Effect of Vegetation Degradation on Soil Nitrogen Components and N-Cycling Enzyme Activities in a Wet Meadow on the Qinghai–Tibetan Plateau
Abstract
1. Introduction
2. Results
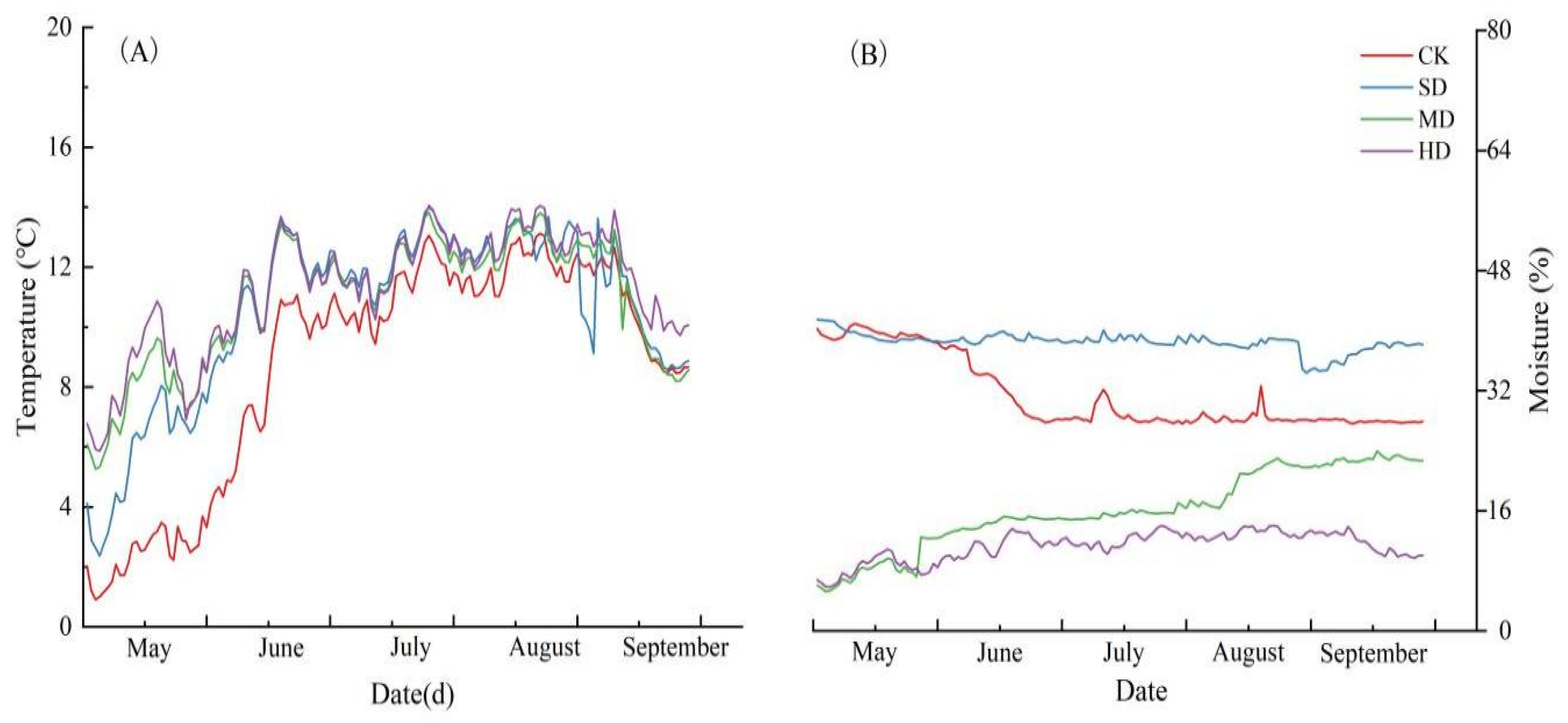
2.1. Environmental Factors During the Growing Season
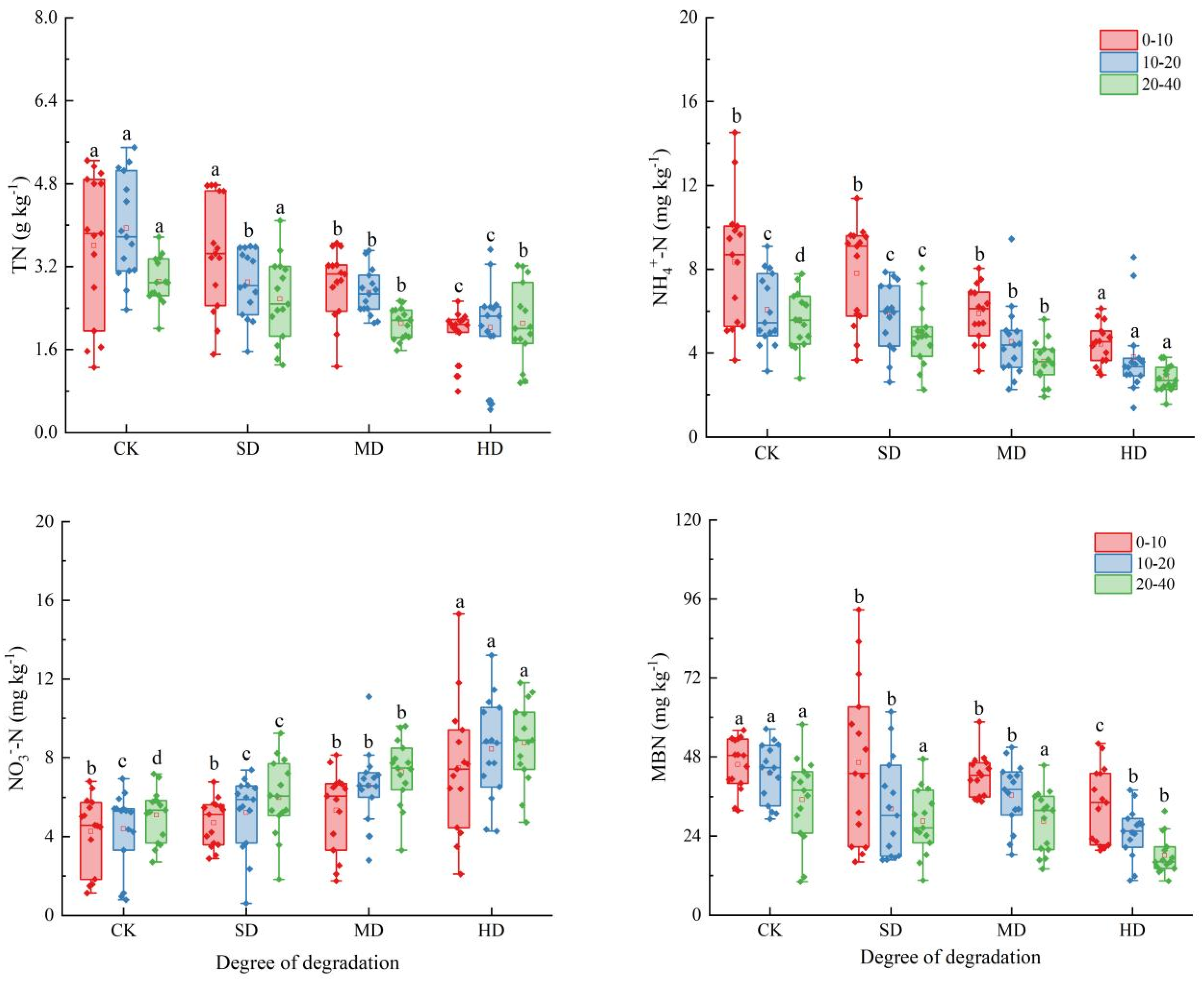
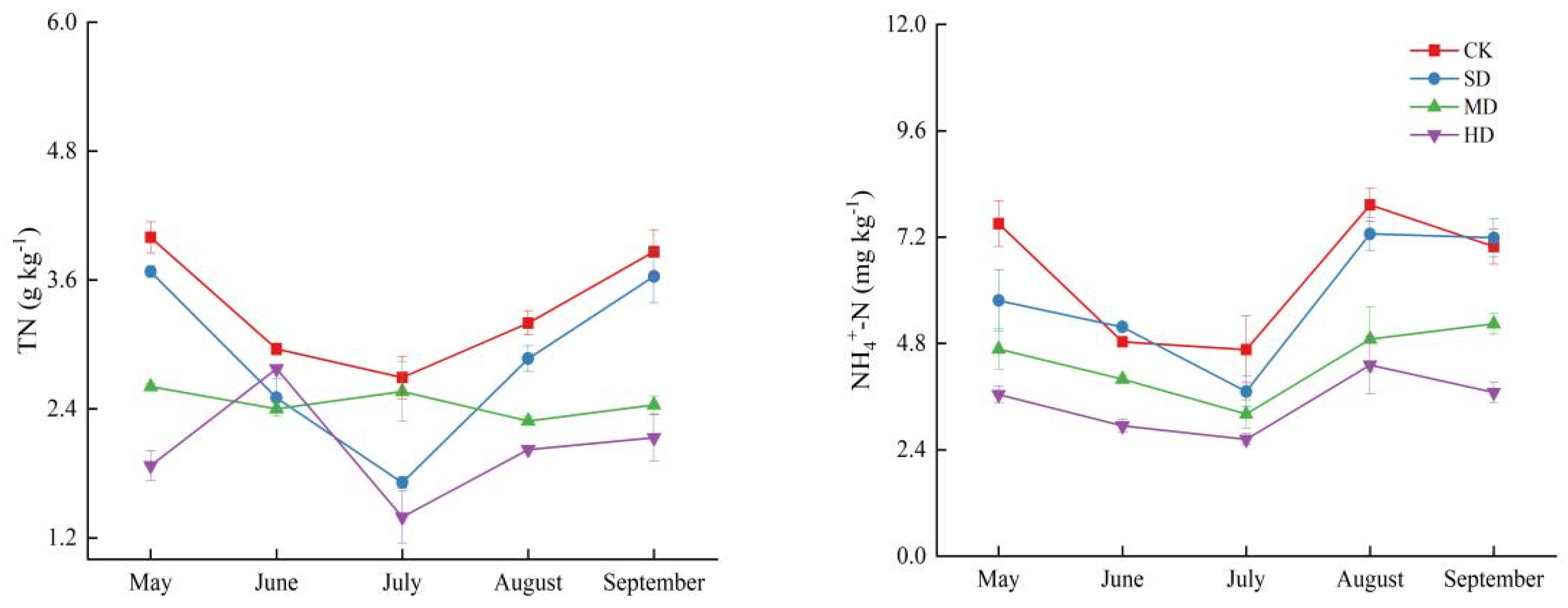
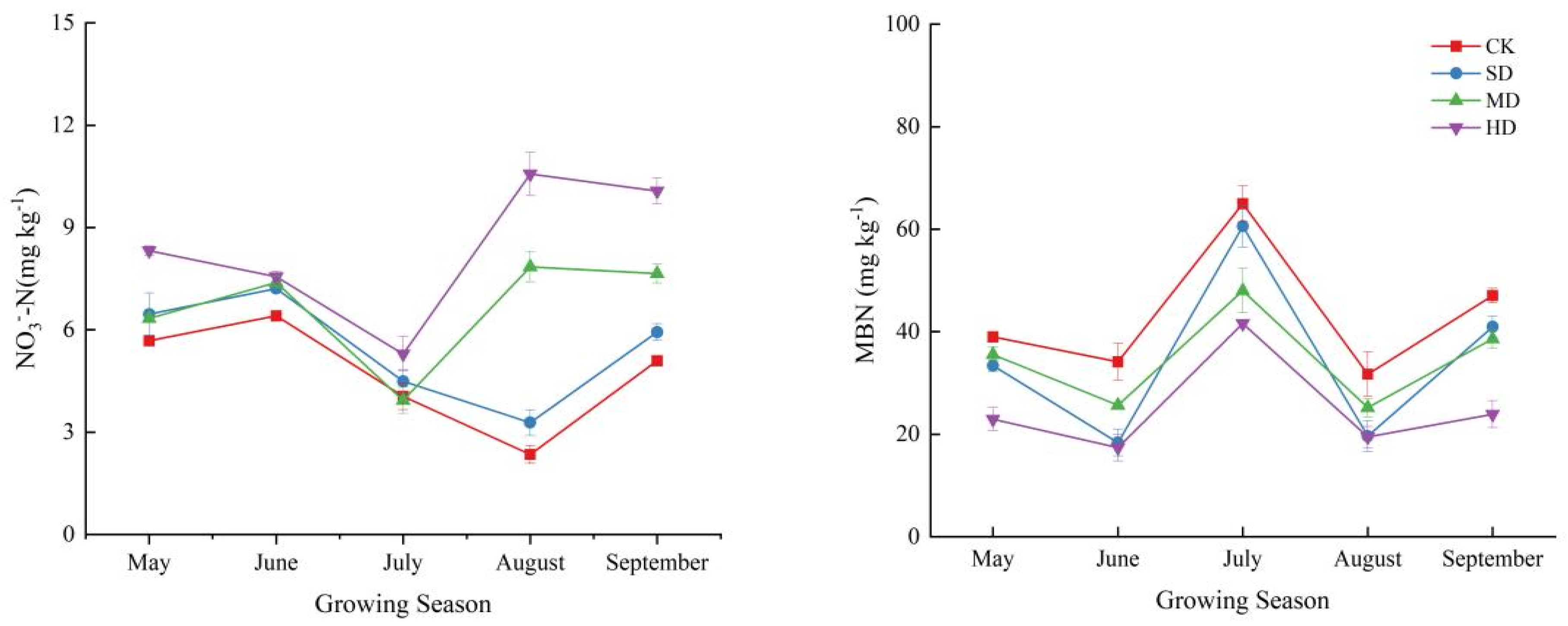
2.2. Spatial and Temporal Variation Characteristics of Soil Nitrogen Components in the Process of Vegetation Degradation
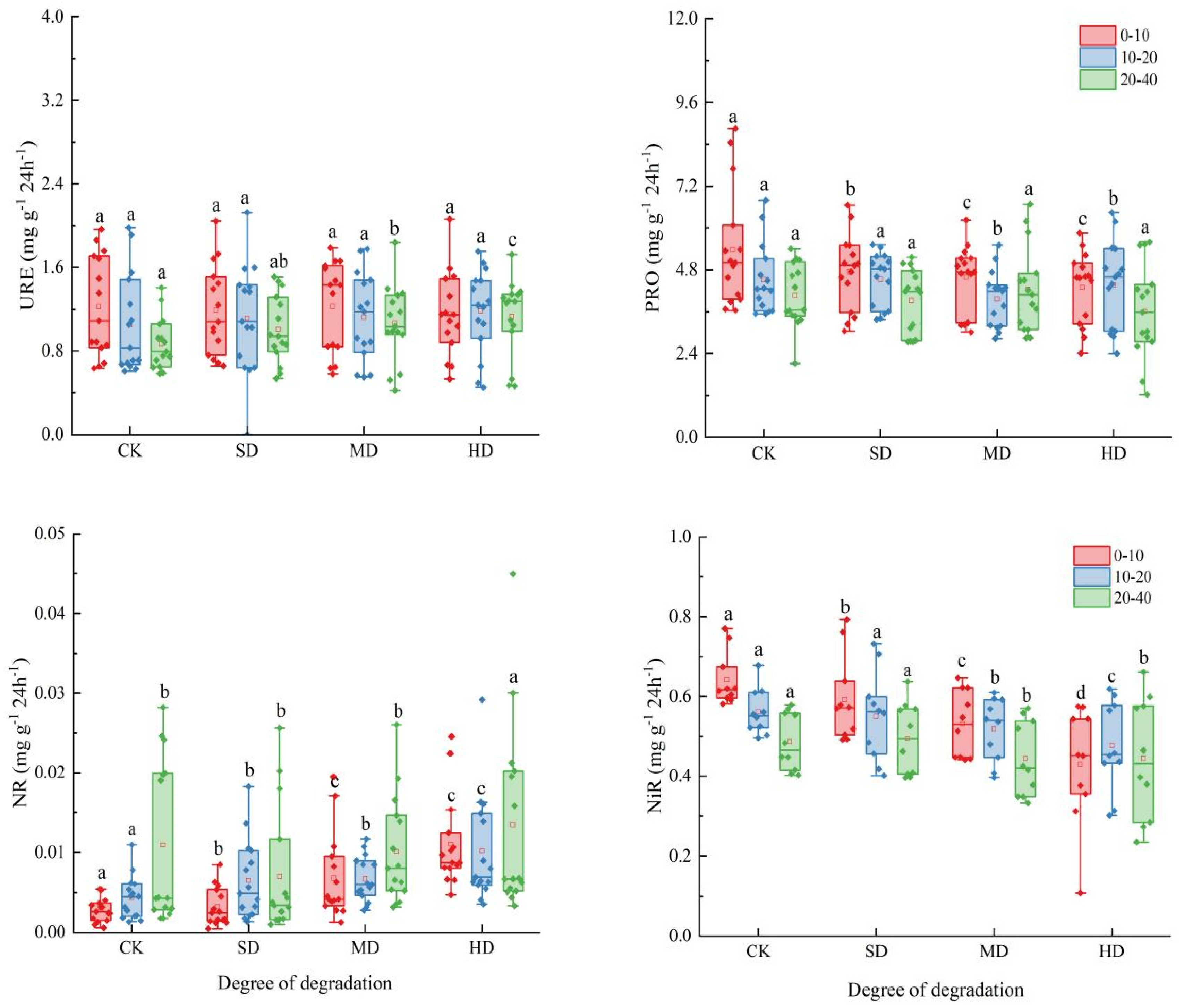
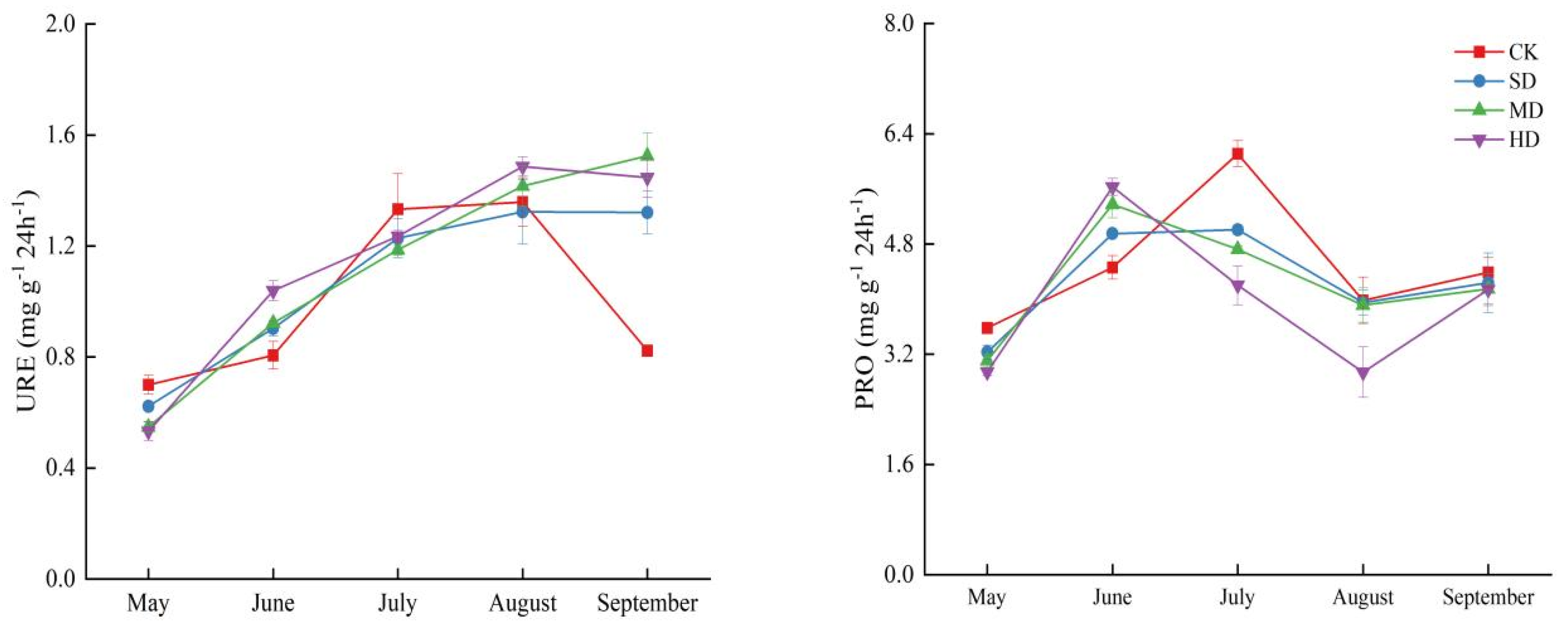
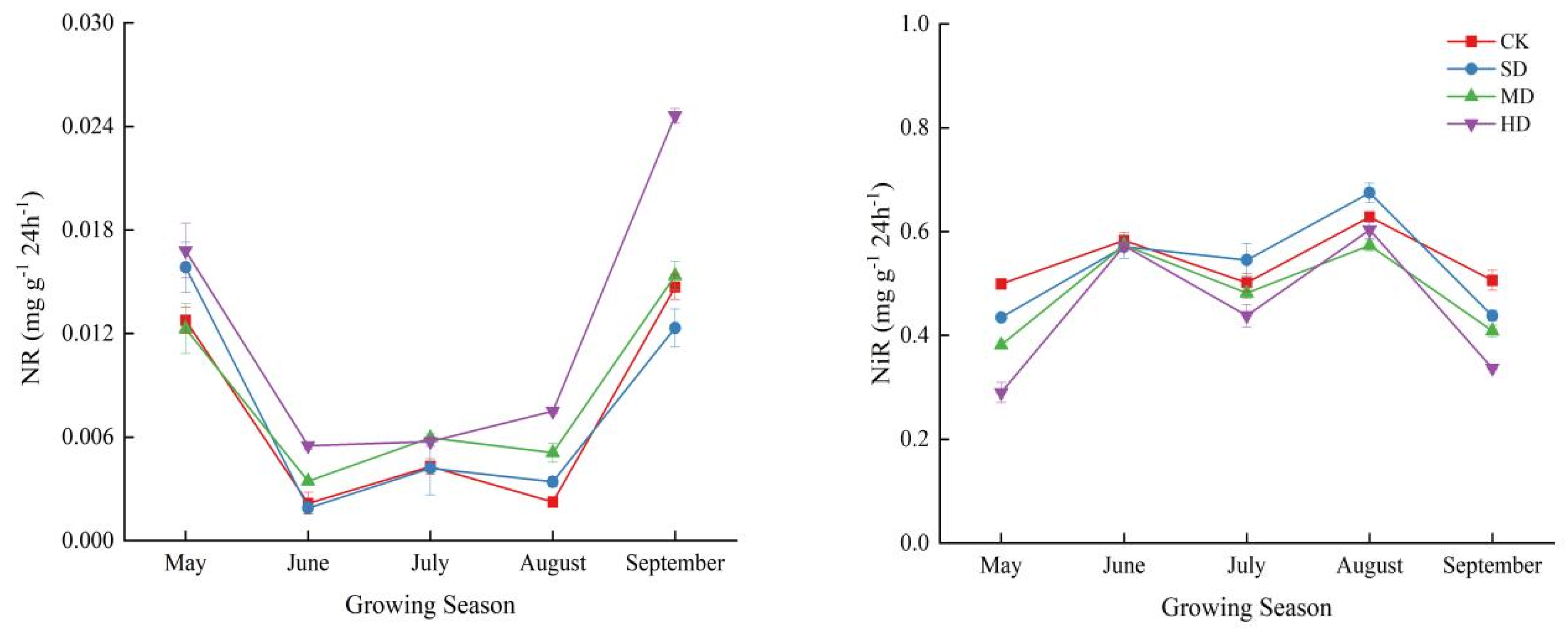
2.3. Spatial and Temporal Variation Characteristics of Soil Enzyme Activities During Vegetation Degradation
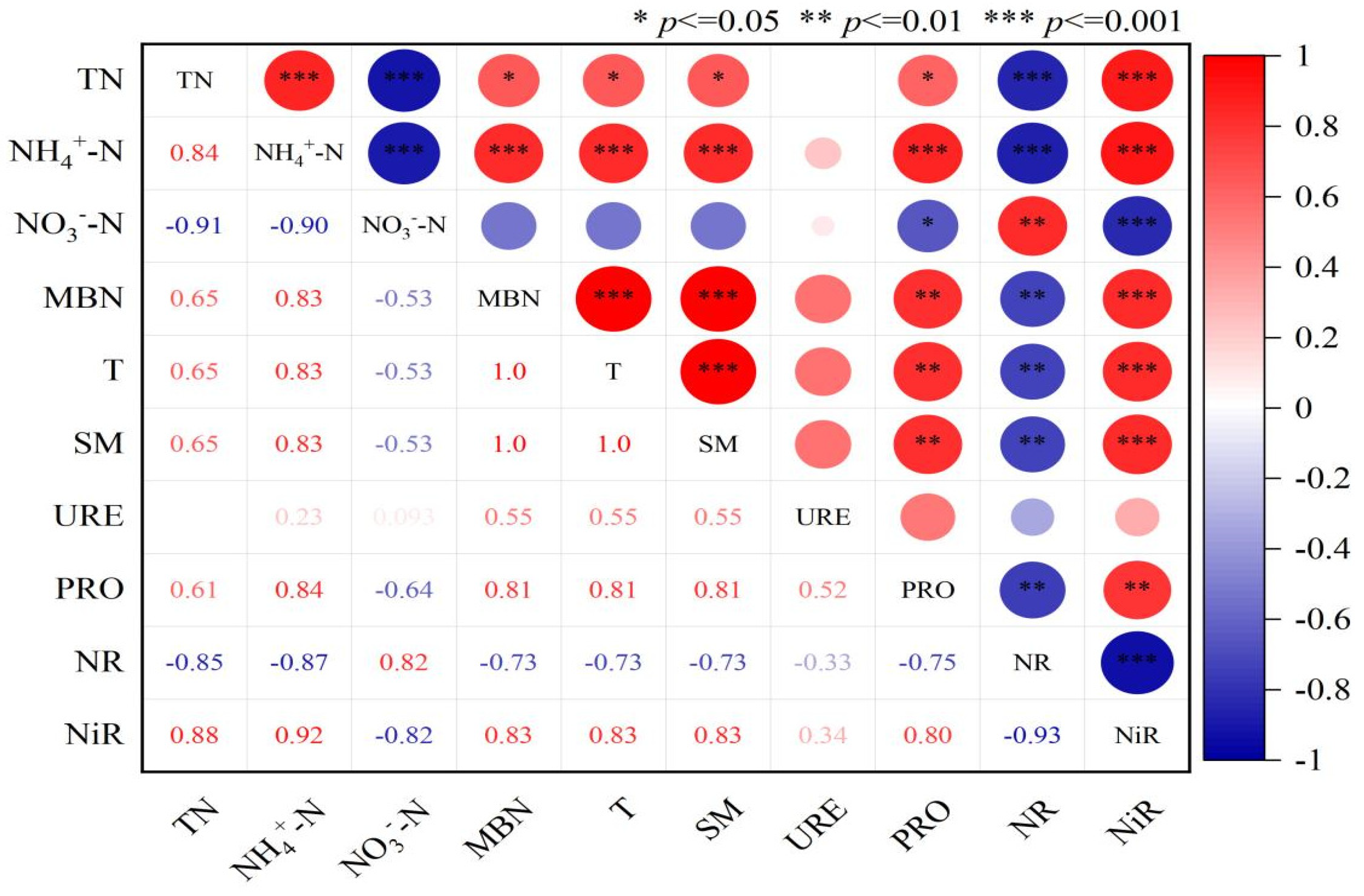
2.4. Correlation Between Soil Nitrogen Components and Enzyme Activity Under Vegetation Degradation
2.5. Relationship Between Environmental Variables and Soil N Components
3. Discussion
3.1. Response of Soil Nitrogen Components to Vegetation Degradation
3.2. Response of Soil Enzyme Activity to Vegetation Degradation
3.3. Relationships Between Soil Nitrogen, Enzyme Activities, and Environmental Factors
4. Materials and Methods
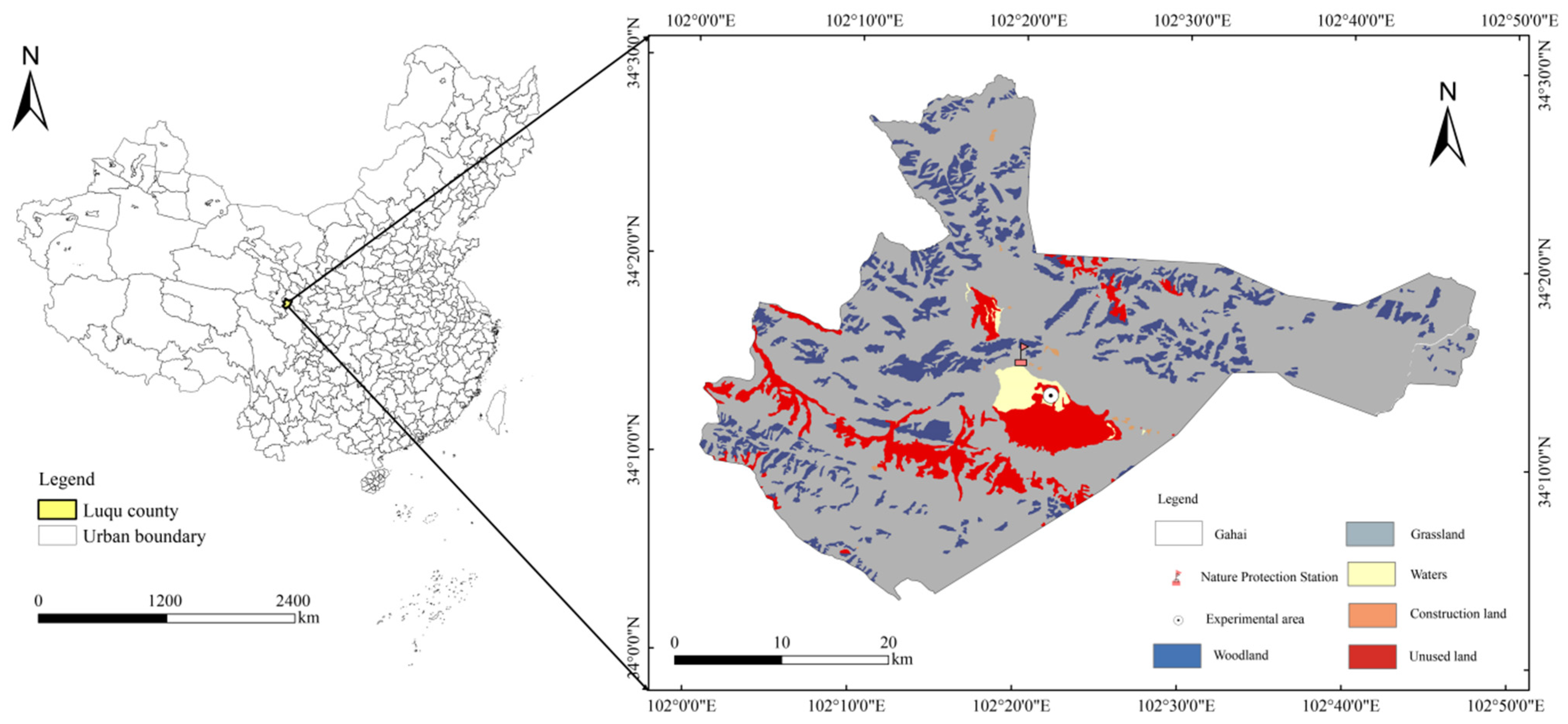
4.1. Study Area
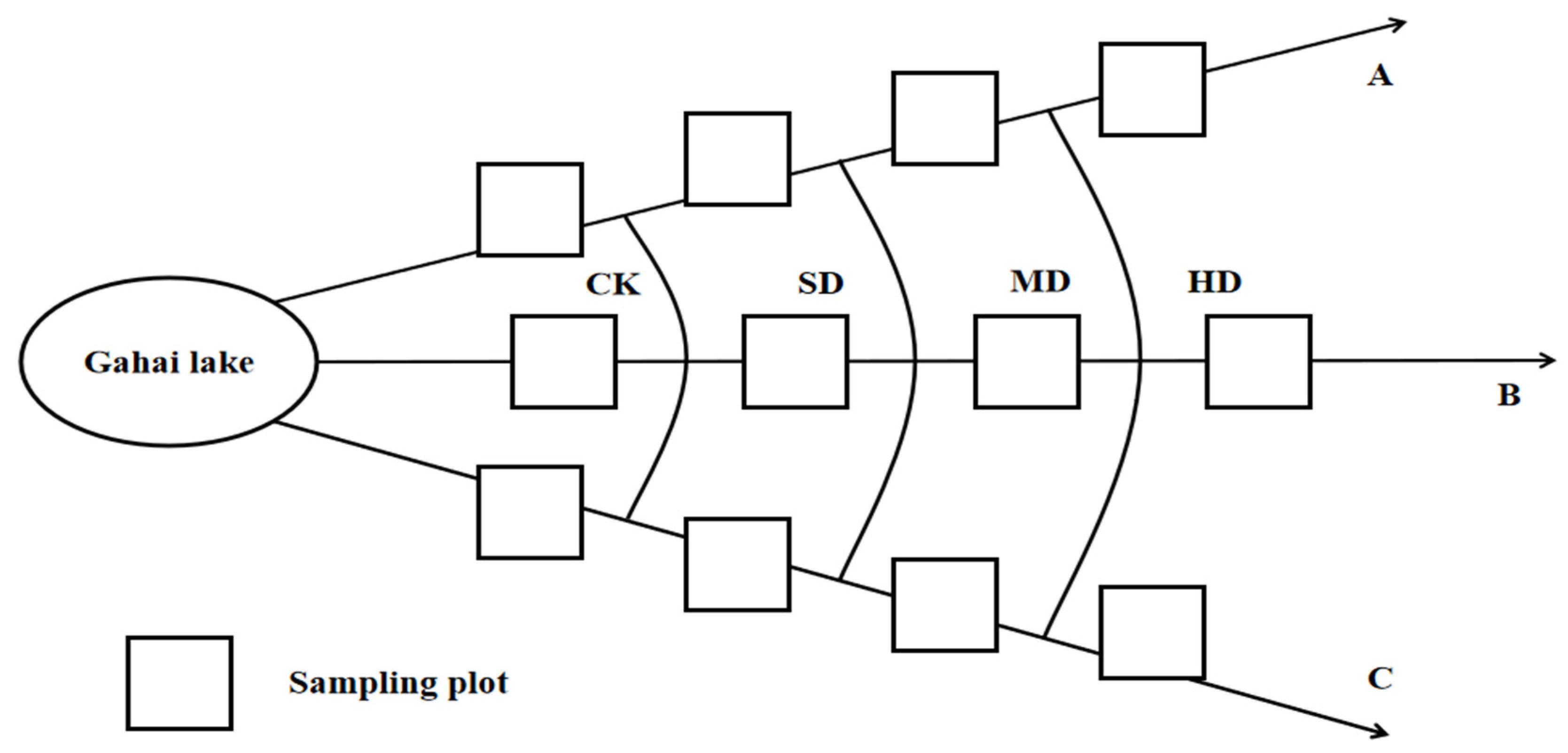
4.2. Materials and Experimental Design
4.3. Soil Sampling and Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordan, S.J.; Nestlerode, S.J.A. Wetlands as Sinks for Reactive Nitrogen at Continental and Global Scales: A Meta-Analysis. Ecosystems 2011, 14, 144–155. [Google Scholar] [CrossRef]
- Wang, H.; Wu, C.; Chen, D.; Liu, H.; Sun, X.; Zhang, S. Changes in soil carbon and nutrients and related extracellular enzymes in successive rotations of Japanese larch plantations. Catena 2021, 204, 105386. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, B.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 91, 3244–3257. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.T.; Hu, F.N.; Xu, C.Y.; Liu, E.; Zhao, S.W. Response of soil aggregate stability and splash erosion to diferent breakdown mechanisms along natural vegetation restoration. Catena 2022, 208, 105–775. [Google Scholar] [CrossRef]
- Yousaf, A.; Khalid, N.; Aqeel, M.; Noman, A.; Naeem, N.; Sarfraz, W.; Ejaz, U.; Qaiser, Z.; Khalid, A. Nitrogen Dynamics in Wetland Systems and Its Impact on Biodiversity. Nitrogen 2021, 2, 196–217. [Google Scholar] [CrossRef]
- Ravi, S.; Breshears, D.D.; Huxman, T.E.; D’Odorico, P. Land degradation in drylands: Interactions among hydrologic–aeolian erosion and vegetation dynamics. Geomorphology 2010, 116, 236–245. [Google Scholar] [CrossRef]
- Katayama, A.; Nanko, K.; Jeong, S.; Kume, T.; Shinohara, Y.; Seitz, S. Short communication: Concentrated impacts by tree canopy drips-hotspots of soil erosion in forests. Esurf 2023, 11, 1275–1282. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Yao, Y.; Dang, P.; Farooq, T.H.; Wu, X.; Wang, J.; Yan, W. Effects of Vegetation Restoration on Soil Nitrogen Fractions and Enzyme Activities in Arable Land on Purple Soil Slopes. Plants 2023, 12, 4188. [Google Scholar] [CrossRef]
- Wang, J.; Fu, X.; Chimire, R.; Sainju, U.M.; Jia, Y.; Zhao, F.Z. Responses of soil bacterial community and enzyme activity to organicmatter components under long-term fertilization on the loess plateau of China. Appl. Soil Ecol. 2021, 166, 103992. [Google Scholar] [CrossRef]
- Yang, C.B.; Ni, H.J.; Su, W.H.; Zhong, Z.K.; Zhang, X.P.; Bian, F.Y.; Li, W. Effects of management measures on organic carbon, nitrogen and chemical structure of diferentsoil fractions in Phyllostachys edulis plantations. Appl. Ecol. 2020, 31, 25–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.D.; Wu, J.J.; Li, J.S.; Feng, J.; Cheng, X.L. Soil nitrogen-hvdrolyzing enzyme activity and stoichiometry following a subtropical land usechange. Land. Degrad. Dev. 2021, 32, 4277–4287. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Cui, Z.J.; Liu, G.N.; Ge, D.K.; Yin, J.R.; Xu, Q.H.; Pang, Q.Q. Quaternary geomorphological evolution of the Kunlun Pass area and uplift of the Qinghai-Xizang (Tibet) Plateau. Geomorphology 2001, 36, 203–216. [Google Scholar] [CrossRef]
- Liu, J.H.; Xin, Z.B.; Huang, Y.Z.; Yu, J. Climate suitability assessment on the Qinghai-Tibet Plateau. Sci. Total Environ. 2022, 816, 151653. [Google Scholar] [CrossRef]
- Wu, J.Q.; Wang, H.Y.; Li, G. Effects of nitrogen deposition on N2O emission in a wet meadow on the Qinghai-Tibet Plateau. Appl. Soil Ecol. 2023, 191, 105049. [Google Scholar] [CrossRef]
- Li, Z.F.; Gao, J.X.; Wen, L.Q.; Zou, C.X.; Feng, C.Y.; Li, D.Q.; Xu, D.L. Dynamics ofsoil respiration in alpine wetland meadows exposed to different levels of degradationin the Oinghai-Tibet Plateau, China. Sci. Rep. 2019, 9, 7469. [Google Scholar] [CrossRef]
- Hirota, M.; Hu, Q.W.; Kato, T.; Hirata, S.; Mo, W.; Cao, G.M.; Mariko, S. The potential importance of grazing to the fluxes of carbon dioxide and methane in an alpine wetland on the Qinghai-Tibetan Plateau. Atmos. Environ. 2005, 39, 5255–5259. [Google Scholar] [CrossRef]
- Nie, Y.; Li, A. Assessment of alpine wetland dynamics from 1976–2006 in the vicinity of Mount Qomolangma(Everest). Wetlands 2011, 31, 875–884. [Google Scholar] [CrossRef]
- Ma, W.W.; Alhassan, A.R.M.; Wang, Y.S.; Li, G.; Wang, H.; Zhao, J.M. Greenhouse gas emissions as influenced by wetland vegetation degradation along a moisture gradient on the eastern Qinghai-Tibet Plateau of North-West China. Nutr. Cycl. Agroecosyst. 2018, 112, 335–354. [Google Scholar] [CrossRef]
- Mishra, U.; Hugelius, G.; Shelef, E.; Yang, Y.; Orr, A. Spatial heterogeneity and environmental predictors of permafrost region soil organic carbon stocks. Sci. Adv. 2021, 7, 2375–2548. [Google Scholar] [CrossRef]
- Li, H.; Cai, J.J.; Liu, M.; Sui, X.; Hu, Y.; Feng, F. Microbial community structure and the relationship with soil carbon and nitroger in an original Korean pine forest of Changbai Mountain, China. BMC Microbiol. 2019, 19, 218. [Google Scholar] [CrossRef]
- Jian, J.; Du, X.; Reiter, M.S.; Stewart, R.D. A meta-analysis of global cropland soil carbon changes due to cover cropping. Soil Biol. Biochem. 2020, 143, 107735. [Google Scholar] [CrossRef]
- Davidson, E.A.; De, C.; Cláudio, J.; Reis, F.; Adelaine, M.; Ishida, F.Y.; Ometto, J.P.H.B.; Nardoto, G.B.; Sabá, R.T.; Hayashi, S.N.; et al. Recuperation of nitrogen cycling in Amazonian forests following agricultural abandonment. Nature 2007, 447, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Nearing, M.A.; Pruski, F.F.; O’Neal, M.R. Expected climate change impacts on soil erosion rates: A review. J. Soil Water Conserv. 2004, 59, 43–50. [Google Scholar] [CrossRef]
- Bationo, A.; Kihara, J.; Vanlauwe, B.; Waswa, B.; Kimetu, J.; Hansen, J.W. Soil organic carbon dynamics, functions and management in West African agro-ecosystems. Agric. Syst. 2004, 94, 13–25. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Knight, R. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Sheng, H.; Wang, X.; Zhao, H.; Feng, K. Excessive Nitrogen Fertilizer Application Causes Rapid Degradation of Greenhouse Soil in China. Pol. J. Environ. Stud. 2022, 31, 1527–1534. [Google Scholar] [CrossRef]
- Olivares, J.; Bedmar, E.J.; Sanjuán, J. Biological nitrogen fixation in the context of global change. Mol. Plant 2013, 26, 486–494. [Google Scholar] [CrossRef]
- Hooper, V. Effects of Plant Composition and Diversity on Nutrient Cycling. Ecol. Monogr. 1998, 68, 121–149. [Google Scholar] [CrossRef]
- Qiu, K.; Xie, Y.; Xu, D.; Pott, R. Ecosystem functions including soil organic carbon, total nitrogen and available potassium are crucial for vegetation recovery. Sci. Rep. 2018, 8, 7607. [Google Scholar] [CrossRef]
- Fu, Y.; Li, R.; Liu, C. Seasonal variations in coupled nitrogen and phosphorus uptake across various NO3−-N/NH4+-N addition ratios in a vegetated stream: The effect of hydromorphology. Ecol. Eng. 2024, 205, 107286. [Google Scholar] [CrossRef]
- Li, Y.J.; Xu, C.L.; Da, Z.L.; Hu, R.M.; Sun, C.C. Alpine Meadow Degradation Changed Soil Nitrogen Characteristics in the Eastern Qilian Mountains Area, Northwest China. Commun. Soil Sci. Plant Anal. 2024, 55, 564–578. [Google Scholar] [CrossRef]
- Cheng, C.; Xie, H.; Yang, E.; Shen, X.; Dai, P.; Zhang, J. Nutrient removal and microbial mechanisms in constructed wetland microcosms treating high nitrate/nitrite polluted river water. RSC Adv. 2016, 6, 70848–70854. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 2007, 53, 203–213. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, S.; Liu, S.; Guo, H.; Wang, T.; Zhou, J.; Peng, X. Changes in plant rhizosphere microbial communities under different vegetation restoration patterns in karst and non-karst ecosystems. Sci. Rep. 2019, 9, 8761. [Google Scholar] [CrossRef]
- Liu, X.; Guo, K.; Huang, L.; Ji, Z.; Zhang, J. Responses of absolute and specific enzyme activity toconsecutive application of composted sewage sludge in a Fluventic Ustochrept. PLoS ONE 2017, 12, e177–e796. [Google Scholar] [CrossRef]
- Wu, G.f.; Chen, Z.H.; Jiang, N.; Jiang, H.; Chen, L.A. Effects of long-term no-tillage with different residue application rates on soil nitrogen cycling. Soil Tillage Res. 2021, 212, 105044. [Google Scholar] [CrossRef]
- Cao, L.; Qin, B.; Gong, Z.; Zhang, Y. Melatonin improves nitrogen metabolism during grain filling under drought stress. Physiol. Mol. Biol. Plants 2022, 28, 1477–1488. [Google Scholar] [CrossRef]
- Sindhu, L.; Song, Y.; Li, L.; Dong, Z.K.; Yang, Q.F.; Mangi, S.S.; Guo, W.X.; Yang, L.J.; Cui, H.Y.; Lv, S.H.; et al. Meta-omics revealed that nitrogen fertilization alters the endophytic fungi divergence in maize field ecosystem. Ecol. Indic. 2024, 160, 111852. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M. Salinity effects on nitrogen metabolism in plants-focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Ma, L. Progress on the Effect of Nitrogen on Transformation of Soil Organic Carbon. Processes 2022, 10, 2425. [Google Scholar] [CrossRef]
- Daniel, R.M.; Danson, M.J.; Eisenthal, R.; Lee, C.K.; Peterson, M.E. The effect of temperature on enzyme activity: New insights and their implications. Extremophiles 2008, 12, 51–59. [Google Scholar] [CrossRef]
- Cardoso, D.O.M.F.; Bufon, V.B.; Leo, T.P. Vegetation indices as a Tool for Mapping Sugarcane Management Zones. Precis. Agric. 2022, 24, 213–234. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Sun, S.; Liu, W.; Zhu, L.; Yan, X. Different Forms and Proportions of Exogenous Nitrogen Promote the Growth of Alfalfa by Increasing Soil Enzyme Activity. Plants 2022, 11, 1057. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Alkorta, I.; Aizpurua, A.; Riga, P.; Albizu, I.; Amézaga, I.; Garbisu, C. Soil Enzyme Activities as Biological Indicators of Soil Health. Rev. Environ. Health 2003, 18, 65–73. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Pinto, M.; Langer, T.M.; Xosé, A.l.-S.; Herndl, G.J. Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean. Nat. Commun. 2018, 9, 1430. [Google Scholar] [CrossRef]
- Frossard, A.; Gerull, L.; Mutz, M.; Gessner, M.O. Disconnect of microbial structure and function: Enzyme activities and bacterial communities in nascent stream corridors. ISME J. 2012, 6, 680–691. [Google Scholar] [CrossRef]
- Wu, J.Q.; Wang, H.; Li, G.; Ma, W.W.; Xu, G.R. Vegetation degradation impacts soil nutrients and enzyme activities in wet meadows on the Qinghai-Tibet Plateau. Sci. Rep. 2020, 10, 21271. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Rafiroiu, A.; Kanthety, R.; Iqbal, S.; Malik, R.; Rahman, M. Factors predicting erythropoietin resistance among maintenance hemodialysis patients. Blood Purif. 2012, 33, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, X.L. Degradation of frigid swampy meadows on the Qinghai-Tibet pla-teau: Current status and future directions of research. Prog. Phys. Geogr. 2016, 40, 794–810. [Google Scholar] [CrossRef]
- Qin, H.; Xing, X.; Tang, Y.; Zhu, B.; Wei, X. Soil moisture and activity of nitrite- and nitrous oxide-reducing microbes enhanced nitrous oxide emissions in fallow paddy soils. Biol. Fertil. Soils 2020, 56, 53–67. [Google Scholar] [CrossRef]
- Kader, M.A.; Yeasmin, S.; Solaiman, Z.M.; De Neve, S.; Sleutel, S. Response of hydrolytic enzyme activities and nitrogen mineralization to fertilizer and organic matter application in subtropical paddy soils. Eur. J. Soil Biol. 2017, 80, 27–34. [Google Scholar] [CrossRef]
- Wu, H.; Cai, A.; Xing, T.; Huai, S.; Lu, C. Fertilization enhances mineralization of soil carbon and nitrogen pools by regulating the bacterial community and biomass. J. Soils Sediments 2021, 21, 1633–1634. [Google Scholar] [CrossRef]
- Brix, J.H. Nitrogen nutrition of Salvinia natans: Effects of inorganic nitrogen form on growth, morphology, nitrate reductase activity and uptake kinetics of ammonium and nitrate. Aquat. Bot. 2022, 90, 67–73. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.K.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. a review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, G.L.; Kuzyakov, Y.; Darrel, J.G.; Ma, Y.; Liu, W.; Wang, Z.F.; Shen, W.J. Soil nitrogen transformation responses to seasonal precipitation changes are regulated by changes in functional microbial abundance in a subtropical forest. Biogeosciences 2017, 14, 2513–2525. [Google Scholar] [CrossRef]
- Naik, D.B. Role of Soil Physical, Chemical and Biological Properties for Soil Health Improvement and Sustainable Agriculture. J. Pharm. Pharmacol. 2019, 8, 1256–1267. [Google Scholar] [CrossRef]
- Piotrowska-Dugosz, A. Significance of the Enzymes Associated with Soil C and N Transformation. Carbon Nitrogen Cycl. Soil 2020, 13, 399–433. [Google Scholar] [CrossRef]
- Chen, X.; Song, B.; Yao, Y.; Wu, H.; Hu, J.; Zhao, L. Aromatic plants play an important role in promoting soil biological activity related to nitrogen cycling in an orchard ecosystem. Sci. Total Environ. 2014, 472, 939–946. [Google Scholar] [CrossRef]
- Ma, W.W.; Li, G.; Wu, J.Q.; Xu, G.R. Response of soil labile organic carbon fractions and carbon-cycle enzyme activities to vegetation degradation in a wet meadow on the Qinghai–Tibet Plateau. Geoderma 2020, 337, 114565. [Google Scholar] [CrossRef]
- Du, J.N.; Ma, W.W.; Li, G.; Wu, J.H.; Chang, W.H. Vegetation degradation and its progressive impact on soil nitrogen mineralization in the Qinghai-Tibet Plateau’s apline wetland: Insights from a three-year study. J. Environ. Manag. 2025, 373, 123668. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, X. Total nitrogen and total phosphorous in urban soils used for different purposes in Beijing, China. Procedia Environ. Sci. 2013, 13, 95–104. [Google Scholar] [CrossRef]
- Dimmock, S.J.; Martinez, J. Nitrite plus nitrate recoveries in piggery slurry by direct distillation and modified Kjeldahl methods. Bioresour. Technol. 1994, 48, 21–24. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, G.; Zhang, T.; Fan, X.; Liu, Z.; Bai, E. Erosion effects on soil microbial carbon use efficiency in the mollisol cropland in northeast China. Soil Ecol. Lett. 2023, 5, 52–64. [Google Scholar] [CrossRef]
- Maria, C.T.; Oliveira, K.A.; Ngelo, d.F.; Coltro, W.K.T.; Josué, C.C.S. Paper-based analytical device with colorimetric detection for urease activity determination in soils and evaluation of potential inhibitors. Talanta 2021, 230, 122301. [Google Scholar] [CrossRef]
- Zhang, X.; Shuai, Y.S.; Tao, H.; Omega, L.H. Novel Method for the Quantitative Analysis of Protease Activity: The Casein Plate Method and Its Applications. ACS Omega 2021, 6, 3675–3680. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Wu, Y.; Yin, X.A.; Zhang, G. Effects of surface-modified biochars and activated carbon on the transformation of soil inorganic nitrogen and growth of maize under chromium stress. Chemosphere 2019, 227, 124–132. [Google Scholar] [CrossRef]

| Factor | TN g kg−1 | NH4+-N mg kg−1 | NO3−-N mg kg−1 | MBN mg kg−1 | URE mg g−1 24 h−1 | PRO mg g−1 24 h−1 | NR mg g−1 24 h−1 | NiR mg g−1 24 h−1 | |
|---|---|---|---|---|---|---|---|---|---|
| V | F | 81.03 | 65.10 | 59.11 | 46.24 | 2.28 | 8.30 | 32.25 | 45.45 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | 0.08 | <0.01 | <0.01 | <0.01 | |
| S | F | 21.70 | 70.11 | 13.98 | 71.67 | 11.55 | 31.43 | 31.07 | 38.75 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| G | F | 38.95 | 41.58 | 24.53 | 100.97 | 99.91 | 79.23 | 82.66 | 142.04 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| V × S | F | 6.81 | 1.82 | 0.64 | 1.63 | 1.69 | 4.37 | 3.89 | 7.83 |
| p | <0.01 | 0.10 | 0.70 | 0.14 | 0.13 | <0.01 | 0.00 | <0.01 | |
| V × G | F | 8.55 | 2.56 | 11.25 | 3.92 | 5.43 | 7.57 | 8.01 | 8.08 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| V × S × G | F | 5.25 | 2.29 | 0.55 | 2.45 | 2.77 | 4.91 | 4.41 | 2.02 |
| p | <0.01 | 0.00 | 0.97 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Degradation Degree | Dominant Species | Coverage (%) | Height (cm) | Aboveground Biomass (g m−2) |
|---|---|---|---|---|
| CK | Kobresia tibetica + Potentilla anserine + Poa annua | 96.25 ± 5.32 A | 16.71 ± 2.98 A | 355.90 ± 174.64 A |
| SD | Carex sp. + Potentilla anserine + Artemisia frigida Willd + Kobresia capilifolia | 86.34 ± 7.36 B | 13.02 ± 2.24 B | 293.02 ± 143.93 B |
| MD | Artemisia frigida Willd + Artemisia frigida willd + Kobresia capilifolia | 45.33 ± 13.34 C | 7.43 ± 0.97 C | 185.73 ± 134.90 C |
| HD | The vegetation is sparse, with only a little Artemisia frigida Willd. and Polygonum viviparum | |||
| Degradation Degree | pH | BD g cm−3 | SOM g kg−1 | TP g kg−1 | TK g kg−1 |
|---|---|---|---|---|---|
| CK | 7.92 ± 0.04 A | 0.36 ± 0.01 C | 65.82 ± 13.64 A | 1.48 ± 0.51 A | 6.03 ± 0.41 A |
| SD | 7.79 ± 0.06 B | 0.39 ± 0.02 C | 65.45 ± 9.67 A | 1.29 ± 0.30 AB | 6.02 ± 0.44 A |
| MD | 7.77 ± 0.08 B | 0.61 ± 0.05 A | 54.39 ± 10.66 A | 1.17 ± 0.08 B | 5.74 ± 0.26 AB |
| HD | 7.76 ± 0.06 B | 0.56 ± 0.03 B | 53.63 ± 10.66 A | 1.15 ± 0.22 B | 5.58 ± 0.42 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Ma, W.; Du, J.; Chang, W.; Li, G. Effect of Vegetation Degradation on Soil Nitrogen Components and N-Cycling Enzyme Activities in a Wet Meadow on the Qinghai–Tibetan Plateau. Plants 2025, 14, 1549. https://doi.org/10.3390/plants14101549
He W, Ma W, Du J, Chang W, Li G. Effect of Vegetation Degradation on Soil Nitrogen Components and N-Cycling Enzyme Activities in a Wet Meadow on the Qinghai–Tibetan Plateau. Plants. 2025; 14(10):1549. https://doi.org/10.3390/plants14101549
Chicago/Turabian StyleHe, Wanpeng, Weiwei Ma, Jianan Du, Wenhua Chang, and Guang Li. 2025. "Effect of Vegetation Degradation on Soil Nitrogen Components and N-Cycling Enzyme Activities in a Wet Meadow on the Qinghai–Tibetan Plateau" Plants 14, no. 10: 1549. https://doi.org/10.3390/plants14101549
APA StyleHe, W., Ma, W., Du, J., Chang, W., & Li, G. (2025). Effect of Vegetation Degradation on Soil Nitrogen Components and N-Cycling Enzyme Activities in a Wet Meadow on the Qinghai–Tibetan Plateau. Plants, 14(10), 1549. https://doi.org/10.3390/plants14101549





