Integrating Genetic Diversity and Agronomic Innovations for Climate-Resilient Maize Systems
Abstract
1. Introduction
1.1. Genetic Diversity for Climate Resilience
1.2. Identification of Resilience Traits
1.2.1. Drought Tolerance (DT)
1.2.2. Heat Stress (HS)
1.2.3. Waterlogging Tolerance (WT)
1.2.4. Cold Tolerance (CT)
1.3. Disease Resistance in Maize Under Changing Climates
1.4. Association Studies for QTL Identification and Candidate Gene Mining
Genome-Wide Association Studies
1.5. Gene Editing Technologies
1.5.1. Gene Editing Unveils Key Regulators in Maize Development
1.5.2. Virus-Induced Gene Silencing (VIGS)
1.6. Breeding Strategies for Climate Adaptation
1.6.1. Utilization of Maize Wild Relatives as a Genetic Resource
1.6.2. Speed Breeding in Maize
1.6.3. Enhancing Maize Breeding with Genomic Selection and Speed Breeding
1.6.4. Marker-Assisted Selection (MAS)
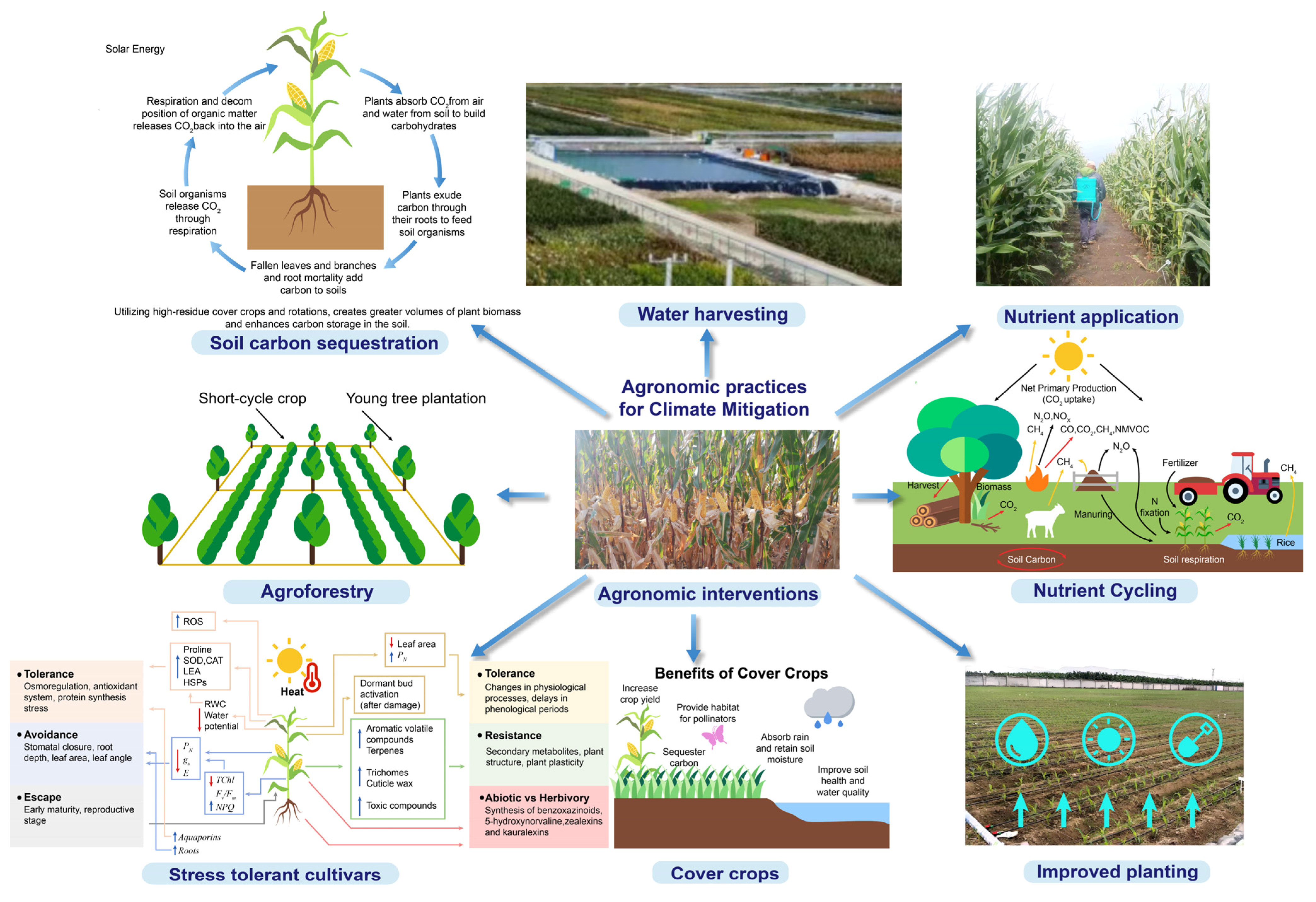
1.7. Agronomic Practices for Climate Mitigation in Maize
1.7.1. Conservation Agriculture
1.7.2. Precision Farming Technologies
1.7.3. Climate-Smart Crop Management
1.7.4. Agroforestry Integration
1.7.5. Cover Cropping Benefits
1.7.6. Policy Support for Sustainable Agriculture
1.8. Technological Innovations for Resilience Enhancement
1.8.1. High-Throughput Phenotyping (HTP)
1.8.2. Omics-Based Approaches for Developing Climate-Resilient Maize
1.8.3. Transcriptomics
Metabolomics
1.9. Integrated Modeling for Climate-Resilient Maize Varieties
1.10. Challenges and Future Directions
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, U.; Hussain, M.A.; Ahmad, W.; Javed, J.; Arshad, Z.; Akram, Z. Impact of Global Climate Change on Maize (Zea mays): Physiological Responses and Modern Breeding Techniques. Trends Biotechnol. Plant Sci. 2024, 2, 62–77. [Google Scholar] [CrossRef]
- Blake, M. Maize for the Gods: Unearthing the 9000-Year History of Corn; Univ of California Press: Oakland, CA, USA, 2015. [Google Scholar]
- Kaushik, P.; Grichar, W.J. New Prospects of Maize; BoD–Books on Demand: Hamburg, Germany, 2024. [Google Scholar]
- Cairns, J.E.; Sonder, K.; Zaidi, P.; Verhulst, N.; Mahuku, G.; Babu, R.; Nair, S.; Das, B.; Govaerts, B.; Vinayan, M. Maize production in a changing climate: Impacts, adaptation, and mitigation strategies. Adv. Agron. 2012, 114, 1–58. [Google Scholar]
- Gong, F.; Wu, X.; Zhang, H.; Chen, Y.; Wang, W. Making better maize plants for sustainable grain production in a changing climate. Front. Plant Sci. 2015, 6, 835. [Google Scholar] [CrossRef]
- Tokatlidis, I.S. Adapting maize crop to climate change. Agron. Sustain. Dev. 2013, 33, 63–79. [Google Scholar] [CrossRef]
- Weis, T. The accelerating biophysical contradictions of industrial capitalist agriculture. J. Agrar. Change 2010, 10, 315–341. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Krupke, C.H.; White, M.A.; Alexander, C.E. Global warming presents new challenges for maize pest management. Environ. Res. Lett. 2008, 3, 044007. [Google Scholar] [CrossRef]
- Carena, M.J. Germplasm enhancement for adaptation to climate changes. Crop Breed. Appl. Biotechnol. 2011, 11, 56–65. [Google Scholar] [CrossRef]
- Prasanna, B. Diversity in global maize germplasm: Characterization and utilization. J. Biosci. 2012, 37, 843–855. [Google Scholar] [CrossRef]
- Rauf, S.; Al-Khayri, J.M.; Zaharieva, M.; Monneveux, P.; Khalil, F. Breeding strategies to enhance drought tolerance in crops. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Springer: Cham, Switzerland, 2016; pp. 397–445. [Google Scholar]
- Babu, R. Molecular marker-assisted breeding for tropical maize improvement. In Proceedings of the 12th Asian Maize Conference, Bangkok, Thailand, 30 October–1 November 2014; Volume 30. [Google Scholar]
- Nepolean, T.; Kaul, J.; Mukri, G.; Mittal, S. Genomics-enabled next-generation breeding approaches for developing system-specific drought tolerant hybrids in maize. Front. Plant Sci. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Duvick, D.N. The contribution of breeding to yield advances in maize (Zea mays L.). Adv. Agron. 2005, 86, 83–145. [Google Scholar]
- Batool, R.; Umer, M.J.; Wang, Y.; He, K.; Zhang, T.; Bai, S.; Zhi, Y.; Chen, J.; Wang, Z. Synergistic effect of Beauveria bassiana and Trichoderma asperellum to induce maize (Zea mays L.) defense against the Asian corn borer, Ostrinia furnacalis (Lepidoptera, Crambidae) and larval immune response. Int. J. Mol. Sci. 2020, 21, 8215. [Google Scholar] [CrossRef] [PubMed]
- Batool, R.; Xuelian, G.; Hui, D.; Xiuzhen, L.; Umer, M.J.; Rwomushana, I.; Ali, A.; Attia, K.A.; Jingfei, G.; Zhenying, W. Endophytic fungi-mediated defense signaling in maize: Unraveling the role of WRKY36 in regulating immunity against Spodoptera frugiperda. Physiol. Plant. 2024, 176, e14243. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Liu, S.; Ferjani, A.; Li, J.; Yan, J.; Yang, X.; Qin, F. Genetic variation in ZmVPP1 contributes to drought tolerance in maize seedlings. Nat. Genet. 2016, 48, 1233–1241. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Leipner, J.; Stamp, P.; Guerra-Peraza, O. Low temperature stress in maize (Zea mays L.) induces genes involved in photosynthesis and signal transduction as studied by suppression subtractive hybridization. Plant Physiol. Biochem. 2009, 47, 116–122. [Google Scholar] [CrossRef]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L. Plant J. 2007, 50, 54–69. [Google Scholar] [CrossRef]
- Guerra-Peraza, O.; Nguyen, H.T.; Stamp, P.; Leipner, J. ZmCOI6. 1, a novel, alternatively spliced maize gene, whose transcript level changes under abiotic stress. Plant Sci. 2009, 176, 783–791. [Google Scholar] [CrossRef]
- Wei, K.; Pan, S. Maize protein phosphatase gene family: Identification and molecular characterization. BMC Genom. 2014, 15, 773. [Google Scholar] [CrossRef]
- Kong, X.; Pan, J.; Zhang, M.; Xing, X.; Zhou, Y.; Liu, Y.; Li, D.; Li, D. ZmMKK4, a novel group C mitogen-activated protein kinase kinase in maize (Zea mays), confers salt and cold tolerance in transgenic Arabidopsis. Plant Cell Environ. 2011, 34, 1291–1303. [Google Scholar] [CrossRef]
- Yuan, Y.; Cairns, J.E.; Babu, R.; Gowda, M.; Makumbi, D.; Magorokosho, C.; Zhang, A.; Liu, Y.; Wang, N.; Hao, Z. Genome-wide association mapping and genomic prediction analyses reveal the genetic architecture of grain yield and flowering time under drought and heat stress conditions in maize. Front. Plant Sci. 2019, 9, 1919. [Google Scholar] [CrossRef]
- Ndlovu, N.; Spillane, C.; McKeown, P.C.; Cairns, J.E.; Das, B.; Gowda, M. Genome-wide association studies of grain yield and quality traits under optimum and low-nitrogen stress in tropical maize (Zea mays L.). Theor. Appl. Genet. 2022, 135, 4351–4370. [Google Scholar] [CrossRef] [PubMed]
- Osuman, A.S.; Badu-Apraku, B.; Karikari, B.; Ifie, B.E.; Tongoona, P.; Danquah, E.Y. Genome-wide association study reveals genetic architecture and candidate genes for yield and related traits under terminal drought, combined heat and drought in tropical maize germplasm. Genes 2022, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.N.; Li, X.H.; George, M.L.; Li, M.S.; Zhang, S.H.; Zheng, Y.L. Quantitative trait locus analysis of drought tolerance and yield in Maize in China. Plant Mol. Biol. Report. 2005, 23, 155–165. [Google Scholar] [CrossRef]
- Edmeades, G.O.; Trevisan, W.; Prasanna, B.; Campos, H. Tropical maize (Zea mays L.). In Genetic Improvement of Tropical Crops; Springer: Cham, Switzerland, 2017; pp. 57–109. [Google Scholar]
- Bolaños, J.; Edmeades, G.; Martinez, L. Eight cycles of selection for drought tolerance in lowland tropical maize. III. Responses in drought-adaptive physiological and morphological traits. Field Crops Res. 1993, 31, 269–286. [Google Scholar] [CrossRef]
- Ribaut, J.M.; Jiang, C.; Gonzalez-de-Leon, D.; Edmeades, G.O.; Hoisington, D.A. Identification of quantitative trait loci under drought conditions in tropical maize. 2. Yield components and marker-assisted selection strategies. Theor. Appl. Genet. 1997, 94, 887–896. [Google Scholar] [CrossRef]
- Agrama, H.A.S.; Moussa, M.E. Mapping QTLs in breeding for drought tolerance in maize (Zea mays L.). Euphytica 1996, 91, 89–97. [Google Scholar] [CrossRef]
- Trachsel, S.; Messmer, R.; Stamp, P.; Hund, A. Mapping of QTLs for lateral and axile root growth of tropical maize. Theor. Appl. Genet. 2009, 119, 1413–1424. [Google Scholar] [CrossRef]
- Semagn, K.; Beyene, Y.; Warburton, M.L.; Tarekegne, A.; Mugo, S.; Meisel, B.; Sehabiague, P.; Prasanna, B.M. Meta-analyses of QTL for grain yield and anthesis silking interval in 18 maize populations evaluated under water-stressed and well-watered environments. BMC Genom. 2013, 14, 313. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Gu, L.; Liu, P.; Zhao, B.; Zhang, J.; Ren, B. The effects of high temperature, drought, and their combined stresses on the photosynthesis and senescence of summer maize. Agric. Water Manag. 2023, 289, 108525. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Cairns, J.E.; Zaidi, P.; Beyene, Y.; Makumbi, D.; Gowda, M.; Magorokosho, C.; Zaman-Allah, M.; Olsen, M.; Das, A. Beat the stress: Breeding for climate resilience in maize for the tropical rainfed environments. Theor. Appl. Genet. 2021, 134, 1729–1752. [Google Scholar] [CrossRef]
- Lizaso, J.; Ruiz-Ramos, M.; Rodríguez, L.; Gabaldon-Leal, C.; Oliveira, J.; Lorite, I.; Sánchez, D.; García, E.; Rodríguez, A. Impact of high temperatures in maize: Phenology and yield components. Field Crops Res. 2018, 216, 129–140. [Google Scholar] [CrossRef]
- Cairns, J.; Hellin, J.; Sonder, K.; Araus, J.; MacRobert, J.; Thierfelder, C.; Prasanna, B. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Edreira, J.R.; Otegui, M.E. Heat stress in temperate and tropical maize hybrids: A novel approach for assessing sources of kernel loss in field conditions. Field Crops Res. 2013, 142, 58–67. [Google Scholar] [CrossRef]
- Frey, F.P.; Presterl, T.; Lecoq, P.; Orlik, A.; Stich, B. First steps to understand heat tolerance of temperate maize at adult stage: Identification of QTL across multiple environments with connected segregating populations. Theor. Appl. Genet. 2016, 129, 945–961. [Google Scholar] [CrossRef]
- Frova, C.; Sari-Gorla, M. Quantitative trait loci (QTLs) for pollen thermotolerance detected in maize. Mol. Gen. Genet. MGG 1994, 245, 424–430. [Google Scholar] [CrossRef]
- Inghelandt, D.V.; Frey, F.P.; Ries, D.; Stich, B. QTL mapping and genome-wide prediction of heat tolerance in multiple connected populations of temperate maize. Sci. Rep. 2019, 9, 14418. [Google Scholar] [CrossRef]
- Yao, Q. Crucial waterlogging-responsive genes and pathways revealed by comparative physiology and transcriptome in tropical and temperate maize (Zea mays L.) inbred lines. J. Plant Biol. 2021, 64, 313–325. [Google Scholar] [CrossRef]
- Subbaiah, C.C.; Sachs, M.M. Molecular and cellular adaptations of maize to flooding stress. Ann. Bot. 2003, 91, 119–127. [Google Scholar] [CrossRef]
- Zaidi, P.; Maniselvan, P.; Srivastava, A.; Yadav, P.; Singh, R. Genetic analysis of water-logging tolerance in tropical maize (Zea mays L.). Maydica 2010, 55, 17–26. [Google Scholar]
- Qiu, F.; Zheng, Y.; Zhang, Z.; Xu, S. Mapping of QTL associated with waterlogging tolerance during the seedling stage in maize. Ann. Bot. 2007, 99, 1067–1081. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F.; Tamaki, H.; Mitsuhashi, S.; Takahashi, W. DNA marker-assisted selection approach for developing flooding-tolerant maize. Jpn. Agric. Res. Q. JARQ 2016, 50, 175–182. [Google Scholar] [CrossRef]
- Mano, Y.; Omori, F.; Takeda, K. Construction of intraspecific linkage maps, detection of a chromosome inversion, and mapping of QTL for constitutive root aerenchyma formation in the teosinte Zea nicaraguensis. Mol. Breed. 2012, 29, 137–146. [Google Scholar] [CrossRef]
- Zhou, X.; Muhammad, I.; Lan, H.; Xia, C. Recent advances in the analysis of cold tolerance in maize. Front. Plant Sci. 2022, 13, 866034. [Google Scholar] [CrossRef]
- Hund, A.; Frascaroli, E.; Leipner, J.; Jompuk, C.; Stamp, P.; Fracheboud, Y. Cold tolerance of the photosynthetic apparatus: Pleiotropic relationship between photosynthetic performance and specific leaf area of maize seedlings. Mol. Breed. 2005, 16, 321–331. [Google Scholar] [CrossRef]
- Rodríguez, V.M.; Butrón, A.; Rady, M.O.; Soengas, P.; Revilla, P. Identification of quantitative trait loci involved in the response to cold stress in maize (Zea mays L.). Mol. Breed. 2014, 33, 363–371. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Fu, J.; Li, L.; Jia, G.; Ren, L.; Lubberstedt, T.; Wang, G.; Wang, J.; Gu, R. QTL mapping in three connected populations reveals a set of consensus genomic regions for low temperature germination ability in Zea mays L. Front. Plant Sci. 2018, 9, 65. [Google Scholar] [CrossRef]
- Strigens, A.; Freitag, N.M.; Gilbert, X.; Grieder, C.; Riedelsheimer, C.; Schrag, T.A.; Messmer, R.; Melchinger, A.E. Association mapping for chilling tolerance in elite flint and dent maize inbred lines evaluated in growth chamber and field experiments. Plant Cell Environ. 2013, 36, 1871–1887. [Google Scholar] [CrossRef]
- Hu, G.; Li, Z.; Lu, Y.; Li, C.; Gong, S.; Yan, S.; Li, G.; Wang, M.; Ren, H.; Guan, H. Genome-wide association study identified multiple genetic loci on chilling resistance during germination in maize. Sci. Rep. 2017, 7, 10840. [Google Scholar] [CrossRef]
- Hung, H.Y.; Holland, J.B. Diallel analysis of resistance to Fusarium ear rot and fumonisin contamination in maize. Crop Sci. 2012, 52, 2173–2181. [Google Scholar] [CrossRef]
- Rose, L.J.; Okoth, S.; Beukes, I.; Ouko, A.; Mouton, M.; Flett, B.C.; Makumbi, D.; Viljoen, A. Determining resistance to Fusarium verticillioides and fumonisin accumulation in African maize inbred lines resistant to Aspergillus flavus and aflatoxins. Euphytica 2017, 213, 93. [Google Scholar] [CrossRef]
- Maschietto, V.; Colombi, C.; Pirona, R.; Pea, G.; Strozzi, F.; Marocco, A.; Rossini, L.; Lanubile, A. QTL mapping and candidate genes for resistance to Fusarium ear rot and fumonisin contamination in maize. BMC Plant Biol. 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-Q.; Wang, X.-M.; Chander, S.; Yan, J.-B.; Li, J.-S. QTL mapping of resistance to Fusarium ear rot using a RIL population in maize. Mol. Breed. 2008, 22, 395–403. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Li, H.; Li, Z.; Sun, X.; Li, J.; Wang, R.; Dai, X.; Dong, H.; Song, W. Detection and verification of quantitative trait loci for resistance to Fusarium ear rot in maize. Mol. Breed. 2012, 30, 1649–1656. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Velten, J.; Xin, Z.; Stout, J. Characterization of maize inbred lines for drought and heat tolerance. J. Soil Water Conserv. 2012, 67, 354–364. [Google Scholar] [CrossRef]
- Zila, C.T.; Samayoa, L.F.; Santiago, R.; Butrón, A.; Holland, J.B. A genome-wide association study reveals genes associated with Fusarium ear rot resistance in a maize core diversity panel. G3 Genes Genomes Genet. 2013, 3, 2095–2104. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Guo, Y.; Yang, Q.; Ye, J.; Chen, S.; Xu, M. Fine-mapping of qRfg2, a QTL for resistance to Gibberella stalk rot in maize. Theor. Appl. Genet. 2012, 124, 585–596. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Ma, C.; Zhang, D.; Wang, C.; Yang, Q. Transcriptome analysis of maize resistance to Fusarium graminearum. BMC Genom. 2016, 17, 477. [Google Scholar]
- Chen, Q.; Song, J.; Du, W.-P.; Xu, L.-Y.; Jiang, Y.; Zhang, J.; Xiang, X.-L.; Yu, G.-R. Identification, mapping, and molecular marker development for Rgsr8. 1: A new quantitative trait locus conferring resistance to Gibberella stalk rot in maize (Zea mays L.). Front. Plant Sci. 2017, 8, 1355. [Google Scholar]
- Beló, A.; Zheng, P.; Luck, S.; Shen, B.; Meyer, D.J.; Li, B.; Tingey, S.; Rafalski, A. Whole genome scan detects an allelic variant of fad2 associated with increased oleic acid levels in maize. Mol. Genet. Genom. 2008, 279, 1–10. [Google Scholar] [CrossRef]
- Shikha, K.; Shahi, J.; Vinayan, M.; Zaidi, P.; Singh, A.; Sinha, B. Genome-wide association mapping in maize: Status and prospects. 3 Biotech 2021, 11, 244. [Google Scholar] [CrossRef]
- Schnable, P.S.; Ware, D.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.; Graves, T.A. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, H.; Wu, L.; Warburton, M.; Yan, J. Genome-wide association studies in maize: Praise and stargaze. Mol. Plant 2017, 10, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Flint-Garcia, S.A.; Thornsberry, J.M.; Buckler IV, E.S. Structure of linkage disequilibrium in plants. Annu. Rev. Plant Biol. 2003, 54, 357–374. [Google Scholar] [CrossRef]
- Warburton, M.L.; Womack, E.D.; Tang, J.D.; Thrash, A.; Smith, J.S.; Xu, W.; Murray, S.C.; Williams, W.P. Genome-wide association and metabolic pathway analysis of corn earworm resistance in maize. Plant Genome 2018, 11, 170069. [Google Scholar] [CrossRef]
- Kuki, M.C.; Scapim, C.A.; Rossi, E.S.; Mangolin, C.A.; Amaral Júnior, A.T.d.; Pinto, R.J.B. Genome wide association study for gray leaf spot resistance in tropical maize core. PLoS ONE 2018, 13, e0199539. [Google Scholar] [CrossRef]
- Zhou, G.; Hao, D.; Mao, Y.; Zhu, Q.; Chen, G.; Lu, H.; Shi, M.; Huang, X.; Zhang, Z.; Zhao, J. Identification of genetic loci conferring partial resistance to southern corn rust through a genome-wide association study. Eur. J. Plant Pathol. 2018, 150, 1083–1090. [Google Scholar] [CrossRef]
- de Jong, G.; Pamplona, A.K.A.; Von Pinho, R.G.; Balestre, M. Genome-wide association analysis of ear rot resistance caused by Fusarium verticillioides in maize. Genomics 2018, 110, 291–303. [Google Scholar] [CrossRef]
- Han, S.; Miedaner, T.; Utz, H.F.; Schipprack, W.; Schrag, T.A.; Melchinger, A.E. Genomic prediction and GWAS of Gibberella ear rot resistance traits in dent and flint lines of a public maize breeding program. Euphytica 2018, 214, 6. [Google Scholar] [CrossRef]
- Samayoa, L.F.; Cao, A.; Santiago, R.; Malvar, R.; Butrón, A. Genome-wide association analysis for fumonisin content in maize kernels. BMC Plant Biol. 2019, 19, 166. [Google Scholar] [CrossRef]
- Sitonik, C.a.; Suresh, L.; Beyene, Y.; Olsen, M.S.; Makumbi, D.; Oliver, K.; Das, B.; Bright, J.M.; Mugo, S.; Crossa, J. Genetic architecture of maize chlorotic mottle virus and maize lethal necrosis through GWAS, linkage analysis and genomic prediction in tropical maize germplasm. Theor. Appl. Genet. 2019, 132, 2381–2399. [Google Scholar] [CrossRef]
- Adewale, S.A.; Badu-Apraku, B.; Akinwale, R.O.; Paterne, A.A.; Gedil, M.; Garcia-Oliveira, A.L. Genome-wide association study of Striga resistance in early maturing white tropical maize inbred lines. BMC Plant Biol. 2020, 20, 203. [Google Scholar] [CrossRef]
- Nyaga, C.; Gowda, M.; Beyene, Y.; Muriithi, W.T.; Makumbi, D.; Olsen, M.S.; Suresh, L.; Bright, J.M.; Das, B.; Prasanna, B.M. Genome-wide analyses and prediction of resistance to MLN in large tropical maize germplasm. Genes 2019, 11, 16. [Google Scholar] [CrossRef]
- Stagnati, L.; Rahjoo, V.; Samayoa, L.F.; Holland, J.B.; Borrelli, V.M.; Busconi, M.; Lanubile, A.; Marocco, A. A genome-wide association study to understand the effect of Fusarium verticillioides infection on seedlings of a maize diversity panel. G3 Genes Genomes Genet. 2020, 10, 1685–1696. [Google Scholar] [CrossRef]
- Rashid, Z.; Sofi, M.; Harlapur, S.I.; Kachapur, R.M.; Dar, Z.A.; Singh, P.K.; Zaidi, P.H.; Vivek, B.S.; Nair, S.K. Genome-wide association studies in tropical maize germplasm reveal novel and known genomic regions for resistance to Northern corn leaf blight. Sci. Rep. 2020, 10, 21949. [Google Scholar] [CrossRef]
- Han, G.; Li, C.; Xiang, F.; Zhao, Q.; Zhao, Y.; Cai, R.; Cheng, B.; Wang, X.; Tao, F. Genome-wide association study leads to novel genetic insights into resistance to Aspergillus flavus in maize kernels. BMC Plant Biol. 2020, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Kibe, M.; Nair, S.K.; Das, B.; Bright, J.M.; Makumbi, D.; Kinyua, J.; Suresh, L.; Beyene, Y.; Olsen, M.S.; Prasanna, B.M. Genetic dissection of resistance to gray leaf spot by combining genome-wide association, linkage mapping, and genomic prediction in tropical maize germplasm. Front. Plant Sci. 2020, 11, 572027. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Pei, Y.; Jiang, X.; Jaqueth, J.S.; Li, B.; Han, J.; Jeffers, D.; Wang, J.; Song, X. Identification of genetic loci associated with rough dwarf disease resistance in maize by integrating GWAS and linkage mapping. Plant Sci. 2022, 315, 111100. [Google Scholar] [CrossRef]
- Okunlola, G.; Badu-Apraku, B.; Ariyo, O.; Agre, P.; Offernedo, Q.; Ayo-Vaughan, M. Genome-wide association studies of Striga resistance in extra-early maturing quality protein maize inbred lines. G3 2023, 13, jkac237. [Google Scholar] [CrossRef]
- Ruiz, M.; Rossi, E.A.; Bonamico, N.C.; Balzarini, M.G. Genome-wide association study for bacterial leaf streak resistance in maize. Agron. J. 2023, 115, 1051–1058. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, M.; Zhang, Z.; Wang, Z.; Wu, N.; Song, Y.; Wang, P. Screening and verification of genes associated with leaf angle and leaf orientation value in inbred maize lines. PLoS ONE 2018, 13, e0208386. [Google Scholar] [CrossRef]
- Lin, M.; Matschi, S.; Vasquez, M.; Chamness, J.; Kaczmar, N.; Baseggio, M.; Miller, M.; Stewart, E.L.; Qiao, P.; Scanlon, M.J. Genome-wide association study for maize leaf cuticular conductance identifies candidate genes involved in the regulation of cuticle development. G3 Genes Genomes Genet. 2020, 10, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zhao, X.; Wang, Y.; Li, C.; Li, Y.; Zhang, D.; Shi, Y.; Song, Y.; Wang, L.; Li, Y. Genome-wide association studies of leaf angle in maize. Mol. Breed. 2021, 41, 50. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, D.; Liu, M.; Cui, Z.; Sun, D.; Li, C.; Zhang, A.; Cao, H.; Ruan, Y. Genome-wide association study identified novel SNPs associated with chlorophyll content in maize. Genes 2023, 14, 1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, J.; He, L.; Lan, H.; Li, L. Genome-wide association analysis of plant height using the maize F1 population. Plants 2019, 8, 432. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Guan, Z.; Zhang, X.; Zhang, Y.; Ma, L.; Yao, Y.; Peng, H.; Zhang, Q.; Zhang, B. Combination of multi-locus genome-wide association study and QTL mapping reveals genetic basis of tassel architecture in maize. Mol. Genet. Genom. 2019, 294, 1421–1440. [Google Scholar] [CrossRef]
- An, Y.; Chen, L.; Li, Y.-X.; Li, C.; Shi, Y.; Zhang, D.; Li, Y.; Wang, T. Genome-wide association studies and whole-genome prediction reveal the genetic architecture of KRN in maize. BMC Plant Biol. 2020, 20, 490. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, L.; Li, Z.; Bi, Y.; Yin, X.; Guo, R.; Wang, J.; Zhang, Y.; Shaw, R.K.; Fan, X. Identification of candidate QTLs and genes for ear diameter by multi-parent population in maize. Genes 2023, 14, 1305. [Google Scholar] [CrossRef]
- Zhu, X.-M.; Shao, X.-Y.; Pei, Y.-H.; Guo, X.-M.; Li, J.; Song, X.-Y.; Zhao, M.-A. Genetic diversity and genome-wide association study of major ear quantitative traits using high-density SNPs in maize. Front. Plant Sci. 2018, 9, 966. [Google Scholar] [CrossRef]
- Xu, S.; Tang, X.; Zhang, X.; Wang, H.; Ji, W.; Xu, C.; Yang, Z.; Li, P. Genome-wide association study identifies novel candidate loci or genes affecting stalk strength in maize. Crop J. 2023, 11, 220–227. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, F.; Huang, Y.; Zhao, Y.; Jia, X.; Zhu, L.; Guo, J. Genome-wide association studies of grain quality traits in maize. Sci. Rep. 2021, 11, 9797. [Google Scholar] [CrossRef]
- Zeng, T.; Meng, Z.; Yue, R.; Lu, S.; Li, W.; Li, W.; Meng, H.; Sun, Q. Genome wide association analysis for yield related traits in maize. BMC Plant Biol. 2022, 22, 449. [Google Scholar] [CrossRef] [PubMed]
- Bhadmus, O.A.; Badu-Apraku, B.; Adeyemo, O.A.; Agre, P.A.; Queen, O.N.; Ogunkanmi, A.L. Genome-wide association analysis reveals genetic architecture and candidate genes associated with grain yield and other traits under low soil nitrogen in early-maturing white quality protein maize inbred lines. Genes 2022, 13, 826. [Google Scholar] [CrossRef]
- Moussa, A.A.; Mandozai, A.; Jin, Y.; Qu, J.; Zhang, Q.; Zhao, H.; Anwari, G.; Khalifa, M.A.S.; Lamboro, A.; Noman, M. Genome-wide association screening and verification of potential genes associated with root architectural traits in maize (Zea mays L.) at multiple seedling stages. BMC Genom. 2021, 22, 558. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, L.-G.; Luo, J.-H.; Xia, A.-A.; Chen, L.-Q.; He, Y. Genome-wide association study reveals the genetic architecture of root hair length in maize. BMC Genom. 2021, 22, 664. [Google Scholar] [CrossRef]
- Xuhui, L.; Siqi, L.; Weiwei, C.; Hang, Z.; Huanzhang, L.; Danwen, F.; Lina, F.; Junteng, F.; Yuanqiang, H.; Xiangbo, Z. Genome-wide association study of root hair length in maize. Trop. Plant Biol. 2023, 16, 67–74. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Yang, M.; Liu, R.; Zhang, X.; Jia, Z.; Li, P. Natural variation in ZmNAC087 contributes to total root length regulation in maize seedlings under salt stress. BMC Plant Biol. 2023, 23, 392. [Google Scholar] [CrossRef]
- Wang, N.; Liu, B.; Liang, X.; Zhou, Y.; Song, J.; Yang, J.; Yong, H.; Weng, J.; Zhang, D.; Li, M. Genome-wide association study and genomic prediction analyses of drought stress tolerance in China in a collection of off-PVP maize inbred lines. Mol. Breed. 2019, 39, 113. [Google Scholar] [CrossRef]
- Chen, S.; Dang, D.; Liu, Y.; Ji, S.; Zheng, H.; Zhao, C.; Dong, X.; Li, C.; Guan, Y.; Zhang, A. Genome-wide association study presents insights into the genetic architecture of drought tolerance in maize seedlings under field water-deficit conditions. Front. Plant Sci. 2023, 14, 1165582. [Google Scholar] [CrossRef]
- Longmei, N.; Gill, G.K.; Zaidi, P.H.; Kumar, R.; Nair, S.K.; Hindu, V.; Vinayan, M.T.; Vikal, Y. Genome wide association mapping for heat tolerance in sub-tropical maize. BMC Genom. 2021, 22, 154. [Google Scholar] [CrossRef]
- Ahmed, Z.; Khalid, M.; Ghafoor, A.; Shah, M.K.N.; Raja, G.K.; Rana, R.M.; Mahmood, T.; Thompson, A.M. SNP-based genome-wide association mapping of pollen viability under heat stress in tropical Zea mays L. inbred lines. Front. Genet. 2022, 13, 819849. [Google Scholar] [CrossRef]
- Gao, J.; Wang, S.; Zhou, Z.; Wang, S.; Dong, C.; Mu, C.; Song, Y.; Ma, P.; Li, C.; Wang, Z. Linkage mapping and genome-wide association reveal candidate genes conferring thermotolerance of seed-set in maize. J. Exp. Bot. 2019, 70, 4849–4864. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, L.; Zhang, L.; Su, A.; Wang, R.; Song, W.; Li, Z.; Zhao, J. Genome-wide association analysis of chilling-tolerant germination in a new maize association mapping panel. Food Energy Secur. 2023, 12, e445. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Z.; Xi, Y.; Yang, Z.; Xiao, Z.; Guan, S.; Qu, J.; Wang, P.; Zhao, R. Identification and functional verification of cold tolerance genes in spring maize seedlings based on a genome-wide association study and quantitative trait locus mapping. Front. Plant Sci. 2021, 12, 776972. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, Y.; Chen, Q.; Zhao, F.; Zhou, S.; Ding, Y.; Song, X.; Li, P.; Wang, B. Genome-wide association analysis of salt tolerance QTLs with SNP markers in maize (Zea mays L.). Genes Genom. 2019, 41, 1135–1145. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Yang, Z.; Xu, C. Genetic mapping of quantitative trait loci in crops. Crop J. 2017, 5, 175–184. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Malenica, N.; Dunić, J.A.; Vukadinović, L.; Cesar, V.; Šimić, D. Genetic approaches to enhance multiple stress tolerance in maize. Genes 2021, 12, 1760. [Google Scholar] [CrossRef]
- Alqudah, A.M.; Sallam, A.; Baenziger, P.S.; Börner, A. GWAS: Fast-forwarding gene identification and characterization in temperate cereals: Lessons from barley—A review. J. Adv. Res. 2020, 22, 119–135. [Google Scholar] [CrossRef]
- Comadran, J.; Kilian, B.; Russell, J.; Ramsay, L.; Stein, N.; Ganal, M.; Shaw, P.; Bayer, M.; Thomas, W.; Marshall, D. Natural variation in a homolog of Antirrhinum CENTRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nat. Genet. 2012, 44, 1388–1392. [Google Scholar] [CrossRef]
- Doll, N.M.; Gilles, L.M.; Gérentes, M.-F.; Richard, C.; Just, J.; Fierlej, Y.; Borrelli, V.M.; Gendrot, G.; Ingram, G.C.; Rogowsky, P.M. Single and multiple gene knockouts by CRISPR–Cas9 in maize. Plant Cell Rep. 2019, 38, 487–501. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, J.; Wang, R.; Liu, Y.; Birchler, J.A.; Han, F. Efficient targeted genome modification in maize using CRISPR/Cas9 system. J. Genet. Genom. 2016, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Yu, L.; Chen, L.; Li, Y.; Zhang, J.; Sheng, H.; Ren, Z.; Li, Y.; Yu, X.; Jin, S. Recent progress and future prospect of CRISPR/Cas-derived transcription activation (CRISPRa) system in plants. Cells 2022, 11, 3045. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, C.; Su, H.; Liu, Y.; Liu, Y.; Han, F. The cohesin complex subunit ZmSMC3 participates in meiotic centromere pairing in maize. Plant Cell 2020, 32, 1323–1336. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Tao, F.; Luo, Y.; Cao, J.; Li, Z.; Xie, R.; Li, S. Planning maize hybrids adaptation to future climate change by integrating crop modelling with machine learning. Environ. Res. Lett. 2021, 16, 124043. [Google Scholar] [CrossRef]
- Zhao, H.; Qin, Y.; Xiao, Z.; Li, Q.; Yang, N.; Pan, Z.; Gong, D.; Sun, Q.; Yang, F.; Zhang, Z. Loss of function of an RNA polymerase III subunit leads to impaired maize kernel development. Plant Physiol. 2020, 184, 359–373. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Du, Y.; Shen, X.; Ning, Q.; Li, Y.; Liu, D.; Xiong, Q.; Zhang, Z. The coordinated KNR6–AGAP–ARF1 complex modulates vegetative and reproductive traits by participating in vesicle trafficking in maize. Cells 2021, 10, 2601. [Google Scholar] [CrossRef]
- Yang, R.; Xu, F.; Wang, Y.; Zhong, W.; Dong, L.; Shi, Y.; Tang, T.; Sheng, H.; Jackson, D.; Yang, F. Glutaredoxins regulate maize inflorescence meristem development via redox control of TGA transcriptional activity. Nat. Plants 2021, 7, 1589–1601. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, Y.; Li, J.; Zhang, P.; Chen, K.; Song, W.; Wang, X.; Yang, J.; Lu, X.; Lu, B. Molecular dissection of maize seedling salt tolerance using a genome-wide association analysis method. Plant Biotechnol. J. 2021, 19, 1937–1951. [Google Scholar] [CrossRef]
- Shi, J.; Gao, H.; Wang, H.; Lafitte, H.R.; Archibald, R.L.; Yang, M.; Hakimi, S.M.; Mo, H.; Habben, J.E. ARGOS 8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 2017, 15, 207–216. [Google Scholar] [CrossRef]
- Wang, F.; Cui, P.J.; Tian, Y.; Huang, Y.; Wang, H.F.; Liu, F.; Chen, Y.F. Maize ZmPT7 regulates Pi uptake and redistribution which is modulated by phosphorylation. Plant Biotechnol. J. 2020, 18, 2406–2419. [Google Scholar] [CrossRef]
- Qi, X.; Gao, H.; Lv, R.; Mao, W.; Zhu, J.; Liu, C.; Mao, L.; Li, X.; Xie, C. CRISPR/dCas-mediated gene activation toolkit development and its application for parthenogenesis induction in maize. Plant Commun. 2023, 4, 100449. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D.C. Fast forward genetics based on virus-induced gene silencing. Curr. Opin. Plant Biol. 1999, 2, 109–113. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Salt, D.E. Plant ionomics: From elemental profiling to environmental adaptation. Mol. Plant 2016, 9, 787–797. [Google Scholar] [CrossRef]
- Liou, M.R.; Huang, Y.W.; Hu, C.C.; Lin, N.S.; Hsu, Y.H. A dual gene-silencing vector system for monocot and dicot plants. Plant Biotechnol. J. 2014, 12, 330–343. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Wang, G.; Zhang, Q.; Zhang, X.; Fan, Z.; Cao, Y.; Wei, L.; Wang, T.; Wang, Z. Maize ZmbZIP33 is involved in drought resistance and recovery ability through an abscisic acid-dependent signaling pathway. Front. Plant Sci. 2021, 12, 629903. [Google Scholar] [CrossRef]
- Doebley, J. Molecular systematics of Zea (Gramineae). Maydica 1990, 35, 143–150. [Google Scholar]
- Blakey, C.; Costich, D.; Sokolov, V.; Islam-Faridi, M. Tripsacum genetics: From observations along a river to molecular genomics. Maydica 2007, 52, 81–99. [Google Scholar]
- Schlesinger, W.H. Carbon sequestration in soils. Science 1999, 284, 2095. [Google Scholar] [CrossRef]
- Gitz III, D.C.; Baker, J.T.; Stout, J.E.; Brauer, D.K.; Lascano, R.J.; Velten, J.P. Suitability of eastern gamagrass for in situ precipitation catchment forage production in playas. Agron. J. 2013, 105, 907–914. [Google Scholar] [CrossRef]
- Shavrukov, Y.; Sokolov, V. Maize-Gamagrass interspecific hybrid, Zea mays × Tripsacum dactyloides, shows better salinity tolerance and higher Na+ exclusion than maize and sorghum. Int. J. Latest Res. Sci. Technol. 2015, 4, 128–133. [Google Scholar]
- Pesqueira, J.; García, M.; Molina, M.C.R. NaCI tolerance in maize (“Zea mays” ssp. “mays”) × “Tripsacum dactyloides” L. hybrid “calli” and regenerated plants. Span. J. Agric. Res. 2003, 1, 59–64. [Google Scholar] [CrossRef]
- Samantara, K.; Reyes, V.P.; Agrawal, N.; Mohapatra, S.R.; Jena, K.K. Advances and trends on the utilization of multi-parent advanced generation intercross (MAGIC) for crop improvement. Euphytica 2021, 217, 189. [Google Scholar] [CrossRef]
- Hickey, L.T.; Germán, S.E.; Pereyra, S.A.; Diaz, J.E.; Ziems, L.A.; Fowler, R.A.; Platz, G.J.; Franckowiak, J.D.; Dieters, M.J. Speed breeding for multiple disease resistance in barley. Euphytica 2017, 213, 64. [Google Scholar] [CrossRef]
- Bernardo, R. Genomewide predictions for backcrossing a quantitative trait from an exotic to an adapted line. Crop Sci. 2016, 56, 1067–1075. [Google Scholar] [CrossRef]
- de Koning, D.-J. Meuwissen et al. on Genomic Selection. Genetics 2016, 203, 5–7. [Google Scholar] [CrossRef]
- Massman, J.M.; Jung, H.J.G.; Bernardo, R. Genomewide selection versus marker-assisted recurrent selection to improve grain yield and stover-quality traits for cellulosic ethanol in maize. Crop Sci. 2013, 53, 58–66. [Google Scholar] [CrossRef]
- Zhang, X.; Pérez-Rodríguez, P.; Burgueño, J.; Olsen, M.; Buckler, E.; Atlin, G.; Prasanna, B.M.; Vargas, M.; San Vicente, F.; Crossa, J. Rapid cycling genomic selection in a multiparental tropical maize population. G3 Genes Genomes Genet. 2017, 7, 2315–2326. [Google Scholar] [CrossRef]
- Della Coletta, R.; Fernandes, S.B.; Monnahan, P.J.; Mikel, M.A.; Bohn, M.O.; Lipka, A.E.; Hirsch, C.N. Importance of genetic architecture in marker selection decisions for genomic prediction. Theor. Appl. Genet. 2023, 136, 220. [Google Scholar] [CrossRef]
- Farooqi, M.Q.U.; Nawaz, G.; Wani, S.H.; Choudhary, J.R.; Rana, M.; Sah, R.P.; Afzal, M.; Zahra, Z.; Ganie, S.A.; Razzaq, A. Recent developments in multi-omics and breeding strategies for abiotic stress tolerance in maize (Zea mays L.). Front. Plant Sci. 2022, 13, 965878. [Google Scholar] [CrossRef]
- Trachsel, S.; Dhliwayo, T.; Gonzalez Perez, L.; Mendoza Lugo, J.A.; Trachsel, M. Estimation of physiological genomic estimated breeding values (PGEBV) combining full hyperspectral and marker data across environments for grain yield under combined heat and drought stress in tropical maize (Zea mays L.). PLoS ONE 2019, 14, e0212200. [Google Scholar] [CrossRef]
- Krishna, T.P.A.; Veeramuthu, D.; Maharajan, T.; Soosaimanickam, M. The era of plant breeding: Conventional breeding to genomics-assisted breeding for crop improvement. Curr. Genom. 2023, 24, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, A.; Kaur, P.; Akhter, N.; Wani, S.H.; Saleem, F. Next-generation breeding strategies for climate-ready crops. Front. Plant Sci. 2021, 12, 620420. [Google Scholar] [CrossRef]
- Ahmad, M. Plant breeding advancements with “CRISPR-Cas” genome editing technologies will assist future food security. Front. Plant Sci. 2023, 14, 1133036. [Google Scholar] [CrossRef]
- Li, J.-F.; Norville, J.E.; Aach, J.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 2013, 31, 688–691. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.-L. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- Beyene, Y.; Semagn, K.; Crossa, J.; Mugo, S.; Atlin, G.N.; Tarekegne, A.; Meisel, B.; Sehabiague, P.; Vivek, B.S.; Oikeh, S. Improving maize grain yield under drought stress and non-stress environments in sub-Saharan Africa using marker-assisted recurrent selection. Crop Sci. 2016, 56, 344–353. [Google Scholar] [CrossRef]
- Ribaut, J.-M.; Ragot, M. Marker-assisted selection to improve drought adaptation in maize: The backcross approach, perspectives, limitations, and alternatives. J. Exp. Bot. 2007, 58, 351–360. [Google Scholar] [CrossRef]
- Tuberosa, R.; Salvi, S. QTL for agronomic traits in maize production. In Handbook of Maize: Its Biology; Springer: New York, NY, USA, 2009; pp. 501–541. [Google Scholar]
- Messmer, R.; Fracheboud, Y.; Bänziger, M.; Vargas, M.; Stamp, P.; Ribaut, J.-M. Drought stress and tropical maize: QTL-by-environment interactions and stability of QTLs across environments for yield components and secondary traits. Theor. Appl. Genet. 2009, 119, 913–930. [Google Scholar] [CrossRef]
- Messmer, R.; Fracheboud, Y.; Bänziger, M.; Stamp, P.; Ribaut, J.-M. Drought stress and tropical maize: QTLs for leaf greenness, plant senescence, and root capacitance. Field Crops Res. 2011, 124, 93–103. [Google Scholar] [CrossRef]
- Almeida, G.D.; Makumbi, D.; Magorokosho, C.; Nair, S.; Borém, A.; Ribaut, J.-M.; Bänziger, M.; Prasanna, B.M.; Crossa, J.; Babu, R. QTL mapping in three tropical maize populations reveals a set of constitutive and adaptive genomic regions for drought tolerance. Theor. Appl. Genet. 2013, 126, 583–600. [Google Scholar] [CrossRef]
- Hao, Z.; Li, X.; Liu, X.; Xie, C.; Li, M.; Zhang, D.; Zhang, S. Meta-analysis of constitutive and adaptive QTL for drought tolerance in maize. Euphytica 2010, 174, 165–177. [Google Scholar] [CrossRef]
- Trachsel, S.; Sun, D.; SanVicente, F.M.; Zheng, H.; Atlin, G.N.; Suarez, E.A.; Babu, R.; Zhang, X. Identification of QTL for early vigor and stay-green conferring tolerance to drought in two connected advanced backcross populations in tropical maize (Zea mays L.). PLoS ONE 2016, 11, e0149636. [Google Scholar]
- Bernier, J.; Atlin, G.N.; Serraj, R.; Kumar, A.; Spaner, D. Breeding upland rice for drought resistance. J. Sci. Food Agric. 2008, 88, 927–939. [Google Scholar] [CrossRef]
- Tuberosa, R.; Salvi, S.; Sanguineti, M.C.; Landi, P.; Maccaferri, M.; Conti, S. Mapping QTLs regulating morpho-physiological traits and yield: Case studies, shortcomings and perspectives in drought-stressed maize. Ann. Bot. 2002, 89, 941–963. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G. The importance of the anthesis-silking interval in breeding for drought tolerance in tropical maize. Field Crops Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Mei, H.; Li, Z.; Shu, Q.; Guo, L.; Wang, Y.; Yu, X.; Ying, C.; Luo, L. Gene actions of QTLs affecting several agronomic traits resolved in a recombinant inbred rice population and two backcross populations. Theor. Appl. Genet. 2005, 110, 649–659. [Google Scholar] [CrossRef]
- Szalma, S.; Hostert, B.; LeDeaux, J.; Stuber, C.; Holland, J. QTL mapping with near-isogenic lines in maize. Theor. Appl. Genet. 2007, 114, 1211–1228. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Poland, J.A.; Brown, P.J.; Sorrells, M.E.; Jannink, J.-L. Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PLoS ONE 2012, 7, e32253. [Google Scholar] [CrossRef]
- Adesete, A.A.; Olanubi, O.E.; Dauda, R.O. Climate change and food security in selected Sub-Saharan African Countries. Environ. Dev. Sustain. 2023, 25, 14623–14641. [Google Scholar] [CrossRef]
- FAO; FAOSTAT. Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2018; 403p, Available online: http://faostat.fao.org (accessed on 9 April 2025).
- Seppelt, R.; Klotz, S.; Peiter, E.; Volk, M. Agriculture and food security under a changing climate: An underestimated challenge. iScience 2022, 25, 105551. [Google Scholar] [CrossRef] [PubMed]
- Pathak, H. Impact, adaptation, and mitigation of climate change in Indian agriculture. Environ. Monit. Assess. 2023, 195, 52. [Google Scholar] [CrossRef]
- Jat, S.; Parihar, C.; Dey, A.; Nayak, H.; Ghosh, A.; Parihar, N.; Goswami, A.; Singh, A. Dynamics and temperature sensitivity of soil organic carbon mineralization under medium-term conservation agriculture as affected by residue and nitrogen management options. Soil Tillage Res. 2019, 190, 175–185. [Google Scholar] [CrossRef]
- Padhan, S.R.; Rathore, S.; Prasad, S.M.; Shekhawat, K.; Singh, V. Influence of precision nutrient and weed management on growth and productivity of direct-seeded upland rice (Oryza sativa) under Eastern Plateau and Hills Region. Indian J. Agron. 2021, 66, 366–369. [Google Scholar] [CrossRef]
- Jat, S.; Parihar, C.; Singh, A.; Nayak, H.; Meena, B.; Kumar, B.; Parihar, M.; Jat, M. Differential response from nitrogen sources with and without residue management under conservation agriculture on crop yields, water-use and economics in maize-based rotations. Field Crops Res. 2019, 236, 96–110. [Google Scholar] [CrossRef]
- Dmuchowski, W.; Baczewska-Dąbrowska, A.H.; Gworek, B. The role of temperate agroforestry in mitigating climate change: A review. For. Policy Econ. 2024, 159, 103136. [Google Scholar] [CrossRef]
- Raj, A.; Jhariya, M.K.; Banerjee, A.; Meena, R.S.; Jha, R.K.; Kittur, B.H.; Singh, K.P. Agroforestry to mitigate the climate change. In Agroforestry for Carbon and Ecosystem Management; Elsevier: Amsterdam, The Netherlands, 2024; pp. 79–96. [Google Scholar]
- Deines, J.M.; Guan, K.; Lopez, B.; Zhou, Q.; White, C.S.; Wang, S.; Lobell, D.B. Recent cover crop adoption is associated with small maize and soybean yield losses in the United States. Glob. Change Biol. 2023, 29, 794–807. [Google Scholar] [CrossRef]
- Won, S.; Rejesus, R.M.; Goodwin, B.K.; Aglasan, S. Understanding the effect of cover crop use on prevented planting losses. Am. J. Agric. Econ. 2024, 106, 659–683. [Google Scholar] [CrossRef]
- Masuka, B.; Araus, J.L.; Das, B.; Sonder, K.; Cairns, J.E. Phenotyping for abiotic stress tolerance in maize F. J. Integr. Plant Biol. 2012, 54, 238–249. [Google Scholar] [CrossRef]
- Arya, S.; Sandhu, K.S.; Singh, J.; Kumar, S. Deep learning: As the new frontier in high-throughput plant phenotyping. Euphytica 2022, 218, 47. [Google Scholar] [CrossRef]
- Sankaran, S.; Zhang, C.; Hurst, J.P.; Marzougui, A.; Veeranampalayam-Sivakumar, A.N.; Li, J.; Schnable, J.; Shi, Y. Investigating the potential of satellite imagery for high-throughput field phenotyping applications. In Proceedings of the Autonomous Air and Ground Sensing Systems for Agricultural Optimization and Phenotyping V, Online, 27 April–9 May 2020; Volume 11414, p. 1141402. [Google Scholar]
- Zhang, L.; Niu, Y.; Zhang, H.; Han, W.; Li, G.; Tang, J.; Peng, X. Maize canopy temperature extracted from UAV thermal and RGB imagery and its application in water stress monitoring. Front. Plant Sci. 2019, 10, 1270. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.; Bort, J.; Slafer, G.; Araus, J. Can wheat yield be assessed by early measurements of Normalized Difference Vegetation Index? Ann. Appl. Biol. 2007, 150, 253–257. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef]
- Rorie, R.L.; Purcell, L.C.; Mozaffari, M.; Karcher, D.E.; King, C.A.; Marsh, M.C.; Longer, D.E. Association of “greenness” in corn with yield and leaf nitrogen concentration. Agron. J. 2011, 103, 529–535. [Google Scholar] [CrossRef]
- Boote, B.W.; Freppon, D.J.; De La Fuente, G.N.; Lübberstedt, T.; Nikolau, B.J.; Smith, E.A. Haploid differentiation in maize kernels based on fluorescence imaging. Plant Breed. 2016, 135, 439–445. [Google Scholar] [CrossRef]
- Zia, S.; Romano, G.; Spreer, W.; Sanchez, C.; Cairns, J.; Araus, J.; Müller, J. Infrared thermal imaging as a rapid tool for identifying water-stress tolerant maize genotypes of different phenology. J. Agron. Crop Sci. 2013, 199, 75–84. [Google Scholar] [CrossRef]
- Gill, M.; Anderson, R.; Hu, H.; Bennamoun, M.; Petereit, J.; Valliyodan, B.; Nguyen, H.T.; Batley, J.; Bayer, P.E.; Edwards, D. Machine learning models outperform deep learning models, provide interpretation and facilitate feature selection for soybean trait prediction. BMC Plant Biol. 2022, 22, 180. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Sarkar, S.; Ganapathysubramanian, B.; Schapaugh, W.; Miguez, F.E.; Carley, C.N.; Carroll, M.E.; Chiozza, M.V.; Chiteri, K.O. High-throughput phenotyping in soybean. In High-Throughput Crop Phenotyping; Springer: Cham, Switzerland, 2021; pp. 129–163. [Google Scholar]
- Jonas, E.; De Koning, D.-J. Does genomic selection have a future in plant breeding? Trends Biotechnol. 2013, 31, 497–504. [Google Scholar] [CrossRef]
- Heffner, E.L.; Sorrells, M.E.; Jannink, J.L. Genomic selection for crop improvement. Crop Sci. 2009, 49, 1–12. [Google Scholar] [CrossRef]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Spindel, J.; Begum, H.; Akdemir, D.; Virk, P.; Collard, B.; Redona, E.; Atlin, G.; Jannink, J.-L.; McCouch, S.R. Genomic selection and association mapping in rice (Oryza sativa): Effect of trait genetic architecture, training population composition, marker number and statistical model on accuracy of rice genomic selection in elite, tropical rice breeding lines. PLoS Genet. 2015, 11, e1004982. [Google Scholar]
- Endelman, J.B.; Atlin, G.N.; Beyene, Y.; Semagn, K.; Zhang, X.; Sorrells, M.E.; Jannink, J.L. Optimal design of preliminary yield trials with genome-wide markers. Crop Sci. 2014, 54, 48–59. [Google Scholar] [CrossRef]
- Solberg Woods, L.C. QTL mapping in outbred populations: Successes and challenges. Physiol. Genom. 2014, 46, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, N.R.; Gould, M.N. The long path from QTL to gene. PLoS Genet. 2012, 8, e1002975. [Google Scholar] [CrossRef]
- Fu, J.; Falke, K.C.; Thiemann, A.; Schrag, T.A.; Melchinger, A.E.; Scholten, S.; Frisch, M. Partial least squares regression, support vector machine regression, and transcriptome-based distances for prediction of maize hybrid performance with gene expression data. Theor. Appl. Genet. 2012, 124, 825–833. [Google Scholar] [CrossRef]
- Frisch, M.; Thiemann, A.; Fu, J.; Schrag, T.A.; Scholten, S.; Melchinger, A.E. Transcriptome-based distance measures for grouping of germplasm and prediction of hybrid performance in maize. Theor. Appl. Genet. 2010, 120, 441–450. [Google Scholar] [CrossRef]
- Zenke-Philippi, C.; Thiemann, A.; Seifert, F.; Schrag, T.; Melchinger, A.E.; Scholten, S.; Frisch, M. Prediction of hybrid performance in maize with a ridge regression model employed to DNA markers and mRNA transcription profiles. BMC Genom. 2016, 17, 262. [Google Scholar] [CrossRef]
- Schrag, T.A.; Schipprack, W.; Melchinger, A.E. Across-years prediction of hybrid performance in maize using genomics. Theor. Appl. Genet. 2019, 132, 933–946. [Google Scholar] [CrossRef]
- Guo, Z.; Magwire, M.M.; Basten, C.J.; Xu, Z.; Wang, D. Evaluation of the utility of gene expression and metabolic information for genomic prediction in maize. Theor. Appl. Genet. 2016, 129, 2413–2427. [Google Scholar] [CrossRef]
- Li, J.-Q.; Fang, J.-S.; Qin, X.-M.; Gao, L. Metabolomics profiling reveals the mechanism of caffeic acid in extending lifespan in Drosophila melanogaster. Food Funct. 2020, 11, 8202–8213. [Google Scholar] [CrossRef]
- Zarringhalam, K.; Degras, D.; Brockel, C.; Ziemek, D. Robust phenotype prediction from gene expression data using differential shrinkage of co-regulated genes. Sci. Rep. 2018, 8, 1237. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-H.; Jeon, Y.-D.; Moon, K.-H.; Lee, J.-H.; Kim, D.-G.; Kim, W.; Myung, H.; Kim, J.-S.; Kim, H.-J.; Bang, K.-S. Aronia berry extract ameliorates the severity of dextran sodium sulfate-induced ulcerative colitis in mice. J. Med. Food 2017, 20, 667–675. [Google Scholar] [CrossRef]
- Medeiros, D.B.; Brotman, Y.; Fernie, A.R. The utility of metabolomics as a tool to inform maize biology. Plant Commun. 2021, 2, 100187. [Google Scholar] [CrossRef]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef]
- Casati, P.; Morrow, D.J.; Fernandes, J.F.; Walbot, V. Rapid maize leaf and immature ear responses to UV-B radiation. Front. Plant Sci. 2011, 2, 33. [Google Scholar] [CrossRef]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol. Plant 2012, 5, 401–417. [Google Scholar] [CrossRef]
- Richter, J.A.; Erban, A.; Kopka, J.; Zörb, C. Metabolic contribution to salt stress in two maize hybrids with contrasting resistance. Plant Sci. 2015, 233, 107–115. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Chen, X.; Fu, J.; Zhang, Y. Metabolic and growth responses of maize to successive drought and re-watering cycles. Agric. Water Manag. 2016, 172, 62–73. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat, and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef]
- Gavaghan, C.L.; Li, J.V.; Hadfield, S.T.; Hole, S.; Nicholson, J.K.; Wilson, I.D.; Howe, P.W.; Stanley, P.D.; Holmes, E. Application of NMR-based metabolomics to the investigation of salt stress in maize (Zea mays). Phytochem. Anal. 2011, 22, 214–224. [Google Scholar] [CrossRef]
- Ganie, A.H.; Ahmad, A.; Pandey, R.; Aref, I.M.; Yousuf, P.Y.; Ahmad, S.; Iqbal, M. Metabolite profiling of low-P tolerant and low-P sensitive maize genotypes under phosphorus starvation and restoration conditions. PLoS ONE 2015, 10, e0129520. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.E.; Prasanna, B.M. Developing and deploying climate-resilient maize varieties in the developing world. Curr. Opin. Plant Biol. 2018, 45, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, S.; Chapman, S.; Mahop, M.T.; Deva, C.; Masamba, K.; Mwamahonje, A. Exploring assumptions in crop breeding for climate resilience: Opportunities and principles for integrating climate model projections. Clim. Change 2021, 164, 38. [Google Scholar] [CrossRef]
- Matthew, O.J.; Abiodun, B.J.; Salami, A.T. Modelling the impacts of climate variability on crop yields in Nigeria: Performance evaluation of RegCM3-GLAM system. Meteorol. Appl. 2015, 22, 198–212. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, W.; Ge, Q. CERES-Maize model-based simulation of climate change impacts on maize yields and potential adaptive measures in Heilongjiang Province, China. J. Sci. Food Agric. 2015, 95, 2838–2849. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Z.; Qiao, S.; Zhang, Z.; Huang, Q.; Su, Z.; Yang, X. How could observed sowing dates contribute to maize potential yield under climate change in Northeast China based on APSIM model. Eur. J. Agron. 2022, 136, 126511. [Google Scholar] [CrossRef]
- Dzotsi, K.; Basso, B.; Jones, J. Parameter and uncertainty estimation for maize, peanut and cotton using the SALUS crop model. Agric. Syst. 2015, 135, 31–47. [Google Scholar] [CrossRef]
- Rai, T.; Kumar, S.; Nleya, T.; Sexton, P.; Hoogenboom, G. Simulation of maize and soybean yield using DSSAT under long-term conventional and no-till systems. Soil Res. 2022, 60, 520–533. [Google Scholar] [CrossRef]
- Falconnier, G.N.; Corbeels, M.; Boote, K.J.; Affholder, F.; Adam, M.; MacCarthy, D.S.; Ruane, A.C.; Nendel, C.; Whitbread, A.M.; Justes, É. Modelling climate change impacts on maize yields under low nitrogen input conditions in sub-Saharan Africa. Glob. Change Biol. 2020, 26, 5942–5964. [Google Scholar] [CrossRef]
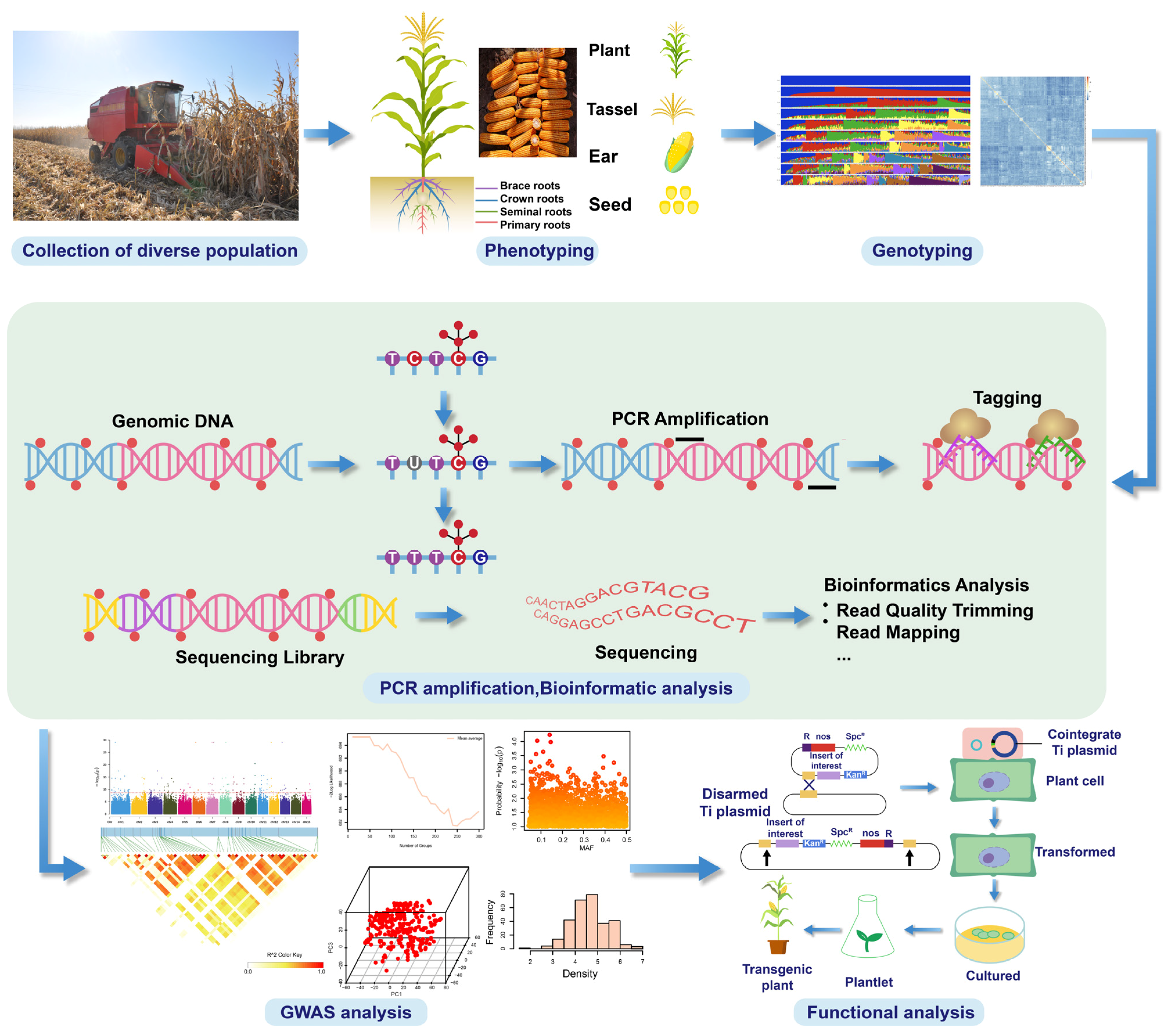


| Gene ID | Gene Description | References |
|---|---|---|
| ZmVPP1 | Vascular pyrophosphatase improves drought tolerance | [18] |
| ZmACA1 | The gene for cold stress tolerance | [19] |
| ZmDREB2A | The gene for cold stress tolerance | [20] |
| ZmERF3 | The gene for cold stress tolerance | [19] |
| ZmCOI6.1 | The gene for cold stress tolerance | [21] |
| ZmPP2C2 | The gene for abiotic stress response | [22] |
| ZmMKK4 | The gene for abiotic stress response | [23] |
| GRMZM2G329229 | Associated with different stress conditions such as heat and drought | [24] |
| GRMZM2G313009 | Associated with different stress conditions such as heat and drought | [24] |
| GRMZM2G043764 | Associated with different stress conditions such as heat and drought | [24] |
| GRMZM2G109651 | Associated with different stress conditions such as heat and drought | [24] |
| GRMZM2G159307 | Encodes ATP binding protein; important for response to stresses | [25] |
| GRMZM2G104325 | Encodes ATP binding protein; important for response to stresses | [25] |
| Zm00001d048531 | Encodes an RNA helicase; associated with improved stress tolerance | [26] |
| Mapped Traits | Population Type | Sample Size | Number of SNPs/QTLs/Genes | Chromosomal Location | References |
|---|---|---|---|---|---|
| Corn earworm resistance | Diverse inbreed lines | 287 | 51 SNPs | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [69] |
| Gray leaf spot resistance | Diverse inbred lines | 157 | 7 SNPs | 1, 2, 3, 4, 5, 6, 7, and 10 | [70] |
| Southern corn rust resistance | Diverse inbred lines | 253 | 7 SNPs | 4, 8, and 10 | [71] |
| Corn ear rot resistance | Diverse inbred lines | 242 | 5 candidate genes | 5, 7, and 10 | [72] |
| Ear rot resistance | Diverse inbred lines | 244 | 8 candidate genes | 1, 2, 3, 5, 7, and 9 | [73] |
| Fumonisin accumulation in kernels | Diverse inbred lines | 270 | 39 SNPs/17 QTLs | 3 and 4 | [74] |
| Maize lethal necrosis (MLN) and maize chlorotic mottle virus (MCMV) | Three double-haploid populations | 965 | 54 SNPs and 40 QTLs | 1, 2, 3, 4, 5, 6, 7, 8, and 9 | [75] |
| Striga resistance | White maize inbred lines | 132 | 24 SNPs | 1, 3, 4, 5, 7, 8, 9, and 10 | [76] |
| Maize leaf necrosis resistance | Diverse inbred lines | 1400 | 32 SNPs and 9 candidate genes | 1, 3, 4, 7, 9, and 10 | [77] |
| Fusarium verticillioides resistance | Maize association population | 230 | 42 SNPs and 25 candidate genes | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [78] |
| Corn leaf blight | Association mapping panel | 419 | 22 SNPs | 1, 6, 7, 8, and 10 | [79] |
| Aspergillus flavus resistance in kernels | Diverse inbred lines | 313 | 4 SNPs and 16 candidate genes | 1, 2, 8, and 9 | [80] |
| Gray leaf spot resistance | Diverse inbred lines | 410 | 22 SNPs | 1, 2, 6, 7, and 8 | [81] |
| Rough dwarf disease resistance | Diverse inbred lines | 292 | 22 SNPs | 1, 3, 4, 7, and 8 | [82] |
| Striga resistance | Diverse inbred lines | 141 | 22 SNPs | 1, 3, 4, 5, 6, 7, 8, 9, and 10 | [83] |
| Leaf streak resistance | Diverse inbred lines | 200 | 11 SNPs | 1, 2, 5, 7, 8 and 9 | [84] |
| Root architecture traits | Diverse inbred lines | 300 | 19 SNPs | 1, 2, 5, 7, and 8 | [69] |
| Leaf angle and leaf orientation | Diverse inbred lines | 80 | 33 SNPs | 1, 3, 4, 5, 6, 7, and 9 | [85] |
| Leaf cuticular conductance | Diverse inbred lines | 468 | 9 SNPs and 7 candidate genes | 1, 4, 7, 8, and 10 | [86] |
| Leaf angle | Diverse inbred lines | 285 | 96 SNPs | 1, 2, 3, 4, 5, 6, 7, 9, and 10 | [87] |
| Chlorophyll content | Diverse inbred lines | 378 | 19 SNPs | 2, 4, 5, 6, and 10 | [88] |
| Plant height | Maize hybrids | 300 | 9 SNPs and 2 candidate genes | 1, 2, 4, 7, 9, and 10 | [89] |
| Tassel architecture | Association panel | 359 | 55 candidate genes/19 QTLs | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [90] |
| Kernal row number | Diverse inbred lines | 639 | 49 candidate genes | 1, 2, 3, 5, 9, and 10 | [91] |
| Ear diameter | Multiple parent population | 162 | 11 SNPs and 3 QTLs | 1, 2, 3, 6, 8, and 9 | [92] |
| Ear traits (ear length, diameter, kernel length and width, cob diameter) | Inbred association population | 292 | 20 SNPs | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [93] |
| Stalk strength | Diverse inbred lines | 345 | 94 QTLs and 241 SNPs | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [94] |
| Grain quality traits | Diverse inbred lines | 248 | 49 SNPs and 29 candidate genes | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [95] |
| Grain yield quality traits | Association mapping population | 410 | 42 SNPs | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [25] |
| Yield related traits | Diverse inbred lines | 291 | 59 SNPs and 66 candidate genes | 1, 2, 3, 4, 6, 7, 8, 9, and 10 | [96] |
| Grain yield and other traits | Diverse inbred lines | 169 | 40 SNPs and 6 candidate genes | 1, 2, 8, and 10 | [97] |
| Grain yield and flowering time | Inbred association panel | 300 | 1549 SNPs and 46 candidate genes | 1, 2, 4, 5, 8, and 10 | [24] |
| Root architecture traits | RIL population | 179 | 8 SNPs | 1, 2, 4, and 10 | [98] |
| Root hair length | Diverse inbred lines | 281 | 11 | 1, 2, 4, 5, 6, and 10 | [99] |
| Root hair length | Association panel | 200 | 88 QTLs | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [100] |
| Total root length | Diverse inbred lines | 280 | 38 candidate genes | 1, 2, 3, 4, 6, 7, 8, and 9 | [101] |
| Drought tolerance | Diverse inbred lines | 210 | 26 QTL promising candidate genes | 1, 2, 5, 8, and 10 | [102] |
| Drought tolerance | Association panel | 379 | 15 candidate genes | 1, 3, 4, 5, 6, 8, and 9 | [103] |
| Drought and heat resistance | Diverse inbred lines | 162 | 117 SNPs and 20 candidate genes | 1, 2, 5, and 7 | [26] |
| Heat tolerance | Double haploid lines | 662 | 46 SNPs | 1, 2, 3, 6, 7, and 8 | [104] |
| Heat resistance | Diverse inbred lines | 375 | 14 SNPs | 1, 2, 4, 5, and 9 | [105] |
| Thermos tolerance of seed | Diverse inbred lines | 261 | 4 QTLs, 17 candidate genes, and 42 SNPs | 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 | [106] |
| Chilling tolerant | Diverse inbred lines | 190 | 26 SNPs and 37 candidate genes | 4, 6, 8, and 9 | [107] |
| Cold tolerance | Diverse inbred lines | 80 | 4 SNPs and 12 QTLs, 1 gene | 3 | [108] |
| Salt tolerance | Diverse inbred lines | 150 | 7 SNPs and 8 candidate genes | 1, 3, and 6 | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, Y.; Sun, Y.; Li, S.; Cai, Q.; Li, S.; Sun, M.; Yu, T.; Meng, X.; Zhang, J. Integrating Genetic Diversity and Agronomic Innovations for Climate-Resilient Maize Systems. Plants 2025, 14, 1552. https://doi.org/10.3390/plants14101552
Li X, Li Y, Sun Y, Li S, Cai Q, Li S, Sun M, Yu T, Meng X, Zhang J. Integrating Genetic Diversity and Agronomic Innovations for Climate-Resilient Maize Systems. Plants. 2025; 14(10):1552. https://doi.org/10.3390/plants14101552
Chicago/Turabian StyleLi, Xin, Yunlong Li, Yan Sun, Sinan Li, Quan Cai, Shujun Li, Minghao Sun, Tao Yu, Xianglong Meng, and Jianguo Zhang. 2025. "Integrating Genetic Diversity and Agronomic Innovations for Climate-Resilient Maize Systems" Plants 14, no. 10: 1552. https://doi.org/10.3390/plants14101552
APA StyleLi, X., Li, Y., Sun, Y., Li, S., Cai, Q., Li, S., Sun, M., Yu, T., Meng, X., & Zhang, J. (2025). Integrating Genetic Diversity and Agronomic Innovations for Climate-Resilient Maize Systems. Plants, 14(10), 1552. https://doi.org/10.3390/plants14101552






