Phytotoxic Effects of the Aqueous Extracts of Magnolia biondii Pamp. Flower Litter and the Joint Action of Allelochemicals
Abstract
1. Introduction
2. Results
2.1. Effects of EMT and EMB on Seed Germination
2.2. Effects of EMT and EMB on Seedlings’ Growth
2.3. LC-HRMS Level 3 Analysis of Active Compounds in Flowers of M. biondii
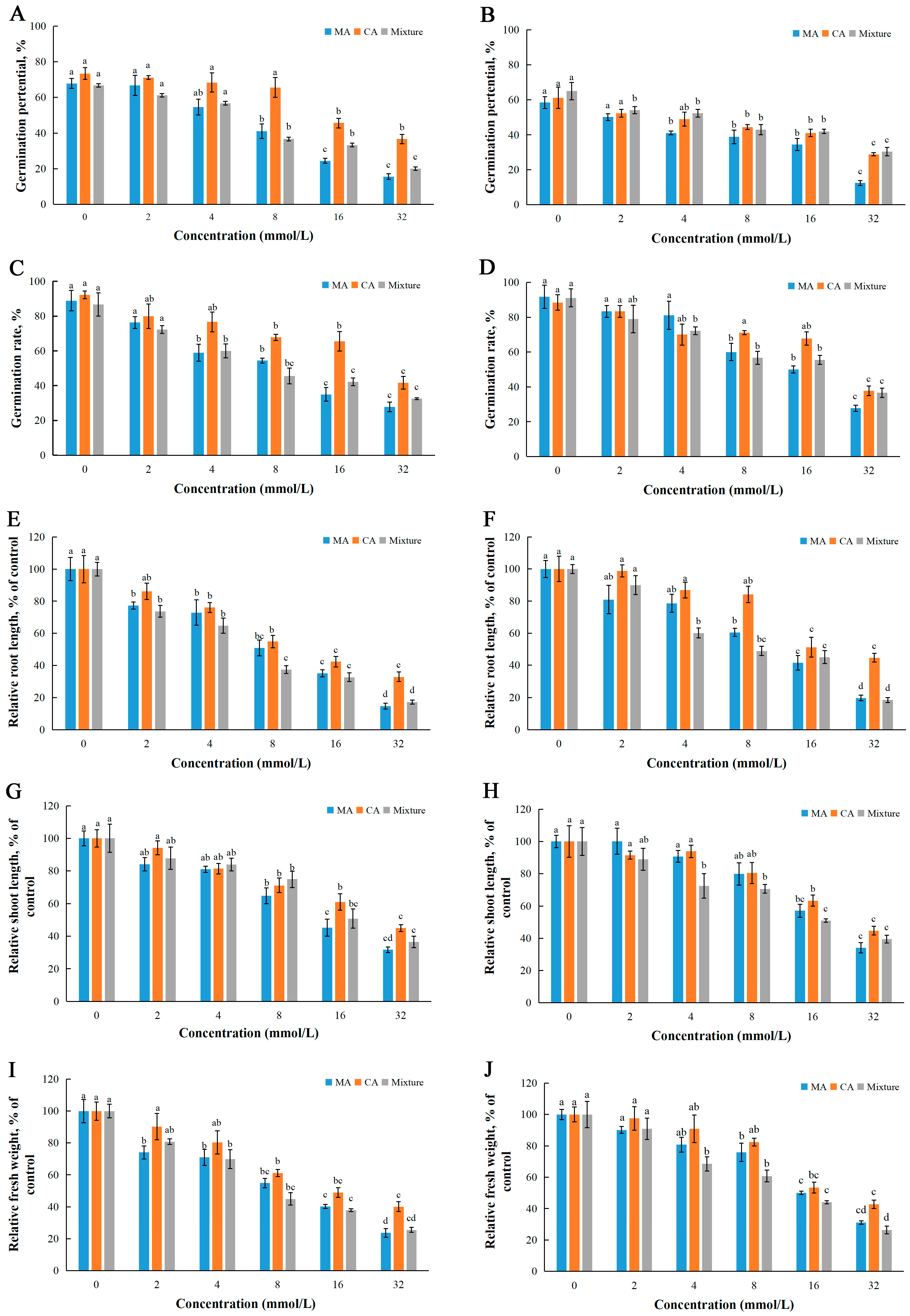
2.4. Joint Action of MA and CA
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Aqueous Extracts of M. biondii Flower Litter
4.3. Seed Germination
4.4. Seedling Growth
4.5. LC-HRMS
4.6. Joint Action of Allelochemicals
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMT | aqueous extracts of M. biondii flower tepal litter |
| EMB | aqueous extracts of M. biondii flower bract litter |
| LC-HRMS | liquid chromatography high-resolution mass spectrometry |
| MA | malic acid |
| CA | citric acid |
| ADM | additive dose model |
References
- Wu, X.P.; Song, L.H.; Yang, Q.; Yang, Y.; Hu, M.L. Assessing the Impact of Climate Change on the Habitat Dynamics of Magnolia biondii in China: A MaxEnt Modelling Approach. Appl. Ecol. Environ. Res. 2024, 22, 2241–2255. [Google Scholar] [CrossRef]
- Fu, D.L. Notes on Yulania spach. Plant Sci. J. 2001, 19, 191–198. [Google Scholar]
- Yin, Z.F.; Ou, X.; Chen, Y.; Yang, A.X.; Sun, L.Y. Research Progress and Prospects of Biological Basis in Magnolia biondii. J. Nanjing For. Univ. 2024, 48, 256–262. [Google Scholar] [CrossRef]
- Song, C.; Liu, H. Habitat Differentiation and Conservation Gap of Magnolia biondii, M. denudata, and M. sprengeri in China. PeerJ 2019, 6, e6126. [Google Scholar] [CrossRef]
- China Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 1st ed.; China Medical Science and Technology Press: Beijing, China, 2020; pp. 189–190.
- Xu, L.; Sun, L.; Chen, Y.; Nie, T.; Zhu, H.; Yin, Z. Magnolia biondii Pamp.: A Comprehensive Review of the Pharmacognosy, Phytochemistry, Pharmacology, and Applications. Ind. Crops Prod. 2024, 222, 119648. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Zhang, D.; Zhao, G.; Liang, Y.; Jia, X.; Guo, L.; Xu, C.; Gao, X. Chemical Constituents and Bioactivities of the Essential Oils of Magnolia biondii Flower Buds from Three Provinces in China. Flavour Fragr. J. 2024, 39, 302–311. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Tang, G.; Li, R.; Guo, H.; Weng, W.; Wang, D.; Ji, S. Development of a Comprehensive Quality Control Method for the Quantitative Analysis of Volatiles and Lignans in Magnolia biondii Pamp. by Near Infrared Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118080. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, X.; Wang, Y.; Zhou, P.; Zhang, X. Volatile Oil of Magnolia biondii Pamp. for Transnasal Administration: Its Preparation, Characterization, and Mechanism of Action in the Treatment of Allergic Rhinitis. Curr. Drug Deliv. 2024, 21, 1408–1421. [Google Scholar] [CrossRef]
- Wang, N.H.; Dai, M.Y.; Zheng, G.; Chang, P.J.; Xuan, L.J.; Liu, Z.G.; Wang, Y.L.; Cheng, S.Y.; Wang, Z.W.; Wang, H.L.; et al. Flavonoid Components and Gene Expression Analysis Reveal Flower Pigmentation Difference Between Magnolia biondii and Its Variety M. biondii var. purpurascens. Trees 2022, 36, 583–591. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, T.; Fan, G.; Chai, Y.; Wu, Y. Isolation and Purification of Lignans from Magnolia biondii Pamp by Isocratic Reversed-Phase Two-Dimensional Liquid Chromatography Following Microwave-Assisted Extraction. J. Sep. Sci. 2015, 30, 2370–2381. [Google Scholar] [CrossRef]
- Feng, W.S.; He, Y.H.; Zheng, X.K.; Wang, J.C.; Cao, Y.G.; Zhang, Y.L.; Song, K. Four New Monoterpenoid Glycosides from the Flower Buds of Magnolia biondii. Molecules 2016, 21, 728. [Google Scholar] [CrossRef] [PubMed]
- Schühly, W.; Skarbina, J.; Kunert, O.; Nandi, O.I.; Bauer, R. Chemical Characterization of Magnolia biondii (Flos Magnoliae, Xin Yi). Nat. Prod. Commun. 2009, 4, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.Y.; Li, R.; Jiang, Z.T.; Wang, Y.; Tan, J.; Tang, S.H.; Zhang, Y. Screening and Evaluation of Radical Scavenging Active Compounds in the Essential Oil from Magnolia biondii Pamp by Electronic Nose Coupled with Chemical Methodology. Ind. Crops Prod. 2020, 144, 112060. [Google Scholar] [CrossRef]
- Aldulaimi, O.; Li, W. Antibacterial Effects of the Essential Oil from Flower Buds of Magnolia biondii Pamp. Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Jia, Q.; Sun, W.; Fu, J.; Lv, Y.; Han, S. Cell Membrane Chromatography Coupled Online with LC-MS to Screen Anti-Anaphylactoid Components from Magnolia biondii Pamp. Targeting on Mas-Related G Protein-Coupled Receptor X2. J. Sep. Sci. 2020, 43, 2571–2578. [Google Scholar] [CrossRef]
- Cao, Y.G.; Li, H.W.; Cao, B.; Wang, J.C.; Zhang, Y.L.; Zhao, X.; Zheng, X.K.; Feng, W.S. Two New Phenylpropanoids and a New Dihydrostilbenoid from the Flower Buds of Magnolia. biondii pamp and their acetylcholinesterase inhibitory activities. Nat. Prod. Res. 2021, 35, 3233–3240. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.; Lee, H.; Lee, B.K.; Min, B.S.; Kim, J. Anti-Allergic and Cytotoxic Effects of Sesquiterpenoids and Phenylpropanoids Isolated from Magnolia biondii. Nat. Prod. Commun. 2017, 12, 1543–1545. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, D.R.; Choi, B.K.; Yang, S.H. Antiperiodontitis Effects of Magnolia biondii Extract on Ligature-Induced Periodontitis in Rats. Nutrients 2019, 11, 934. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic Applications of Compounds in the Magnolia Family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar] [CrossRef]
- Dias, L.S.; Pereira, I.P.; Dias, A.S. Allelopathy, Seed Germination, Weed Control and Bioassay Methods. Allelopath. J. 2016, 37, 31–40. [Google Scholar]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant Allelochemicals and Their Various Applications. In Co-Evolution of Secondary Metabolites; Merillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, M.; Xu, P. Allelochemicals: A Source for Developing Economically and Environmentally Friendly Plant Growth Regulators. Biochem. Biophys. Res. Commun. 2024, 690, 149248. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Xuan, T.D.; Khanh, T.D.; Tran, H.D.; Trung, N.T. Allelochemicals and Signaling Chemicals in Plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant Allelochemicals: Agronomic, Nutritional and Ecological Relevance in the Soil System. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The Ecological Importance of Allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Wan, H.H.; Liu, W.X.; Wan, F.H. Allelopathic Effect of Ageratina adenophora (Spreng.) Leaf Litter on Four Herbaceous Plants in Invaded Regions. Chin. J. Eco-Agric. 2011, 19, 130–134. [Google Scholar] [CrossRef]
- Xiao, Y.; Tan, J.; Yu, Y.; Dong, J.; Cao, L.; Yao, L.; Zhang, Y.; Yan, Z. Phytotoxic Effects and Potential Allelochemicals from aqueous extracts of Paulownia tomentosa Flower Litter. Agronomy 2024, 14, 367. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Hu, T.; ur Rehman, H.; Chen, H.; Li, Z.; Hu, H. Allelopathic Activity and Chemical Constituents of Walnut (Juglans regia) Leaf Litter in Walnut–Winter Vegetable Agroforestry System. Nat. Prod. Res. 2014, 28, 2017–2020. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, Q.; Huang, L.X.; Xin, P.Y.; Wang, Q. Effects of Leaf Litter Water Extract of Two Tree Species on the Growth and Root Exudates of Ryegrass. Pratacult. Sci. 2025, 42, 396–410. [Google Scholar] [CrossRef]
- Wu, H.B.; Liu, T.T.; Zhang, Z.X.; Wang, W.S.; Zhu, W.W.; Li, L.F.; Li, Y.R.; Chen, X. Leaves of Magnolia liliflora Desr. as a High-Potential By-Product: Lignans Composition, Antioxidant, Anti-Inflammatory, Anti-Phytopathogenic Fungal and Phytotoxic Activities. Ind. Crops Prod. 2018, 125, 416–424. [Google Scholar] [CrossRef]
- Jacyno, J.M.; Montemurro, N.; Bates, A.D.; Cutler, H.G. Phytotoxic and Antimicrobial Properties of Cyclocolorenone from Magnolia grandiflora L. J. Agric. Food Chem. 1991, 39, 1166–1168. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Hashinaga, F. Allelopathic Potential of Two Sesquiterpene Lactones from Magnolia grandiflora L. Biochem. Syst. Ecol. 2007, 35, 737–742. [Google Scholar] [CrossRef]
- Yahaya, A.A.H.; Salleh, W.M.N.H.W.; Ghani, N. Magnolia Genus-A Systematic Review on the Composition and Biological Properties of Its Essential Oils. Riv. Ital. Delle Sostanze Grasse 2022, 99, 249–261. [Google Scholar]
- Schandry, N.; Becker, C. Allelopathic Plants: Models for Studying Plant-Interkingdom Interactions. Trends Plant Sci. 2020, 25, 176–185. [Google Scholar] [CrossRef]
- Dechoum, M.S.; Zenni, R.D.; Castellani, T.T.; Zalba, S.M.; Rejmánek, M. Invasions Across Secondary Forest Successional Stages: Effects of Local Plant Community, Soil, Litter, and Herbivory on Hovenia dulcis Seed Germination and Seedling Establishment. Plant Ecol. 2015, 216, 823–833. [Google Scholar] [CrossRef]
- Kimura, F.; Sato, M.; Kato-Noguchi, H. Allelopathy of Pine Litter: Delivery of Allelopathic Substances into Forest Floor. J. Plant Biol. 2015, 58, 61–67. [Google Scholar] [CrossRef]
- Ahmed, R.; Hoque, A.T.M.R.; Hossain, M.K. Allelopathic Effects of Leucaena leucocephala Leaf Litter on Some Forest and Agricultural Crops Grown in Nursery. J. For. Res. 2008, 19, 298–302. [Google Scholar] [CrossRef]
- Huang, W.; Reddy, G.V.P.; Shi, P.; Huang, J.; Hu, H.; Hu, T. Allelopathic Effects of Cinnamomum septentrionale Leaf Litter on Eucalyptus grandis Saplings. Glob. Ecol. Conserv. 2020, 21, e00872. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Wang, D.; Liu, Y.; Song, H. Allelopathic Effects, Physiological Responses and Phenolic Compounds in Litter Extracts of Juniperus rigida Sieb. et Zucc. Chem. Biodivers. 2017, 14, e1700088. [Google Scholar] [CrossRef]
- Bengyella, L.; Russell, T.R.; Kaminski, J.E. Stimulatory and Inhibitory Role of Allelopathic Chlorogenic Acid in Seed Germination and Seedling Growth of Tall Fescue Grass (Festuca arundinaceae Schreb.) via pH Reprogramming. J. Plant Growth Regul. 2023, 42, 6969–6979. [Google Scholar] [CrossRef]
- Voll, E.; Voll, C.E.; Filho, R.V. Allelopathic Effects of Aconitic Acid on Wild Poinsettia (Euphorbia heterophylla) and Morningglory (Ipomoea grandifolia). J. Environ. Sci. Health Part B 2005, 40, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bortolo, T.D.S.C.; Marchiosi, R.; Viganó, J.; Siqueira-Soares, R.C.; Ferro, A.P.; Barreto, G.E.; Bido, G.S.; Abrahão, J.; Dos Santos, W.D.; Ferrarese-Filho, O. Trans-Aconitic Acid Inhibits the Growth and Photosynthesis of Glycine max. Plant Physiol. Biochem. 2018, 132, 490–496. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, S.; Araniti, F.; Teijeira, M.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Azelaic Acid Can Efficiently Compete for the Auxin Binding Site TIR1, Altering Auxin Polar Transport, Gravitropic Response, and Root Growth and Architecture in Arabidopsis thaliana Roots. Plant Physiol. Biochem. 2024, 210, 108592. [Google Scholar] [CrossRef]
- Inderjit; Streibig, J.C.; Olofsdotter, M. Joint Action of Phenolic Acid Mixtures and Its Significance in Allelopathy Research. Physiol. Plant. 2002, 114, 422–428. [Google Scholar] [CrossRef]
- Jia, C.; Kudsk, P.; Mathiassen, S.K. Joint Action of Benzoxazinone Derivatives and Phenolic Acids. J. Agric. Food Chem. 2006, 54, 1049–1057. [Google Scholar] [CrossRef]
- Zuo, S.; Zhou, S.; Ye, L.; Ma, S. Synergistic and Antagonistic Interactions Among Five Allelochemicals with Antialgal Effects on Bloom-Forming Microcystis aeruginosa. Ecol. Eng. 2016, 97, 486–492. [Google Scholar] [CrossRef]
- Syed, S.; Ahmed, Z.I.; Al-Haq, M.I.; Mohammad, A.; Fujii, Y. The Possible Role of Organic Acids as Allelochemicals in Tamarindus indica L. Leaves. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 511–517. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Sun, Z.H.; Chen, W.H.; Lin, W.X. Allelopathic Effects of Organic Acid Allelochemicals on Melon. Acta Ecol. Sin. 2013, 33, 4591–4598. [Google Scholar] [CrossRef]
- Xing, S.Z.; Wang, J.F.; Cai, D. Effects of Citrate and Malate on the Seed Germination and Membrane Permeability of Pakchoi. Chin. Agric. Sci. Bull. 2007, 23, 312–316. [Google Scholar]
- Summer, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
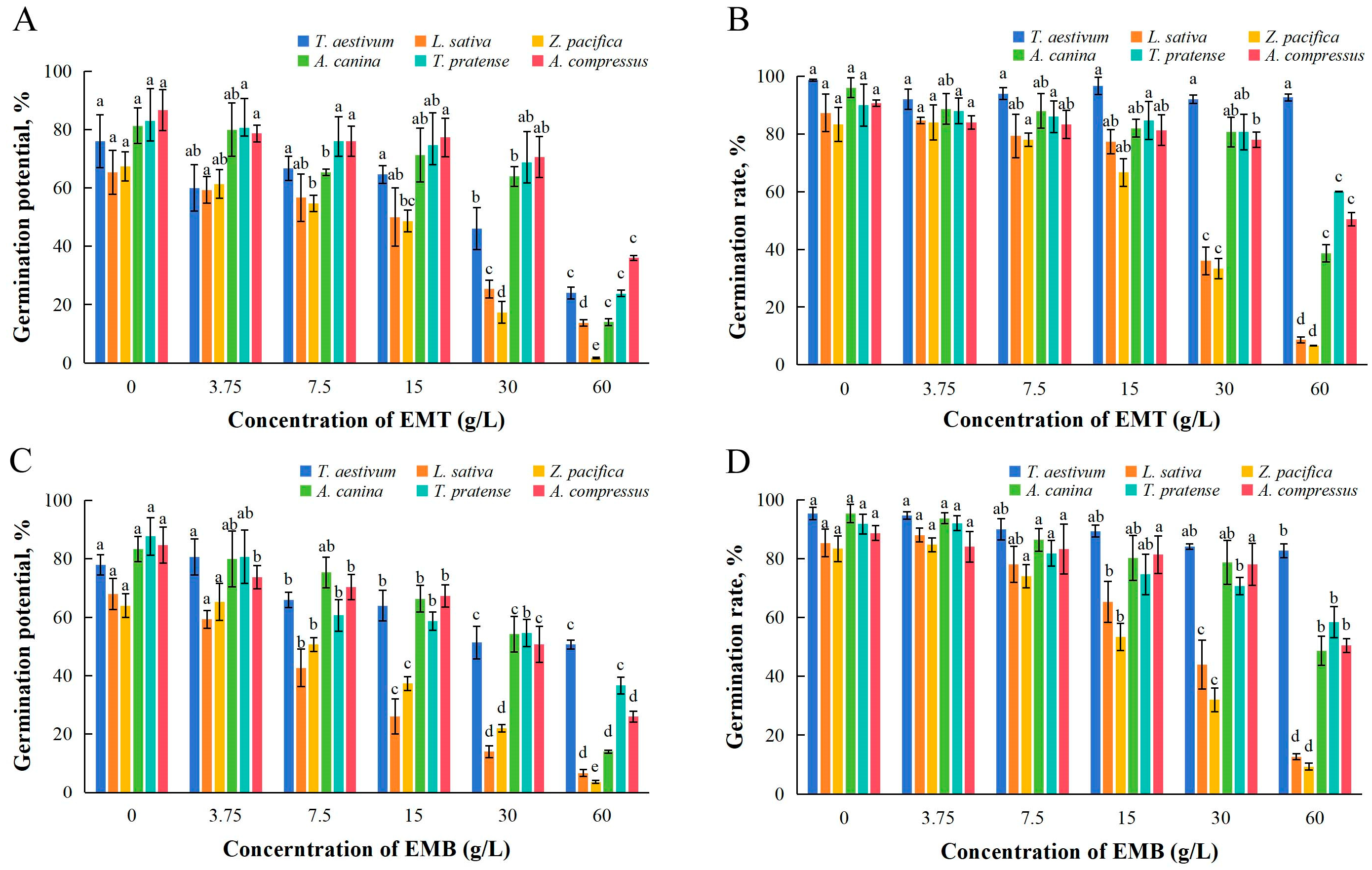
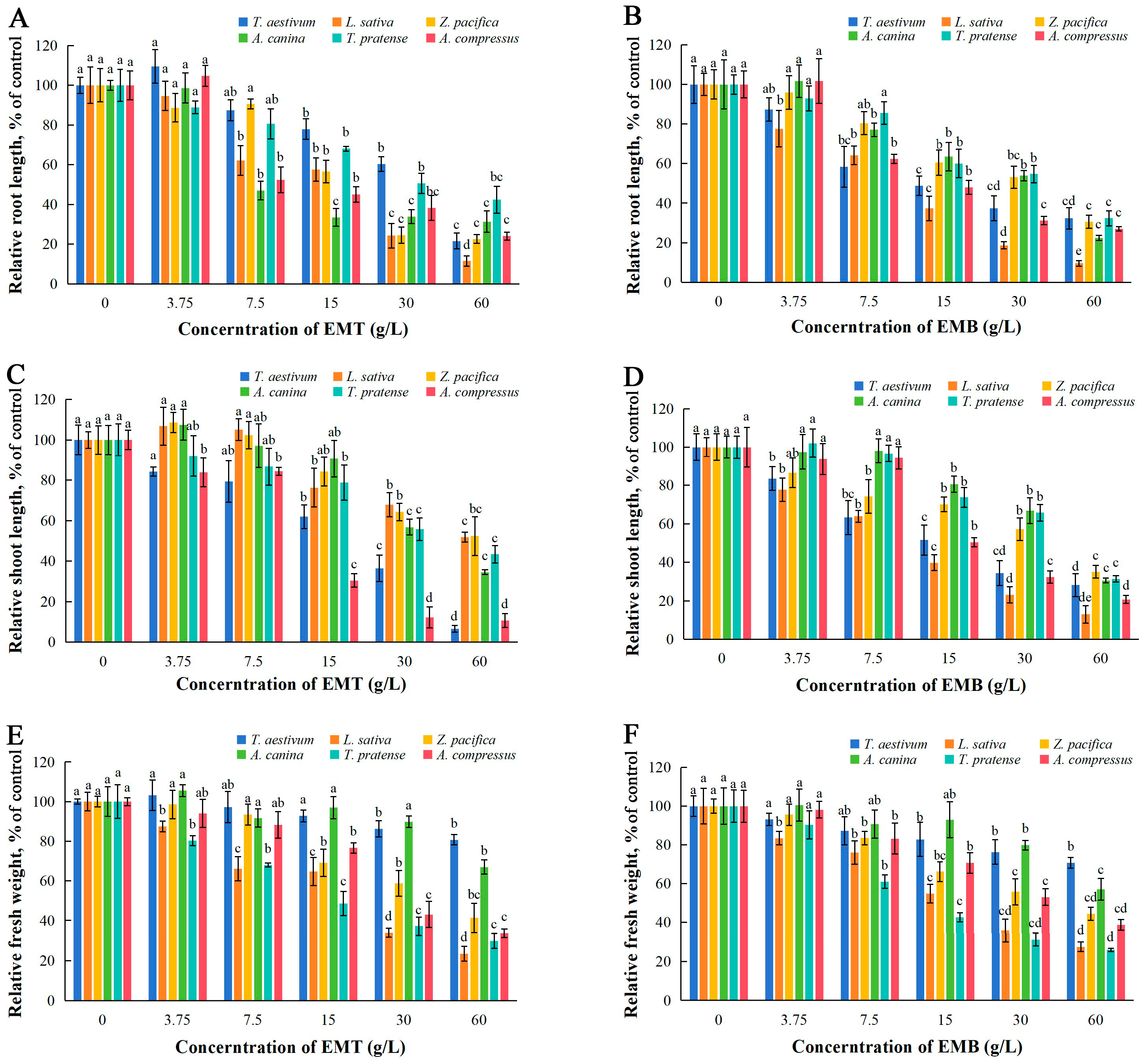
| Materials | Test Plants | Germination Potential | Germination Rate | Root Length | Stem Length | Fresh Weight |
|---|---|---|---|---|---|---|
| EMT | T. aestivum | 41.69 | – | 32.02 | 17.42 | – |
| L. sativa | 25.22 | 26.37 | 15.03 | 68.89 | 19.09 | |
| Z. pacifica | 17.75 | 24.92 | 19.57 | 60.71 | 39.94 | |
| A. canina | 37.62 | 66.33 | 14.19 | 39.99 | – | |
| T. pratense | 46.79 | – | 36.71 | 44.03 | 17.49 | |
| A. compressus | 67.85 | – | 9.96 | 12.08 | 31.31 | |
| EMB | T. aestivum | – | – | 17.61 | 16.85 | – |
| L. sativa | 11.64 | 27.82 | 10.44 | 11.21 | 19.67 | |
| Z. pacifica | 17.66 | 21.62 | 28.65 | 34.77 | 39.8 | |
| A. canina | 32.28 | 72.66 | 25.4 | 39.76 | – | |
| T. pratense | 42.01 | – | 30.46 | 38.3 | 15.11 | |
| A. compressus | 36.97 | – | 13.52 | 20.72 | 35.48 |
| No. | Proposed Compounds | Molecular Formula | MW | Mass Error (ppm) | Main Fragment MS2 | RT (min) | Peak Area (%) | |
|---|---|---|---|---|---|---|---|---|
| EMT | EMB | |||||||
| 1 | Asparagine | C4H8N2O3 | 132.0532 | −2 | 88.0403, 72.009 | 0.85 | 0.42 | 0.55 |
| 2 | Choline | C5H13NO | 103.0993 | −3.72 | 60.0809, 58.0650 | 0.861 | 3.16 | 0.14 |
| 3 | Mannitol | C6H14O6 | 182.0788 | −1.5 | 181.0722, 180.0597 | 0.868 | 0.29 | 0.77 |
| 4 | Raffinose | C18H32O16 | 504.1678 | −0.39 | 504.165, 503.1616, 543.1309 | 0.871 | 0.02 | 0.52 |
| 5 | Sucrose | C12H22O11 | 342.1158 | −1.23 | 179.056, 119.0348 | 0.878 | 10.32 | 16.03 |
| 6 | Adonitol | C5H12O5 | 152.0682 | −2.13 | 89.0243, 101.0243, 59.0138 | 0.895 | 0.48 | 0.92 |
| 7 | Trigonelline HCl | C7H7NO2 | 137.0473 | −3.06 | 137.0445, 136.0616 | 0.901 | 2.00 | 0.11 |
| 8 | 4-Guanidinobutyric acid | C5H11N3O2 | 145.0847 | −3.02 | 88.0759, 104.1834, 146.1761 | 0.924 | 0.59 | 0.01 |
| 9 | Quebrachitol | C7H14O6 | 194.0787 | −1.87 | 217.0799, 411.1486 | 0.94 | 0.41 | 1.19 |
| 10 | Shikimic acid | C7H10O5 | 174.0525 | −1.85 | 173.0049, 175.0611 | 0.946 | 0.57 | 2.64 |
| 11 | Malic acid | C4H6O5 | 134.0213 | −1.51 | 115.0036, 71.0137 | 0.96 | 10.65 | 24.13 |
| 12 | D-(+)-Mannose | C6H12O6 | 180.0632 | −1.14 | 127.0626 | 0.982 | 15.87 | 22.36 |
| 13 | Citric acid | C6H8O7 | 192.0268 | −0.86 | 111.0086, 87.00864 | 1.002 | 3.91 | 6.03 |
| 14 | Arabinofuranosyluracil | C9H12N2O6 | 244.0691 | −1.65 | 245.0431, 110.0246, 189.2093 | 1.002 | 0.47 | 0.60 |
| 15 | 4-Oxoproline | C5H7NO3 | 129.0425 | −1.12 | 55.0188, 82.0298, 99.9256 | 1.015 | 0.40 | 0.57 |
| 16 | Aconitic acid | C6H6O6 | 174.0162 | −1.35 | 129.0192, 111.0087, 85.0294 | 1.107 | 0.83 | 0.20 |
| 17 | L-Leucine | C6H13NO2 | 131.0942 | −2.96 | 132.1018, 86.0963, 69.0699, 70.0651 | 1.202 | 0.67 | 0.01 |
| 18 | D-(+)-Glucose | C6H12O6 | 180.0629 | −2.22 | 179.056, 101.0234, 71.0137, 161.0454, 143.0343 | 1.343 | 0.79 | 2.71 |
| 19 | Chlorogenic acid | C16H18O9 | 354.0949 | −0.61 | 193.0494, 164.0418, 107.049 | 3.087 | 6.13 | 1.77 |
| 20 | Scopolin | C16H18O9 | 354.0949 | −0.28 | 192.0595, 179.0347, 165.0511, 150.0345, 149.0583, 137.0573 | 3.179 | 0.21 | 0.79 |
| 21 | Caffeic acid | C9H8O4 | 180.0422 | −0.3 | 179.0349, 134.037, 135.0450, 92.9199 | 4.304 | 0.50 | 0.14 |
| 22 | Echinacoside | C35H46O20 | 786.2571 | −1.45 | 161.0243, 477.1613, 162.0277 | 4.571 | 0.18 | 0.80 |
| 23 | Magnoflorine | C20H23NO4 | 341.1617 | −3.03 | 297.113, 265.0851, | 4.778 | 1.71 | 1.06 |
| 24 | p-Hydroxybenzaldehyde | C7H6O2 | 122.0367 | −0.89 | 121.0294, 92.2206, | 4.857 | 0.73 | 1.10 |
| 25 | Purpureaside C | C35H46O20 | 786.2571 | −1.47 | 471.1538, 785,623 | 5.066 | 1.23 | 0.25 |
| 26 | 2-Hydroxycinnamic acid | C9H8O3 | 164.0472 | −0.7 | 116.9285, 120.0534, 121.0292 | 5.559 | 0.88 | 0.15 |
| 27 | Rutin | C27H30O16 | 610.1530 | −0.68 | 300.0274, 271.0249 | 5.588 | 2.08 | 0.59 |
| 28 | Lariciresinol 4-O-glucoside | C26H34O11 | 522.2106 | −1.03 | 329.1392, 359.1498 | 5.781 | 0.79 | 0.28 |
| 29 | Isoacteoside | C29H36O15 | 624.2048 | −1.02 | 623.1974, 461.1665 | 5.84 | 2.14 | 0.38 |
| 30 | Poliumoside | C35H46O19 | 770.2622 | −1.42 | 162.0277, 161.0234 | 5.911 | 3.49 | 0.86 |
| 31 | Pinoresinol 4-O-glucoside | C26H32O11 | 520.1941 | −0.71 | 357.1343,151.0399 | 6.183 | 0.70 | 0.35 |
| 32 | Azelaic acid | C9H16O4 | 188.1047 | −0.63 | 187.0974, 99.9256 | 6.74 | 2.69 | 0.71 |
| 33 | Tiliroside | C30H26O13 | 594.1370 | −0.6 | 284.0324, 255.0301, 227.0346, 145.0293 | 7.431 | 0.01 | 2.28 |
| 34 | Abscisic acid | C15H20O4 | 264.1361 | −0.1 | 219.1389, 204.1154 | 7.53 | 0.73 | 0.07 |
| 35 | (15Z)-9,12,13-Trihydroxy-15-octadecenoic acid | C18H34O5 | 330.2406 | −0.2 | 171.1026, 229.1442, 127.1127, 211.134 | 8.764 | 5.38 | 1.06 |
| 36 | (10E,12Z)-9-Hydroxyoctadeca-10,12-dienoic acid | C18H32O4 | 312.2300 | −0.33 | 310.1715, 293.2123, | 11.634 | 1.42 | 0.16 |
| 37 | Veraguensin | C22H28O5 | 372.1915 | −5.91 | 151.0750, 179.1062, 217.1217 | 12.053 | 1.07 | 0.01 |
| 38 | 9-Hydroxy-10,12-octadecadienoic acid | C18H32O3 | 296.2348 | −1.16 | 295.2275, 277.2171, 195.1387 | 12.895 | 7.03 | 2.65 |
| 39 | 13S-Hydroxy-6Z,9Z,11E-octadecatrienoic acid | C18H30O3 | 294.2194 | −0.27 | 147.0972, 125.097 | 13.547 | 0.27 | 0.59 |
| Test Plants | Growth Indicators | IC50 Values of MA | IC50 Values of CA | IC50 Values of Mixture | Joint Action of MA and CA |
|---|---|---|---|---|---|
| L. sativa | Germination potential | 11.81 | 29.21 | 14.15 | synergism |
| Germination rate | 11.54 | 33.56 | 13.54 | synergism | |
| Root length | 8.06 | 12.43 | 6.23 | synergism | |
| Stem length | 14.34 | 24.59 | 18.78 | synergism | |
| Fresh weight | 9.35 | 17.01 | 8.8 | synergism | |
| Z. pacifica | Germination potential | 13.86 | 34.32 | 30.69 | addition |
| Germination rate | 16.6 | 32.57 | 20.04 | synergism | |
| Root length | 10.76 | 22.54 | 9.03 | synergism | |
| Stem length | 20.05 | 27.19 | 18.41 | synergism | |
| Fresh weight | 16.78 | 22.32 | 11.86 | synergism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Du, Y.; Dong, J.; Yin, Z.; Chen, P.; Cao, L.; Yan, Z. Phytotoxic Effects of the Aqueous Extracts of Magnolia biondii Pamp. Flower Litter and the Joint Action of Allelochemicals. Plants 2025, 14, 1577. https://doi.org/10.3390/plants14111577
Yu Y, Du Y, Dong J, Yin Z, Chen P, Cao L, Yan Z. Phytotoxic Effects of the Aqueous Extracts of Magnolia biondii Pamp. Flower Litter and the Joint Action of Allelochemicals. Plants. 2025; 14(11):1577. https://doi.org/10.3390/plants14111577
Chicago/Turabian StyleYu, Yi, Yalei Du, Jiajia Dong, Zhigang Yin, Peiyu Chen, Lingling Cao, and Zhiqiang Yan. 2025. "Phytotoxic Effects of the Aqueous Extracts of Magnolia biondii Pamp. Flower Litter and the Joint Action of Allelochemicals" Plants 14, no. 11: 1577. https://doi.org/10.3390/plants14111577
APA StyleYu, Y., Du, Y., Dong, J., Yin, Z., Chen, P., Cao, L., & Yan, Z. (2025). Phytotoxic Effects of the Aqueous Extracts of Magnolia biondii Pamp. Flower Litter and the Joint Action of Allelochemicals. Plants, 14(11), 1577. https://doi.org/10.3390/plants14111577








