Understanding the Brassinosteroid-Dependent Environmental Adaption in Brassicaceae Plants
Abstract
1. Introduction
2. The Synthesis and Metabolism of BRs
2.1. The Synthesis of BRs
2.2. The Degradation of BRs
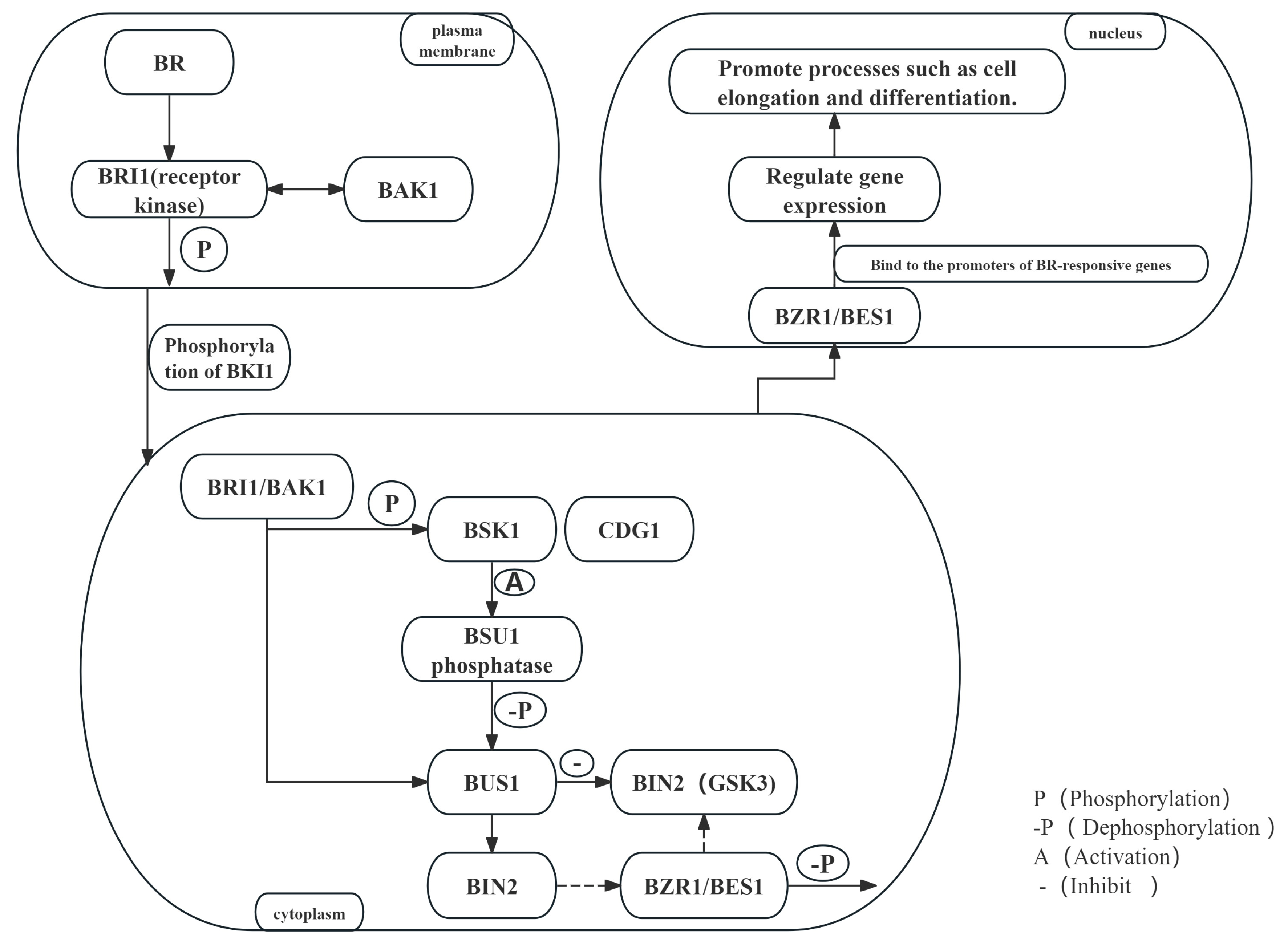
3. BR Signal Transduction
3.1. BR Receptor Activation and Recognition
3.2. The Downstream Signaling Cascade of BRs
3.3. BR-Mediated Transcriptional Regulation and Gene Expression
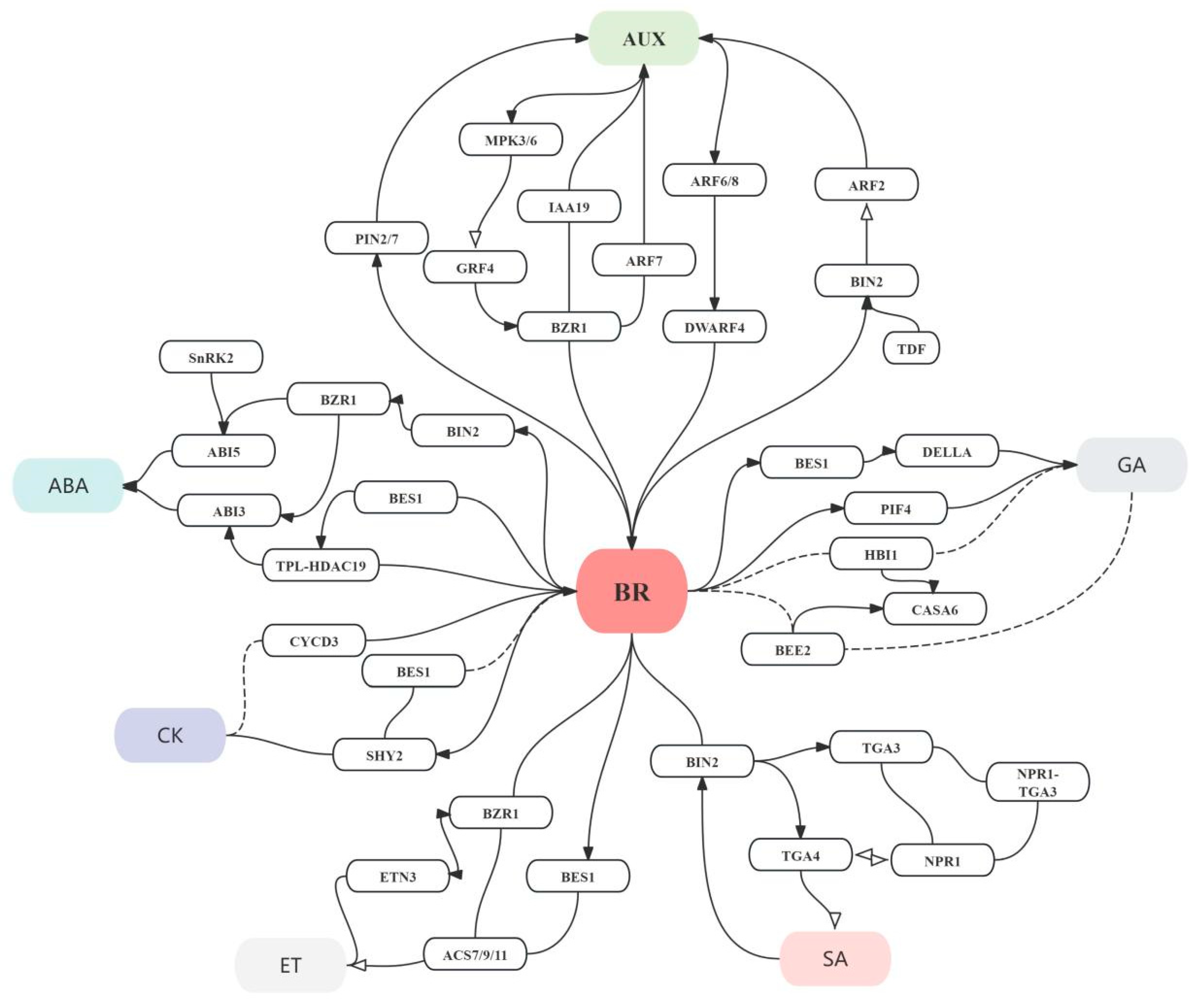
4. The Crosstalk Among BRs and Other Phytohormones in Brassicaceae Plants
4.1. The Synergistic Interaction Between BRs and Auxins
4.2. The Interaction Between BR and ABA
4.3. The Interaction Between BR and Gibberellins
4.4. The Interaction Between BR and Cytokinins
4.5. The Interactions Between BRs and Other Phytohormones
5. BRs Shape the Environmental Adaptability in Brassicaceae Crops
5.1. BRs Regulate Temperature Stress Responses in Brassicaceae Crops
5.2. BRs Regulate Drought Stress Responses in Brassicaceae Crops
5.3. BRs Regulate Salt Stress Responses in Brassicaceae Crops
5.4. BRs Respond to Metal Stresses in Brassicaceae Crops
6. Conclusions
7. Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [PubMed]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D., Jr.; Steffens, G.L.; Flippen-Anderson, J.L.; Cook, J.C., Jr. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Poppenberger, B.; Russinova, E.; Savaldi-Goldstein, S. Brassinosteroids in Focus. Plant Cell Physiol. 2024, 65, 1495–1499. [Google Scholar] [CrossRef]
- Bajguz, A.; Chmur, M.; Gruszka, D. Comprehensive Overview of the Brassinosteroid Biosynthesis Pathways: Substrates, Products, Inhibitors, and Connections. Front. Plant Sci. 2020, 11, 1034. [Google Scholar] [CrossRef]
- Oh, M.H.; Honey, S.H.; Tax, F.E. The Control of Cell Expansion, Cell Division, and Vascular Development by Brassinosteroids: A Historical Perspective. Int. J. Mol. Sci. 2020, 21, 1743. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Corvalán, C.; Choe, S. Identification of brassinosteroid genes in Brachypodium distachyon. BMC Plant Biol. 2017, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Ackerman-Lavert, M.; Savaldi-Goldstein, S. Growth models from a brassinosteroid perspective. Curr. Opin. Plant Biol. 2020, 53, 90–97. [Google Scholar] [CrossRef]
- Furuya, T.; Ohashi-Ito, K.; Kondo, Y. Multiple Roles of Brassinosteroid Signaling in Vascular Development. Plant Cell Physiol. 2024, 65, 1601–1607. [Google Scholar] [CrossRef]
- Hacham, Y.; Holland, N.; Butterfield, C.; Ubeda-Tomas, S.; Bennett, M.J.; Chory, J.; Savaldi-Goldstein, S. Brassinosteroid perception in the epidermis controls root meristem size. Development 2011, 138, 839–848. [Google Scholar] [CrossRef]
- Li, B.; Liu, X.; Guo, Y.; Deng, L.; Qu, L.; Yan, M.; Li, M.; Wang, T. BnaC01.BIN2, a GSK3-like kinase, modulates plant height and yield potential in Brassica napus. Theor. Appl. Genet. 2023, 136, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ou, Y.; Zhang, Z.; Li, J.; He, Y. Brassinosteroid Signaling Recruits Histone 3 Lysine-27 Demethylation Activity to FLOWERING LOCUS C Chromatin to Inhibit the Floral Transition in Arabidopsis. Mol. Plant 2018, 11, 1135–1146. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q. Enhanced photosynthetic capacity and antioxidant potential mediate brassinosteriod-induced phenanthrene stress tolerance in tomato. Environ. Pollut. 2015, 201, 58–66. [Google Scholar] [CrossRef]
- Zhou, B.; Luo, Q.; Shen, Y.; Wei, L.; Song, X.; Liao, H.; Ni, L.; Shen, T.; Du, X.; Han, J.; et al. Coordinated regulation of vegetative phase change by brassinosteroids and the age pathway in Arabidopsis. Nat. Commun. 2023, 14, 2608. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, J.; Chen, Y.; Zhang, D.; Zhou, D.; Zhang, J.; Yan, M. The phytohormone brassinosteroid (BR) promotes early seedling development via auxin signaling pathway in rapeseed. BMC Plant Biol. 2025, 25, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, J. Regulation of brassinosteroid biosynthesis and inactivation. J. Integr. Plant Biol. 2012, 54, 746–759. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukasinovic, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef]
- Asami, T.; Yoshida, S. Brassinosteroid biosynthesis inhibitors. Trends Plant Sci. 1999, 4, 348–353. [Google Scholar] [CrossRef]
- Khan, T.A.; Kappachery, S.; Karumannil, S.; AlHosani, M.; Almansoori, N.; Almansoori, H.; Yusuf, M.; Tran, L.P.; Gururani, M.A. Brassinosteroid Signaling Pathways: Insights into Plant Responses under Abiotic Stress. Int. J. Mol. Sci. 2023, 8, 17246. [Google Scholar] [CrossRef]
- Yu, L.; Cai, W.J.; Ye, T.; Feng, Y.Q. A new boronic acid reagent for the simultaneous determination of C27-, C28-, and C29-brassinosteroids in plant tissues by chemical labeling-assisted liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 1623–1632. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Dockter, C.; Gruszka, D.; Braumann, I.; Druka, A.; Druka, I.; Franckowiak, J.; Gough, S.P.; Janeczko, A.; Kurowska, M.; Lundqvist, J.; et al. Induced variations in brassinosteroid genes define barley height and sturdiness, and expand the green revolution genetic toolkit. Plant Physiol. 2014, 166, 1912–1927. [Google Scholar] [CrossRef]
- Shah, S.H.; Parrey, Z.A.; Barwal, S.K.; Mohammad, F.; Siddiqui, M.H. Deciphering the mechanism of action and crosstalk of brassinosteroids with other plant growth regulators in orchestrating physio-biochemical responses in plants under salt stress. Plant Growth Regul. 2024, 104, 1285–1306. [Google Scholar] [CrossRef]
- Delesalle, C.; Vert, G.; Fujita, S. The cell surface is the place to be for brassinosteroid perception and responses. Nat. Plants 2024, 10, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2004, 2, 505–513. [Google Scholar] [CrossRef]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Clouse, S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, J.; Wang, J.; Chen, L.; Fan, S.L.; Wu, J.W.; Wang, X.; Wang, Z.X. Structural insights into the negative regulation of BRI1 signaling by BRI1-interacting protein BKI1. Cell Res. 2014, 24, 1328–1341. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.-X.; Sun, Y.; Burlingame, A.L.; Wang, Z.-Y. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef]
- Ying, W.; Wang, Y.; Wei, H.; Luo, Y.; Ma, Q.; Zhu, H.; Janssens, H.; Vukašinović, N.; Kvasnica, M.; Winne, J.M.; et al. Structure and function of the Arabidopsis ABC transporter ABCB19 in brassinosteroid export. Science 2024, 383, eadj4591. [Google Scholar] [CrossRef]
- Tang, W.; Kim, T.; Oses-Prieto, J.; Sun, Y.; Deng, Z.; Zhu, S.; Wang, R.; Burlingame, A.L.; Wang, Z.-Y. BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 2008, 321, 557–560. [Google Scholar] [CrossRef]
- Kim, T.; Guan, S.; Burlingame, A.; Wang, Z. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell 2011, 43, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Shen, B.; Li, W.; Liu, L.; Li, J. Post-translational Regulation of BRI1-EMS Suppressor 1 and Brassinazole-Resistant 1. Plant Cell Physiol. 2024, 65, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Espinosa-Ruíz, A.; de Lucas, M.; Bernardo-García, S.; Franco-Zorrilla, J.M.; Prat, S. PIF4-induced BR synthesis is critical to diurnal and thermomorphogenic growth. EMBO J. 2018, 37, e99552. [Google Scholar] [CrossRef]
- Vert, G.; Walcher, C.L.; Chory, J.; Nemhauser, J.L. Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc. Natl. Acad. Sci. USA 2008, 105, 9829–9834. [Google Scholar] [CrossRef] [PubMed]
- Gampala, S.S.; Kim, T.-W.; He, J.-X.; Tang, W.; Deng, Z.; Bai, M.-Y.; Guan, S.; Lalonde, S.; Sun, Y.; Gendron, J.M.; et al. An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell 2007, 13, 177–189. [Google Scholar] [CrossRef]
- Zhao, B.; Lv, M.; Feng, Z.; Campbell, T.; Liscum, E.; Li, J. TWISTED DWARF 1 Associates with BRASSINOSTEROID-INSENSITIVE 1 to Regulate Early Events of the Brassinosteroid Signaling Pathway. Mol. Plant 2016, 9, 582–592. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, D.; Wang, P.; Ma, X.; Lin, W.; Chen, S.; Mishev, K.; Lu, D.; Kumar, R.; Vanhoutte, I.; et al. Regulation of Arabidopsis brassinosteroid receptor BRI1 endocytosis and degradation by plant U-box PUB12/PUB13-mediated ubiquitination. Proc. Natl. Acad. Sci. USA 2018, 115, E1906–E1915. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, F.; Yao, X.; Zhao, Y.; Wen, Y.; Lin, H.; Guo, H.; Yin, Y.; Zhang, D. The deubiquitinating enzymes UBP12 and UBP13 positively regulate recovery after carbon starvation by modulating BES1 stability in Arabidopsis thaliana. Plant Cell 2022, 34, 4516–4530. [Google Scholar] [CrossRef]
- Park, S.H.; Jeong, J.S.; Zhou, Y.; Binte Mustafa, N.F.; Chua, N.H. Deubiquitination of BES1 by UBP12/UBP13 promotes brassinosteroid signaling and plant growth. Plant Commun. 2022, 3, 100348. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Y.; Cao, D.; Yang, H.; Oh, E.; Bi, Y.; Zhu, S.; Wang, Z.Y. The F-box Protein KIB1 Mediates Brassinosteroid-Induced Inactivation and Degradation of GSK3-like Kinases in Arabidopsis. Mol. Cell 2017, 66, 648–657.e4. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Z.; Wang, Y.; Liang, J. Scaffold protein RACK1 regulates BR signaling by modulating the nuclear localization of BZR1. New Phytol. 2023, 239, 1804–1818. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Fu, Y.; Zhou, H.; Zhou, Y.; Zhang, D.; Wang, Y.; Su, Y.; Li, Z.; Liang, J. RACK1 links phyB and BES1 to coordinate brassinosteroid-dependent root meristem development. New Phytol. 2024, 244, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Soares, T.F.S.N.; dos Santos Dias, D.C.F.; Oliveira, A.M.S.; Ribeiro, D.M.; dos Santos Dias, L.A. Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol. Environ. Saf. 2020, 193, 110296. [Google Scholar] [CrossRef]
- Lanza, M.; Garcia-Ponce, B.; Castrillo, G.; Catarecha, P.; Sauer, M.; Rodriguez-Serrano, M.; Páez-García, A.; Sánchez-Bermejo, E.; Tc, M.; del Puerto, Y.L.; et al. Role of actin cytoskeleton in brassinosteroid signaling and in its integration with the auxin response in plants. Dev. Cell 2012, 22, 1275–1285. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Xu, Z.-H.; Xue, H.-W. Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell 2005, 17, 2738–2753. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wu, B.; Du, F.; Guo, X.; Tian, C.; Hu, J.; Lü, S.; Long, M.; Zhang, L.; Wang, Y.; et al. A crosstalk between auxin and brassinosteroid regulates leaf shape by modulating growth anisotropy. Mol. Plant 2021, 14, 949–962. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Song, L.; Xue, H.W. Brassinosteroids regulate the differential growth of Arabidopsis hypocotyls through auxin signaling components IAA19 and ARF7. Mol. Plant 2013, 6, 887–904. [Google Scholar] [CrossRef]
- Yu, Z.; Ma, J.; Zhang, M.; Li, X.; Sun, Y.; Zhang, M.; Ding, Z. Auxin promotes hypocotyl elongation by enhancing BZR1 nuclear accumulation in Arabidopsis. Sci. Adv. 2023, 9, eade2493. [Google Scholar] [CrossRef]
- Cho, H.; Ryu, H.; Rho, S.; Hill, K.; Smith, S.; Audenaert, D.; Park, J.; Han, S.; Beeckman, T.; Bennett, M.J.; et al. A secreted peptide acts on BIN2-mediated phosphorylation of ARFs to potentiate auxin response during lateral root development. Nat. Cell Biol. 2014, 16, 66–76. [Google Scholar] [CrossRef]
- Sun, L.; Feraru, E.; Feraru, M.I.; Waidmann, S.; Wang, W.; Passaia, G.; Wang, Z.Y.; Wabnik, K.; Kleine-Vehn, J. PIN-LIKES Coordinate Brassinosteroid Signaling with Nuclear Auxin Input in Arabidopsis thaliana. Curr. Biol. 2020, 30, 1579–1588.e6. [Google Scholar] [CrossRef]
- Retzer, K.; Akhmanova, M.; Konstantinova, N.; Malínská, K.; Leitner, J.; Petrášek, J.; Luschnig, C. Brassinosteroid signaling delimits root gravitropism via sorting of the Arabidopsis PIN2 auxin transporter. Nat. Commun. 2019, 10, 5516. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Singh, I.K.; Singh, A. ABCB19 transporter: Fostering brassinosteroid transport through membrane flexibility. Trends Plant Sci. 2024, 29, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque-Martins, R.; Szakonyi, D.; Rowe, J.; Jones, A.M.; Duque, P. ABA signaling prevents phosphodegradation of the SR45 splicing factor to alleviate inhibition of early seedling development in Arabidopsis. Plant Commun. 2023, 4, 100495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liao, Q.; Ding, X.; Jia, Q.; Ding, Z. Research progress on the regulation of plant growth, development and stress resistance by brassinosteroids. Agric. Sci. 2020, 10, 407–418. [Google Scholar]
- Ha, Y.; Shang, Y.; Nam, K.H. Brassinosteroids modulate ABA-induced stomatal closure in Arabidopsis. J. Exp. Bot. 2016, 67, 6297–6308. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, D. BRASSINOSTEROID INSENSITIVE2 interacts with ABSCISIC ACID INSENSITIVE5 to mediate the antagonism of brassinosteroids to abscisic acid during seed germination in Arabidopsis. Plant Cell 2014, 26, 4394–4408. [Google Scholar] [CrossRef]
- Zhao, X.; Dou, L.; Gong, Z.; Wang, X.; Mao, T. BES1 hinders ABSCISIC ACID INSENSITIVE5 and promotes seed germination in Arabidopsis. New Phytol. 2019, 221, 908–918. [Google Scholar] [CrossRef]
- Chi, Y.; Yu, M.; Wang, Z.; Zhou, M.; Zhao, L.; Shi, J.; Wang, F.; Wang, C. Birch (Betula platyphylla) BES/BZR transcription factor BpBZR1-6 improves salt tolerance in transgenic Arabidopsis thaliana. BMC Plant Biol. 2024, 24, 1136. [Google Scholar] [CrossRef]
- Li, J.; Terzaghi, W.; Gong, Y.; Li, C.; Ling, J.J.; Fan, Y.; Qin, N.; Gong, X.; Zhu, D.; Deng, X.W. Modulation of BIN2 kinase activity by HY5 controls hypocotyl elongation in the light. Nat. Commun. 2020, 11, 1592. [Google Scholar] [CrossRef]
- Yuan, X.P.; Zhao, Y. SnRK2 kinases sense mol ecular crowding and form condensates to disrupt ABI1 inhibition. Sci. Adv. 2025, 11, eadr8250. [Google Scholar] [CrossRef]
- Ye, H.; Li, L.; Yin, Y. Recent advances in the regulation of brassinosteroid signaling and biosynthesis pathways. J. Integr. Plant Biol. 2011, 53, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.Y.; Shang, J.X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.P.; Wang, Z.Y. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, C.; Jiang, L.; Li, S.; Sun, S.S.; He, J.X. An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci. Signal. 2012, 5, ra72. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Patra, B.; Tang, Y.; Li, X.; Yuan, L.; Wang, X. A transcriptional hub integrating gibberellin-brassinosteroid signals to promote seed germination in Arabidopsis. J. Exp. Bot. 2021, 72, 4708–4720. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Chen, X.; Li, L.; Liu, K.; Zhao, H.; Wang, Y.; Han, S. ERF72 interacts with ARF6 and BZR1 to regulate hypocotyl elongation in Arabidopsis. J. Exp. Bot. 2018, 69, 3933–3947. [Google Scholar] [CrossRef]
- Bernardo-García, S.; de Lucas, M.; Martínez, C.; Espinosa-Ruiz, A.; Davière, J.M.; Prat, S. BR-dependent phosphorylation modulates PIF4 transcriptional activity and shapes diurnal hypocotyl growth. Genes. Dev. 2014, 28, 1681–1694. [Google Scholar] [CrossRef]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, I.; Pati, P.K. Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front. Plant Sci. 2015, 6, 950. [Google Scholar] [CrossRef]
- Hu, Y.; Bao, F.; Li, J. Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. Plant J. 2000, 24, 693–701. [Google Scholar] [CrossRef]
- Li, T.; Kang, X.; Lei, W.; Yao, X.; Zou, L.; Zhang, D.; Lin, H. SHY2 as a node in the regulation of root meristem development by auxin, brassinosteroids, and cytokinin. J. Integr. Plant Biol. 2020, 62, 1500–1517. [Google Scholar] [CrossRef] [PubMed]
- Zu, S.H.; Jiang, Y.T.; Chang, J.H.; Zhang, Y.J.; Xue, H.W.; Lin, W.H. Interaction of brassinosteroid and cytokinin promotes ovule initiation and increases seed number per silique in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, M.; Tian, Y.; Wang, Y.; Han, C.; Fan, M.; Guo, H.; Bai, M.Y. Interaction between BZR1 and EIN3 mediates signalling crosstalk between brassinosteroids and ethylene. New Phytol. 2021, 232, 2308–2323. [Google Scholar] [CrossRef]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Lone, W.; Majeed, N.; Yaqoob, U.; John, R. Exogenous brassinosteroid and jasmonic acid improve drought tolerance in Brassica rapa L. genotypes by modulating osmolytes, antioxidants and photosynthetic system. Plant Cell Rep. 2022, 41, 603–617. [Google Scholar] [CrossRef]
- Liao, K.; Peng, Y.J.; Yuan, L.B.; Dai, Y.S.; Chen, Q.F.; Yu, L.J.; Bai, M.Y.; Zhang, W.Q.; Xie, L.J.; Xiao, S. Brassinosteroids Antagonize Jasmonate-Activated Plant Defense Responses through BRI1-EMS-SUPPRESSOR1 (BES1). Plant Physiol. 2020, 182, 1066–1108. [Google Scholar] [CrossRef]
- Kim, B.; Fujioka, S.; Kwon, M.; Jeon, J.; Choe, S. Arabidopsis brassinosteroid-overproducing gulliver3-D/dwarf4-D mutants exhibit altered responses to jasmonic acid and pathogen. Plant Cell Rep. 2013, 32, 1139–1149. [Google Scholar] [CrossRef]
- Peng, Z.; Han, C.; Yuan, L.; Zhang, K.; Huang, H.; Ren, C. Brassinosteroid enhances jasmonate-induced anthocyanin accumulation in Arabidopsis seedlings. J. Integr. Plant Biol. 2011, 53, 632–640. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, L.; Li, X.; Zhang, Y. Salicylic acid: The roles in plant immunity and crosstalk with other hormones. J. Integr. Plant Biol. 2025, 67, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Park, C.H.; Hsu, C.C.; Kim, Y.W.; Ko, Y.W.; Zhang, Z.; Zhu, J.Y.; Hsiao, Y.C.; Branon, T.; Kaasik, K.; et al. Mapping the signaling network of BIN2 kinase using TurboID-mediated biotin labeling and phosphoproteomics. Plant Cell 2023, 35, 975–993. [Google Scholar] [CrossRef]
- Kim, Y.W.; Youn, J.H.; Roh, J.; Kim, J.M.; Kim, S.K.; Kim, T.W. Brassinosteroids enhance salicylic acid-mediated immune responses by inhibiting BIN2 phosphorylation of clade I TGA transcription factors in Arabidopsis. Mol. Plant 2022, 15, 991–1007. [Google Scholar] [CrossRef]
- Han, Q.; Tan, W.; Zhao, Y.; Yang, F.; Yao, X.; Lin, H.; Zhang, D. Salicylic acid-activated BIN2 phosphorylation of TGA3 promotes Arabidopsis PR gene expression and disease resistance. EMBO J. 2022, 41, e110682. [Google Scholar] [CrossRef]
- Mishra, S.; Spaccarotella, K.; Gido, J.; Samanta, I.; Chowdhary, G. Effects of Heat Stress on Plant-Nutrient Relations: An Update on Nutrient Uptake, Transport, and Assimilation. Int. J. Mol. Sci. 2023, 24, 15670. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Halder, K.; Abdin, M.Z.; Majee, M.; Datta, A. Abiotic Stress Tolerance in Plants: Brassinosteroids Navigate Competently. Int. J. Mol. Sci. 2022, 23, 14577. [Google Scholar] [CrossRef]
- He, Z.; Zhou, M.; Feng, X.; Di, Q.; Meng, D.; Yu, X.; Yan, Y.; Sun, M.; Li, Y. The Role of Brassinosteroids in Plant Cold Stress Response. Life 2024, 14, 1015. [Google Scholar] [CrossRef]
- Xia, X.; Fang, P.; Guo, X.; Qian, X.; Zhou, J.; Shi, K.; Zhou, Y.; Yu, J. Brassinosteroid-Mediated Apoplastic H2O2-Glutaredoxin 12/14 Cascade Regulates Antioxidant Capacity in Response to Chilling in Tomato. Plant Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef]
- Zhao, M.; Yuan, L.; Wang, J.; Xie, S.; Zheng, Y.; Nie, L.; Zhu, S.; Hou, J.; Chen, G.; Wang, C. Transcriptome analysis reveals a positive effect of brassinosteroids on the photosynthetic capacity of wucai under low temperature. BMC Genom. 2019, 20, 810. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Ma, C.; Kang, X.; Wang, J.; Zhang, T. 24-epibrassinolide enhanced cold tolerance of winter turnip rape (Brassica rapa L.). Biologia 2021, 76, 2859–2877. [Google Scholar] [CrossRef]
- Gao, X.; Ma, J.; Tie, J.; Li, Y.; Hu, L.; Yu, J. BR-Mediated Protein S-Nitrosylation Alleviated Low-Temperature Stress in Mini Chinese Cabbage (Brassica rapa ssp. pekinensis). Int. J. Mol. Sci. 2022, 23, 10964. [Google Scholar] [CrossRef]
- Stachurska, J.; Rys, M.; Pociecha, E.; Kalaji, H.M.; Dąbrowski, P.; Oklestkova, J.; Jurczyk, B.; Janeczko, A. Deacclimation-Induced Changes of Photosynthetic Efficiency, Brassinosteroid Homeostasis and BRI1 Expression in Winter Oilseed Rape (Brassica napus L.)-Relation to Frost Tolerance. Int. J. Mol. Sci. 2022, 23, 5224. [Google Scholar] [CrossRef]
- Stachurska, J.; Sadura, I.; Jurczyk, B.; Rudolphi-Szydło, E.; Dyba, B.; Pociecha, E.; Ostrowska, A.; Rys, M.; Kvasnica, M.; Oklestkova, J.; et al. Cold Acclimation and Deacclimation of Winter Oilseed Rape, with Special Attention Being Paid to the Role of Brassinosteroids. Int. J. Mol. Sci. 2024, 25, 6010. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR Signaling Cascade and Its Regulation in Plants Responding to Cold Stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef] [PubMed]
- Kagale, S.; Divi, U.; Krochko, J.; Keller, W.; Krishna, P. Brassinosteroid Confers Tolerance in Arabidopsis Thaliana and Brassica Napus to a Range of Abiotic Stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The Physiological and Molecular Mechanism of Brassinosteroid in Response to Stress: A Review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, X.; Shi, Z.; He, F.; Qi, G.; Li, X.; Niu, Y.; Zhou, W. Exogenous 24-Epibrassinolide Improves Low-Temperature Tolerance of Maize Seedlings by Influencing Sugar Signaling and Metabolism. Int. J. Mol. Sci. 2025, 26, 585. [Google Scholar] [CrossRef]
- Nie, W.; He, Q.; Ma, J.; Guo, H.; Shi, Q. Exogenous 2,4-Epibrassinolide Alleviates Alkaline Stress in Cucumber by Modulating Photosynthetic Performance. Plants 2024, 14, 54. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant 2021, 172, 696–706. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Z.; Feng, K.; Ji, Y.; Xu, Y.; Tu, D.; Teng, B.; Liu, Q.; Liu, J.; Zhou, Y.; et al. Strategies for indica rice adapted to high-temperature stress in the middle and lower reaches of the Yangtze River. Front. Plant Sci. 2023, 13, 1081807. [Google Scholar] [CrossRef]
- Dhaubhadel, S.; Browning, K.S.; Gallie, D.R.; Krishna, P. Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J. 2002, 29, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.-J.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Chen, X.; Xue, H.; Zhu, L.; Wang, H.; Long, H.; Zhao, J.; Meng, F.; Liu, Y.; Ye, Y.; Luo, X.; et al. ERF49 mediates brassinosteroid regulation of heat stress tolerance in Arabidopsis thaliana. BMC Biol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Krishna, P.; Prasad, B.D.; Rahman, T. Brassinosteroid Action in Plant Abiotic Stress Tolerance. Methods Mol. Biol. 2017, 1564, 193–202. [Google Scholar] [PubMed]
- Sahni, S.; Prasad, B.D.; Liu, Q.; Grbic, V.; Sharpe, A.; Singh, S.P.; Krishna, P. Overexpression of the brassinosteroid biosynthetic gene DWF4 in Brassica napus simultaneously increases seed yield and stress tolerance. Sci. Rep. 2016, 6, 28298. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694, Erratum in Nat. Rev. Mol. Cell Biol. 2022, 23, 516. [Google Scholar] [CrossRef]
- Pavlović, I.; Petřík, I.; Tarkowská, D.; Lepeduš, H.; Vujčić Bok, V.; Radić Brkanac, S.; Novák, O.; Salopek-Sondi, B. Correlations between Phytohormones and Drought Tolerance in Selected Brassica Crops: Chinese Cabbage, White Cabbage and Kale. Int. J. Mol. Sci. 2018, 19, 2866. [Google Scholar] [CrossRef] [PubMed]
- Mira, M.; Ibrahim, S.; So, K.; Kowatsch, R.; Duncan, R.W.; Hill, R.D.; Stasolla, C. Specificity in root domain accumulation of Phytoglobin1 and nitric oxide (NO) determines meristematic viability in water-stressed Brassica napus roots. Ann. Bot. 2023, 131, 475–490. [Google Scholar] [CrossRef]
- Surina, S.; Yamagami, A.; Miyaji, T.; Chagan, Z.; Chung, K.; Mitsuda, N.; Nishida, K.; Tachibana, R.; Zhu, Z.; Miyakawa, T.; et al. BIL9 Promotes Both Plant Growth via BR Signaling and Drought Stress Resistance by Binding with the Transcription Factor HDG11. Plant Cell Physiol. 2024, 65, 1640–1654. [Google Scholar] [CrossRef]
- Saha, G.; Park, J.I.; Jung, H.J.; Ahmed, N.U.; Kayum, M.A.; Kang, J.G.; Nou, I.S. Molecular characterization of BZR transcription factor family and abiotic stress induced expression profiling in Brassica rapa. Plant Physiol. Biochem. 2015, 92, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Göre, M. Mitigation of salt stress in Camelina sativa by epibrassinolide and salicylic acid treatments. Sci. Rep. 2025, 15, 7965. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zheng, X.; Tian, Y.; Wang, C. Exogenous Brassinolide Alleviates Salt Stress in Malus hupehensis Rehd. by Regulating the Transcription of NHX-Type Na+(K+)/H+ Antiporters. Front. Plant Sci. 2020, 11, 38. [Google Scholar] [CrossRef]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Bok, V.V.; Brkanac, S.R.; Strnad, M.; Salopek-Sondi, B. Early Brassica Crops Responses to Salinity Stress: A Comparative Analysis Between Chinese Cabbage, White Cabbage, and Kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef]
- Wang, X.; Chai, J.; Liu, W.; Zhu, X.; Liu, H.; Wei, X. Promotion of Ca2+ Accumulation in Roots by Exogenous Brassinosteroids as a Key Mechanism for Their Enhancement of Plant Salt Tolerance: A Meta-Analysis and Systematic Review. Int. J. Mol. Sci. 2023, 24, 16123. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Fang, S.; Chen, H.; Cai, W. The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. 2020, 104, 59–75. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, X.; Wang, X.N.; Liu, H.F.; Zhang, T.T.; Wang, D.R.; Liu, G.D.; Liu, Y.Q.; Song, X.H.; Zhang, Z.; et al. Brassinosteroids biosynthetic gene MdBR6OX2 regulates salt stress tolerance in both apple and Arabidopsis. Plant Physiol. Biochem. 2024, 212, 108767. [Google Scholar] [CrossRef]
- Liu, J.; Yang, R.; Jian, N.; Wei, L.; Ye, L.; Wang, R.; Gao, H.; Zheng, Q. Putrescine metabolism modulates the biphasic effects of brassinosteroids on canola and Arabidopsis salt tolerance. Plant Cell Environ. 2020, 43, 1348–1359. [Google Scholar] [CrossRef]
- Kaur, H.; Sirhindi, G.; Bhardwaj, R.; Alyemeni, M.N.; Siddique, K.H.M.; Ahmad, P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci. Rep. 2018, 8, 8735. [Google Scholar] [CrossRef]
- Liu, X.; Liang, D.; Song, W.; Wang, X.; Duan, W.; Wang, C.; Wang, P. Tobacco roots increasing diameter and secondary lateral density in response to drought stress. Plant Physiol. Biochem. 2023, 204, 108122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Wang, L.; Zhang, Y.; Zhou, D.; Anwar, A.; Li, J.; Wang, F.; Li, C.; Zhang, Y.; et al. Comparative Transcriptome and Co-Expression Network Analyses Reveal the Molecular Mechanism of Calcium-Deficiency-Triggered Tipburn in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). Plants 2022, 11, 3555. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Tang, Z.; Xiao, X.; Gao, X.; Qiao, Y.; Ma, J.; Hu, L.; Yu, J. Exogenous brassinosteroid alleviates calcium deficiency induced tip-burn by regulating calcium transport in Brassica rapa L. ssp. pekinensis. Ecotoxicol. Environ. Saf. 2023, 251, 114534. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2018, 255, 11–24. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Bhardwaj, R.; Gupta, B.D.; Dutt, P.; Gupta, R.K.; Biondi, S.; Kanwar, M. Epibrassinolide induces changes in indole-3-acetic acid, abscisic acid and polyamine concentrations and enhances antioxidant potential of radish seedlings under copper stress. Physiol. Plant 2010, 140, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.P.; Oral, H.V.; Bhardwaj, R.; Yu, J.-Q.; Tran, L.-S.P. Interaction of Brassinosteroids and Polyamines Enhances Copper Stress Tolerance in Raphanus sativus. J. Exp. Bot. 2012, 63, 5659–5675. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, T.A.; Fariduddin, Q. Interaction of epibrassinolide and selenium ameliorates the excess copper in Brassica juncea through altered proline metabolism and antioxidants. Ecotoxicol. Environ. Saf. 2016, 129, 25–34. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Kanwar, M.; Bhardwaj, R.; Yu, J.Q.; Tran, L.S. Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE 2012, 7, e33210. [Google Scholar] [CrossRef]
- Sharma, I.; Pati, P.K.; Bhardwaj, R. Effect of 28-homobrassinolide on antioxidant defence system in Raphanus sativus L. under chromium toxicity. Ecotoxicology 2011, 20, 862–874. [Google Scholar] [CrossRef]
- Ramakrishna, B.; Rao, S.S. Foliar application of brassinosteroids alleviates adverse effects of zinc toxicity in radish (Raphanus sativus L.) plants. Protoplasma 2015, 252, 665–677. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Fonne-Pfister, R.; Screpanti, C.; De Mesmaeker, A. Strigolactones: Plant Hormones with Promising Features. Angew. Chem. Int. Ed. Engl. 2019, 58, 12778–12786. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, J.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 2002, 110, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Liu, X.; Gong, W.; Zhang, C. Synthesis of brassinosteroids analogues from laxogenin and their plant growth promotion. Nat. Prod. Res. 2015, 29, 149–157. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, W.; Cui, X.; Chen, M.; Yin, C.; Luo, Z.; Zhu, J.; Lucas, W.J.; Wang, Z.; Zhang, D. Brassinosteroids promote development of rice pollen grains and seeds by triggering expression of Carbon Starved Anther, a MYB domain protein. Plant J. 2015, 82, 570–581. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, W.; Liu, D.; Pan, D.; Yang, Y.; Chen, Z.; Ma, X.; Yin, W.; Niu, M.; Dong, N.; et al. Enhancing rice panicle branching and grain yield through tissue-specific brassinosteroid inhibition. Science 2024, 383, eadk8838. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Ma, C.; Chen, B.; Sun, Z.; Tian, Y.; Wang, C. A mutation in the promoter of the arabinogalactan protein 7-like gene PcAGP7-1 affects cell morphogenesis and brassinolide content in pear (Pyrus communis L.) stems. Plant J. 2022, 109, 47–63. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Ma, C.; Xie, Y.; Zeng, Y.; Peng, J.; Zhou, D.; Wu, J. Understanding the Brassinosteroid-Dependent Environmental Adaption in Brassicaceae Plants. Plants 2025, 14, 1554. https://doi.org/10.3390/plants14101554
Lu Z, Ma C, Xie Y, Zeng Y, Peng J, Zhou D, Wu J. Understanding the Brassinosteroid-Dependent Environmental Adaption in Brassicaceae Plants. Plants. 2025; 14(10):1554. https://doi.org/10.3390/plants14101554
Chicago/Turabian StyleLu, Zhenni, Changrui Ma, Yuzhen Xie, Yuqing Zeng, Jiashi Peng, Dinggang Zhou, and Jinfeng Wu. 2025. "Understanding the Brassinosteroid-Dependent Environmental Adaption in Brassicaceae Plants" Plants 14, no. 10: 1554. https://doi.org/10.3390/plants14101554
APA StyleLu, Z., Ma, C., Xie, Y., Zeng, Y., Peng, J., Zhou, D., & Wu, J. (2025). Understanding the Brassinosteroid-Dependent Environmental Adaption in Brassicaceae Plants. Plants, 14(10), 1554. https://doi.org/10.3390/plants14101554





