Characterization of a New Stripe Rust Resistance Gene on Chromosome 2StS from Thinopyrum intermedium in Wheat
Abstract
1. Introduction
2. Results
2.1. Chromosomal Composition of Line Th93-1-6
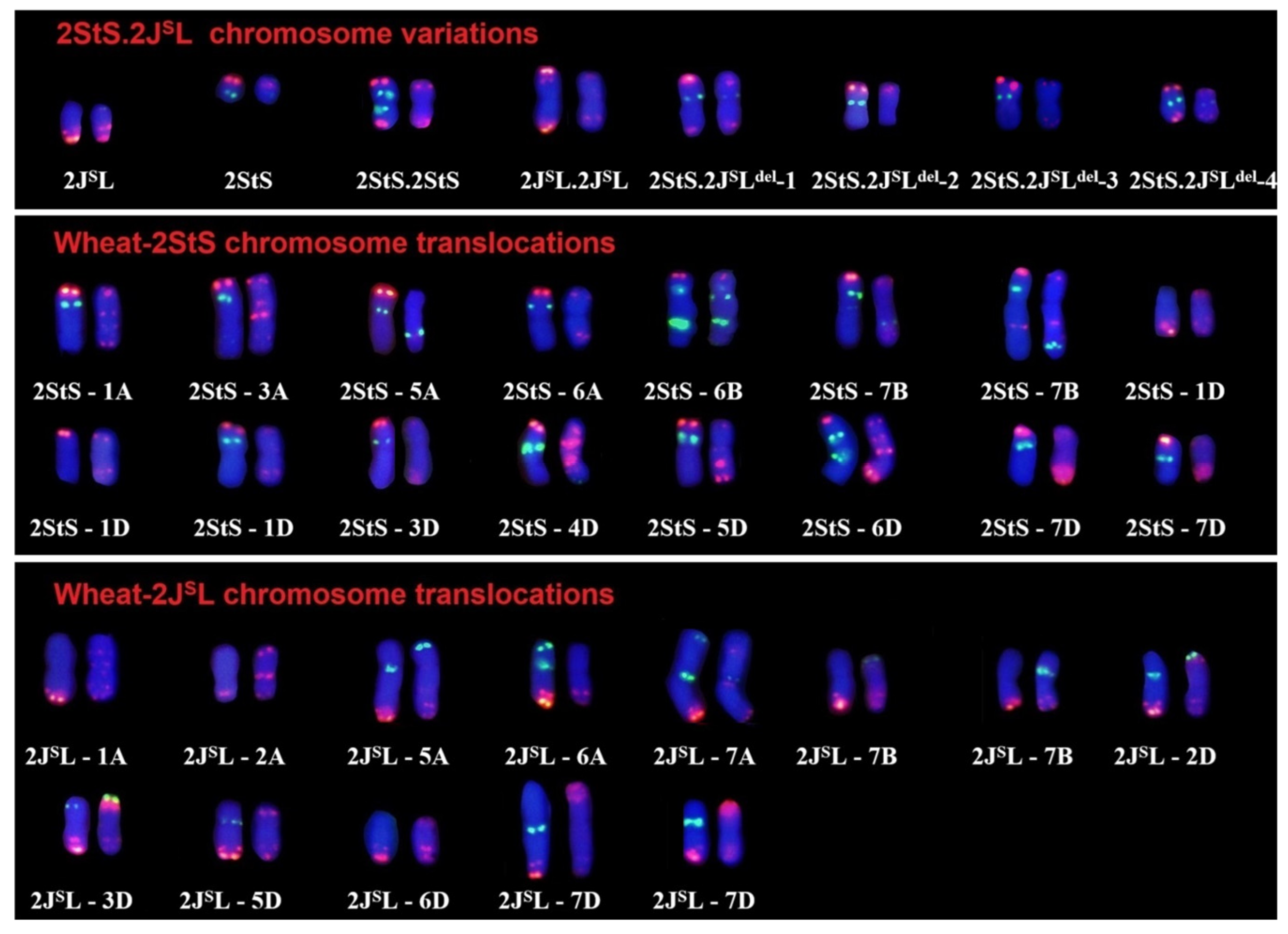
2.2. Chromosome Variations in M1 Population of Th93-1-6
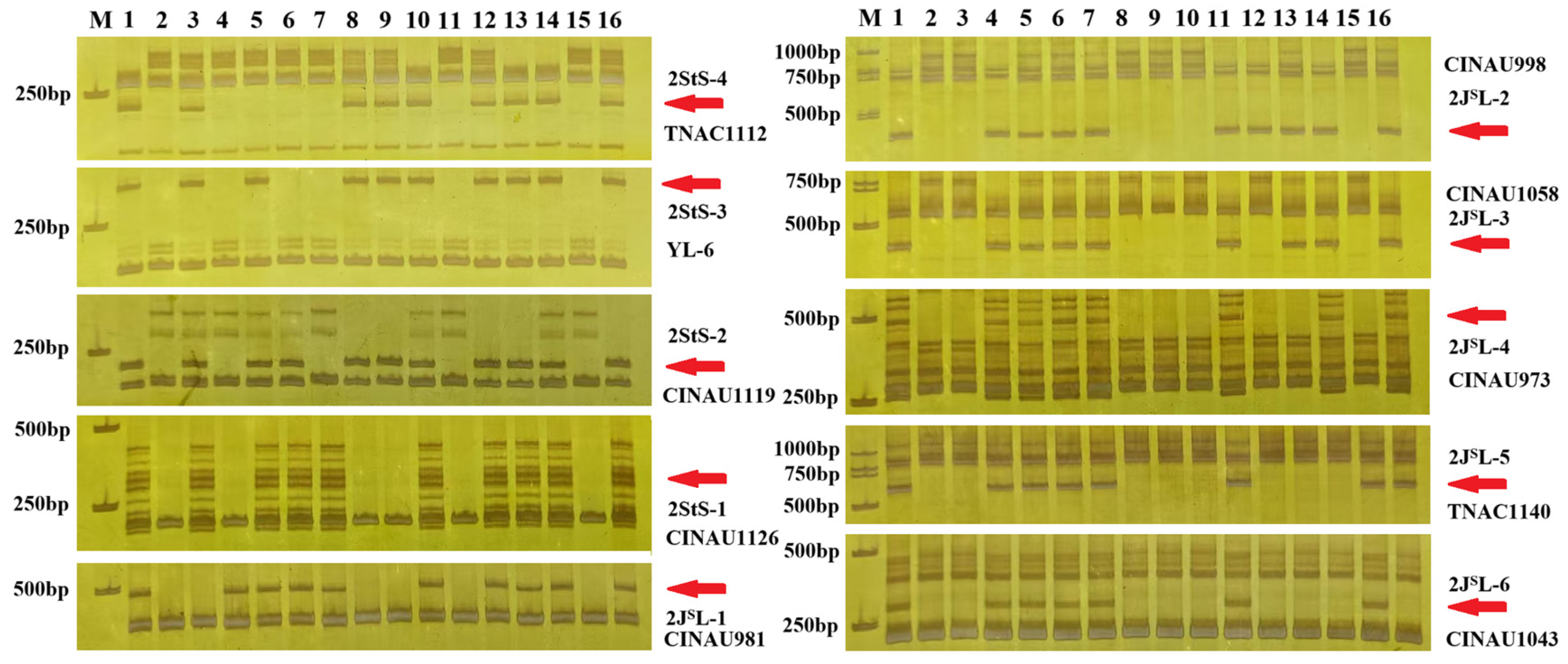
2.3. Identification of Wheat–Th. intermedium 2StS.2JSL Deletions and Translocations
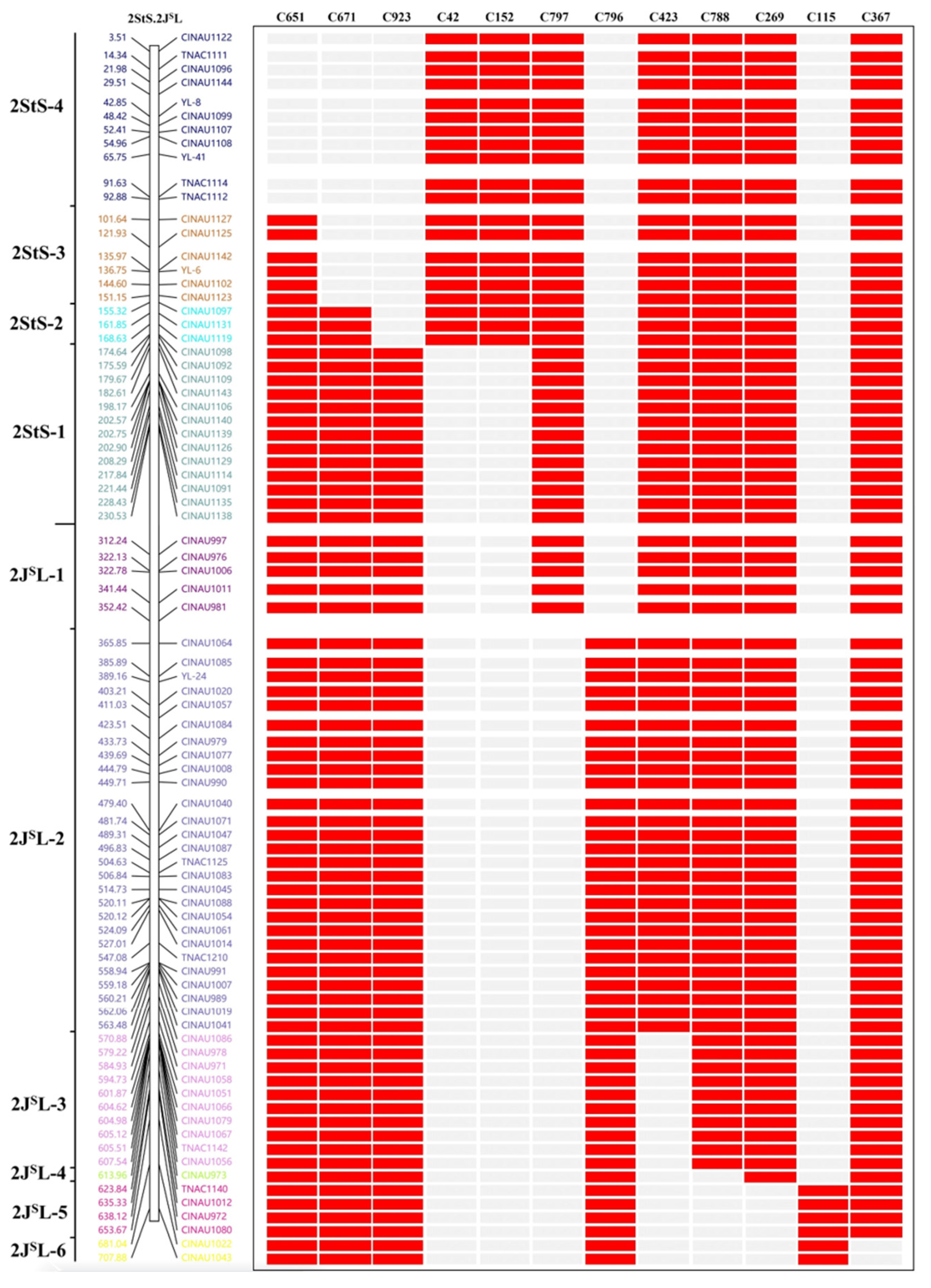
2.4. Construction of a Physical Map of 2StS.2JSL Chromosomes
2.5. Responses of Th93-1-6 and Derived Lines to Stripe Rust
2.6. Evaluation of Major Agronomic Traits of Th93-1-6 and Its Derived Lines
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. ND-FISH and Oligo-FISH Painting
4.3. Molecular Marker Analysis
4.4. Rust Resistance Tests
4.5. Evaluation of Agronomic Traits
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.Z.; Huang, L.; Zhang, H.F. An ancestral NB-LRR with duplicated 3′UTRs confers stripe rust resistance in wheat and barley. Nat. Commun. 2019, 10, 4023. [Google Scholar] [CrossRef] [PubMed]
- Li, J.B.; Dundas, I.; Dong, C.M.; Zhang, P. Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 2020, 133, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M. Integration of cultivar resistance and fungicide application for control of wheat stripe rust. Can. J. Plant Pathol. 2014, 36, 311–326. [Google Scholar] [CrossRef]
- Wellings, C.R. Global status of stripe rust: A review of historical and current threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Cao, Q.; Han, D.J.; Hao, Y.F. Molecular characterization and validation of adult-plant stripe rust resistance gene Yr86 in Chinese wheat cultivar Zhongmai 895. Theor. Appl. Genet. 2023, 136, 142. [Google Scholar] [CrossRef]
- Lai, H.L.; Shen, Y.Y.; Yang, H.; Li, Y. Comparative analysis of stripe rust resistance in seedling stage and Yr gene incidence in spring and winter wheat from Xinjiang; China. Front. Plant Sci. 2024, 15, 1394213. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Mu, J.M.; Han, D.J.; Kang, Z.S. Wheat stripe rust resistance gene Yr24/Yr26: A retrospective review. Crop J. 2018, 6, 321–329. [Google Scholar] [CrossRef]
- Yang, Z.J.; Li, G.R.; Chang, Z.J.; Zhou, J.P.; Ren, Z.L. Characterization of a partial amphiploid between Triticum aestivum cv. Chinese Spring and Thinopyrum intermedium ssp. trichophorum. Euphytica 2006, 149, 11–17. [Google Scholar]
- Li, H.J.; Wang, X.M. Thinopyrum ponticum and Th. intermedium: The promising source of resistance to fungal and viral diseases of wheat. J. Genet. Genom. 2009, 36, 557–565. [Google Scholar] [CrossRef]
- Pototskaya, I.V.; Shamanin, V.P.; Aydarov, A.N.; Morgounov, A.I. The use of wheatgrass (Thinopyrum intermedium) in breeding. Vavilov J. Genet. Breed. 2020, 26, 413–421. [Google Scholar] [CrossRef]
- Friebe, B.; Jiang, J.M.; Gill, B.S.; Dyck, P.L. Radiation-induced nonhomoeologous wheat-Agropyron intermedium chromosomal translocations conferring resistance to leaf rust. Theor. Appl. Genet. 1993, 86, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Friebe, B.; Jiang, J.M.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Luo, P.G.; Luo, H.Y.; Chang, Z.J.; Zhang, H.Y.; Zhang, M.; Ren, Z.L. Characterization and chromosomal location of Pm40 in common wheat: A new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor. Appl. Genet. 2009, 118, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- He, R.L.; Chang, Z.J.; Yang, Z.J.; Zhan, H.X.; Zhang, X.J.; Liu, J.X. Inheritance and mapping of a powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor. Appl. Genet. 2009, 118, 1173–1180. [Google Scholar] [CrossRef]
- Liu, J.; Chang, Z.J.; Zhang, X.J.; Yang, Z.J.; Li, X.; Jia, J.Q.; Zhan, H.X.; Guo, H.J.; Wang, J.M. Putative Thinopyrum intermedium-derived stripe rust resistance gene Yr50 maps on wheat chromosome arm 4BL. Theor. Appl. Genet. 2013, 126, 265–274. [Google Scholar] [CrossRef]
- Luo, X.Q.; He, Y.J.; Feng, X.L.; Ren, Y. Molecular and cytological identification of wheat-Thinopyrum intermedium partial amphiploid line 92048 with resistance to stripe rust and Fusarium head blight. Plants 2024, 13, 1198. [Google Scholar] [CrossRef]
- Yu, Z.H.; Li, G.R.; Zheng, Z.Q.; Yang, Z.J. Characterization of new wheat-Thinopyrum intermedium derivative lines with superior genes for stripe rust and powdery mildew resistance. Plants 2024, 13, 2333. [Google Scholar] [CrossRef]
- Hu, L.J.; Li, G.R.; Zeng, Z.X.; Chang, Z.J.; Liu, C.; Zhou, J.P.; Yang, Z.J. Molecular cytogenetic identification of a new wheat-Thinopyrum substitution line with stripe rust resistance. Euphytica 2011, 177, 169–177. [Google Scholar] [CrossRef]
- Nie, L.M.; Yang, Y.N.; Fu, T.H. Disomic chromosome addition from Thinopyrum intermedium to bread wheat appears to confer stripe rust resistance. Euphytica 2019, 215, 56. [Google Scholar] [CrossRef]
- Li, J.B.; Lang, T.; Li, B.; Yu, Z.H.; Wang, H.J.; Li, G.R.; Yang, E.N.; Yang, Z.J. Introduction of Thinopyrum intermedium ssp. trichophorum chromosomes to wheat by trigeneric hybridization involving Triticum; Secale and Thinopyrum genera. Planta 2017, 245, 1121–1135. [Google Scholar]
- Li, J.B.; Chen, Q.H.; Zhang, P.; Lang, T.; Hoxha, S.; Li, G.R.; Yang, Z.J. Comparative FISH and molecular identification of new stripe rust resistant wheat-Thinopyrum intermedium ssp. trichophorum introgression line. Crop J. 2019, 7, 819–829. [Google Scholar]
- Lang, T.; La, S.X.; Li, B.; Yang, Z.J. Precise identification of wheat-Thinopyrum intermedium translocation chromosomes carrying resistance to wheat stripe rust in line Z4 and its derived progenies. Genome 2018, 61, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Conner, R.L.; Tomas, J.B. Genome analysis of Thinopyrum intermedium and Thinopyrum ponticum using genomic in situ hybridization. Genome 1998, 41, 4. [Google Scholar] [CrossRef]
- Zhan, H.X.; Zhang, X.J.; Li, G.R.; Yang, Z.J. Molecular characterization of a new wheat-Thinopyrum intermedium translocation line with resistance to powdery mildew and stripe rust. Int. J. Mol. Sci. 2015, 16, 2162–2173. [Google Scholar] [CrossRef]
- Zhang, X.J.; Li, J.B.; Ge, Y.D.; Liu, C. Molecular cytogenetic characterization of a new wheat-Thinopyrum intermedium homoeologous group-6 chromosome disomic substitution line with resistance to leaf rust and stripe rust. Front. Plant Sci. 2022, 13, 106281. [Google Scholar] [CrossRef]
- Wang, L.; Liang, S.; Qi, F.; Li, X.F. The construction of a standard karyotype of intermediate wheatgrass and its potential progenitor species. Plants 2025, 14, 196. [Google Scholar] [CrossRef]
- Cuadrado, A.; Golczyk, H.; Jouve, N. A novel; simple and rapid nondenaturing FISH (ND-FISH) technique for the detection of plant telomeres. Potential used and possible target structures detected. Chromosome Res. 2009, 17, 755–762. [Google Scholar]
- Li, G.R.; Zhang, T.; Yu, Z.H. An efficient Oligo-FISH painting system for revealing chromosome rearrangements and polyploidization in Triticeae. Plant J. 2020, 105, 978–993. [Google Scholar] [CrossRef]
- Yu, Z.H.; Wang, H.J.; Yang, Z.J. Precise identification of chromosome constitution and rearrangements in wheat–Thinopyrum intermedium derivatives by ND-FISH and Oligo-FISH painting. Plants 2022, 11, 2109. [Google Scholar] [CrossRef]
- Bariana, H.S.; McIntosh, R.A. Cytogenetic studies in wheat. XV. Location of rust resistance genes in VPM1 and their genetic linkage with other disease resistance genes in chromosome 2A. Genome 1993, 36, 476–482. [Google Scholar] [CrossRef]
- Faria, R.; Navarro, A. Chromosomal speciation revisited: Rearranging theory with pieces of evidence. Trends Ecol. Evol. 2021, 25, 60–669. [Google Scholar] [CrossRef]
- Shen, W.J.; Liu, B.; Guo, J.L.; Dou, Q.W. Chromosome-scale assembly of the wild cereal relative Elymus sibiricus. Sci. Data 2024, 11, 823. [Google Scholar] [CrossRef]
- Chen, C.; Han, Y.S.; Xiao, H. Chromosome-specific painting in Thinopyrum species using bulked oligonucleotides. Theor. Appl. Genet. 2023, 136, 177. [Google Scholar] [CrossRef]
- Kruppa, K.; Türkösi, E.; Holušová, K.; Kalapos, B. Genotyping-by-sequencing uncovers a Thinopyrum 4StS.1JVSS Robertsonian translocation linked to multiple stress tolerances in bread wheat. Theor. Appl. Genet. 2025, 138, 13. [Google Scholar] [CrossRef]
- Larkin, P.J.; Banks, P.M.; McIntosh, R.A. Disomic Thinopyrum intermedium addition lines in wheat with barley yellow dwarf virus resistance and with rust resistances. Genome 1995, 38, 385–394. [Google Scholar] [CrossRef]
- Liu, L.Q.; Luo, Q.L.; Li, H.W.; Zheng, Q. Physical mapping of the blue-grained gene from Thinopyrum ponticum chromosome 4Ag and development of blue-grain-related molecular markers and a FISH probe based on SLAF-seq technology. Theor. Appl. Genet. 2018, 131, 2359–2370. [Google Scholar] [CrossRef]
- Yao, H.; Tang, C.G.; Zhao, J. Isolation of Thinopyrum ponticum genome specific repetitive sequences and their application for effective detection of alien segments in wheat. Sci. Agric. Sin. 2016, 49, 3683–3693. [Google Scholar]
- Li, G.R.; Chen, Q.H.; Jiang, W.X.; Yang, Z.J. Molecular and cytogenetic identification of wheat-Thinopyrum intermedium double substitution line-derived progenies for stripe rust resistance. Plants 2023, 12, 28. [Google Scholar] [CrossRef]
- Friebe, B.; Hatchett, J.H.; Mukai, Y.; Gill, B.B.; Sebesta, E.E. Transfer of Hessian fly resistance from rye to wheat via radiation-induced terminal and intercalary chromosomal translocations. Theor. Appl. Genet. 1991, 83, 33–40. [Google Scholar] [CrossRef]
- Copete-parada, A.; Palomino, C.; Cabrera, A. Development and characterization of wheat-Agropyron cristatum introgression lines induced by gametocidal genes and wheat ph1b mutant. Agronomy 2021, 11, 277. [Google Scholar] [CrossRef]
- Han, G.H.; Liu, H.; Zhu, S.Y.; An, D.G. Two functional CC-NBS-LRR proteins from rye chromosome 6RS confer differential age-related powdery mildew resistance to wheat. Plant Biotechnol. J. 2024, 2, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Men, W.Q.; Ma, C.; Liu, W.Q. Wheat powdery mildew resistance gene Pm13 encodes a mixed lineage kinase domain-like protein. Nat. Commun. 2024, 15, 2449. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Zhang, H.J.; Zhang, Y.; Wang, X.E. Cytological mapping of a powdery mildew resistance locus PmRc1 based on wheat-Roegneria ciliaris structural rearrangement library. Theor. Appl. Genet. 2024, 137, 276. [Google Scholar] [CrossRef]
- Wang, H.J.; Yu, Z.H.; Li, G.R. Diversified chromosome rearrangements detected in a wheat-Dasypyrum breviaristatum substitution line induced by gamma-ray irradiation. Plants 2019, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.D.; Zhou, S.H.; Yang, W.J.; Li, L.H. Chromosomal mapping of a major genetic locus from Agropyron cristatum chromosome 6P that influences grain number and spikelet number in wheat. Theor. Appl. Genet. 2024, 137, 82. [Google Scholar] [CrossRef]
- Yang, G.T.; Zhang, N.; Zheng, Q. Identification and introgression of a novel leaf rust resistance gene from Thinopyrum intermedium chromosome 7JS into wheat. Theor. Appl. Genet. 2023, 136, 231. [Google Scholar] [CrossRef]
- Hou, L.Y.; Jia, J.Q.; Zhang, X.J. Molecular mapping of the stripe rust resistance gene Yr69 on wheat chromosome 2AS. Plant Dis. 2016, 100, 1717–1724. [Google Scholar] [CrossRef]
- Hang, Q.; Li, X.; Chen, W.Q.; Luo, P.G. Genetic mapping of a putative Thinopyrum intermedium-derived stripe rust resistance gene on wheat chromosome 1B. Theor. Appl. Genet. 2014, 127, 843–853. [Google Scholar] [CrossRef]
- Guo, X.R.; Huang, Y.H.; Wang, J.; Han, F.P. Development and cytological characterization of wheat–Thinopyrum intermedium translocation lines with novel stripe rust resistance gene. Front. Plant Sci. 2023, 14, 1135321. [Google Scholar] [CrossRef]
- Bansal, U.K.; Bariana, H. Mapping of stripe rust resistance gene Yr56 in durum wheat cultivar Wollaroi. In Proceedings of the BGRI 2014 Technical Workshop, Obregon, Mexico, 25 March 2014. [Google Scholar]
- Jang, C.Z.; Luo, Y.J.; Li, G.R.; Yang, Z.J. Characterization of novel wheat-Thinopyrum ponticum introgression lines derived from the partial amphiploid AUS6770 carrying resistance to stripe rust. Crop J. 2024, 12, 1735–1744. [Google Scholar] [CrossRef]
- Wang, S.W.; Wang, C.Y.; Feng, X.B.; Ji, W.Q. Molecular cytogenetics and development of St-chromosome-specific molecular markers of novel stripe rust resistant wheat–Thinopyrum intermedium and wheat–Thinopyrum ponticum substitution lines. BMC Plant Biol. 2022, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Han, F.P.; Lamb, J.C.; Birchler, J.A. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc. Natl. Acad. Sci. USA 2006, 103, 3238–3243. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.X.; Yang, Z.J.; Fu, S.L. Oligonucleotides replacing the roles of repetitive sequences pAs1; pSc119.2; pTa-535; pTa71; CCS1; and pAWRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Xi, W.; Tang, Z.X.; Tang, S.Y. New ND-FISH-Positive Oligo probes for identifying Thinopyrum chromosomes in wheat backgrounds. Int. J. Mol. Sci. 2019, 20, 2031. [Google Scholar] [CrossRef]
- Lang, T.; Li, G.R.; Wang, H.J. Physical location of tandem repeats in the wheat genome and application for chromosome identification. Planta 2019, 249, 663–675. [Google Scholar] [CrossRef]
- Fu, S.L.; Chen, L.; Wang, Y.Y.; Li, M.; Yang, Z.J.; Qiu, L.; Yan, B.J.; Ren, Z.L.; Tang, Z.X. Oligonucleotide probes for ND-FISH analysis to identify rye and wheat chromosomes. Sci. Rep. 2015, 5, 10552. [Google Scholar] [CrossRef]
- Cheng, M.H.; Miroslava, K.; Sun, H.J. Development of oligonucleotide probes specific to Roegneria ciliaris chromosomes based on satellite repeats. J. Nanjing Agric. Univ. 2022, 45, 442–452. [Google Scholar]
- Yu, Z.H.; Wang, H.J.; Xu, Y.F. Characterization of chromosomal rearrangement in new wheat-Thinopyrum intermedium addition lines carrying Thinopyrum-specific grain hardness genes. Agronomy 2019, 9, 18. [Google Scholar] [CrossRef]
- Li, G.R.; Yang, Z.J. Oligo-FISH paints in Triticeae. Curr. Protoc. 2022, 2, e364. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wei, X.; Xiao, J. Whole genome development of intron targeting (IT) markers specific for Dasypyrum villosum chromosomes based on next-generation sequencing technology. Mol. Breed. 2017, 37, 115. [Google Scholar] [CrossRef]
- Ishikawa, G.; Nakamura, T.; Ashida, T. Localization of anchor loci representing five hundred annotated rice genes to wheat chromosomes using PLUG markers. Theor. Appl. Genet. 2008, 118, 499–514. [Google Scholar] [CrossRef]
- Hu, L.J.; Li, G.R.; Zeng, Z.X. Molecular characterization of a wheat-Thinopyrum ponticum partial amphiploid and its derived substitution line for resistance to stripe rust. J. Appl. Genet. 2011, 52, 279–285. [Google Scholar] [CrossRef]



| Materials | Plant Height (cm) | Tiller Number | Spike Length (cm) | Spikelets per Spike | Grains per Spike | 1000-Grain Weight (g) |
|---|---|---|---|---|---|---|
| Th93-1-6 | 89.1 ± 2.0 a | 10.6 ± 2.1 a | 12.1 ± 0.8 a | 24.1 ± 2.1 a | 48.9 ± 2.1 a | 37.8 ± 0.6 a |
| MY11 | 86.4 ± 3.1 b | 9.6 ± 1.8 b | 10.1 ± 1.0 c | 20.6 ± 1.7 c | 48.2 ± 1.9 b | 35.6 ± 0.5 b |
| 2StS carriers | 87.5 ± 2.0 b | 10.8 ± 2.2 a | 11.3 ± 1.0 b | 21.4 ± 2.7 b | 48.7 ± 2.1 ab | 36.3 ± 0.6 c |
| 2JSL carriers | 87.6 ± 2.7 b | 11.3 ± 1.7 a | 9.8 ± 0.8 c | 20.4 ± 1.4 c | 48.4 ± 2.5 b | 35.4 ± 0.5 c |
| C152 | 87.3 ± 4.0 b | 10.9 ± 2.0 a | 11.1 ± 0.8 b | 21.4 ± 2.3 b | 49.1 ± 2.6 a | 37.2 ± 0.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Luo, Y.; Huang, D.; Chen, M.; Yang, E.; Li, G.; Yang, Z. Characterization of a New Stripe Rust Resistance Gene on Chromosome 2StS from Thinopyrum intermedium in Wheat. Plants 2025, 14, 1538. https://doi.org/10.3390/plants14101538
Jiang C, Luo Y, Huang D, Chen M, Yang E, Li G, Yang Z. Characterization of a New Stripe Rust Resistance Gene on Chromosome 2StS from Thinopyrum intermedium in Wheat. Plants. 2025; 14(10):1538. https://doi.org/10.3390/plants14101538
Chicago/Turabian StyleJiang, Chengzhi, Yujie Luo, Doudou Huang, Meiling Chen, Ennian Yang, Guangrong Li, and Zujun Yang. 2025. "Characterization of a New Stripe Rust Resistance Gene on Chromosome 2StS from Thinopyrum intermedium in Wheat" Plants 14, no. 10: 1538. https://doi.org/10.3390/plants14101538
APA StyleJiang, C., Luo, Y., Huang, D., Chen, M., Yang, E., Li, G., & Yang, Z. (2025). Characterization of a New Stripe Rust Resistance Gene on Chromosome 2StS from Thinopyrum intermedium in Wheat. Plants, 14(10), 1538. https://doi.org/10.3390/plants14101538








