Upregulation of an IAA-Glucosyltransferase OsIAGLU in Rice (Oryza sativa L.) Impairs Root Gravitropism by Disrupting Starch Granule Homeostasis
Abstract
1. Introduction
2. Results
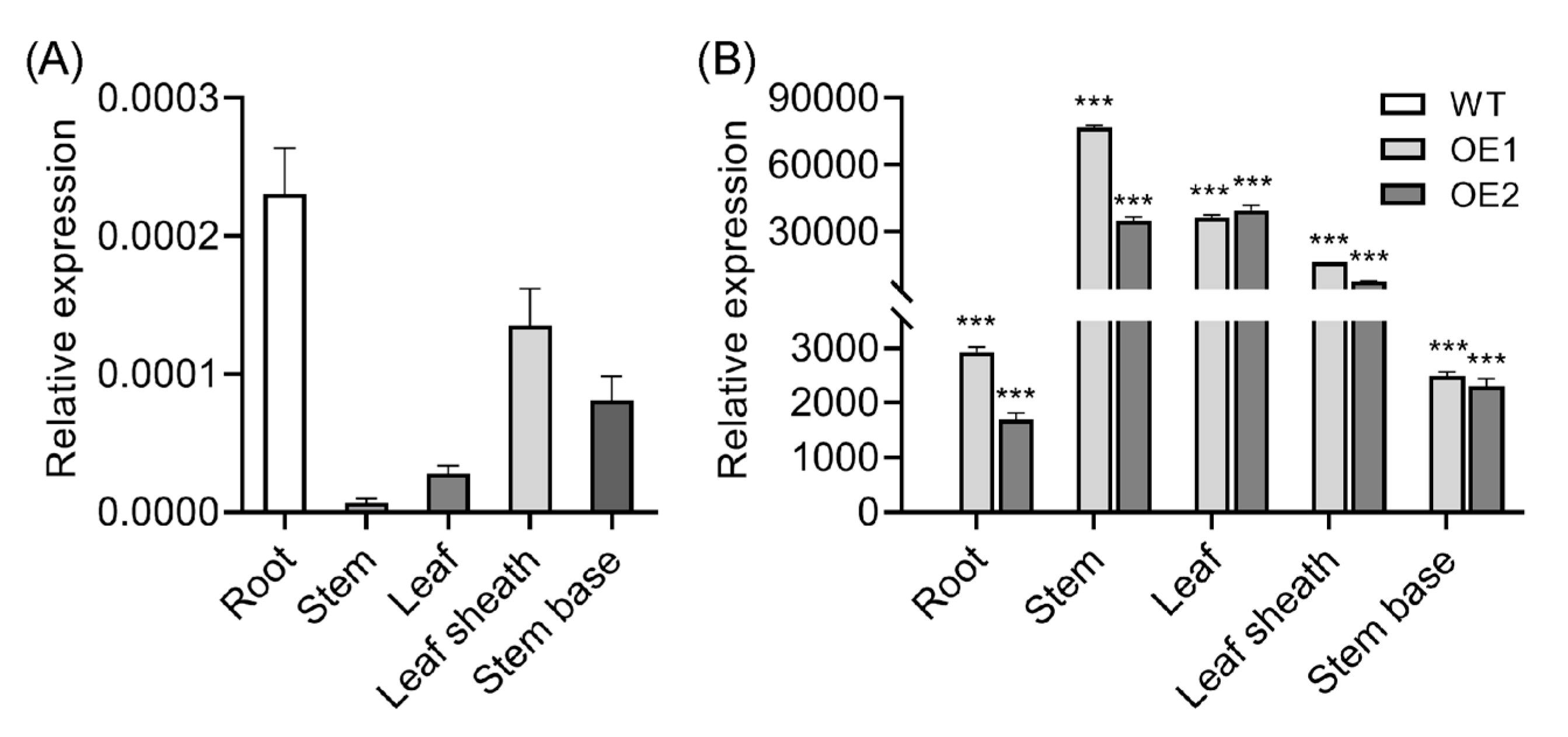
2.1. Tissue-Specific Analysis of OsIAGLU in Wild-Type (WT) and OsIAGLU-Overexpressing Seedlings
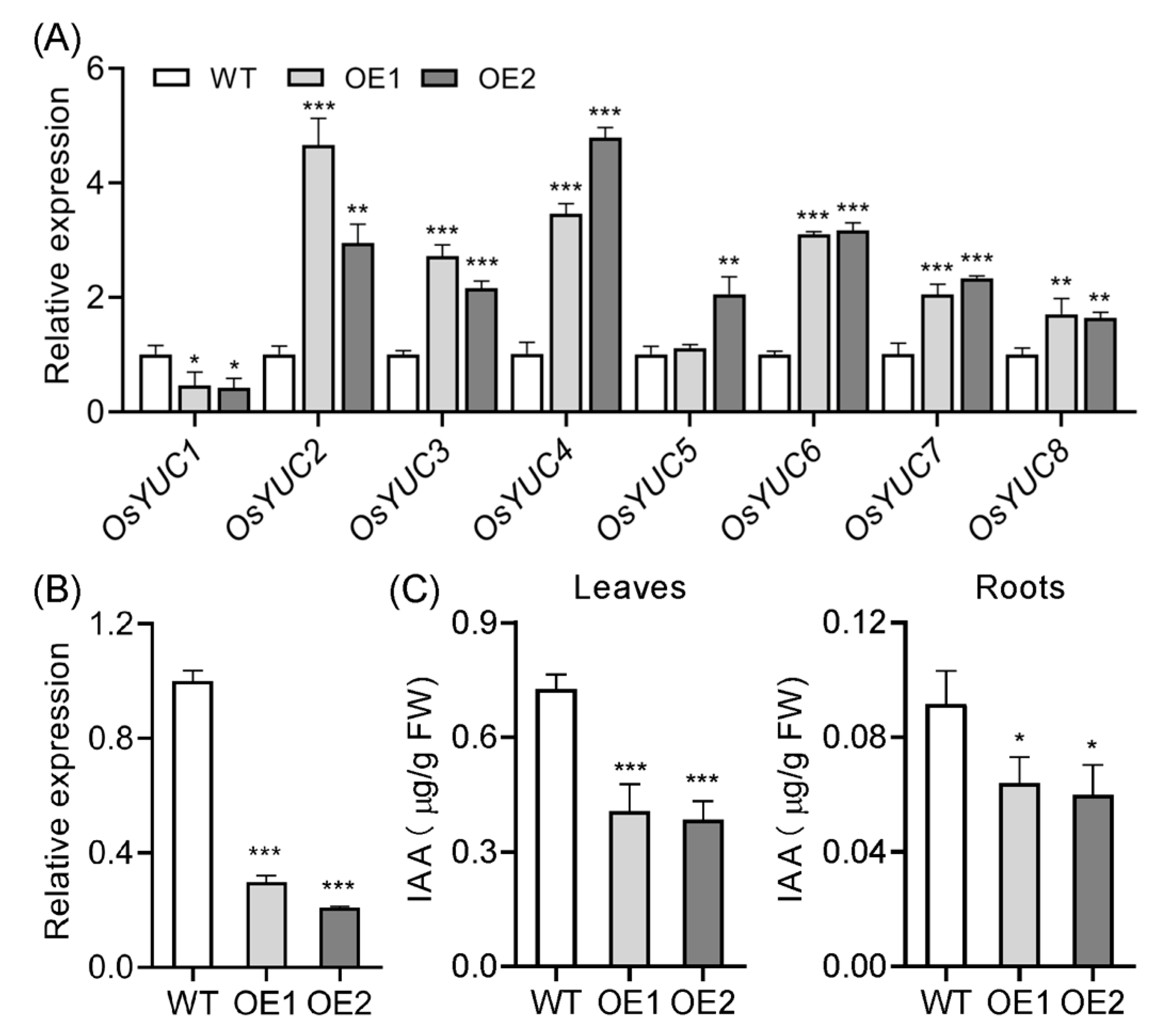
2.2. Overexpression of OsIAGLU Disrupts Auxin Homeostasis
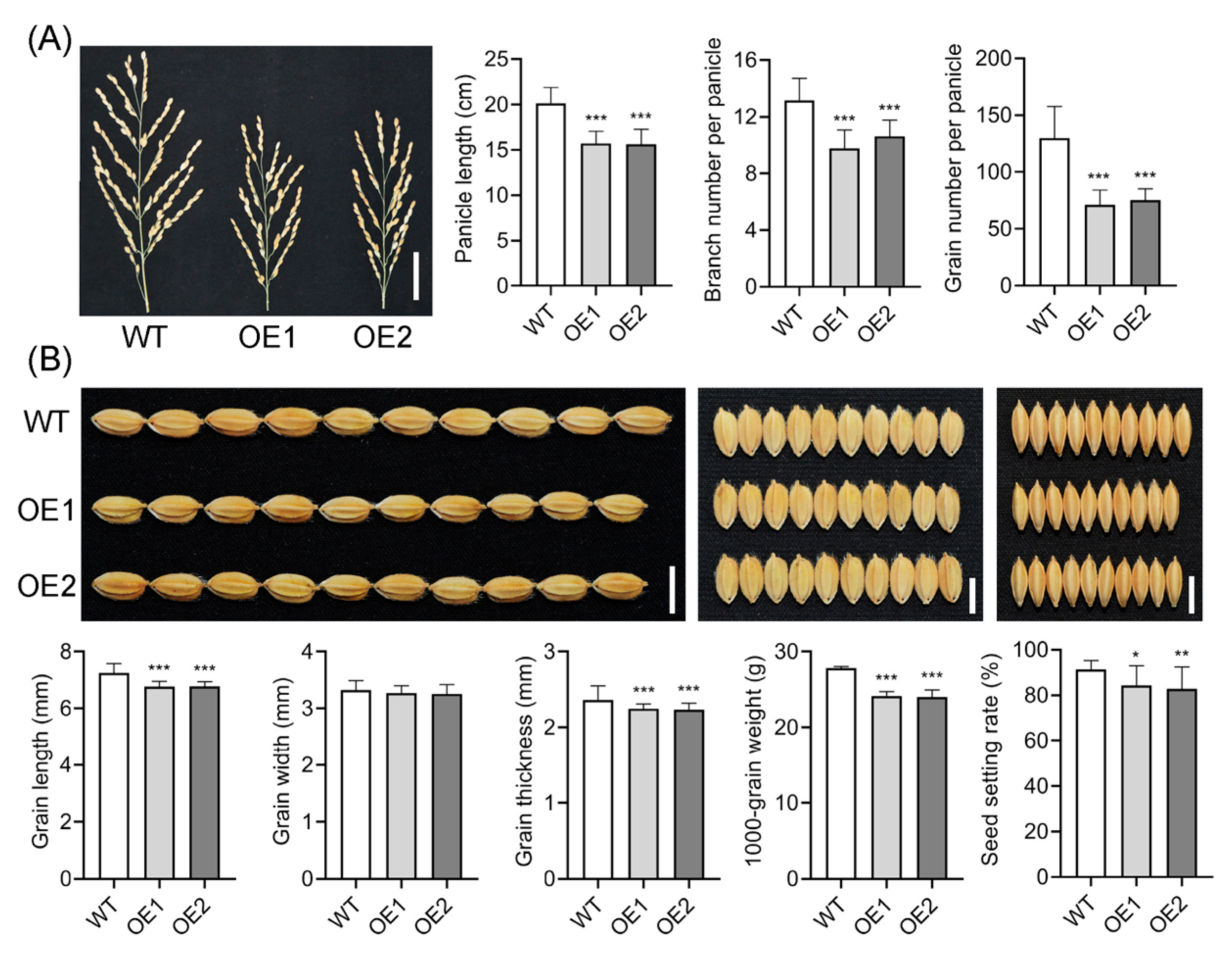
2.3. Overexpression of OsIAGLU Adversely Impacts Rice Agrinomic Traits
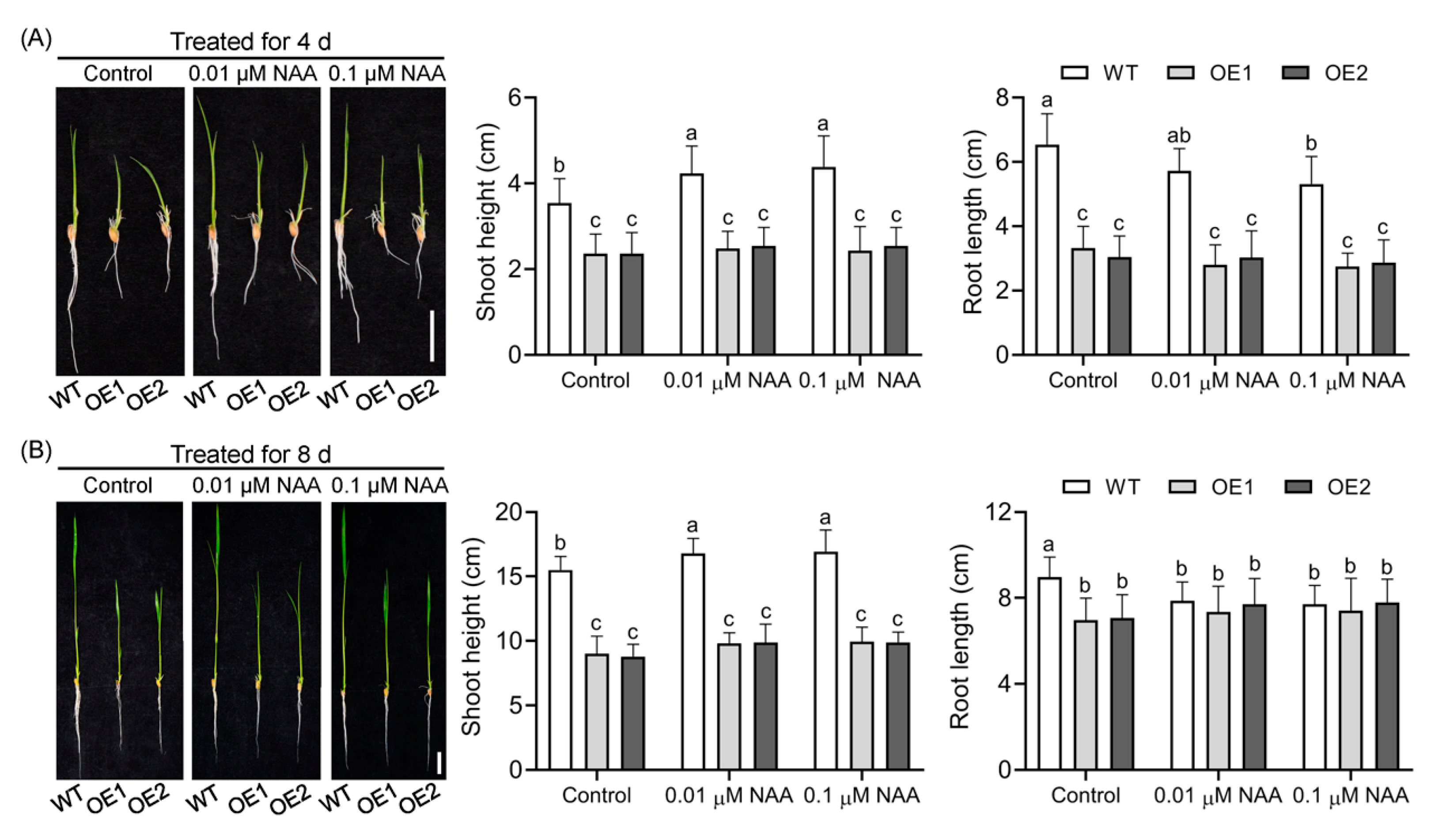
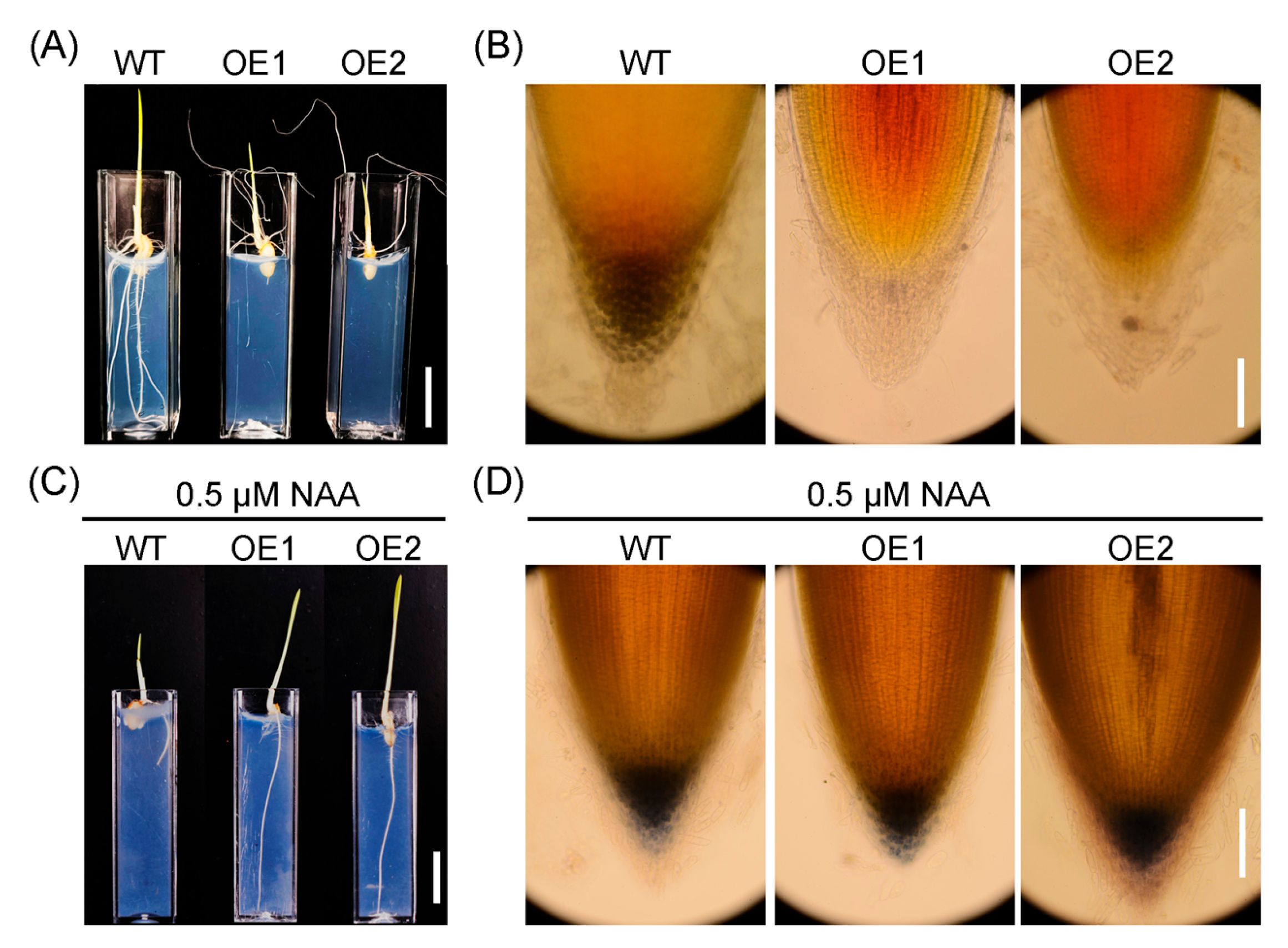
2.4. Upregulation of OsIAGLU Impairs Root Gravitropism by Disruping Starch Granule Accumulation in the Root Caps
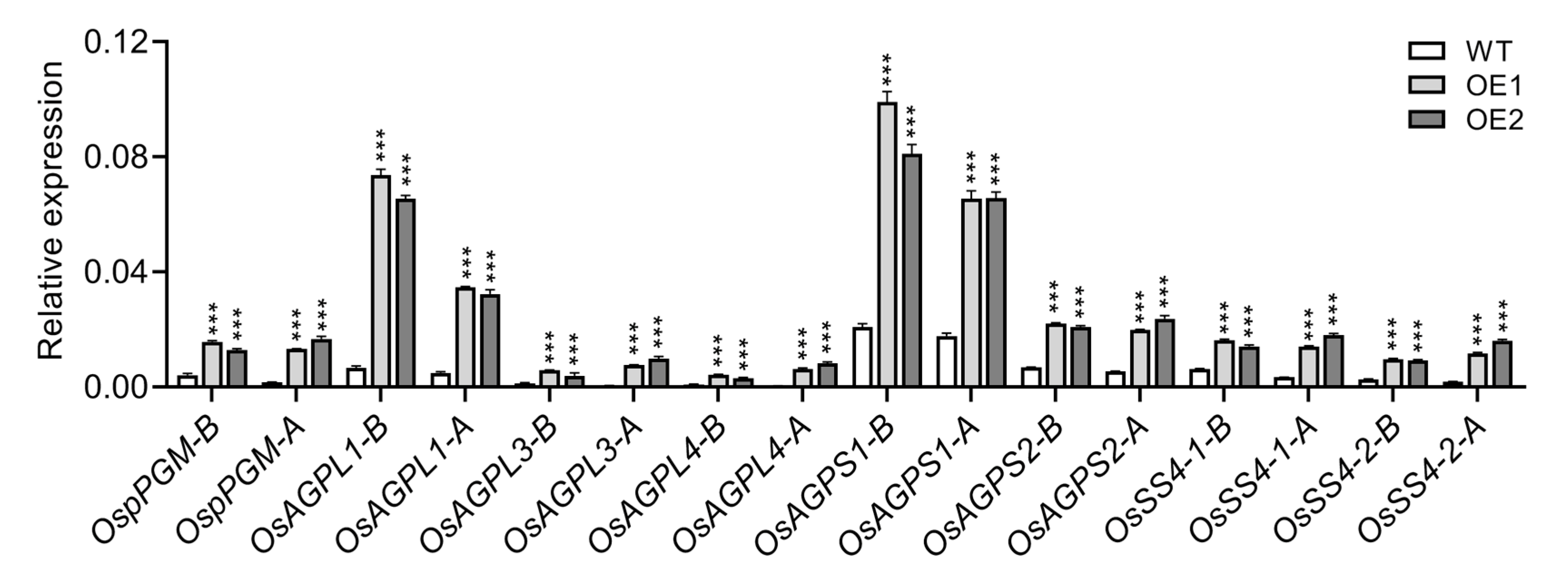
2.5. Starch Biosynthesis Genes Are Upregulated in the OE Root Tips
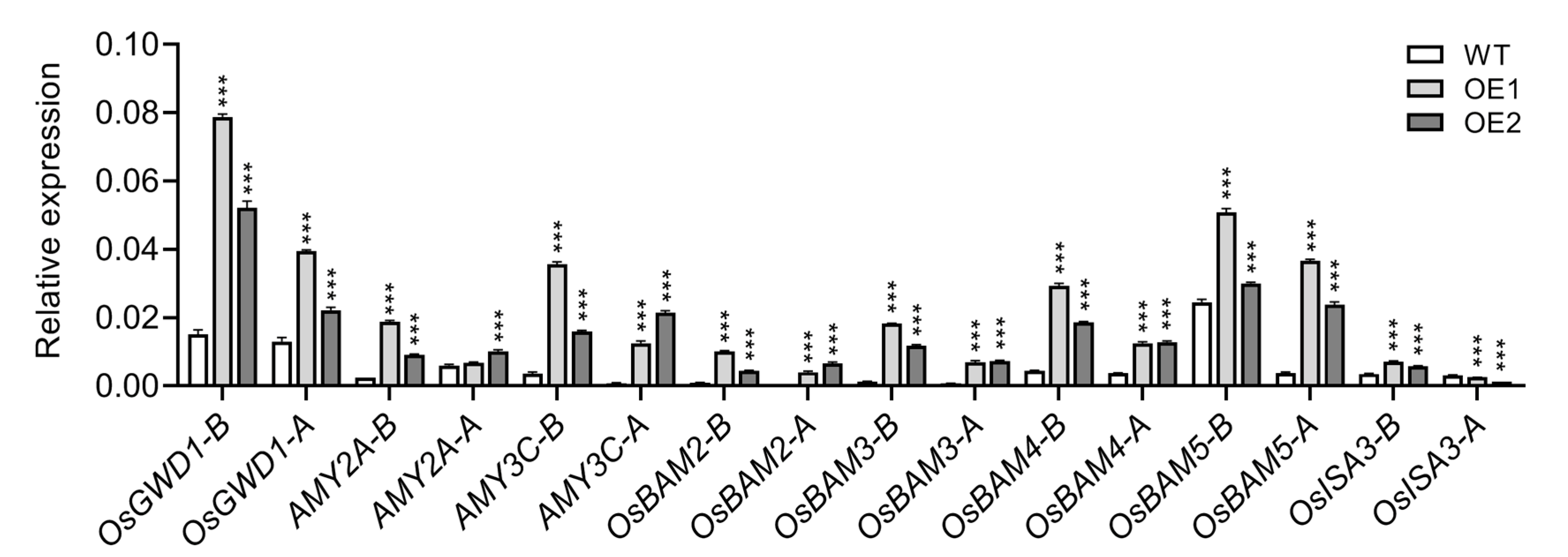
2.6. Starch Degradation Genes Likely Play a More Prominent Role in Regulating Starch Granule Accumulation in OE Rice Root Tips
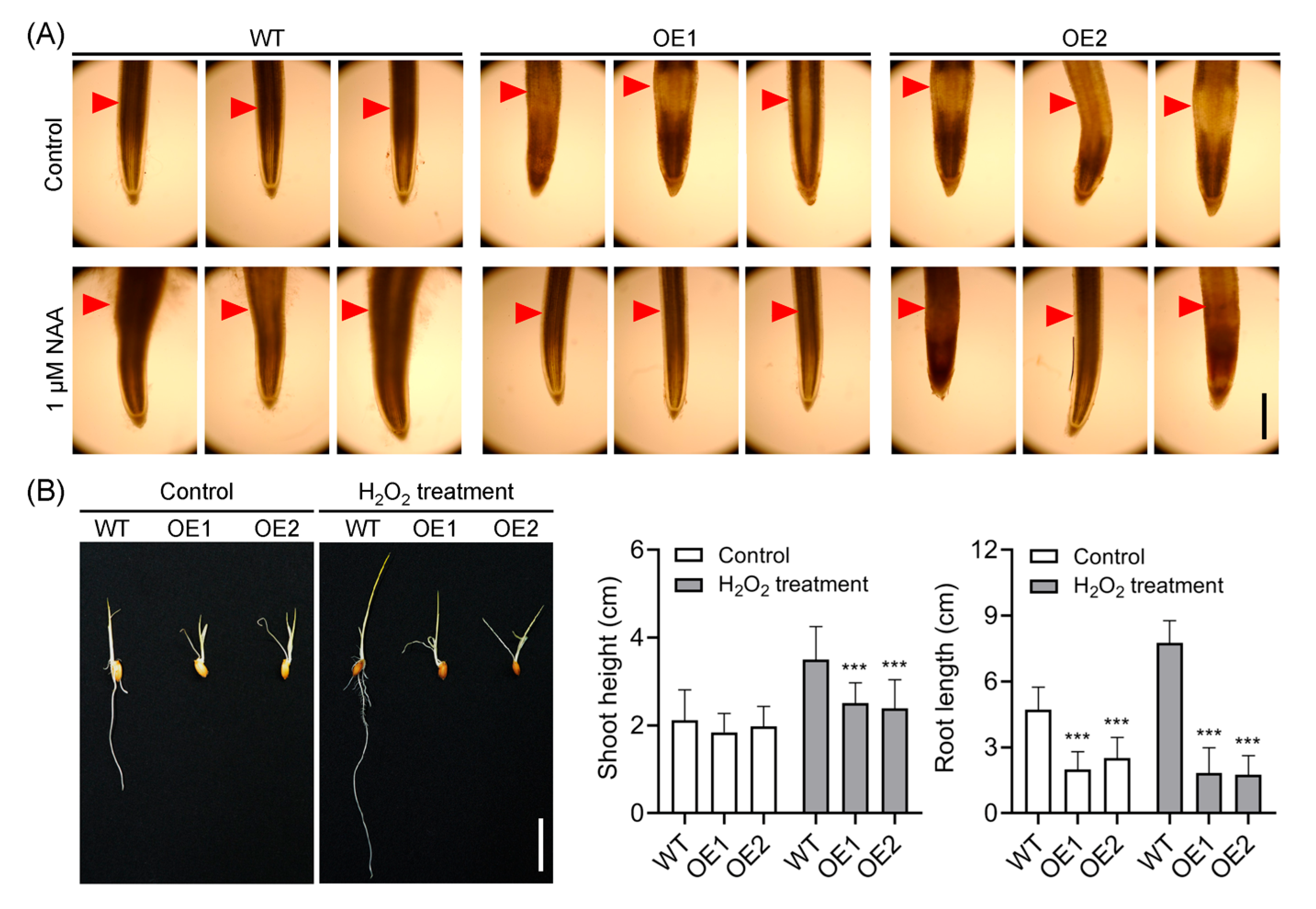
2.7. Decreased Accumulation of Hydrogen Peroxide (H2O2) in the Root Elongation Zone Correlates with Reduced Auxin Levels
3. Discussion
4. Materials and Methods
4.1. Plant Lines and Growth Conditions
4.2. NAA and H2O2 Treatment
4.3. IAA Measurement
4.4. Root Gravitropism Observation
4.5. Starch Granule Staining Assay
4.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7. Expression Analysis of Starch Biosynthesis and Degradation Genes
4.8. H2O2 Staining
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benkova, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertova, D.; Jurgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Lavy, M.; Estelle, M. Mechanisms of auxin signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef]
- Mroue, S.; Simeunovic, A.; Robert, H.S. Auxin production as an integrator of environmental cues for developmental growth regulation. J. Exp. Bot. 2018, 69, 201–212. [Google Scholar] [CrossRef]
- Friml, J. Auxin transport—Shaping the plant. Curr. Opin. Plant Biol. 2003, 6, 7–12. [Google Scholar] [CrossRef]
- Kimura, M.; Kagawa, T. Phototropin and light-signaling in phototropism. Curr. Opin. Plant Biol. 2006, 9, 503–508. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; He, P.; Ma, X.F.; Yang, Z.R.; Pang, C.Y.; Yu, J.N.; Wang, G.D.; Friml, J.; Xiao, G.H. Auxin-mediated statolith production for root gravitropism. New Phytol. 2019, 224, 761–774. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhang, M.J.; Qiao, L.; Chen, Y.B.; Zhang, D.P.; Jing, X.Q.; Gan, P.F.; Huang, Y.B.; Gao, J.R.; Liu, W.T.; et al. Characterization of wavy root 1, an agravitropism allele, reveals the functions of OsPIN2 in fine regulation of auxin transport and distribution and in ABA biosynthesis and response in rice (Oryza sativa L.). Crop J. 2022, 10, 980–992. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, H.; Yu, H.; Cai, Y.; Huang, L.; Xu, C.; Xiong, G.; Meng, X.; Wang, J.; Chen, H.; et al. A core regulatory pathway controlling rice tiller angle mediated by the LAZY1-dependent asymmetric distribution of auxin. Plant Cell 2018, 30, 1461–1475. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Zhang, Y.; Wang, H.; Wu, S.; Zhang, Z.; Ahammed, G.J.; Zhao, C.; Liu, H. CRISPR/Cas9-mediated mutation in auxin efflux carrier OsPIN9 confers chilling tolerance by modulating reactive oxygen species homeostasis in rice. Front. Plant Sci. 2022, 13, 967031. [Google Scholar] [CrossRef] [PubMed]
- Korasick, D.A.; Enders, T.A.; Strader, L.C. Auxin biosynthesis and storage forms. J. Exp. Bot. 2013, 64, 2541–2555. [Google Scholar] [CrossRef]
- Zhang, J.; Peer, W.A. Auxin homeostasis: The DAO of catabolism. J. Exp. Bot. 2017, 68, 3145–3154. [Google Scholar] [CrossRef]
- Kramer, E.M.; Ackelsberg, E.M. Auxin metabolism rates and implications for plant development. Front. Plant Sci. 2015, 6, 150. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, T.; Wang, R.; Zhao, Y. Recent advances in auxin research in rice and their implications for crop improvement. J. Exp. Bot. 2018, 69, 255–263. [Google Scholar] [CrossRef]
- Ludwig-Muller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Seidel, C.; Walz, A.; Park, S.; Cohen, J.D.; Ludwig-Muller, J. Indole-3-acetic acid protein conjugates: Novel players in auxin homeostasis. Plant Biol. 2006, 8, 340–345. [Google Scholar] [CrossRef]
- Szerszen, J.B.; Szczyglowski, K.; Bandurski, R.S. iaglu, a gene from Zea mays involved in conjugation of growth hormone indole-3-acetic acid. Science 1994, 265, 1699–1701. [Google Scholar] [CrossRef]
- Jackson, R.G.; Lim, E.K.; Li, Y.; Kowalczyk, M.; Sandberg, G.; Hoggett, J.; Ashford, D.A.; Bowles, D.J. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J. Biol. Chem. 2001, 276, 4350–4356. [Google Scholar] [CrossRef]
- Jackson, R.G.; Kowalczyk, M.; Li, Y.; Higgins, G.; Ross, J.; Sandberg, G.; Bowles, D.J. Over-expression of an Arabidopsis gene encoding a glucosyltransferase of indole-3-acetic acid: Phenotypic characterisation of transgenic lines. Plant J. 2002, 32, 573–583. [Google Scholar] [CrossRef]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Koh, E.B.; Woo, M.O.; Piao, R.; Oh, C.S.; Koh, H.J. Tiller formation in rice is altered by overexpression of gene encoding an IAA-conjugating enzyme or exogenous treatment of free IAA. J. Plant Biol. 2012, 55, 429–435. [Google Scholar] [CrossRef]
- Yu, X.L.; Wang, H.Y.; Leung, D.W.M.; He, Z.D.; Zhang, J.J.; Peng, X.X.; Liu, E.E. Overexpression of OsIAAGLU reveals a role for IAA-glucose conjugation in modulating rice plant architecture. Plant Cell Rep. 2019, 38, 731–739. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, T.T.; Xiao, D.W.; Zhao, S.M.; Lin, J.S.; Wang, T.; Li, Y.J.; Hou, B.K. OsIAGT1 is a glucosyltransferase gene involved in the glucose conjugation of auxins in rice. Rice 2019, 12, 92. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, B.; Li, W.J.; Sun, S.; Peng, L.L.; Feng, D.F.; Li, L.; Di, H.; He, Y.Q.; Wang, Z.F. A genome-wide association study reveals that the glucosyltransferase OsIAGLU regulates root growth in rice. J. Exp. Bot. 2021, 72, 1119–1134. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Yang, B.; Sun, S.; Peng, L.; Wang, Z. Indole-3-acetate beta-glucosyltransferase OsIAGLU regulates seed vigour through mediating crosstalk between auxin and abscisic acid in rice. Plant Biotechnol. J. 2020, 18, 1933–1945. [Google Scholar] [CrossRef]
- He, Y.; Sun, S.; Zhao, J.; Huang, Z.; Peng, L.; Huang, C.; Tang, Z.; Huang, Q.; Wang, Z. UDP-glucosyltransferase OsUGT75A promotes submergence tolerance during rice seed germination. Nat. Commun. 2023, 14, 2296. [Google Scholar] [CrossRef]
- Bailey, P.H.; Currey, J.D.; Fitter, A.H. The role of root system architecture and root hairs in promoting anchorage against uprooting forces in Allium cepa and root mutants of Arabidopsis thaliana. J. Exp. Bot. 2002, 53, 333–340. [Google Scholar] [CrossRef]
- Moulia, B.; Fournier, M. The power and control of gravitropic movements in plants: A biomechanical and systems biology view. J. Exp. Bot. 2009, 60, 461–486. [Google Scholar] [CrossRef]
- Su, S.H.; Gibbs, N.M.; Jancewicz, A.L.; Masson, P.H. Molecular mechanisms of root gravitropism. Curr. Biol. 2017, 27, R964–R972. [Google Scholar] [CrossRef]
- Morita, M.T. Directional gravity sensing in gravitropism. Annu. Rev. Plant Biol. 2010, 61, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Zádníková, P.; Smet, D.; Zhu, Q.; Van Der Straeten, D.; Benková, E. Strategies of seedlings to overcome their sessile nature: Auxin in mobility control. Front. Plant Sci. 2015, 6, 218. [Google Scholar] [CrossRef]
- Swarup, R.; Peret, B. AUX/LAX family of auxin influx carriers—An overview. Front. Plant Sci. 2012, 3, 225. [Google Scholar] [CrossRef]
- Konstantinova, N.; Korbei, B.; Luschnig, C. Auxin and root gravitropism: Addressing basic cellular processes by exploiting a defined growth response. Int. J. Mol. Sci. 2021, 22, 2749. [Google Scholar] [CrossRef]
- Barlow, P.W. Gravity perception in plants: A multiplicity of systems derived by evolution? Plant Cell Environ. 1995, 18, 951–962. [Google Scholar] [CrossRef]
- Kolesnikov, Y.S.; Kretynin, S.V.; Volotovsky, I.D.; Kordyum, E.L.; Ruelland, E.; Kravets, V.S. Molecular mechanisms of gravity perception and signal transduction in plants. Protoplasma 2016, 253, 987–1004. [Google Scholar] [CrossRef]
- Leitz, G.; Kang, B.H.; Schoenwaelder, M.E.; Staehelin, L.A. Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity-sensing Arabidopsis columella cells. Plant Cell 2009, 21, 843–860. [Google Scholar] [CrossRef]
- Band, L.R.; Wells, D.M.; Larrieu, A.; Sun, J.Y.; Middleton, A.M.; French, A.P.; Brunoud, G.; Sato, E.M.; Wilson, M.H.; Peret, B.; et al. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc. Natl. Acad. Sci. USA 2012, 109, 4668–4673. [Google Scholar] [CrossRef]
- Sato, E.M.; Hijazi, H.; Bennett, M.J.; Vissenberg, K.; Swarup, R. New insights into root gravitropic signalling. J. Exp. Bot. 2015, 66, 2155–2165. [Google Scholar] [CrossRef]
- Friml, J.; Wisniewska, J.; Benkova, E.; Mendgen, K.; Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002, 415, 806–809. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Ding, Z.; Jones, A.R.; Tasaka, M.; Morita, M.T.; Friml, J. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proc. Natl. Acad. Sci. USA 2010, 107, 22344–22349. [Google Scholar] [CrossRef] [PubMed]
- Abas, L.; Benjamins, R.; Malenica, N.; Paciorek, T.; Wisniewska, J.; Moulinier-Anzola, J.C.; Sieberer, T.; Friml, J.; Luschnig, C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Vehn, J.; Leitner, J.; Zwiewka, M.; Sauer, M.; Abas, L.; Luschnig, C.; Friml, J. Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc. Natl. Acad. Sci. USA 2008, 105, 17812–17817. [Google Scholar] [CrossRef]
- Wang, H.; Ouyang, Q.; Yang, C.; Zhang, Z.; Hou, D.; Liu, H.; Xu, H. Mutation of OsPIN1b by CRISPR/Cas9 reveals a role for auxin transport in modulating rice architecture and root gravitropism. Int. J. Mol. Sci. 2022, 23, 8965. [Google Scholar] [CrossRef]
- Terrile, M.C.; París, R.; Calderón-Villalobos, L.I.A.; Iglesias, M.J.; Lamattina, L.; Estelle, M.; Casalongué, C.A. Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J. 2012, 70, 492–500. [Google Scholar] [CrossRef]
- Li, X.; Zhao, R.; Liu, J.; Li, Z.; Chen, A.; Xu, S.; Sheng, X. Dynamic changes in calcium signals during root gravitropism. Plant Physiol. Biochem. 2024, 208, 108481. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, H.; Yang, T.; Lian, Y.; Sun, Y.; Bian, Z.; Wang, C. Exogenous hydrogen peroxide inhibits primary root gravitropism by regulating auxin distribution during Arabidopsis seed germination. Plant Physiol. Biochem. 2018, 128, 126–133. [Google Scholar] [CrossRef]
- Li, S.; Su, L.R.; Ma, S.Y.; Shi, Z.Z.; Yang, X.M. Initial exploration of the mechanism underlying H2O2-induced root horizontal bending in pea. Sci. Bull. 2015, 60, 1298–1300. [Google Scholar] [CrossRef]
- Joo, J.H.; Bae, Y.S.; Lee, J.S. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001, 126, 1055–1060. [Google Scholar] [CrossRef]
- Krieger, G.; Shkolnik, D.; Miller, G.; Fromm, H. Reactive oxygen species tune root tropic responses. Plant Physiol. 2016, 172, 1209–1220. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Huang, H.; Li, L.; Xu, S.; Shen, W. Molecular hydrogen positively influences root gravitropism involving auxin signaling and starch accumulation. Plant J. 2024, 120, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant 2012, 5, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef]
- Sang, D.; Chen, D.; Liu, G.; Liang, Y.; Huang, L.; Meng, X.; Chu, J.; Sun, X.; Dong, G.; Yuan, Y.; et al. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Yuan, Y.D.; Wang, L.; Meng, X.B.; Xiong, G.S.; Zhou, J.; Cai, Y.Y.; Han, N.P.; Hua, L.K.; et al. OsBRXL4 regulates shoot gravitropism and rice tiller angle through affecting LAZY1 nuclear localization. Mol. Plant 2019, 12, 1143–1156. [Google Scholar] [CrossRef]
- Manna, M.; Rengasamy, B.; Sinha, A.K. A rapid and robust colorimetric method for measuring relative abundance of auxins in plant tissues. Phytochem. Anal. 2024, 35, 1052–1062. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, W.; Hu, X.; Shi, X.; Chen, L.; Dai, X.; Qu, H.; Xia, Y.; Liu, W.; Gu, M.; et al. Two ADP-glucose pyrophosphorylase subunits, OsAGPL1 and OsAGPS1, modulate phosphorus homeostasis in rice. Plant J. 2020, 104, 1269–1284. [Google Scholar] [CrossRef]
- Sonnewald, U.; Kossmann, J. Starches-from current models to genetic engineering. Plant Biotechnol. J. 2013, 11, 223–232. [Google Scholar] [CrossRef]
- Silver, D.M.; Kotting, O.; Moorhead, G.B. Phosphoglucan phosphatase function sheds light on starch degradation. Trends Plant Sci. 2014, 19, 471–478. [Google Scholar] [CrossRef]
- Peer, W.A.; Cheng, Y.; Murphy, A.S. Evidence of oxidative attenuation of auxin signalling. J. Exp. Bot. 2013, 64, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.L.; Su, M.; Wang, L.Y.; Jiao, C.J.; Sun, Z.X.; Cheng, W.; Li, F.M.; Wang, C.Y. Exogenous hydrogen peroxide reversibly inhibits root gravitropism and induces horizontal curvature of primary root during grass pea germination. Plant Physiol. Bioch 2012, 53, 84–93. [Google Scholar] [CrossRef]
- Li, S.; Su, L.R.; Ma, S.Y.; Shi, Z.Z.; Zhang, Z.; Liu, H.J.; Zhang, J.L.; Yang, X.M.; Sun, Z.W. The impacts of exogenous H2O2 on primary root horizontal bending of pea (Pisum sativum L.). Plant Growth Regul. 2016, 78, 287–296. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, Y.; Fu, G.; Tao, L. Novel roles of hydrogen peroxide (H2O2) in regulating pectin synthesis and demethylesterification in the cell wall of rice (Oryza sativa) root tips. New Phytol. 2015, 206, 118–126. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamazaki, C.; Mitsui, M.; Kakei, Y.; Mitani, Y.; Nakamura, A.; Ishii, T.; Soeno, K.; Shimada, Y. Transcriptional feedback regulation of YUCCA genes in response to auxin levels in Arabidopsis. Plant Cell Rep. 2015, 34, 1343–1352. [Google Scholar] [CrossRef]
- Luo, P.; Di, D.W. Precise regulation of the TAA1/TAR-YUCCA auxin biosynthesis pathway in plants. Int. J. Mol. Sci. 2023, 24, 8514. [Google Scholar] [CrossRef]
- Cao, X.; Yang, H.; Shang, C.; Ma, S.; Liu, L.; Cheng, J. The roles of auxin biosynthesis YUCCA gene family in plants. Int. J. Mol. Sci. 2019, 20, 6343. [Google Scholar] [CrossRef]
- Han, X.L.; Zhao, F.Y. OsYUCCA2 deficiency in rice growth and development. Cienc. Rural. 2022, 52, e20210327. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhiguo, E.; Zhang, D.P.; Yun, Q.B.; Zhou, Y.; Niu, B.X.; Chen, C. OsYUC11-mediated auxin biosynthesis is essential for endosperm development of rice. Plant Physiol. 2021, 185, 934–950. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.M.; Mawia, A.M.; Wei, X.J.; Cao, R.J.; Jiao, G.A.I.; Wu, Y.W.; Zhang, J.; Xie, L.H.; Sheng, Z.H.; et al. A novel transcription factor OsMYB73 affects grain size and chalkiness by regulating endosperm storage substances’ accumulation-mediated auxin biosynthesis signalling pathway in rice. Plant Biotechnol. J. 2025, 23, 1021–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Liu, X.; Zhang, X.; Liu, S.; Yu, X.; Ren, Y.; Zheng, X.; Zhou, K.; Jiang, L.; et al. A role for a dioxygenase in auxin metabolism and reproductive development in rice. Dev. Cell 2013, 27, 113–122. [Google Scholar] [CrossRef] [PubMed]
- McAdam, E.L.; Meitzel, T.; Quittenden, L.J.; Davidson, S.E.; Dalmais, M.; Bendahmane, A.I.; Thompson, R.; Smith, J.J.; Nichols, D.S.; Urquhart, S.; et al. Evidence that auxin is required for normal seed size and starch synthesis in pea. New Phytol. 2017, 216, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Meitzel, T.; Radchuk, R.; McAdam, E.L.; Thormählen, I.; Feil, R.; Munz, E.; Hilo, A.; Geigenberger, P.; Ross, J.J.; Lunn, J.E.; et al. Trehalose 6-phosphate promotes seed filling by activating auxin biosynthesis. New Phytol. 2021, 229, 1553–1565. [Google Scholar] [CrossRef]
- Bernardi, J.; Battaglia, R.; Bagnaresi, P.; Lucini, L.; Marocco, A. Transcriptomic and metabolomic analysis of ZmYUC1 mutant reveals the role of auxin during early endosperm formation in maize. Plant Sci. 2019, 281, 133–145. [Google Scholar] [CrossRef]
- Zhang, D.P.; Zhang, M.Y.; Liang, J.S. RGB1 regulates grain development and starch accumulation through its effect on OsYUC11-mediated auxin biosynthesis in rice endosperm cells. Front. Plant Sci. 2021, 12, 585174. [Google Scholar] [CrossRef]
- Wu, D.X.; Cao, Y.N.; Wang, D.J.; Zong, G.X.N.; Han, K.X.; Zhang, W.; Qi, Y.H.; Xu, G.H.; Zhang, Y.L. Auxin receptor OsTIR1 mediates auxin signaling during seed filling in rice. Plant Physiol. 2024, 194, 2434–2448. [Google Scholar] [CrossRef]
- Amanda, D.; Frey, F.P.; Neumann, U.; Przybyl, M.; Simura, J.; Zhang, Y.J.; Chen, Z.L.; Gallavotti, A.; Fernie, A.R.; Ljung, K.; et al. Auxin boosts energy generation pathways to fuel pollen maturation in barley. Curr. Biol. 2022, 32, 1798–1811.e8. [Google Scholar] [CrossRef]
- Liu, H.B.; Wu, Y.; Cai, J.H.; Xu, L.L.; Zhou, C.; Wang, C.L. Auxin controls root gravitropic response by affecting starch granule accumulation and cell wall modification in tomato. Plants 2025, 14, 1020. [Google Scholar] [CrossRef]
- Sack, F.D. Plastids and gravitropic sensing. Planta 1997, 203, S63–S68. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Wright, J.B.; Caspar, T. Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol. Plant 1996, 97, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Caspar, T.; Pickard, B.G. Gravitropism in a starchless mutant of Arabidopsis: Implications for the starch-statolith theory of gravity sensing. Planta 1989, 177, 185–197. [Google Scholar] [CrossRef]
- Kiss, J.Z. Statoliths function in gravity perception in plants: Yes, no, yes! Planta 2025, 261, 45. [Google Scholar] [CrossRef]
- Baldwin, K.L.; Strohm, A.K.; Masson, P.H. Gravity sensing and signal transduction in vascular plant primary roots. Am. J. Bot. 2013, 100, 126–142. [Google Scholar] [CrossRef]
- Xu, H.W.; Ji, X.M.; He, Z.H.; Shi, W.P.; Zhu, G.H.; Niu, J.K.; Li, B.S.; Peng, X.X. Oxalate accumulation and regulation is independent of glycolate oxidase in rice leaves. J. Exp. Bot. 2006, 57, 1899–1908. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Yang, X.; Wang, H.; Hou, D. Tissue specificity and responses to abiotic stresses and hormones of PIN genes in rice. Biologia 2022, 77, 1459–1470. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, W.; Zhao, Y.; Xu, Y.; Song, S.; Chong, K. Comparative metabolomic analysis reveals a reactive oxygen species-dominated dynamic model underlying chilling environment adaptation and tolerance in rice. New Phytol. 2016, 211, 1295–1310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Fu, X.; Ruan, X.; Yu, X.; Hou, D.; Xu, H. Upregulation of an IAA-Glucosyltransferase OsIAGLU in Rice (Oryza sativa L.) Impairs Root Gravitropism by Disrupting Starch Granule Homeostasis. Plants 2025, 14, 1557. https://doi.org/10.3390/plants14101557
Chen G, Fu X, Ruan X, Yu X, Hou D, Xu H. Upregulation of an IAA-Glucosyltransferase OsIAGLU in Rice (Oryza sativa L.) Impairs Root Gravitropism by Disrupting Starch Granule Homeostasis. Plants. 2025; 14(10):1557. https://doi.org/10.3390/plants14101557
Chicago/Turabian StyleChen, Guo, Xiaoyu Fu, Xinya Ruan, Xiaolu Yu, Dianyun Hou, and Huawei Xu. 2025. "Upregulation of an IAA-Glucosyltransferase OsIAGLU in Rice (Oryza sativa L.) Impairs Root Gravitropism by Disrupting Starch Granule Homeostasis" Plants 14, no. 10: 1557. https://doi.org/10.3390/plants14101557
APA StyleChen, G., Fu, X., Ruan, X., Yu, X., Hou, D., & Xu, H. (2025). Upregulation of an IAA-Glucosyltransferase OsIAGLU in Rice (Oryza sativa L.) Impairs Root Gravitropism by Disrupting Starch Granule Homeostasis. Plants, 14(10), 1557. https://doi.org/10.3390/plants14101557






