Integrated Analyses Reveal the Physiological and Molecular Mechanisms of Brassinolide in Modulating Salt Tolerance in Rice
Abstract
1. Introduction
2. Results
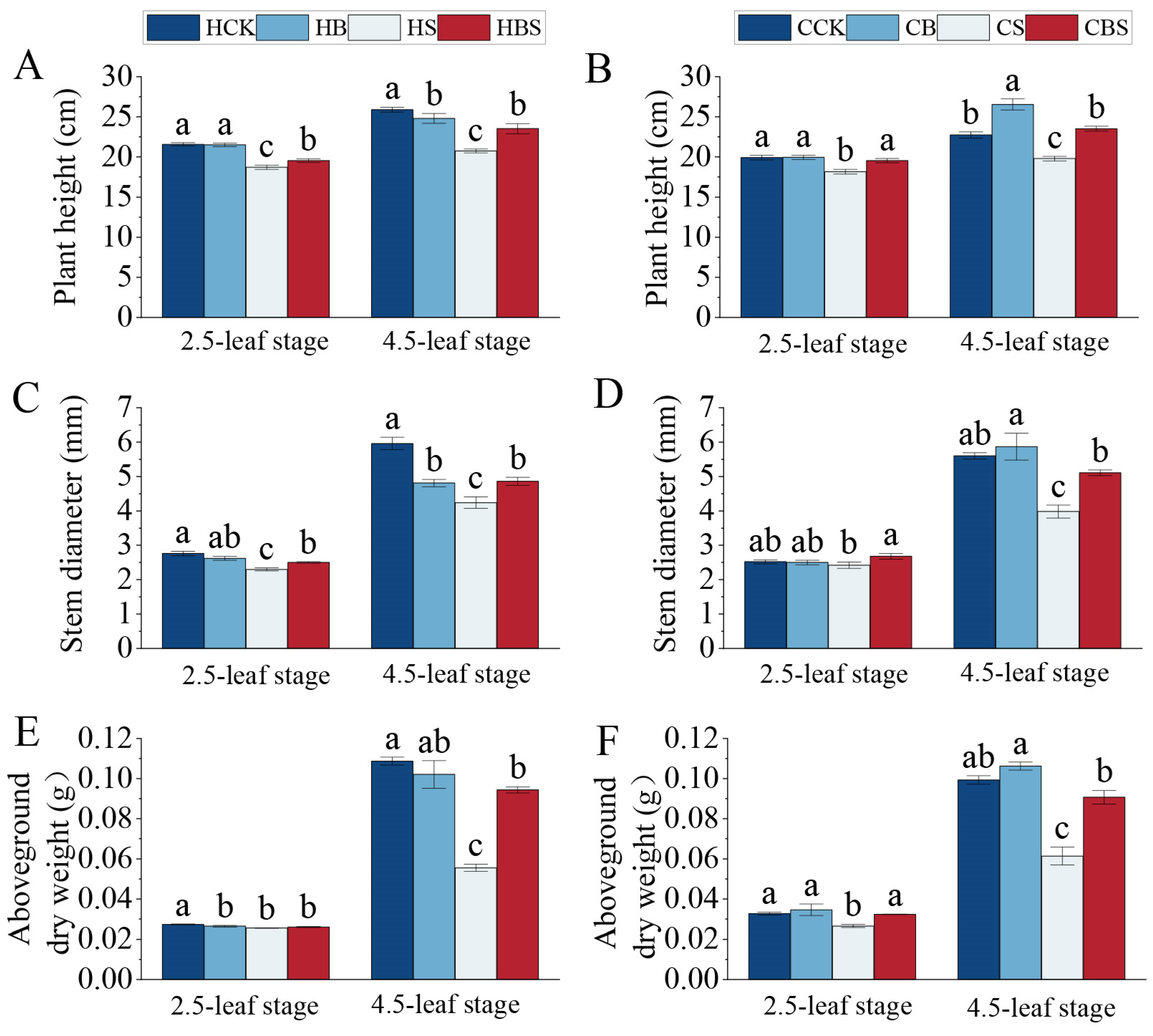
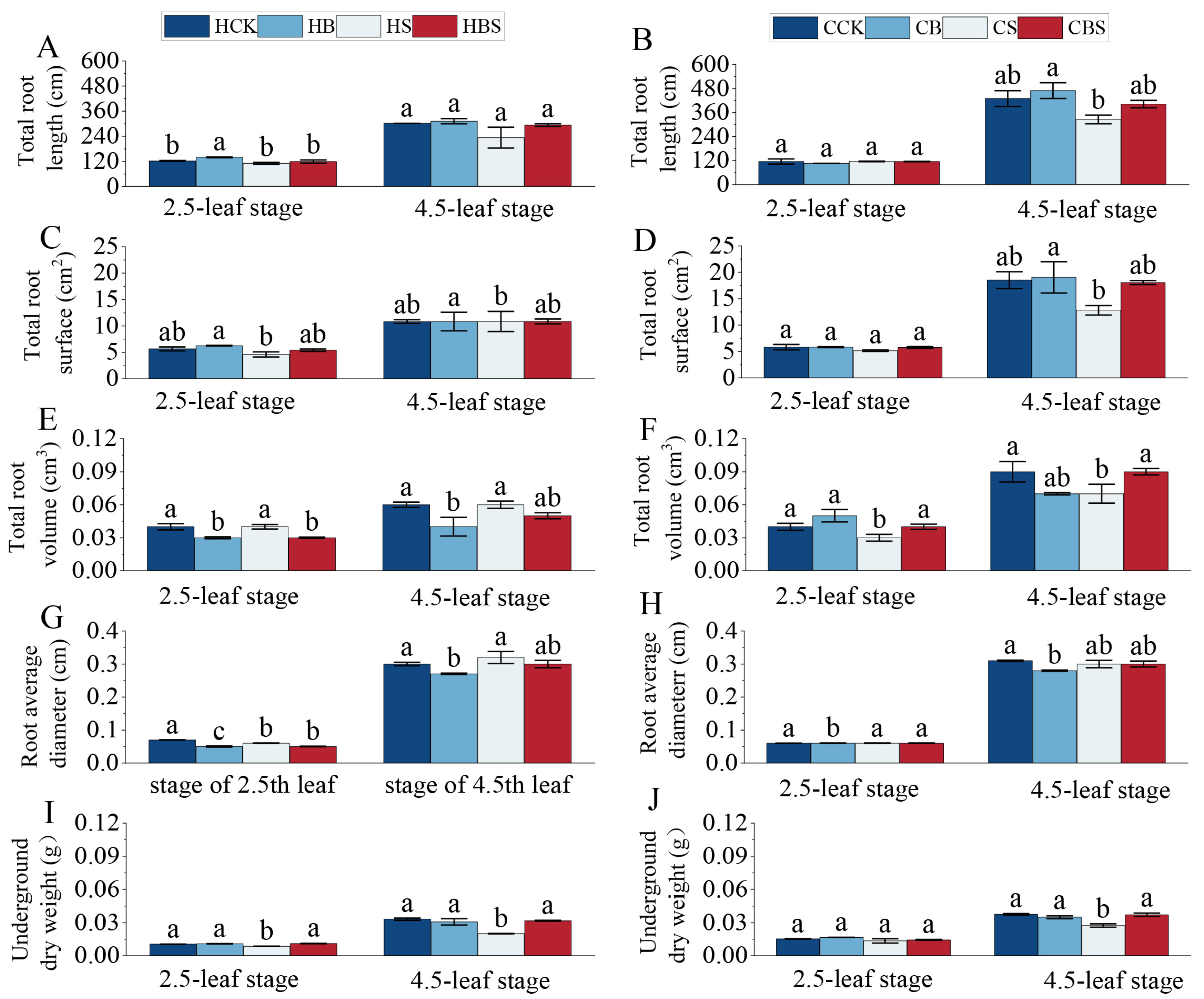
2.1. Effect of BR on Rice Seedling Phenotypes Under Salt Stress
2.2. Effect of BR on Membrane Peroxidation and the Antioxidant System of Rice Seedlings Under Salt Stress
2.3. Effect of BR on the Ion Homeostasis of Rice Seedling Leaves Under Salt Stress
2.4. Effects of BR on Photosynthesis and the Fluorescence-Related Parameters of Rice Leaves Under Salt Stress
2.5. RT-qPCR Verification
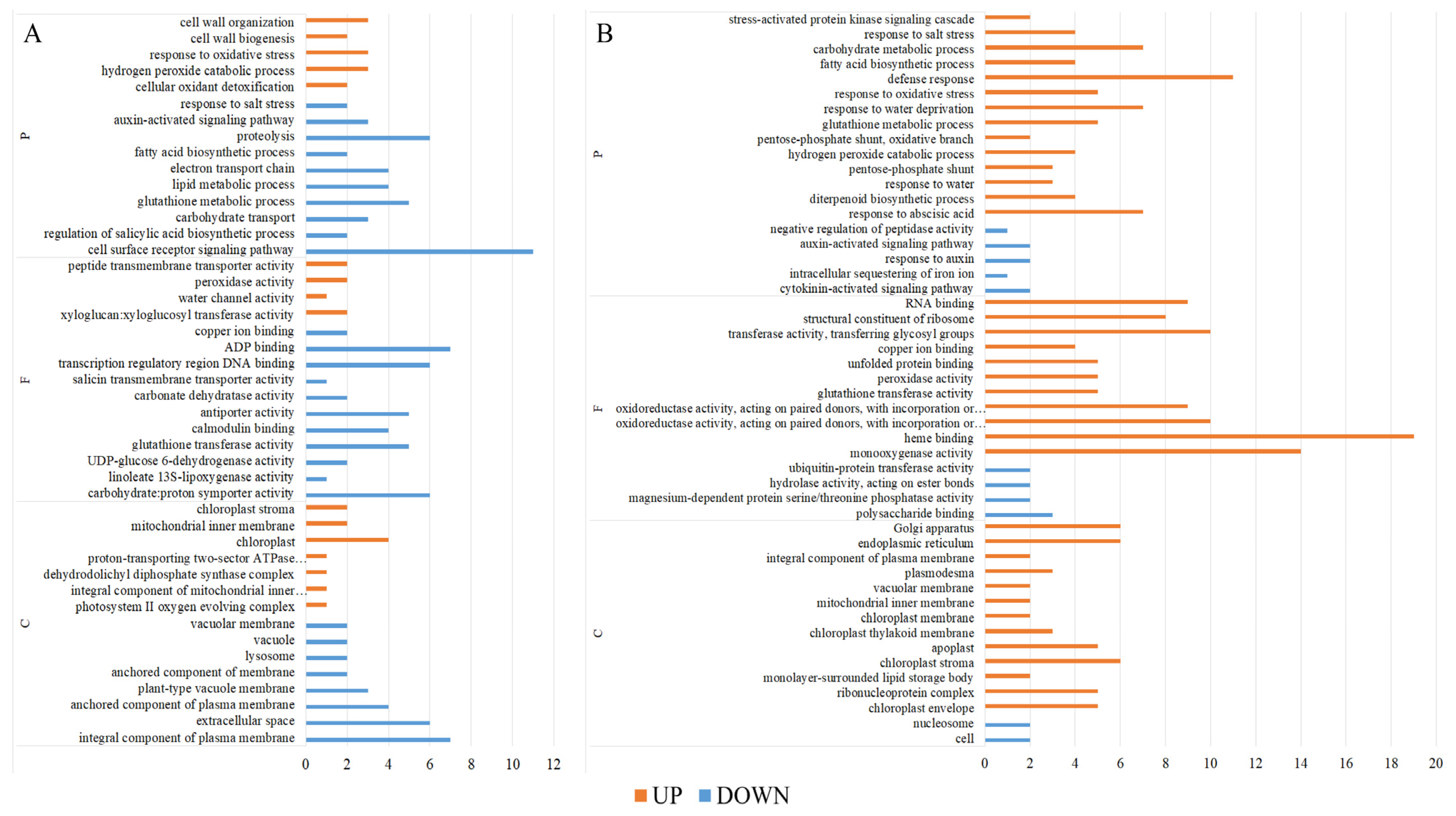
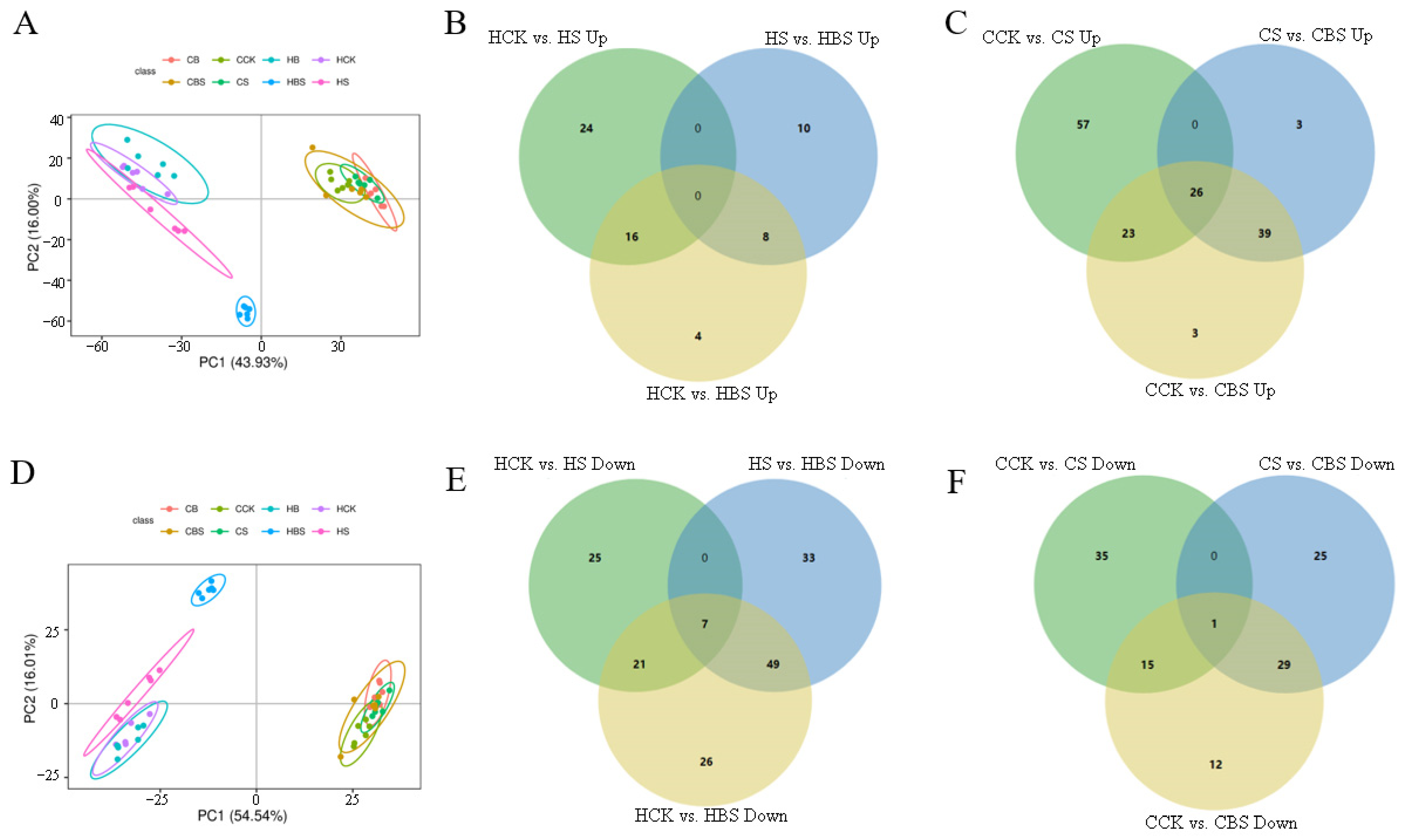
2.6. Transcriptomic Response of Rice Seedlings Under Salt Stress and Brassinolide Treatment
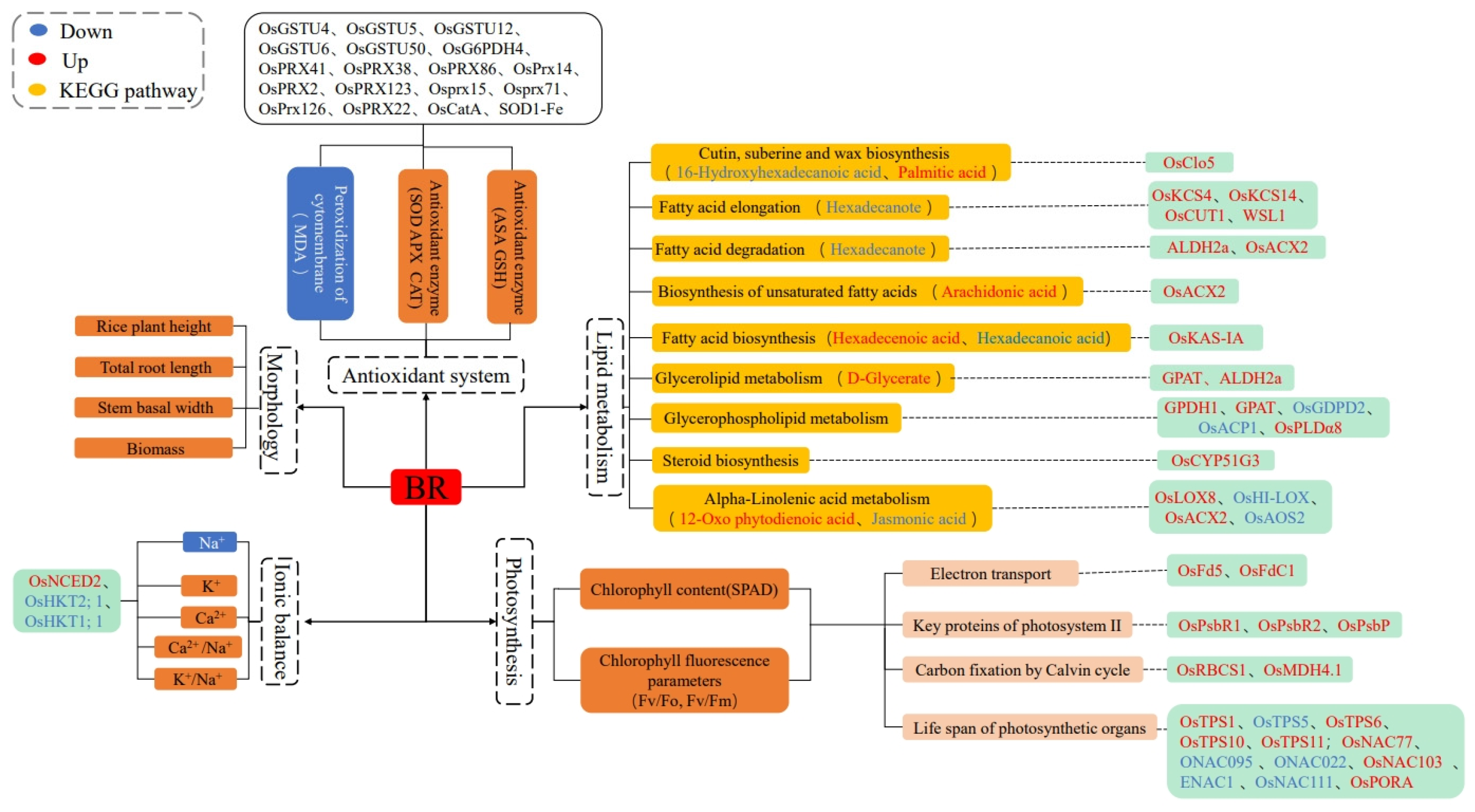
2.7. BR Regulates Functional Genes to Alleviate Salt Stress in Rice
2.8. Effect of BR Treatment on the Metabolome of Rice Seedlings Under Salt Stress
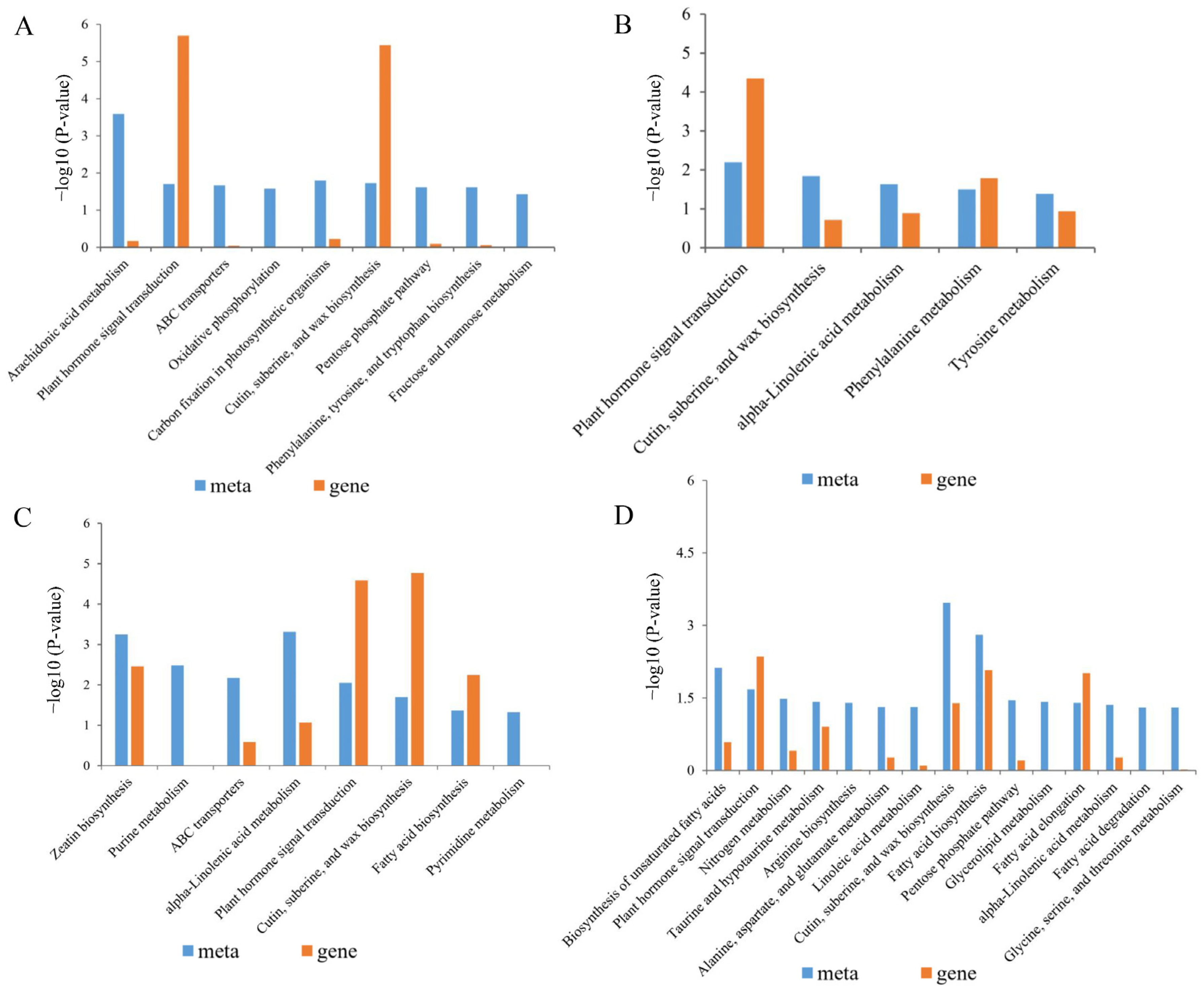
2.9. Combined Analysis of the Transcriptome and Metabolome of Rice Treated with BR Under Salt Stress
3. Discussion
3.1. Exogenous Application of BR to Mitigate the Effects of Salt Stress on Rice Phenotypes
3.2. Exogenous Application of BR Improves Membrane Peroxidation and the Antioxidant System in Rice Under Salt Stress
3.3. Exogenous Application of BR Improves Ionic Homeostasis in Rice Under Salt Stress
3.4. The Exogenous Application of BR Improves Photosynthesis in Rice Under Salt Stress
3.5. Roles of JA and Lipid Metabolism in Salt Stress
4. Materials and Methods
4.1. Plant Growth and Treatment
4.2. Measurement of Rice Seedling Growth Indicators
4.3. Determination of the Root Morphology of Rice Seedlings
4.4. Measurement of Oxidative Stress and Antioxidant Indices
4.5. Determination of the Ion Contents in the Leaves of Rice Seedlings
4.6. Determination of the SPAD Value of Rice Seedling Leaves
4.7. Determination of Chlorophyll Fluorescence Parameters
4.8. Total RNA Isolation and Transcriptome Analysis
4.9. Real-Time Quantitative Fluorescence PCR (qRT-PCR) Validation
4.10. Metabolite Extraction and Metabolomic Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.; Shabala, S.; Niu, Y.; Chen, Z.-H.; Shabala, L.; Meinke, H.; Venkataraman, G.; Pareek, A.; Xu, J.; Zhou, M. Molecular mechanisms of salinity tolerance in rice. Crop J. 2021, 9, 506–520. [Google Scholar] [CrossRef]
- Xu, Y.; Bu, W.; Xu, Y.; Fei, H.; Zhu, Y.; Ahmad, I.; Nimir, N.E.A.; Zhou, G.; Zhu, G. Effects of salt stress on physiological and agronomic traits of rice genotypes with contrasting salt tolerance. Plants 2024, 13, 1157. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Luo, N.; Meng, X.; Song, X.; Jing, Y.; Kou, L.; Liu, G.; Huang, X.; Wang, Y.; Li, J.; et al. OsMPK4 promotes phosphorylation and degradation of IPA1 in response to salt stress to confer salt tolerance in rice. J. Genet. Genom. 2022, 49, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Huang, S.-C.; Chen, Y.-F.; Kuo, C.-W.; Chen, Y.-H.; Chang, M.-C. Overexpression of OsERF106MZ promotes parental root growth in rice seedlings by relieving the ABA-mediated inhibition of root growth under salinity stress conditions. BMC Plant Biol. 2023, 23, 144. [Google Scholar] [CrossRef]
- Shakri, T.; Che-Othman, M.H.; Md Isa, N.; Sukiran, N.L.; Zainal, Z. Morpho-physiological and stress-related gene expression of rice varieties in response to salinity stress at early vegetative stage. Agriculture 2022, 12, 638. [Google Scholar] [CrossRef]
- Ye, X.; Tie, W.; Xu, J.; Ding, Z.; Hu, W. Comparative transcriptional analysis of two contrasting rice genotypes in response to salt stress. Agronomy 2022, 12, 1163. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Bajguz, A.; Zhou, J.; Bhardwaj, R. Analysis of brassinosteroids in plants. J. Plant Growth Regul. 2017, 36, 1002–1030. [Google Scholar] [CrossRef]
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R. Brassins—A new family of plant hormones from rape pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Al-Taey, D.K.; Al-Musawi, Z.J.; Kadium, S.M.A.; Abbas, A.K.; Alsaffar, M.F.; Mahmood, S.S. Brassinolides’ Function and Involvement in Salt Stress Response: A Review. IOP Conf. Ser. Earth Environ. Sci. 2024, 1371, 042032. [Google Scholar] [CrossRef]
- Mu, D.; Feng, N.; Zheng, D.; Zhou, H.; Liu, L.; Chen, G. Studies on the physiological mechanism of brassinolide to improve the resistance of rice seedlings to NaCl stress. Water Air Soil Pollut. 2022, 233, 238. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Z.; Zhang, D.; Liu, S.; Chen, Z.; Wu, Q.; Shang, Z.; Wang, J.; Wang, J. BR regulates wheat root salt tolerance by maintaining ROS homeostasis. Planta 2024, 260, 5. [Google Scholar] [CrossRef]
- Yang, C.; Shen, S.; Zhou, S.; Li, Y.; Mao, Y.; Zhou, J.; Shi, Y.; An, L.; Zhou, Q.; Peng, W. Rice metabolic regulatory network spanning the entire life cycle. Mol. Plant 2022, 15, 258–275. [Google Scholar] [CrossRef]
- Du, Y.-W.; Liu, L.; Feng, N.-J.; Zheng, D.-F.; Liu, M.-L.; Zhou, H.; Deng, P.; Wang, Y.-X.; Zhao, H.-M. Combined transcriptomic and metabolomic analysis of alginate oligosaccharides alleviating salt stress in rice seedlings. BMC Plant Biol. 2023, 23, 455. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, J.; Meng, W.; Sun, Z.; Tan, Y.; Liu, Y.; Tan, M.; Wang, B.; Yang, J. Integrated Analysis of Transcriptome and Metabolome Reveals Molecular Mechanisms of Rice with Different Salinity Tolerances. Plants 2023, 12, 3359. [Google Scholar] [CrossRef]
- Nassar, R.M.; Kamel, H.A.; Ghoniem, A.E.; Alarcón, J.J.; Sekara, A.; Ulrichs, C.; Abdelhamid, M.T. Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants 2020, 9, 237. [Google Scholar] [CrossRef]
- Sun, X.; Zheng, H.X.; Li, S.; Gao, Y.; Dang, Y.; Chen, Z.; Wu, F.; Wang, X.; Xie, Q.; Sui, N. MicroRNAs balance growth and salt stress responses in sweet sorghum. Plant J. 2023, 113, 677–697. [Google Scholar] [CrossRef]
- Liu, A.; Xiao, Z.; Li, M.W.; Wong, F.L.; Yung, W.S.; Ku, Y.S.; Wang, Q.; Wang, X.; Xie, M.; Yim, A.K.; et al. Transcriptomic reprogramming in soybean seedlings under salt stress. Plant Cell Environ. 2019, 42, 98–114. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.-h.; Zhong, C.; Zhu, L.-F.; Cao, X.-C.; Yu, S.-M.; Bohr, J.A.; Hu, J.-J.; Jin, Q.-Y. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, D.; Feng, N.; Qiu, Q.-S.; Zhou, H.; Meng, F.; Huang, X.; Huang, A.; Li, Y. Prohexadione-calcium alleviates the leaf and root damage caused by salt stress in rice (Oryza sativa L.) at the tillering stage. PLoS ONE 2023, 18, e0279192. [Google Scholar] [CrossRef]
- Shi, B.; Li, K.; Xu, R.; Zhang, F.; Yu, Z.; Ding, Z.; Tian, H. Me thionine-mediated trade-off between plant growth and salt tolerance. Plant Physiol. 2025, 197, kiaf074. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Kappachery, S.; Karumannil, S.; AlHosani, M.; Almansoori, N.; Almansoori, H.; Yusuf, M.; Tran, L.-S.P.; Gururani, M.A. Brassinosteroid signaling pathways: Insights into plant responses under abiotic stress. Int. J. Mol. Sci. 2023, 24, 17246. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.P.; Lima, M.D.R.; da Silva, B.R.S.; Batista, B.L.; da Silva Lobato, A.K. Brassinosteroids confer tolerance to salt stress in Eucalyptus urophylla plants enhancing homeostasis, antioxidant metabolism and leaf anatomy. J. Plant Growth Regul. 2019, 38, 557–573. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef]
- Khan, Z.; Jan, R.; Asif, S.; Farooq, M.; Jang, Y.-H.; Kim, E.-G.; Kim, N.; Kim, K.-M. Exogenous melatonin induces salt and drought stress tolerance in rice by promoting plant growth and defense system. Sci. Rep. 2024, 14, 1214. [Google Scholar] [CrossRef]
- Kordrostami, M.; Rabiei, B.; Kumleh, H.H. Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at the seedling stage. Physiol. Mol. Biol. Plants 2017, 23, 529–544. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef]
- Gao, W.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef]
- Valério, L.; De Meyer, M.; Penel, C.; Dunand, C. Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 2004, 65, 1331–1342. [Google Scholar] [CrossRef]
- Liu, L.; Han, T.; Liu, W.; Han, G.; Di, P.; Yu, X.; Yan, J.; Zhang, A. Thr420 and Ser454 of ZmCCaMK play a crucial role in brassinosteroid-induced antioxidant defense in maize. Biochem. Biophys. Res. Commun. 2020, 525, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Liu, L.; Rupasinghe, T.W.T.; Roessner, U.; Barkla, B.J. Salt stress alters membrane lipid content and lipid biosynthesis pathways in the plasma membrane and tonoplast. Plant Physiol. 2022, 189, 805–826. [Google Scholar] [CrossRef] [PubMed]
- Rudolphi-Szydo, E.; Dyba, B.; Janeczko, A.; Latowski, D.; Sadura, I.; Filek, M. Brassinosteroid-lipid membrane interaction under low and high temperature stress in model systems. BMC Plant Biol. 2022, 22, 246. [Google Scholar] [CrossRef]
- Sun, M.; Liu, X.; Gao, H.; Zhang, B.; Peng, F.; Xiao, Y. Phosphatidylcholine enhances homeostasis in peach seedling cell membrane and increases its salt stress tolerance by phosphatidic acid. Int. J. Mol. Sci. 2022, 23, 2585. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in Plant Tolerance to Abiotic Stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Cimini, S.; Locato, V.; Giacinti, V.; Molinari, M.; De Gara, L. A multifactorial regulation of glutathione metabolism behind salt tolerance in rice. Antioxidants 2022, 11, 1114. [Google Scholar] [CrossRef]
- Tang, J.; Li, M.; Mao, P.; Jiang, Y. Effects of gamma-aminobutyric acid on seed germination, ion balance, and metabolic activity in perennial ryegrass under salinity stress. J. Plant Growth Regul. 2022, 41, 1835–1844. [Google Scholar] [CrossRef]
- Huang, L.; Wu, D.-Z.; Zhang, G.-P. Advances in studies on ion transporters involved in salt tolerance and breeding crop cultivars with high salt tolerance. J. Zhejiang Univ.-Sci. B 2020, 21, 426–441. [Google Scholar] [CrossRef]
- Yang, C.; Shi, D.; Wang, D. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Manishankar, P.; Wang, N.; Köster, P.; Alatar, A.A.; Kudla, J. Calcium signaling during salt stress and in the regulation of ion homeostasis. J. Exp. Bot. 2018, 69, 4215–4226. [Google Scholar] [CrossRef] [PubMed]
- Haubrick, L.L.; Torsethaugen, G.; Assmann, S.M. Effect of brassinolide, alone and in concert with abscisic acid, on control of stomatal aperture and potassium currents of Vicia faba guard cell protoplasts. Physiol. Plant. 2006, 128, 134–143. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular mechanism for the regulation of ABA homeostasis during plant development and stress responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, N.; Zhang, J. Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol. 2009, 50, 644–651. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, Y.; Xie, Z.; Yang, Y.; Lu, G.; Jin, Y.; Wang, M.; Liu, M.; Yang, H.; Li, W. Overexpression of abscisic acid biosynthesis gene OsNCED3 enhances survival rate and tolerance to alkaline stress in rice seedlings. Plants 2024, 13, 1713. [Google Scholar] [CrossRef]
- Huang, L.; Bao, Y.; Qin, S.; Ning, M.; Li, Q.; Li, Q.; Zhang, S.; Huang, G.; Zhang, J.; Wang, W. The aba synthesis enzyme allele OsNCED2T promotes dryland adaptation in upland rice. Crop J. 2024, 12, 68–78. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Jabnoune, M.; Espeout, S.; Mieulet, D.; Fizames, C.; Verdeil, J.-L.; Conéjéro, G.; Rodríguez-Navarro, A.; Sentenac, H.; Guiderdoni, E.; Abdelly, C. Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol. 2009, 150, 1955–1971. [Google Scholar] [CrossRef]
- Yao, X.; Horie, T.; Xue, S.; Leung, H.-Y.; Katsuhara, M.; Brodsky, D.E.; Wu, Y.; Schroeder, J.I. Differential sodium and potassium transport selectivities of the rice OsHKT2; 1 and OsHKT2; 2 transporters in plant cells. Plant Physiol. 2010, 152, 341–355. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of low-temperature stress and brassinolide application on the photosynthesis and leaf structure of tung tree seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Zhao, H.-H.; Zhao, L.-M.; Gu, C.-M.; Na, Y.-G.; Baosheng, X.; Cheng, S.-H.; Pan, G.-J. Application of brassinolide alleviates cold stress at the booting stage of rice. J. Integr. Agric. 2020, 19, 975–987. [Google Scholar] [CrossRef]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yang, D. Exogenous Brassinolide Enhances the Growth and Cold Resistance of Maize (Zea mays L.) Seedlings under Chilling Stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef]
- Kozuleva, M.A.; Ivanov, B.N.; Vetoshkina, D.V.; Borisova-Mubarakshina, M.M. Minimizing an electron flow to molecular oxygen in photosynthetic electron transfer chain: An evolutionary view. Front. Plant Sci. 2020, 11, 211. [Google Scholar] [CrossRef]
- Zhang, M.M.; Fan, D.Y.; Murakami, K.; Badger, M.R.; Chow, W.S. Partially Dissecting Electron Fluxes in Both Photosystems in Spinach Leaf Disks during Photosynthetic Induction. Plant Cell Physiol. 2019, 60, 2206–2219. [Google Scholar] [CrossRef]
- Ifuku, K.; Noguchi, T. Structural Coupling of Extrinsic Proteins with the Oxygen-Evolving Center in Photosystem II. Front. Plant Sci. 2016, 7, 84. [Google Scholar] [CrossRef]
- Fei, L.; Chu, J.; Zhang, X.; Dong, S.; Dai, X.; He, M. Physiological and Proteomic Analyses Indicate Delayed Sowing Improves Photosynthetic Capacity in Wheat Flag Leaves Under Heat Stress. Front. Plant Sci. 2022, 13, 848464. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.Y.; Fan, Y.Z.; Wang, W.; Zhao, Y.Y. Comparative chloroplast proteome analysis of exogenously supplied trehalose to wheat seedlings under heat stress. Photosynthetica 2018, 56, 1123–1133. [Google Scholar] [CrossRef]
- Spreitzer, R.J.; Salvucci, M.E. RUBISCO: Structure, Regulatory Interactions, and Possibilities for a Better Enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–475. [Google Scholar] [CrossRef]
- Nan, N.; Wang, J.; Shi, Y.; Qian, Y.; Jiang, L.; Huang, S.; Liu, Y.; Wu, Y.; Liu, B.; Xu, Z.Y. Rice plastidial NAD-dependent malate dehydrogenase 1 negatively regulates salt stress response by reducing the vitamin B6 content. Plant Biotechnol. J. 2020, 18, 172–184. [Google Scholar] [CrossRef]
- Carmo-Silva, E.; Andralojc, P.J.; Scales, J.C.; Driever, S.M.; Mead, A.; Lawson, T.; Raines, C.A.; Parry, M.A. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 2017, 68, 3473–3486. [Google Scholar] [CrossRef] [PubMed]
- Kamal, N.M.; Gorafi, Y.S.A.; Abdelrahman, M.; Abdellatef, E.; Tsujimoto, H. Stay-green trait: A prospective approach for yield potential, and drought and heat stress adaptation in globally important cereals. Int. J. Mol. Sci. 2019, 20, 5837. [Google Scholar] [CrossRef]
- Sekhon, R.S.; Saski, C.; Kumar, R.; Flinn, B.S.; Luo, F.; Beissinger, T.M.; Ackerman, A.J.; Breitzman, M.W.; Bridges, W.C.; de Leon, N. Integrated genome-scale analysis identifies novel genes and networks underlying senescence in maize. Plant Cell 2019, 31, 1968–1989. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef]
- Thilakarathne, A.S.; Liu, F.; Zou, Z. Plant Signaling Hormones and Transcription Factors: Key Regulators of Plant Responses to Growth, Development, and Stress. Plants 2025, 14, 1070. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Cai, P.; Duan, X.; Sang, S.; Qiu, Z. Jasmonic acid pretreatment improves salt tolerance of wheat by regulating hormones biosynthesis and antioxidant capacity. Front. Plant Sci. 2022, 13, 968477. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Jwa, N.S.; Shibato, J.; Han, O.; Iwahashi, H.; Rakwal, R. Diverse environmental cues transiently regulate OsOPR1 of the “octadecanoid pathway” revealing its importance in rice defense/stress and development. Biochem. Biophys. Res. Commun. 2003, 310, 1073–1082. [Google Scholar] [CrossRef]
- Wakuta, S.; Suzuki, E.; Saburi, W.; Matsuura, H.; Nabeta, K.; Imai, R.; Matsui, H. OsJAR1 and OsJAR2 are jasmonyl-L-isoleucine synthases involved in wound- and pathogen-induced jasmonic acid signalling. Biochem. Biophys. Res. Commun. 2011, 409, 634–639. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H.; Ma, S.; Xiang, D.; Liu, R.; Xiong, L. OsJAZ1 Attenuates Drought Resistance by Regulating JA and ABA Signaling in Rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Li, J.; Javed, H.U.; Wu, Z.; Wang, L.; Han, J.; Zhang, Y.; Ma, C.; Jiu, S.; Zhang, C.; Wang, S. Improving berry quality and antioxidant ability in ‘Ruidu Hongyu’grapevine through preharvest exogenous 2, 4-epibrassinolide, jasmonic acid and their signaling inhibitors by regulating endogenous phytohormones. Front. Plant Sci. 2022, 13, 1035022. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, C.; Feng, N.; Zheng, D.; Shen, X.; Rao, G.; Huang, Y.; Cai, W.; Liu, Y.; Zhang, R. Transcriptomic and Lipidomic Analysis Reveals Complex Regulation Mechanisms Underlying Rice Roots’ Response to Salt Stress. Metabolites 2024, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Halford, N.G.; Guo, G.; Chen, Z.; Li, Y.; Zhou, L.; Liu, C.; Xu, R. Transcriptomic and metabolomic analyses reveal the importance of lipid metabolism and photosynthesis regulation in high salinity tolerance in barley (Hordeum vulgare L.) leaves derived from mutagenesis combined with microspore culture. Int. J. Mol. Sci. 2023, 24, 16757. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific Roles of Lipoxygenases in Development and Responses to Stress in Plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef]
- Yi, R.; Li, Y.; Shan, X. OPDA/dn-OPDA actions: Biosynthesis, metabolism, and signaling. Plant Cell Rep. 2024, 43, 206. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Zhang, A.; Chen, Z.; Luo, X.; Kong, D.; Zhang, F.; Yu, X.; Liu, G.; Luo, L. Improvement of salinity tolerance in water-saving and drought-resistance rice (WDR). Int. J. Mol. Sci. 2023, 24, 5444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Hussain, S.; Yang, S.; Li, R.; Liu, S.; Chen, Y.; Wei, H.; Dai, Q.; Hou, H. Study on the effect of salt stress on yield and grain quality among different rice varieties. Front. Plant Sci. 2022, 13, 918460. [Google Scholar] [CrossRef]
- Spychalla, J.P.; Desborough, S.L. Superoxide dismutase, catalase, and α-tocopherol content of stored potato tubers. Plant Physiol. 1990, 94, 1214–1218. [Google Scholar] [CrossRef]
- Ekinci, M.; Ors, S.; Yildirim, E.; Turan, M.; Sahin, U.; Dursun, A.; Kul, R. Determination of physiological indices and some antioxidant enzymes of chard exposed to nitric oxide under drought stress. Russ. J. Plant Physiol. 2020, 67, 740–749. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. J. Exp. Bot. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Arakawa, N.; Tsutsumi, K.; Sanceda, N.G.; Kurata, T.; Inagaki, C.J.A.; Chemistry, B. A rapid and sensitive method for the determination of ascorbic acid using 4, 7-diphenyl-l, 10-phenanthroline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1981, 45, 1289–1290. [Google Scholar] [CrossRef]
- Cai, K.; Chen, X.; Han, Z.; Wu, X.; Zhang, S.; Li, Q.; Nazir, M.M.; Zhang, G.; Zeng, F. Screening of worldwide barley collection for drought tolerance: The assessment of various physiological measures as the selection criteria. Front. Plant Sci. 2020, 11, 1159. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Tang, X.; Yang, Z.; Wang, J.; Zhang, X.; Yan, X.; Li, P.; Xu, N.; Liao, Z. Integration of the transcriptome and proteome provides insights into the mechanism calcium regulated of Ulva prolifera in response to high-temperature stress. Aquaculture 2022, 557, 738344. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Variety | Stage | Treatment | Na+ (mg·g−1) | K+ (mg·g−1) | Ca2+ (mg·g−1) | K+/Na+ (mg·g−1) | Ca2+/Na+ (mg·g−1) |
|---|---|---|---|---|---|---|---|
| Huang | 2.5-leaf stage | CK | 0.70 ± 0.08 b | 2.48 ± 0.03 a | 3.63 ± 0.03 a | 3.64 ± 0.39 a | 5.33 ± 0.52 a |
| huazhan | B | 0.69 ± 0.01 b | 1.97 ± 0.03 c | 3.42 ± 0.01 b | 2.87 ± 0.039 b | 4.99 ± 0.01 a | |
| S | 1.24 ± 0.02 a | 1.81 ± 0.02 d | 2.89 ± 0.02 d | 1.46 ± 0.035 c | 2.33 ± 0.05 b | ||
| BS | 1.16 ± 0.01 a | 2.10 ± 0.01 b | 3.05 ± 0.07 c | 1.81 ± 0.01 c | 2.63 ± 0.07 b | ||
| 4.5-leaf stage | CK | 0.51 ± 0.00 c | 1.89 ± 0.01 a | 3.12 ± 0.01 b | 3.71 ± 0.03 b | 6.12 ± 0.01 b | |
| B | 0.37 ± 0.00 c | 1.71 ± 0.01 b | 3.69 ± 0.01 a | 4.59 ± 0.04 a | 9.91 ± 0.03 a | ||
| S | 11.12 ± 0.11 a | 1.21 ± 0.01 c | 2.53 ± 0.02 d | 0.11 ± 0.00 d | 0.23 ± 0.01 d | ||
| BS | 5.43 ± 0.05 b | 1.69 ± 0.00 b | 3.02 ± 0.01 c | 0.31 ± 0.00 c | 0.55 ± 0.01 c | ||
| Chaoyou | 2.5-leaf stage | CK | 0.72 ± 0.04 c | 2.41 ± 0.02 c | 3.66 ± 0.03 a | 3.35 ± 0.18 a | 5.09 ± 0.23 a |
| qianhao | B | 0.89 ± 0.03 b | 2.48 ± 0.015 b | 3.68 ± 0.01 a | 2.79 ± 0.08 b | 4.13 ± 0.14 b | |
| S | 1.86 ± 0.02 a | 1.66 ± 0.00 d | 3.01 ± 0.03 b | 0.89 ± 0.01 d | 1.62 ± 0.01 c | ||
| BS | 1.83 ± 0.01 a | 2.54 ± 0.02 a | 3.00 ± 0.01 b | 1.39 ± 0.02 c | 1.64 ± 0.01 c | ||
| 4.5-leaf stage | CK | 0.68 ± 0.03 c | 2.13 ± 0.00 a | 3.96 ± 0.01 b | 4.07 ± 0.01 a | 7.58 ± 0.01 b | |
| B | 0.81 ± 0.03 c | 2.01 ± 0.02 b | 4.58 ± 0.07 a | 3.41 ± 0.02 b | 7.80 ± 0.02 a | ||
| S | 10.63 ± 0.07 a | 1.64 ± 0.01 d | 2.97 ± 0.01 d | 0.16 ± 0.00 d | 0.29 ± 0.01 d | ||
| BS | 5.25 ± 0.01 b | 1.85 ± 0.02 c | 3.27 ± 0.03 c | 0.37 ± 0.01 c | 0.64 ± 0.01 c |
| Variety | Stage | Treatment | SPAD | Fv/Fo | Fv/Fm |
|---|---|---|---|---|---|
| Huanghuazhan | 2.5-leaf stage | CK | 32.56 ± 0.27 a | 1.17 ± 0.05 a | 0.52 ± 0.01 a |
| B | 31.03 ± 0.40 b | 1.16 ± 0.05 a | 0.51 ± 0.01 a | ||
| S | 27.93 ± 0.48 c | 0.46 ± 0.03 c | 0.40 ± 0.02 a | ||
| BS | 31.36 ± 0.38 ab | 0.84 ± 0.13 b | 0.50 ± 0.08 a | ||
| 4.5-leaf stage | CK | 22.31 ± 1.16 a | 1.84 ± 0.01 b | 0.65 ± 0.02 b | |
| B | 23.99 ± 0.72 a | 1.99 ± 0.03 a | 0.74 ± 0.04 a | ||
| S | 18.09 ± 0.32 b | 1.13 ± 0.01 c | 0.57 ± 0.02 b | ||
| BS | 21.92 ± 0.23 a | 1.49 ± 0.02 b | 0.62 ± 0.01 b | ||
| Chaoyouqianhao | 2.5-leaf stage | CK | 32.43 ± 0.08 a | 0.93 ± 0.03 b | 0.48 ± 0.02 a |
| B | 30.96 ± 0.17 b | 1.35 ± 0.03 a | 0.47 ± 0.02 a | ||
| S | 27.60 ± 0.26 c | 0.64 ± 0.02 c | 0.46 ± 0.01 a | ||
| BS | 29.96 ± 0.17 ab | 0.84 ± 0.05 b | 0.52 ± 0.03 a | ||
| 4.5-leaf stage | CK | 23.15 ± 0.57 a | 0.62 ± 0.10 a | 0.35 ± 0.04 a | |
| B | 24.05 ± 0.78 a | 0.62 ± 0.05 a | 0.33 ± 0.01 a | ||
| S | 17.82 ± 0.37 b | 0.32 ± 0.01 b | 0.27 ± 0.02 a | ||
| BS | 22.59 ± 0.46 a | 0.51 ± 0.05 ab | 0.34 ± 0.04 a |
| Combinations | Upregulation | Downregulation | All DEGs |
|---|---|---|---|
| HCK vs. HS | 636 | 418 | 1054 |
| HCK vs. HBS | 471 | 571 | 1042 |
| HS vs. HBS | 31 | 353 | 384 |
| HB vs. HCK | 80 | 27 | 107 |
| CCK vs. CS | 435 | 157 | 592 |
| CCK vs. CBS | 636 | 190 | 826 |
| CS vs. CBS | 114 | 59 | 203 |
| CB vs. CCK | 156 | 39 | 195 |
| Group | Total | Up | Down |
|---|---|---|---|
| HCK vs. HS | 146 | 40 | 106 |
| HCK vs. HBS | 119 | 28 | 91 |
| HS vs. HBS | 80 | 18 | 68 |
| CCK vs. CS | 104 | 53 | 51 |
| CCK vs. CBS | 160 | 103 | 57 |
| CS vs. CBS | 144 | 89 | 55 |
| Varieties | Pathway | Metabolite ID | Metabolite Name | Log2FC |
|---|---|---|---|---|
| Huanghuazhan | Cutin, suberine, and wax biosynthesis (ko00073) | 19.007_272.23482 | 16-Hydroxyhexadecanoic acid | −1.34 |
| Plant hormone signal transduction (ko04075) | 13.729_210.12555 | Jasmonic acid | −7.10 | |
| α-Linolenic acid metabolism (ko00592) | 13.729_210.12555 | Jasmonic acid | −7.10 | |
| Chaoyouqianhao | Cutin, suberine, and wax biosynthesis (ko00073) | 19.007_272.23482 | 16-Hydroxyhexadecanoic acid | −1.21 |
| 21.193_256.24002 | Palmitic acid | −0.51 | ||
| alpha-Linolenic acid metabolism (ko00592) | 14.49_292.20360 | 12-Oxo phytodienoic acid | 1.14 | |
| Plant hormone signal transduction (ko04075) | 10.37_210.12588 | Jasmonic acid | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-S.; Mu, D.-W.; Feng, N.-J.; Zheng, D.-F.; Sun, Z.-Y.; Khan, A.; Zhou, H.; Song, Y.-W.; Liu, J.-X.; Luo, J.-Q. Integrated Analyses Reveal the Physiological and Molecular Mechanisms of Brassinolide in Modulating Salt Tolerance in Rice. Plants 2025, 14, 1555. https://doi.org/10.3390/plants14101555
Wu J-S, Mu D-W, Feng N-J, Zheng D-F, Sun Z-Y, Khan A, Zhou H, Song Y-W, Liu J-X, Luo J-Q. Integrated Analyses Reveal the Physiological and Molecular Mechanisms of Brassinolide in Modulating Salt Tolerance in Rice. Plants. 2025; 14(10):1555. https://doi.org/10.3390/plants14101555
Chicago/Turabian StyleWu, Jia-Shuang, De-Wei Mu, Nai-Jie Feng, Dian-Feng Zheng, Zhi-Yuan Sun, Aaqil Khan, Hang Zhou, Yi-Wen Song, Jia-Xin Liu, and Jia-Qi Luo. 2025. "Integrated Analyses Reveal the Physiological and Molecular Mechanisms of Brassinolide in Modulating Salt Tolerance in Rice" Plants 14, no. 10: 1555. https://doi.org/10.3390/plants14101555
APA StyleWu, J.-S., Mu, D.-W., Feng, N.-J., Zheng, D.-F., Sun, Z.-Y., Khan, A., Zhou, H., Song, Y.-W., Liu, J.-X., & Luo, J.-Q. (2025). Integrated Analyses Reveal the Physiological and Molecular Mechanisms of Brassinolide in Modulating Salt Tolerance in Rice. Plants, 14(10), 1555. https://doi.org/10.3390/plants14101555






