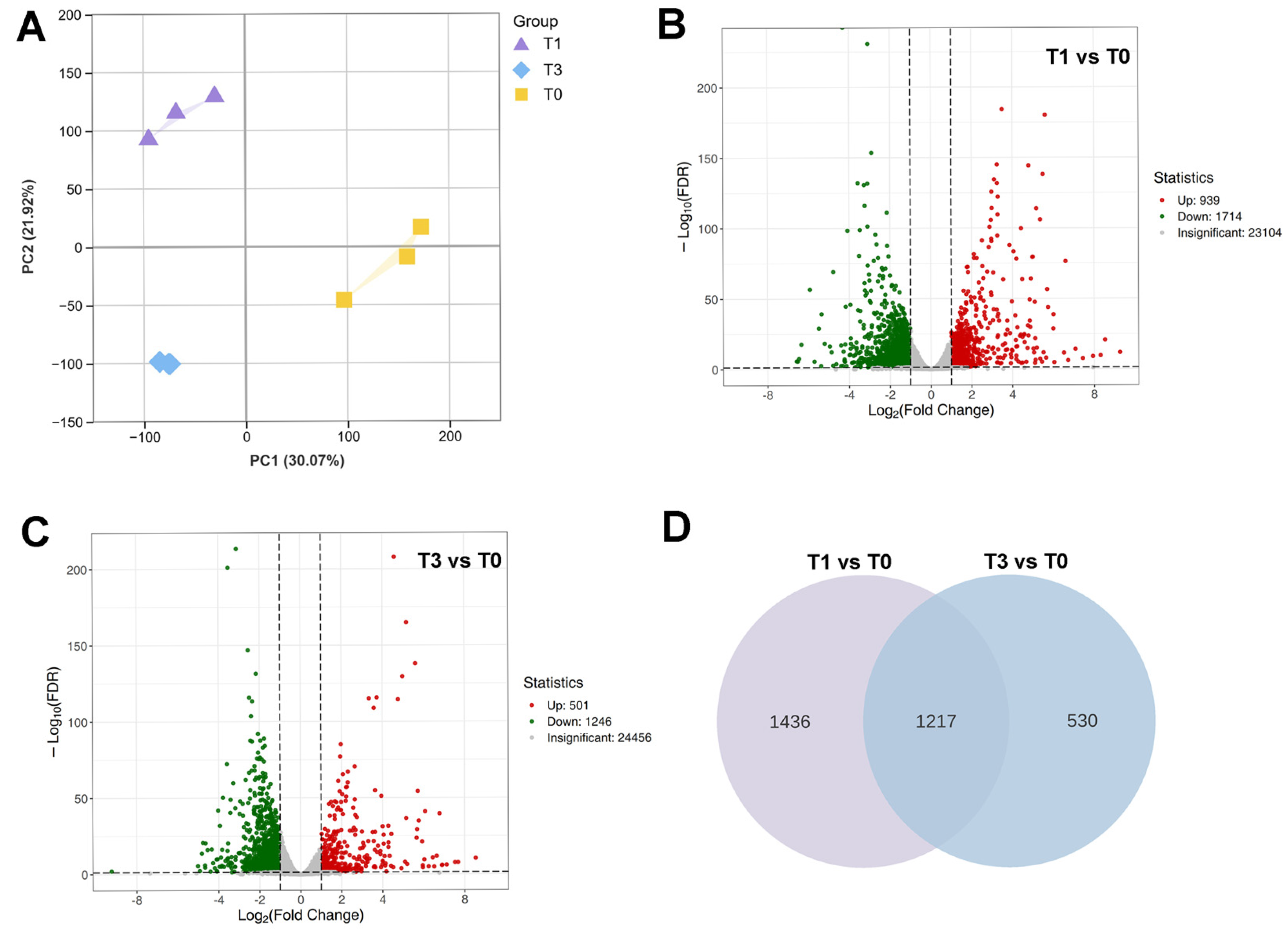
3.3. Differential Expression Genes in Three P. aegyptiaca Haustorium Development Stages
3.4. GO and KEGG Enrichment Analyses of DEGs in P. aegyptiaca Haustorium Development Stages
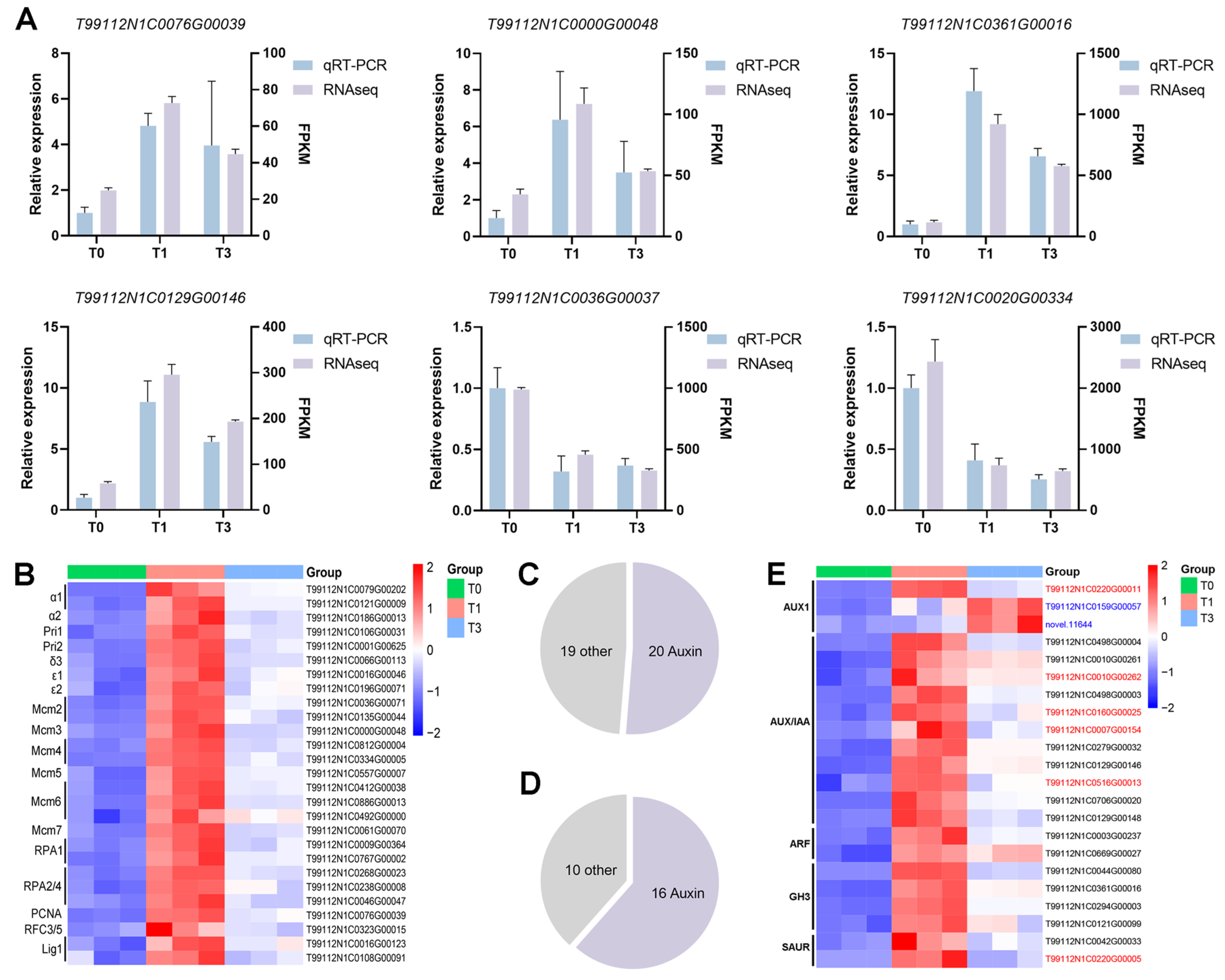
3.5. Analysis of Key Pathways Based on KEGG Enrichment Results
3.6. Auxin Signaling Regulates the Parasitic Capacity of P. aegyptiaca
4. Discussion
4.1. IAA-Driven P. aegyptiaca Haustorium Development
4.2. Cell Proliferative Characteristics During P. aegyptiaca Haustorium Development
4.3. Transcriptional Insights into P. aegyptiaca Haustorium Development
4.4. Anti-Parasitic Strategies Against P. aegyptiaca
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Joel, D.M.; Hershenhorn, J.; Eizenberg, H.; Aly, R.; Ejeta, G.; Rich, P.J.; Ransom, J.K.; Sauerborn, J.; Rubiales, D. Biology and management of weedy root parasites. In Horticultural Reviews; Janick, J., Ed.; Wiley: Hoboken, NJ, USA, 2007; pp. 267–349. [Google Scholar]
- Bürger, M.; Chory, J. A potential role of heat-moisture couplings in the range expansion of Striga asiatica. Ecol. Evol. 2024, 14, e11332. [Google Scholar] [CrossRef] [PubMed]
- Eizenberg, H.; Goldwasser, Y. Control of egyptian broomrape in processing tomato: A summary of 20 years of research and successful implementation. Plant Dis. 2018, 102, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Cao, X.; Fu, C.; Zhao, S. Review of the Distribution and Management Technology of Various Orobanche L. Species in Xinjiang, China. J. Biosaf. 2017, 26, 23–29. [Google Scholar] [CrossRef]
- Pérez, D.E.L.A.; Rubiales, D.; Cubero, J.I.; Press, M.C.; Scholes, J.; Yoneyama, K.; Takeuchi, Y.; Plakhine, D.; Joel, D.M. Interaction between Orobanche crenata and its host legumes: Unsuccessful haustorial penetration and necrosis of the developing parasite. Ann. Bot. 2005, 95, 935–942. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, X.; Yao, Z.; Dong, X.; Chen, M.; Xiao, L.; Zhao, S. Identification of risk areas for Orobanche cumana and Phelipanche aegyptiaca in China, based on the major host plant and CMIP6 climate scenarios. Ecol. Evol. 2022, 12, e8824. [Google Scholar] [CrossRef]
- Ephrath, J.; Hershenhorn, J.; Achdari, G.; Bringer, S.; Eizenberg, H. Use of logistic equation for detection of the initial parasitism phase of egyptian broomrape (Phelipanche aegyptiaca) in tomato. Weed Sci. 2012, 60, 57–63. [Google Scholar] [CrossRef]
- Cochavi, A.; Rubin, B.; Achdari, G.; Eizenberg, H. Thermal time model for egyptian broomrape (Phelipanche aegyptiaca) parasitism dynamics in carrot (Daucus carota L.): Field validation. Front. Plant Sci. 2016, 7, 1807. [Google Scholar] [CrossRef]
- Hosseini, P.; Ahmadvand, G.; Oveisi, M.; Morshedi, P.; Gonzalez-Andujar, J.L. A modelling approach for predicting the initial phase of egyptian broomrape (Phelipanche aegyptiaca) parasitism in potato. Crop Prot. 2017, 100, 51–56. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Ma, C.; Lei, Y.; Shen, G.; Jin, J.; Eaton, D.A.R.; Wu, J. Comparative genomics of orobanchaceous species with different parasitic lifestyles reveals the origin and stepwise evolution of plant parasitism. Mol. Plant 2022, 15, 1384–1399. [Google Scholar] [CrossRef]
- Hearne, S.J. Control—The Striga conundrum. Pest Manag. Sci. 2009, 65, 603–614. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Scalon, M.C.; Liu, J.-X.; Song, X.-Y.; Yang, D.; Zhang, Y.-J.; Ellsworth, D.S.; Zhang, J.-L. You are what you eat: Nutrient and water relations between mistletoes and hosts. New Phytol. 2023, 238, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Rouamba, A.; Shimelis, H.; Drabo, I.; Shamuyarira, K.W.; Mrema, E. Management of the Striga Epidemics in Pearl Millet Production: A review. CABI Agric. Biosci. 2024, 5, 11. [Google Scholar] [CrossRef]
- Yoneyama, K.; Bennett, T. Whispers in the Dark: Signals Regulating Underground Plant–Plant Interactions. Curr. Opin. Plant Biol. 2024, 77, 102456. [Google Scholar] [CrossRef]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The Haustorium, a Specialized Invasive Organ in Parasitic Plants. Annu. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef]
- Rodenburg, J.; Demont, M.; Zwart, S.J.; Bastiaans, L. Parasitic weed Incidence and Related Economic Losses in Rice in Africa. Agric. Ecosyst. Environ. 2016, 235, 306–317. [Google Scholar] [CrossRef]
- Honaas, L.A.; Wafula, E.K.; Yang, Z.; Der, J.P.; Wickett, N.J.; Altman, N.S.; Taylor, C.G.; Yoder, J.I.; Timko, M.P.; Westwood, J.H.; et al. Functional genomics of a generalist parasitic plant: Laser microdissection of host-parasite interface reveals host-specific patterns of parasite gene expression. BMC Plant Biol. 2013, 13, 9. [Google Scholar] [CrossRef]
- Hood, M.E.; Condon, J.M.; Timko, M.P.; Riopel, J.L. Primary haustorial development of Striga asiatica on host and nonhost species. Phytopathology 1998, 88, 70–75. [Google Scholar] [CrossRef]
- Xiao, T.T.; Kirschner, G.K.; Kountche, B.A.; Jamil, M.; Maria, S.; Lube, V.; Mironova, V.; Al Babili, S.; Blilou, I. A PLETHORA/PIN-FORMED/Auxin Network Mediates Prehaustorium Formation in the Parasitic Plant Striga hermonthica. Plant Physiol. 2022, 189, 2281–2297. [Google Scholar] [CrossRef]
- Joel, D.M. Functional structure of the mature haustorium. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–60. [Google Scholar]
- Goyet, V.; Billard, E.; Pouvreau, J.-B.; Lechat, M.-M.; Pelletier, S.; Bahut, M.; Monteau, F.; Spichal, L.; Delavault, P.; Montiel, G.; et al. Haustorium initiation in the obligate parasitic plant Phelipanche ramosa involves a host-exudated cytokinin signal. J. Exp. Bot. 2017, 68, 5539–5552. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Masi, M.; Maddau, L.; Cimmino, A.; Evidente, M.; Rubiales, D.; Evidente, A. Induction of haustorium development by sphaeropsidones in radicles of the parasitic weeds Striga and Orobanche. A structure-activity relationship study. J. Agric. Food Chem. 2016, 64, 5188–5196. [Google Scholar] [CrossRef]
- Joel, D.M.; Losner-Goshen, D. The attachment organ of the parasitic angiosperms Orobanche cumana and O. aegyptiaca and its development. Can. J. Bot. 1994, 72, 564–574. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A. Haustorium Invasion into Host Tissues. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 75–86. [Google Scholar]
- Bian, P.; Sun, C.; Cao, X.; Yao, Z.; Zhang, X.; Zhao, S. Screening of haustorium induction factors of Phelipanche aegyptiaca Pers. based on metabolome analysis of Cucumis melo L. root exudates. Agronomy 2023, 13, 2648. [Google Scholar] [CrossRef]
- Zeng, X.; Cao, X.; Zhao, Q.; Hou, S.; Hu, X.; Yang, Z.; Hao, T.; Zhao, S.; Yao, Z. Isolation of haustorium protoplasts optimized by orthogonal design for transient gene expression in Phelipanche aegyptiaca. Plants 2024, 13, 2163. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.K.; Wakatake, T.; Yoshida, S.; Takebayashi, Y.; Kasahara, H.; Wafula, E.; dePamphilis, C.W.; Namba, S.; Shirasu, K. Local auxin biosynthesis mediated by a YUCCA Flavin Monooxygenase regulates haustorium development in the parasitic plant Phtheirospermum japonicum. Plant Cell 2016, 28, 1795–1814. [Google Scholar] [CrossRef]
- Wakatake, T.; Ogawa, S.; Yoshida, S.; Shirasu, K. An Auxin Transport Network Underlies Xylem Bridge Formation between the Hemi-parasitic Plant Phtheirospermum japonicum and Host Arabidopsis. Development 2020, 147, dev187781. [Google Scholar] [CrossRef]
- Tomilov, A.A.; Tomilova, N.B.; Abdallah, I.; Yoder, J.I. Localized Hormone Fluxes and Early Haustorium Development in the Hemiparasitic Plant Triphysaria versicolor. Plant Physiol. 2005, 138, 1469–1480. [Google Scholar] [CrossRef]
- Ogawa, S.; Cui, S.; White, A.R.F.; Nelson, D.C.; Yoshida, S.; Shirasu, K. Strigolactones are chemoattractants for host tropism in Orobanchaceae parasitic plants. Nat. Commun. 2022, 13, 4653. [Google Scholar] [CrossRef]
- Gonzalez-Verdejo, C.I.; Die, J.V.; Nadal, S.; Jimenez-Marin, A.; Moreno, M.T.; Roman, B. Selection of housekeeping genes for normalization by real-time RT-PCR: Analysis of Or-MYB1 gene expression in Orobanche ramosa development. Anal. Biochem. 2008, 379, 176–181. [Google Scholar] [CrossRef]
- Cao, X.; Xiao, L.; Zhang, L.; Chen, M.; Bian, P.; Ma, Q.; Chen, S.; He, Q.; Ma, X.; Yao, Z.; et al. Phenotypic and histological analyses on the resistance of melon to Phelipanche aegyptiaca. Front. Plant Sci. 2023, 14, 1070319. [Google Scholar] [CrossRef]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid auxin-mediated cell expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef]
- Dai, Y.; Luo, L.; Zhao, Z. Genetic robustness control of auxin output in priming organ initiation. Proc. Natl. Acad. Sci. USA 2023, 120, e2221606120. [Google Scholar] [CrossRef] [PubMed]
- Majda, M.; Robert, S. The role of auxin in cell wall expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef]
- Lin, W.; Zhou, X.; Tang, W.; Takahashi, K.; Pan, X.; Dai, J.; Ren, H.; Zhu, X.; Pan, S.; Zheng, H.; et al. TMK-based cell-surface auxin signalling activates cell-wall acidification. Nature 2021, 599, 278–282. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Z.; Sun, B. KNUCKLES regulates floral meristem termination by controlling auxin distribution and cytokinin activity. Plant Cell 2024, 37, koae312. [Google Scholar] [CrossRef]
- Dequeker, B.J.H.; Scherr, M.J.; Brandao, H.B.; Gassler, J.; Powell, S.; Gaspar, I.; Flyamer, I.M.; Lalic, A.; Tang, W.; Stocsits, R.; et al. MCM complexes are barriers that restrict cohesin-mediated loop extrusion. Nature 2022, 606, 197–203. [Google Scholar] [CrossRef]
- Meagher, M.; Epling, L.B.; Enemark, E.J. DNA translocation mechanism of the MCM complex and implications for replication initiation. Nat. Commun. 2019, 10, 3117. [Google Scholar] [CrossRef]
- Muzi-Falconi, M.; Giannattasio, M.; Foiani, M.; Plevani, P. The DNA polymerase alpha-primase complex: Multiple functions and interactions. Sci. World J. 2003, 3, 21–33. [Google Scholar] [CrossRef]
- Yates, L.A.; Aramayo, R.J.; Pokhrel, N.; Caldwell, C.C.; Kaplan, J.A.; Perera, R.L.; Spies, M.; Antony, E.; Zhang, X. A structural and dynamic model for the assembly of replication protein a on single-stranded DNA. Nat. Commun. 2018, 9, 5447. [Google Scholar] [CrossRef]
- Tsuzuki, K.; Suzuki, T.; Kuruma, M.; Nishiyama, K.; Hayashi, K.I.; Hagihara, S.; Seto, Y. Radicle growth regulation of root parasitic plants by auxin-related compounds. Plant Cell Physiol. 2024, 65, 1377–1387. [Google Scholar] [CrossRef]
- Islam, F.; Wang, J.; Farooq, M.A.; Khan, M.S.S.; Xu, L.; Zhu, J.; Zhao, M.; Muños, S.; Li, Q.X.; Zhou, W. Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ. Int. 2018, 111, 332–351. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Cao, X.; Zhao, Q.; Zeng, X.; Wei, Y.; Yao, Z.; Zhao, S. IAA-Mediated Haustorium Formation in Phelipanche aegyptiaca: Transcriptional Insights and Anti-Parasitic Strategies. Plants 2025, 14, 1591. https://doi.org/10.3390/plants14111591
Hu X, Cao X, Zhao Q, Zeng X, Wei Y, Yao Z, Zhao S. IAA-Mediated Haustorium Formation in Phelipanche aegyptiaca: Transcriptional Insights and Anti-Parasitic Strategies. Plants. 2025; 14(11):1591. https://doi.org/10.3390/plants14111591
Chicago/Turabian StyleHu, Xin, Xiaolei Cao, Qiuyue Zhao, Xiaojian Zeng, Yingming Wei, Zhaoqun Yao, and Sifeng Zhao. 2025. "IAA-Mediated Haustorium Formation in Phelipanche aegyptiaca: Transcriptional Insights and Anti-Parasitic Strategies" Plants 14, no. 11: 1591. https://doi.org/10.3390/plants14111591
APA StyleHu, X., Cao, X., Zhao, Q., Zeng, X., Wei, Y., Yao, Z., & Zhao, S. (2025). IAA-Mediated Haustorium Formation in Phelipanche aegyptiaca: Transcriptional Insights and Anti-Parasitic Strategies. Plants, 14(11), 1591. https://doi.org/10.3390/plants14111591





