Drought-Induced Zinc Finger Transcription Factor OsDi19-3 Positively Regulates Drought Stress Acclimatization in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Results
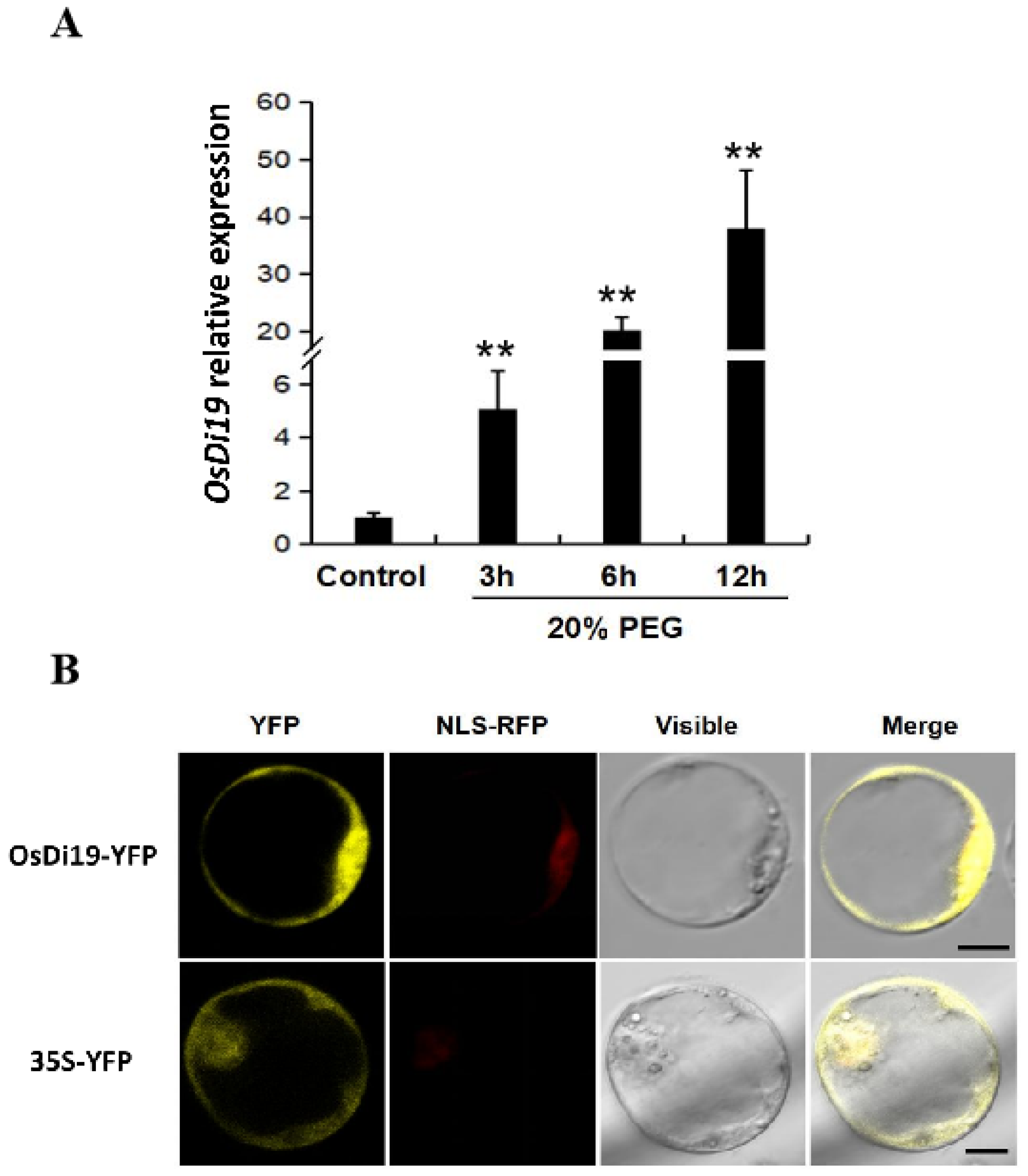
2.1. Characterization of OsDi19-3
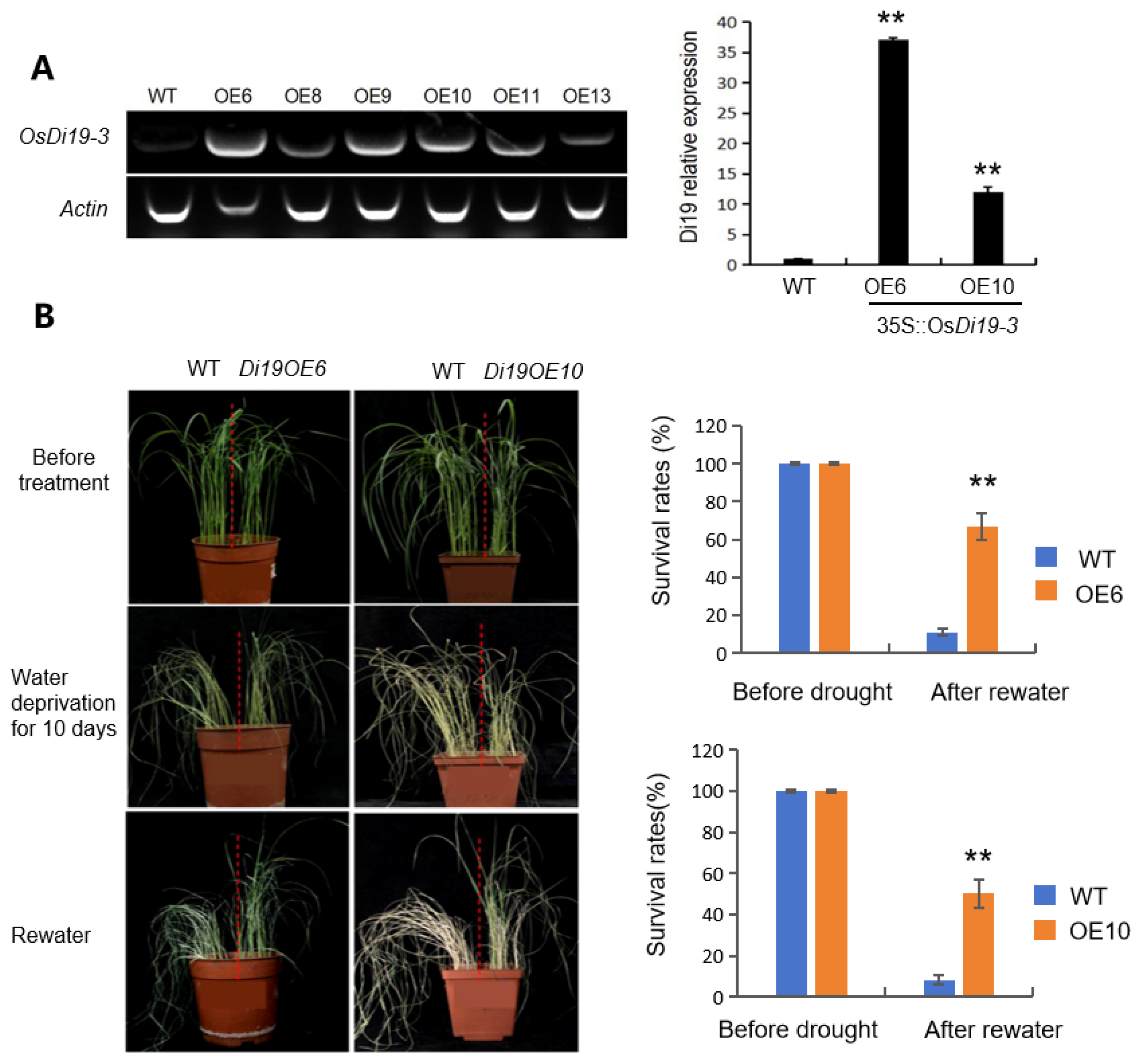
2.2. OsDi19-3 Functions as a Positive Regulator of Drought Stress Acclimatization
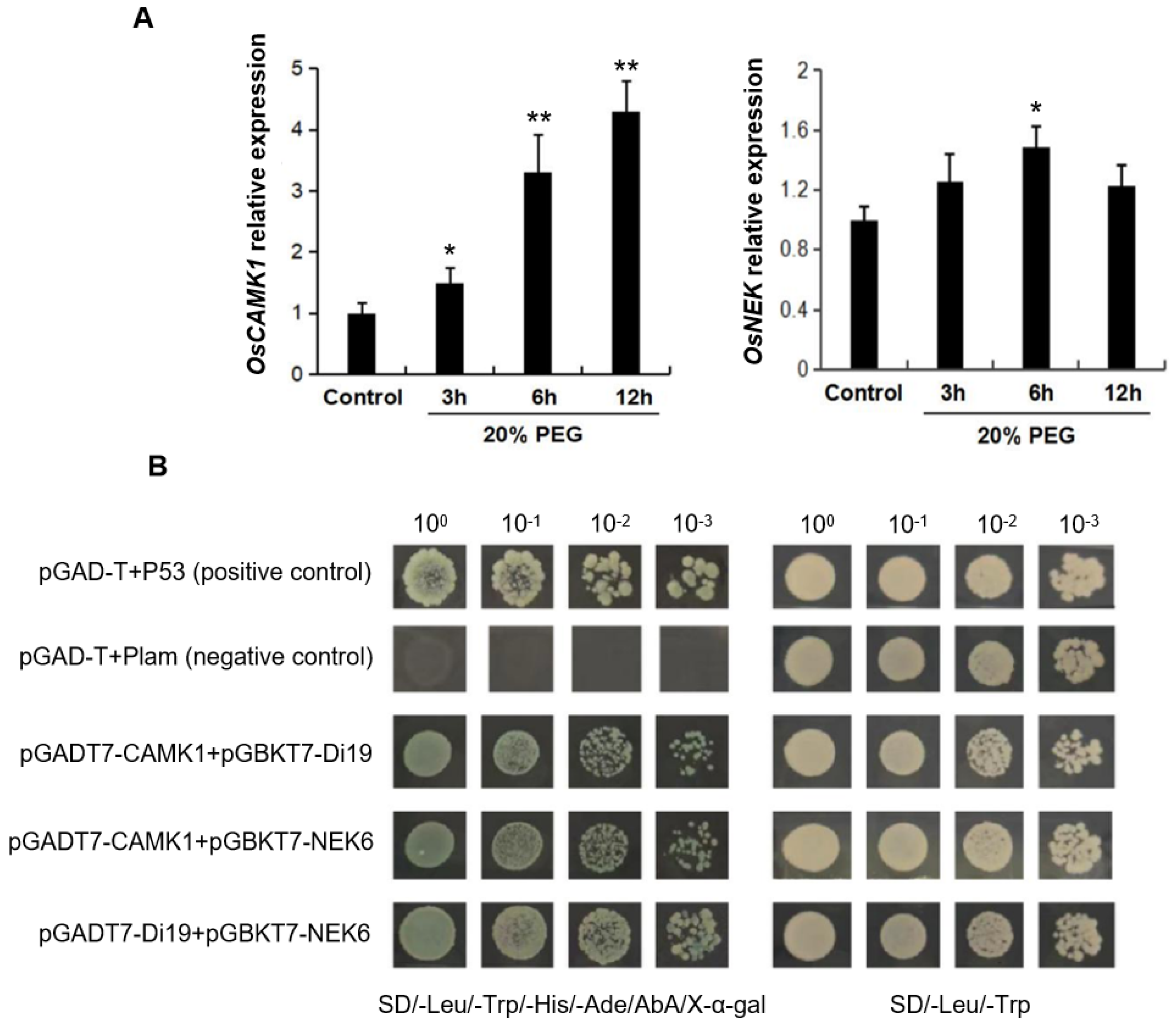
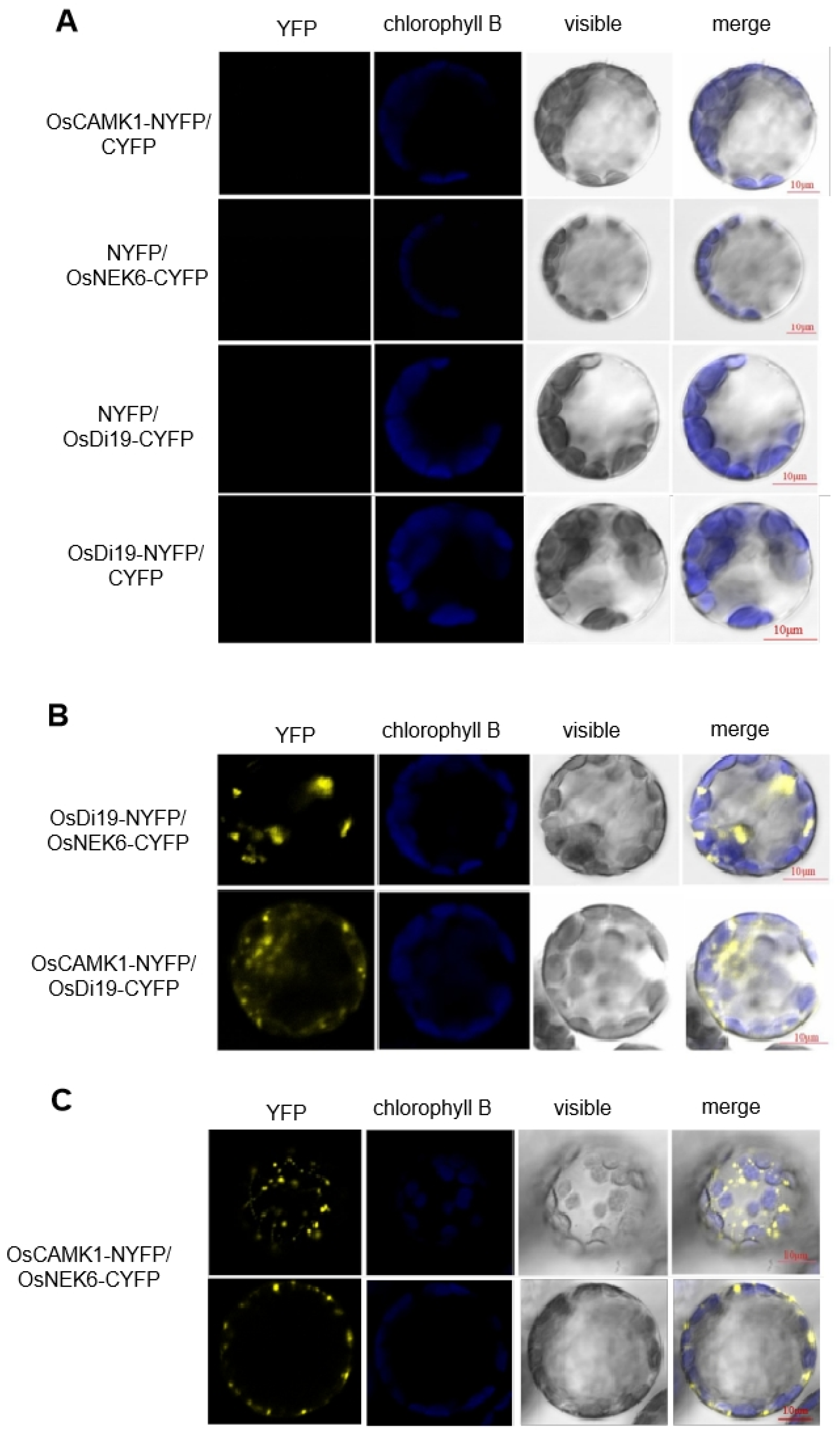
2.3. OsDi19-3 Interacts with OsCAMK1 and OsNEK6 Proteins
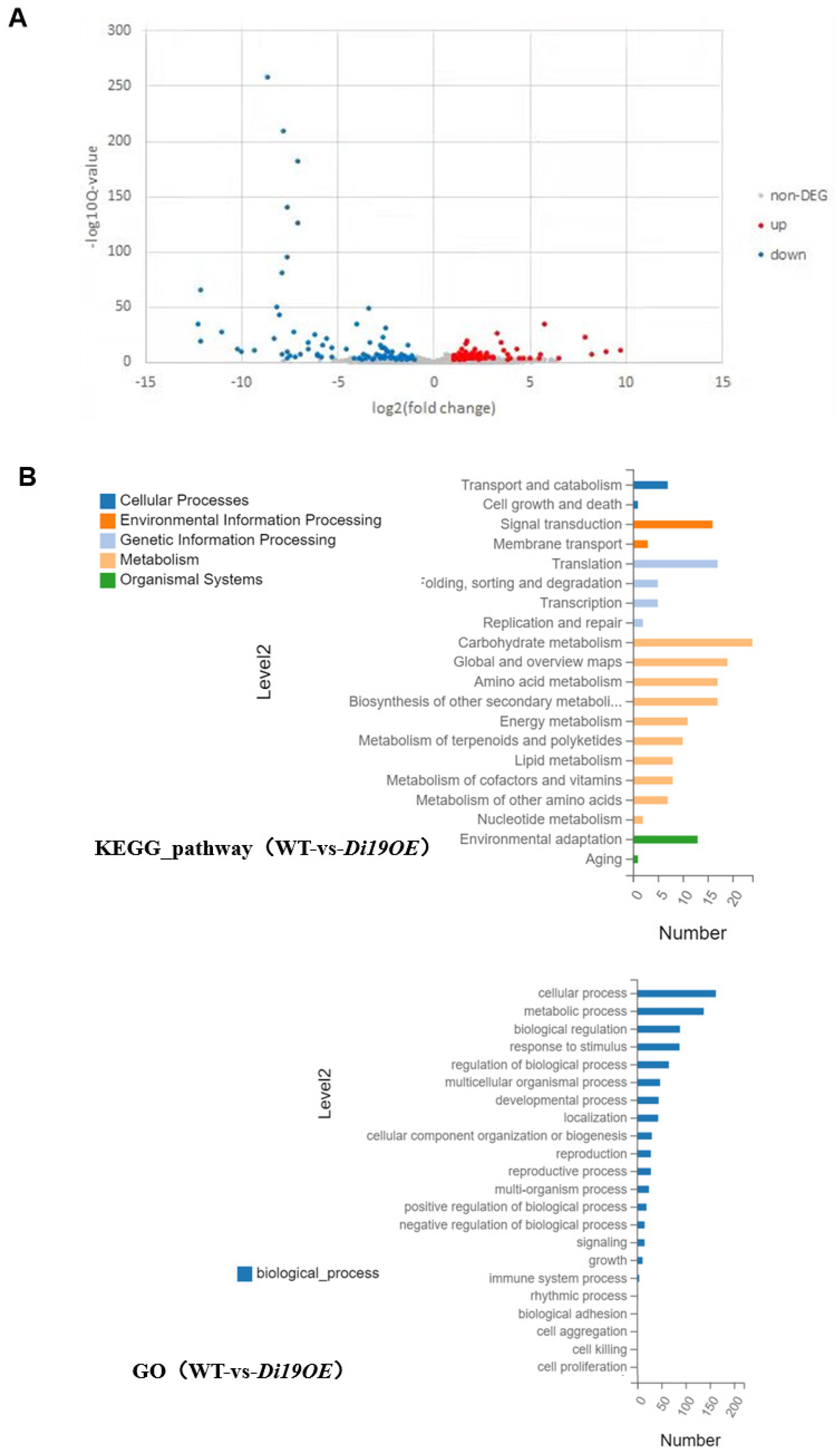
2.4. Transcriptomic Profiling of OsDi19-3 Overexpression Lines Reveals Stress-Responsive Regulatory Networks
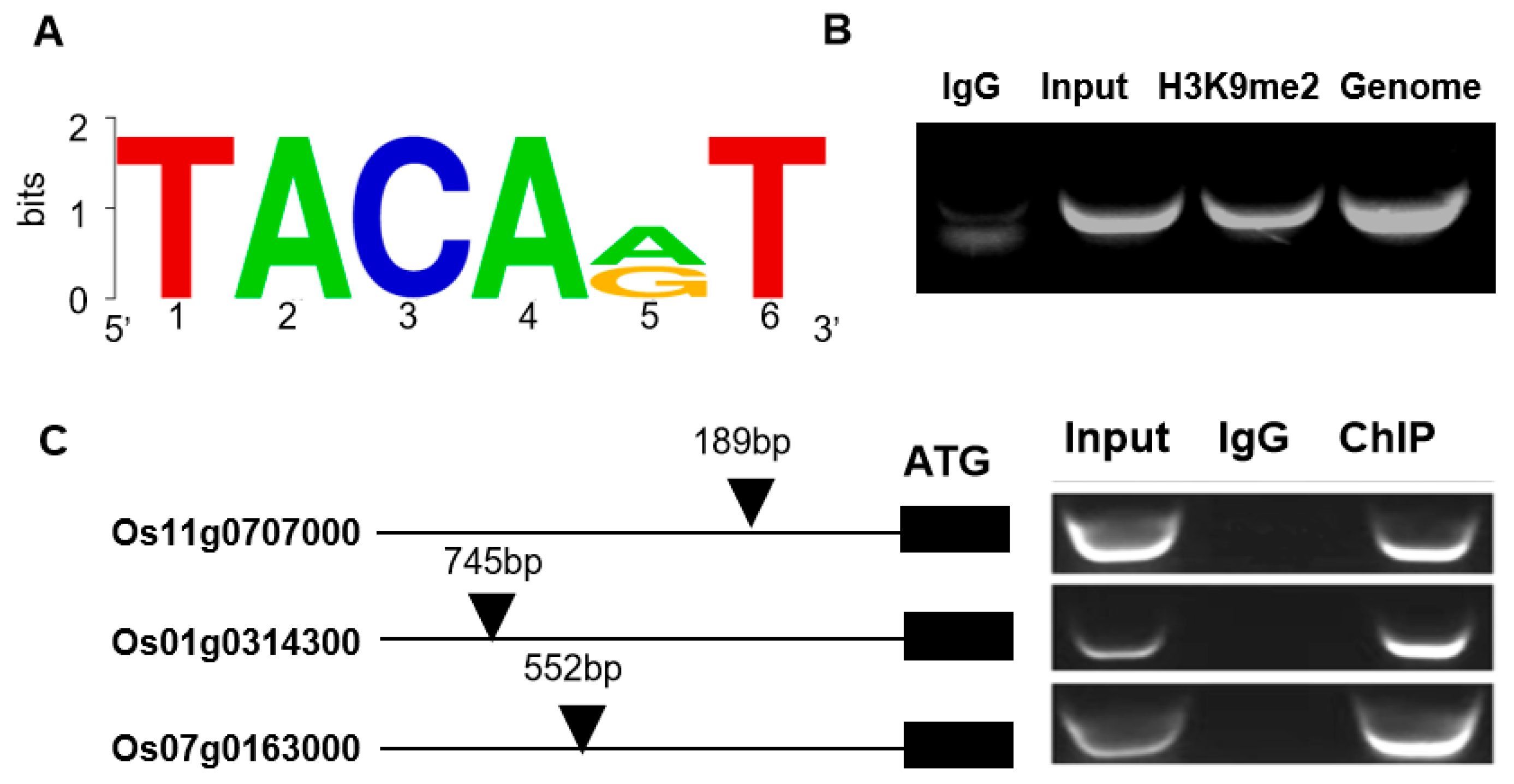
2.5. Identification of OsDi19-3 Downstream Target Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials and Growth Conditions
5.2. Determination of the Subcellular Localization
5.3. Gene Cloning, Vector Construction, and Plant Transformation
5.4. Electron Microscopy Scanning for Stomatal Aperture
5.5. Yeast Two-Hybrid and BiFC Experiment
5.6. Transcriptome Profiling
5.7. Quantitative Real-Time PCR
5.8. ChIP-PCR Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodriguez Milla, M.A.; Uno, Y.; Chang, I.F.; Townsend, J.; Maher, E.A.; Quilici, D.; Cushman, J.C. A novel yeast two-hybrid approach to identify CDPK substrates: Characterization of the interaction between AtCPK11 and AtDi19, a nuclear zinc finger protein. FEBS Lett. 2006, 580, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.X.; Li, Y.; Li, D.D.; Xu, W.L.; Zheng, Y.; Li, X.B. Arabidopsis drought-induced protein Di19-3 participates in plant response to drought and high salinity stresses. Plant Mol. Biol. 2014, 86, 609–625. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.; Chen, C.; He, C.; Zhu, Y.; Huang, W. Identification of rice Di19 family reveals OsDi19-4 involved in drought resistance. Plant Cell Rep. 2014, 33, 2047–2062. [Google Scholar] [CrossRef]
- Li, G.; Tai, F.J.; Zheng, Y.; Luo, J.; Gong, S.Y.; Zhang, Z.T.; Li, X.B. Two cotton Cys2/His2-type zinc-finger proteins, GhDi19-1 and GhDi19-2, are involved in plant response to salt/drought stress and abscisic acid signaling. Plant Mol. Biol. 2010, 74, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Li, Y.; Chen, W.; Yao, J.; Fang, S.; Lv, Y.; Zhang, Y.; Zhu, S. Systematical Characterization of the Cotton Di19 Gene Family and the Role of GhDi19-3 and GhDi19-4 as Two Negative Regulators in Response to Salt Stress. Antioxidants 2022, 11, 2225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, H.; Lu, M.; Wei, Q.; Xu, L.; Bo, C.; Ma, Q.; Zhao, Y.; Cheng, B. A Maize Stress-Responsive Di19 Transcription Factor, ZmDi19-1, Confers Enhanced Tolerance to Salt in Transgenic Arabidopsis. Plant Cell Rep. 2019, 38, 1563–1578. [Google Scholar] [CrossRef]
- Li, S.; Xu, C.; Yang, Y.; Xia, G. Functional analysis of TaDi19A, a salt-responsive gene in wheat. Plant Cell Environ. 2010, 33, 117–129. [Google Scholar]
- Wu, C.; Lin, M.; Chen, F.; Chen, J.; Liu, S.; Yan, H.; Xiang, Y. Correction: Wu et al. Homologous Drought-Induced 19 Proteins, PtDi19-2 and PtDi19-7, Enhance Drought Tolerance in Transgenic Plants. Int. J. Mol. Sci. 2022, 23, 16023. [Google Scholar] [CrossRef]
- Guo, W.; Li, X.; Yang, T.; Huang, C.; Zhao, B.; Wang, P. Identification and expression of the Di19 gene family in response to abiotic stress in common bean (Phaseolus vulgaris L.). Front. Genet. 2024, 15, 1401011. [Google Scholar] [CrossRef]
- Xiao, S.; Wan, Y.; Guo, S.; Fan, J.; Lin, Q.; Zheng, C.; Wu, C. Transcription Factor SiDi19-3 Enhances Salt Tolerance of Foxtail Millet and Arabidopsis. Int. J. Mol. Sci. 2023, 24, 2592. [Google Scholar] [CrossRef]
- Milla, M.A.; Townsend, J.; Chang, I.F.; Cushman, J.C. The Arabidopsis AtDi19 gene family encodes a novel type of Cys2/His2 zinc-finger protein implicated in ABA-independent dehydration, high-salinity stress and light signaling pathways. Plant Mol. Biol. 2006, 61, 13–30. [Google Scholar] [CrossRef]
- Kang, X.; Chong, J.; Ni, M. HYPERSENSITIVE TO RED AND BLUE 1, a ZZ-type zinc finger protein, regulates phytochrome B-mediated red and cryptochrome-mediated blue light responses. Plant Cell 2005, 17, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Maitra Majee, S.; Sharma, E.; Singh, B.; Khurana, J.P. Drought-induced protein (Di19-3) plays a role in auxin signaling by interacting with IAA14 in Arabidopsis. Plant Direct 2020, 4, e00234. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Kong, D.; Ji, L.; Kong, L.; Wang, Y.; Peng, L.; Xie, G. OsClo5 functions as a transcriptional co-repressor by interacting with OsDi19-5 to negatively affect salt stress tolerance in rice seedlings. Plant J. 2021, 105, 800–815. [Google Scholar] [CrossRef]
- Feng, Z.J.; Cui, X.Y.; Cui, X.Y.; Chen, M.; Yang, G.X.; Ma, Y.Z.; He, G.Y.; Xu, Z.S. The soybean GmDi19-5 interacts with GmLEA3.1 and increases sensitivity of transgenic plants to abiotic stresses. Front. Plant Sci. 2015, 6, 179. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, M.; Li, Z.; Jiang, S.; Xu, S.; Wang, M.; Chu, Z.; Zhu, M.; Zhang, Z.; Huang, W. OsCactin positively regulates the drought stress response in rice. Plant Cell Rep. 2024, 43, 281. [Google Scholar] [CrossRef]
- Curran, A.; Chang, I.F.; Chang, C.L.; Garg, S.; Miguel, R.M.; Barron, Y.D.; Li, Y.; Romanowsky, S.; Cushman, J.C.; Gribskov, M.; et al. Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates. Front. Plant Sci. 2011, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cao, J.; Chen, Q.; He, J.; Liu, Z.; Wang, J.; Li, X.; Yang, Y. The Kinase CIPK11 Functions as a Negative Regulator in Drought Stress Response in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 2422. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.; Xu, S.; Zhu, Y.; Huang, W. OsDi19-4 acts downstream of OsCDPK14 to positively regulate ABA response in rice. Plant Cell Environ. 2016, 39, 2740–2753. [Google Scholar] [CrossRef]
- Qin, L.X.; Nie, X.Y.; Hu, R.; Li, G.; Xu, W.L.; Li, X.B. Phosphorylation of serine residue modulates cotton Di19-1 and Di19-2 activities for responding to high salinity stress and abscisic acid signaling. Sci. Rep. 2016, 6, 20371. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, H.W.; Mu, R.L.; Zhang, W.K.; Zhao, M.Y.; Wei, W.; Wang, F.; Yu, H.; Lei, G.; Zou, H.F.; et al. NIMA-related kinase NEK6 affects plant growth and stress response in Arabidopsis. Plant J. 2011, 68, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Jantasuriyarat, C.; Zhao, Q.; Zhang, H.; Chen, S.; Liu, J.; Liu, L.; Tang, S.; Park, C.H.; Wang, X.; et al. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 2011, 157, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Jeong, H.I.; Bang, S.W.; Jung, S.E.; Kim, G.; Kim, Y.S.; Redillas, M.C.F.R.; Oh, S.J.; Seo, J.S.; Kim, J.K. DROUGHT-INDUCED BRANCHED-CHAIN AMINO ACID AMINOTRANSFERASE enhances drought tolerance in rice. Plant Physiol. 2023, 191, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Park, J.R.; Jang, Y.H.; Kim, E.G.; Kim, K.M. Rice Cultivars Under Salt Stress Show Differential Expression of Genes Related to the Regulation of Na+/K+ Balance. Front. Plant Sci. 2021, 12, 680131. [Google Scholar] [CrossRef]
- Wang, T.; Ma, Y.Q.; Huang, X.X.; Mu, T.J.; Li, Y.J.; Li, X.K.; Liu, X.; Hou, B.K. Overexpression of OsUGT3 enhances drought and salt tolerance through modulating ABA synthesis and scavenging ROS in rice. Environ. Exp. Bot. 2021, 192, 104653. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhang, F.C.; Zhang, W.Z.; Song, L.F.; Wu, W.H.; Chen, Y.F. Arabidopsis Di19 functions as a transcription factor and modulates PR1, PR2, and PR5 expression in response to drought stress. Mol. Plant 2013, 6, 1487–1502. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.M.; Zhou, L.; He, Y.; Wang, D.; Qi, Y.H.; Jiang, D.A. Rubisco Activase Is Also a Multiple Responder to Abiotic Stresses in Rice. PLoS ONE 2015, 10, e0140934. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Fan, B.; Liao, K.; Wang, L.N.; Shi, L.L.; Zhang, Y.; Xu, L.J.; Zhou, Y.; Li, J.F.; Chen, Y.Q.; Chen, Q.F.; et al. Calcium-dependent activation of CPK12 facilitates its cytoplasm-to-nucleus translocation to potentiate plant hypoxia sensing by phosphorylating ERF-VII transcription factors. Mol. Plant 2023, 16, 979–998. [Google Scholar] [CrossRef]
- Oakley, B.R.; Morris, N.R. A mutation in Aspergillus nidulans that blocks the transition from interphase to prophase. J. Cell Biol. 1983, 96, 1155–1158. [Google Scholar] [CrossRef]
- Osmani, S.A.; May, G.S.; Morris, N.R. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J. Cell Biol. 1987, 104, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Vigneault, F.; Lachance, D.; Cloutier, M.; Pelletier, G.; Levasseur, C.; Séguin, A. Members of the plant NIMA-related kinases are involved in organ development and vascularization in poplar, Arabidopsis and rice. Plant J. 2007, 51, 575–588. [Google Scholar] [CrossRef]
- Pan, W.J.; Tao, J.J.; Cheng, T.; Shen, M.; Ma, J.B.; Zhang, W.K.; Lin, Q.; Ma, B.; Chen, S.Y.; Zhang, J.S. Soybean NIMA-Related Kinase1 Promotes Plant Growth and Improves Salt and Cold Tolerance. Plant Cell Physiol. 2017, 58, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and Metabolic Maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Sparrow-Muñoz, I.; Chen, T.C.; Burgess, S.J. Recent developments in the engineering of Rubisco activase for enhanced crop yield. Biochem. Soc. Trans. 2023, 51, 627–637. [Google Scholar] [CrossRef]
- Ogbaga, C.C.; Stepien, P.; Athar, H.U.; Ashraf, M. Engineering Rubisco activase from thermophilic cyanobacteria into high-temperature sensitive plants. Crit. Rev. Biotechnol. 2018, 38, 559–572. [Google Scholar] [CrossRef]

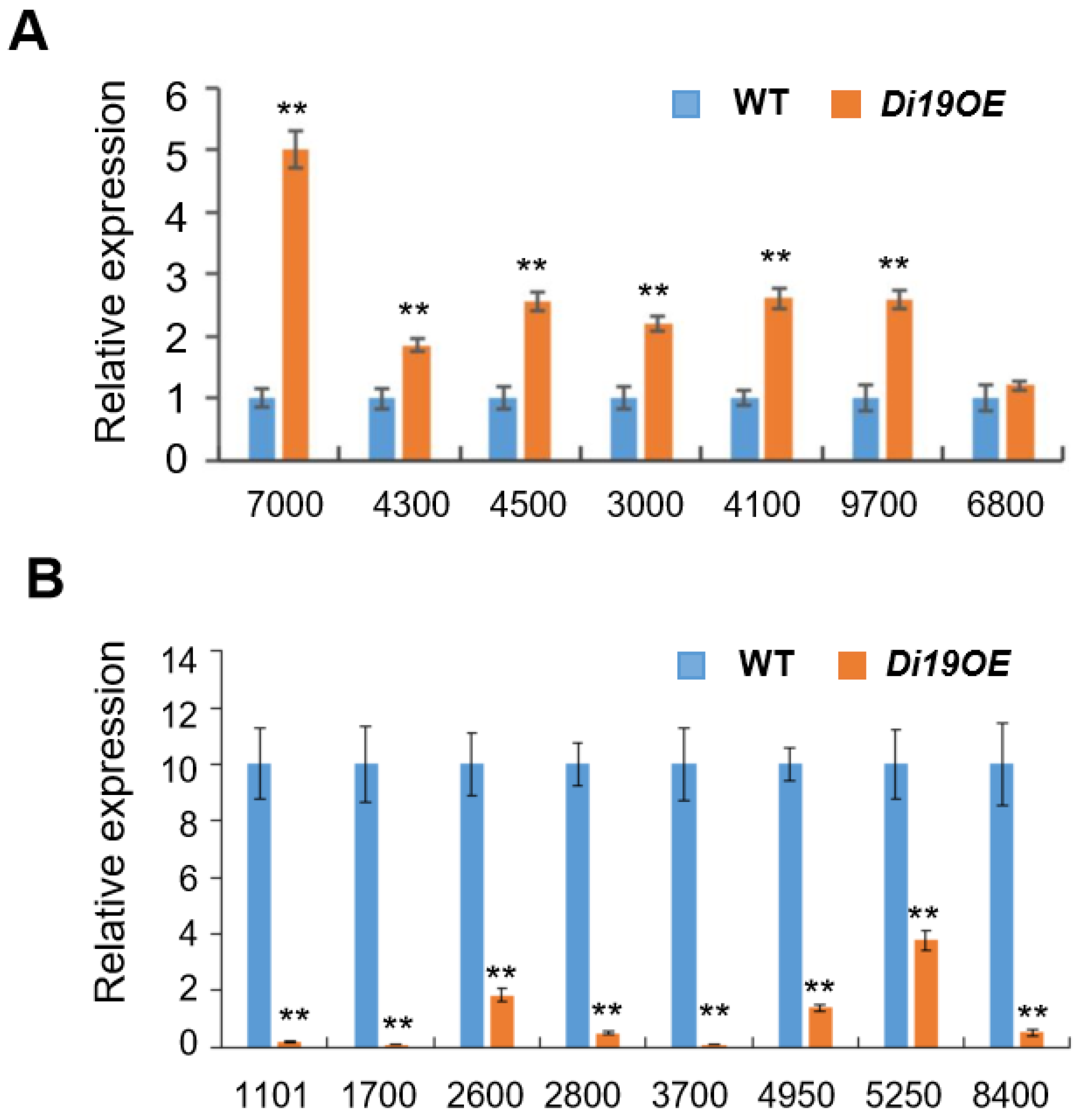
| Gene ID | Log2 (Di19OE/WT) | Gene Name/Functional Description | Stress Regulation a |
|---|---|---|---|
| Os11g0707000 | 27.93 | Rubisco activase | Heat, salt, drought |
| Os01g0314300 | 21.18 | Translation machinery-associated protein | Not available |
| Os01g0800300 | 20.68 | Unknown | Not available |
| Os06g0116800 | 8.41 | OsDjA9 | Biotic stress |
| Os05g0244700 | 7.97 | Aminotransferase, OsDIAT | Drought |
| Os07g0163000 | 7.64 | D-lactate dehydrogenase | Not available |
| Os07g0633100 | 7.55 | X8 domain containing protein | Drought |
| Os01g0672400 | 6.81 | OsDi19-3 | Drought, salt |
| Os01g0692400 | 4.96 | Unknown | Drought |
| Os05g0317900 | 3.81 | OsCrRLK1L2 | Abiotic and biotic stress |
| Os08g0496700 | 3.64 | Myb/SANT-like domain containing protein | Not available |
| Os11g0694100 | 3.39 | OsWAK123 | Biotic and abiotic stress |
| Os09g0396900 | 2.87 | Vacuolar Iron Transporter 2 | Drought |
| Os04g0677300 | 2.86 | Harpin-induced protein 1 domain-containing protein | Drought |
| Os04g0584500 | 2.66 | OsPUB77, OsEnS-73 | Drought |
| Os01g0638000 | 2.51 | Anthocyanin 3-O-beta-glucosyltransferase | Drought |
| Os01g0635200 | 2.40 | MYB family transcription factor | Abiotic stress |
| Os01g0227800 | 2.39 | cytochrome P450 | Drought |
| Os01g0227100 | 2.22 | NON-YELLOW COLORING 1 | Drought |
| Os03g0218400 | 2.21 | MONOSACCHARIDE TRANSPORTER 4 | Drought |
| Os04g0447700 | 2.07 | Oxidoreductase, aldo/keto reductase family protein | Abiotic stress |
| Os02g0755900 | 1.97 | Cytokinin-O-glucosyltransferase, OsUGT3 | Drought |
| Os01g0200700 | 1.92 | Metallothionein-like protein | Tolerant to salinity and heavy metal stress |
| Os06g0701600 | 1.90 | Na+ and K+ transport, OsHKT9 | Salt tolerance |
| Os01g0822800 | 1.84 | RING-H2 finger protein | Drought |
| Os11g0106700 | 1.80 | Ferritin 1 | Drought |
| Os03g0268600 | 1.75 | Protein phosphatase 48 | Drought |
| Os05g0373900 | 1.71 | Unknown | Drought |
| Os01g0160800 | 1.63 | RIBOSOME-INACTIVATING PROTEIN 1.1 | Drought |
| Os12g0106000 | 1.60 | FERRITIN 2 | Drought |
| Os04g0632100 | 1.59 | S-Domain kinase-7 | Drought |
| Os04g0684800 | 1.52 | OsUEV1D | Abiotic stress |
| Os03g0729000 | 1.50 | Peptidase | Drought |
| Gene ID | Log2 (Di19OE/WT) | Gene Name/Functional Description | Locus of TACAA(G)T from the ATG (bp) |
|---|---|---|---|
| Os11g0707000 | 27.93 | Rubisco activase | −189, −318, −755 |
| Os01g0314300 | 21.28 | Uncharacterized | −745 |
| Os01g0606400 | 9.70 | Uncharacterized | −219, −339, −430 |
| Os07g0163000 | 7.64 | D-lactate dehydrogenase | −552 |
| Os09g0560700 | 7.37 | MOSC domain-containing protein | −253, −540 |
| Os03g0218400 | 2.21 | MONOSACCHARIDE TRANSPORTER 4 | −521, −584 |
| Os06g0701600 | 1.90 | OsHKT9 | −166, −536, −592 |
| Os10g0502400 | 1.88 | Glutamyl-tRNA reductase 2 | −615 |
| Os03g0268600 | 1.75 | Protein phosphatase | −229 |
| Os04g0632100 | 1.60 | S-Domain kinase-7 | −248 |
| Os10g0209700 | 1.54 | Heavy-metal-associated isoprenylated plant protein 28 | −317 |
| Os04g0684800 | 1.52 | OsUEV1D | −290, −304, −612 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Mu, T.; Ren, T.; Li, P. Drought-Induced Zinc Finger Transcription Factor OsDi19-3 Positively Regulates Drought Stress Acclimatization in Rice (Oryza sativa L.). Plants 2025, 14, 1560. https://doi.org/10.3390/plants14101560
Li Y, Mu T, Ren T, Li P. Drought-Induced Zinc Finger Transcription Factor OsDi19-3 Positively Regulates Drought Stress Acclimatization in Rice (Oryza sativa L.). Plants. 2025; 14(10):1560. https://doi.org/10.3390/plants14101560
Chicago/Turabian StyleLi, Yanjie, Tianjiao Mu, Tianying Ren, and Pan Li. 2025. "Drought-Induced Zinc Finger Transcription Factor OsDi19-3 Positively Regulates Drought Stress Acclimatization in Rice (Oryza sativa L.)" Plants 14, no. 10: 1560. https://doi.org/10.3390/plants14101560
APA StyleLi, Y., Mu, T., Ren, T., & Li, P. (2025). Drought-Induced Zinc Finger Transcription Factor OsDi19-3 Positively Regulates Drought Stress Acclimatization in Rice (Oryza sativa L.). Plants, 14(10), 1560. https://doi.org/10.3390/plants14101560






