Determination of Optimal Harvest Time in Cannabis sativa L. Based upon Stigma Color Transition
Abstract
1. Introduction
2. Results and Discussion
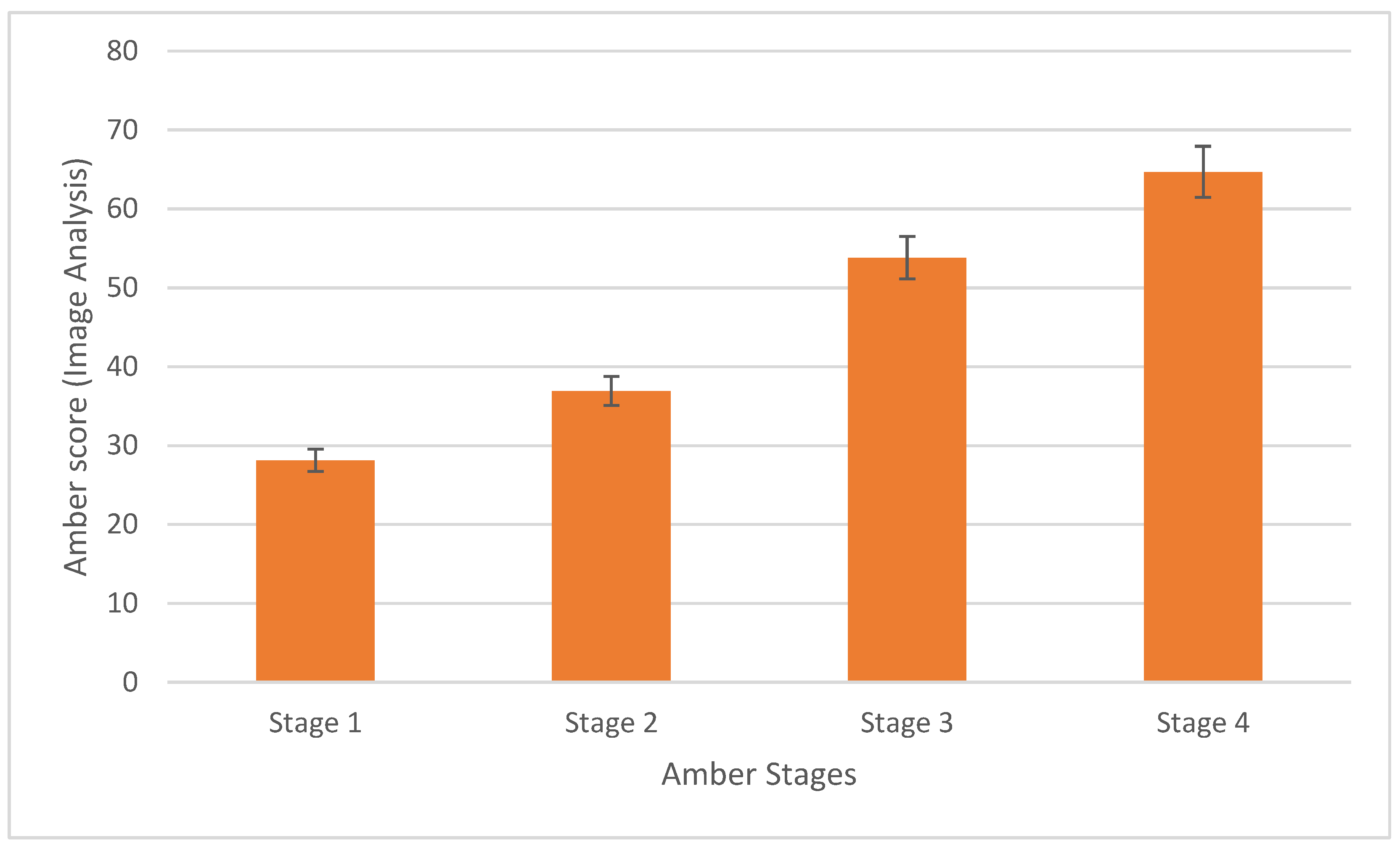
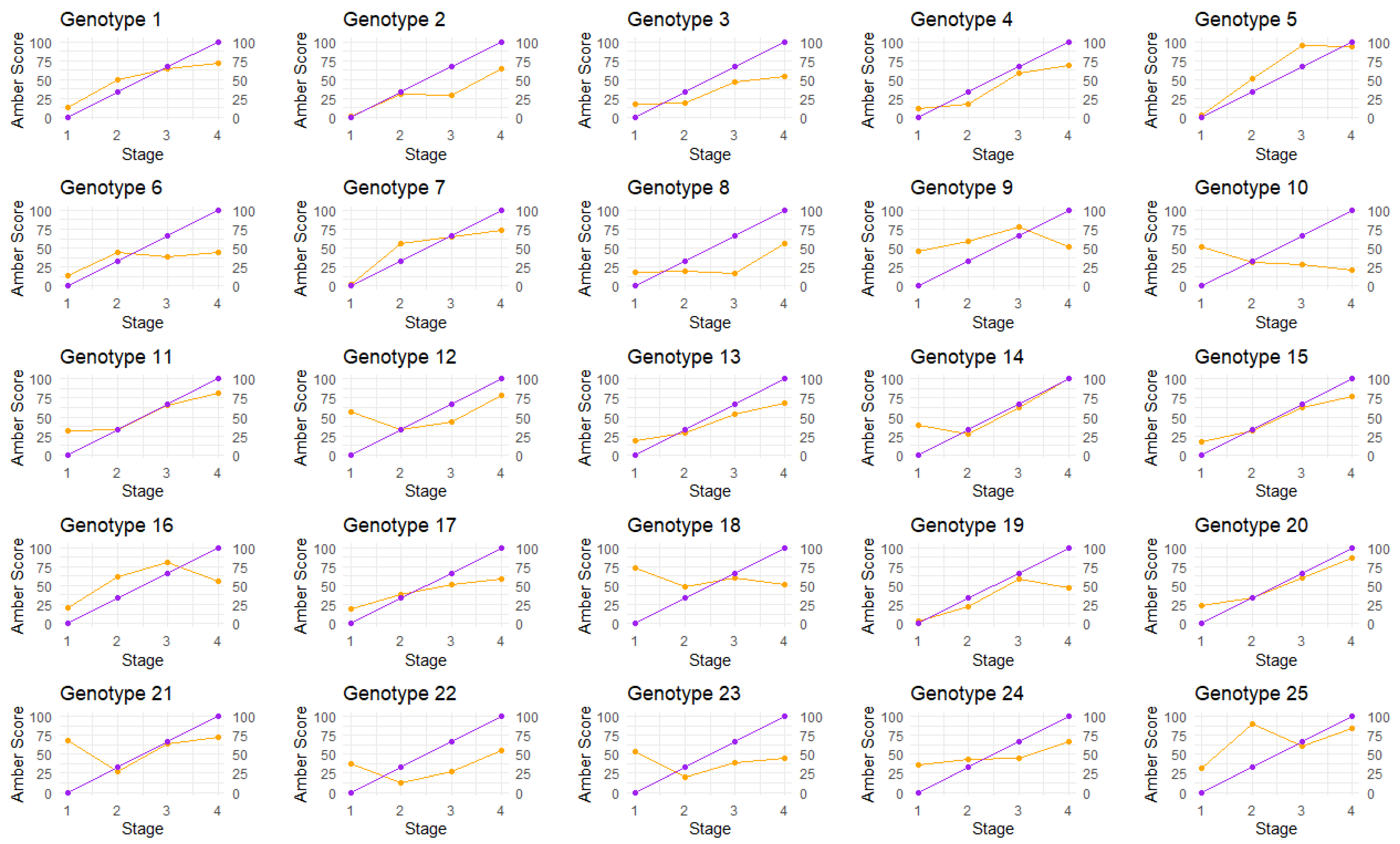
2.1. Calculation of Amber Score
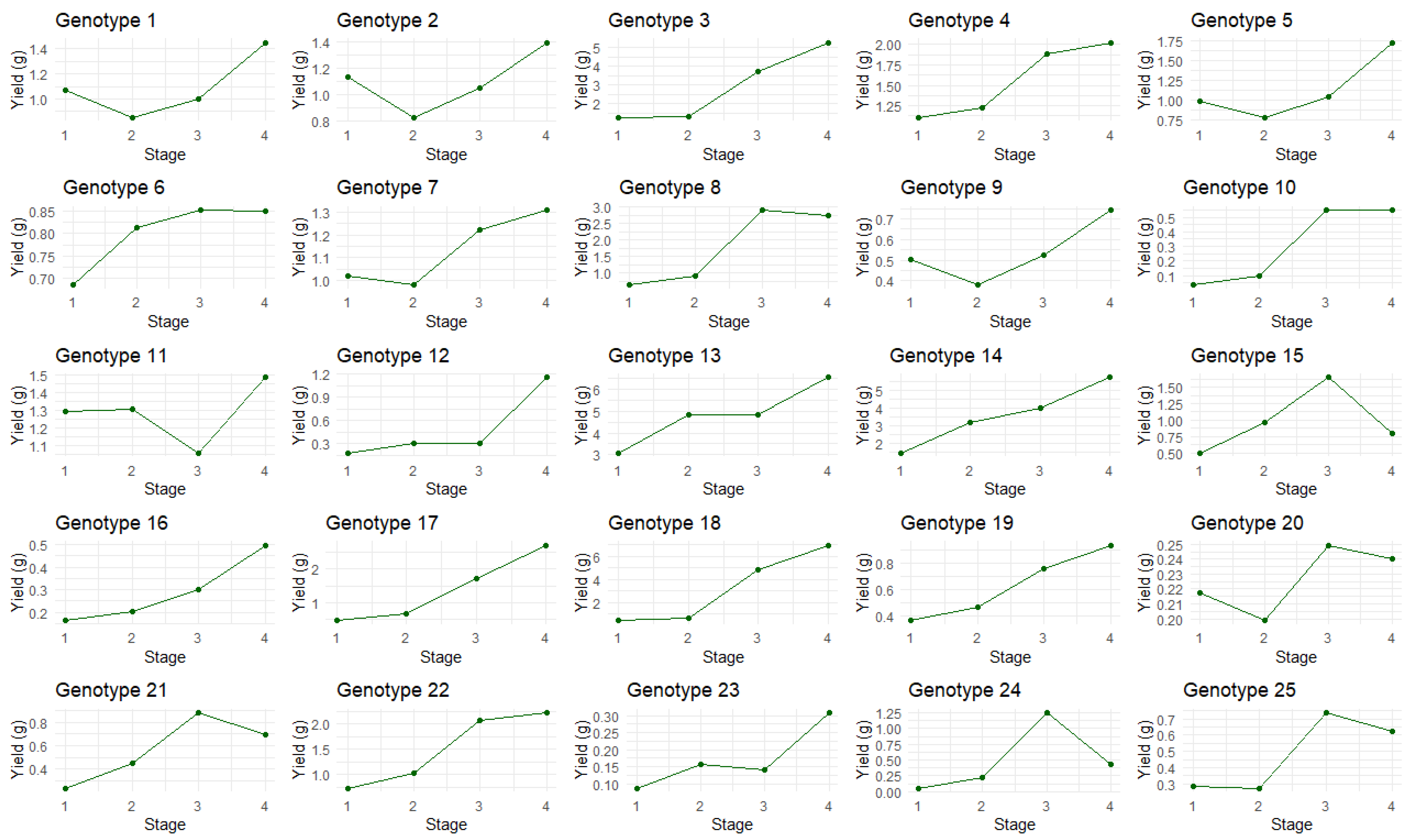
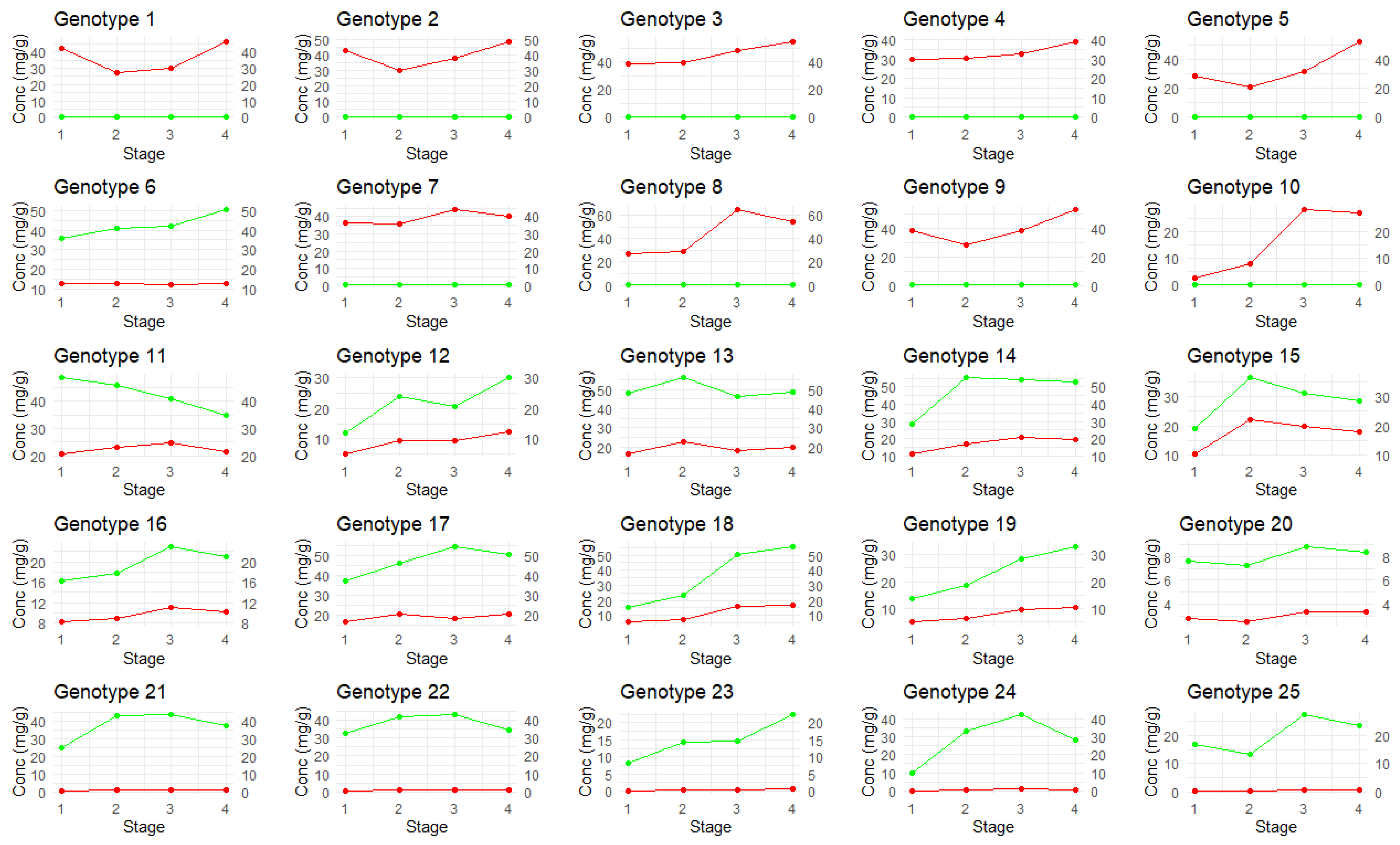
2.2. Total Cannabinoid Concentration Versus Amber Score
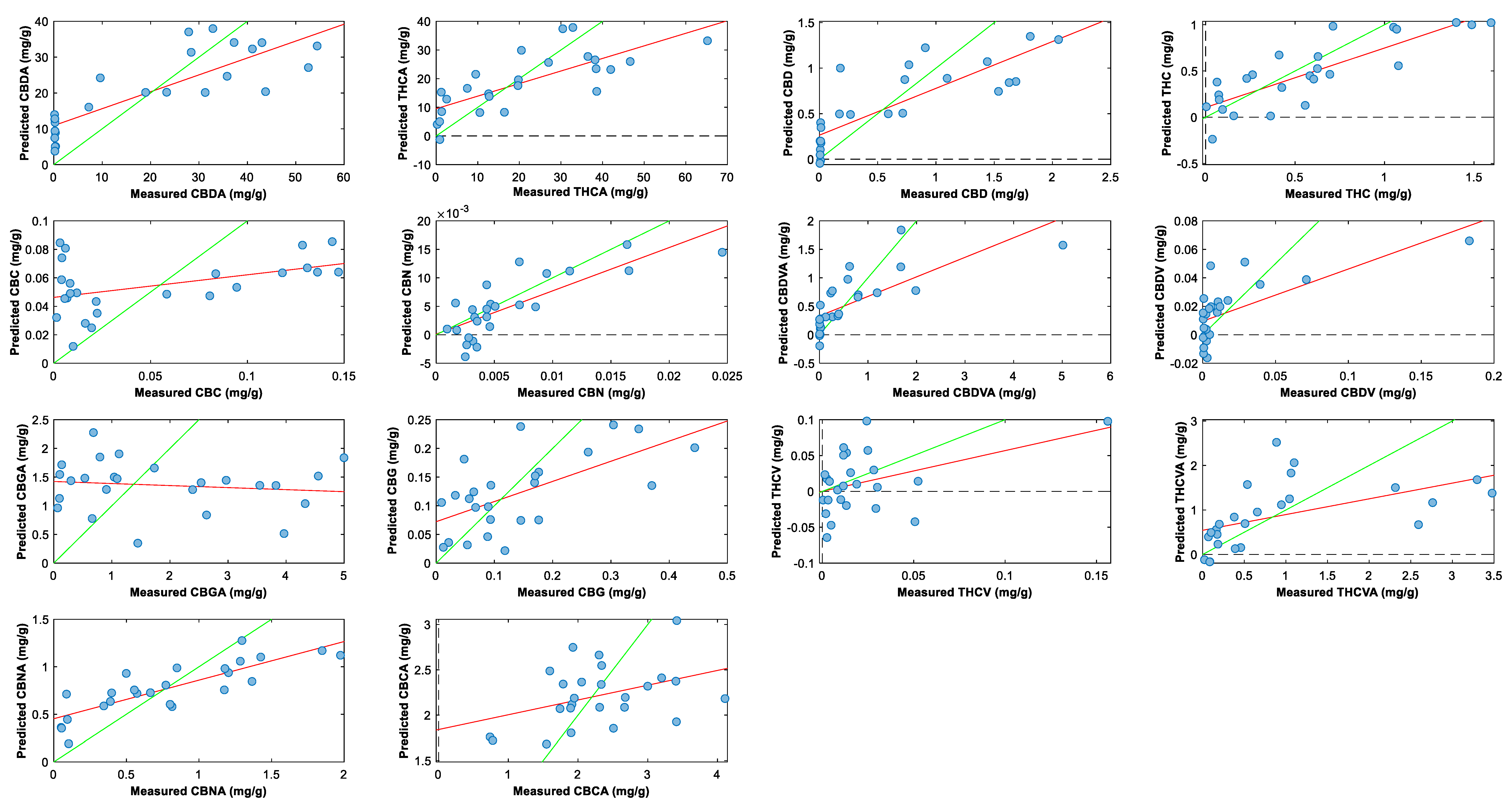
2.3. Cannabinoid Prediction Models
3. Materials and Methods
3.1. Cultivation and Sample Preparation
3.2. Image Analysis and Color Quantification
3.3. LCMS Analysis
3.4. NIR Spectroscopy
3.5. Statistical Analysis
3.6. Cannabinoid Prediction Models Using NIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBC | Cannabichromene |
| CBCA | Cannabichromenic acid |
| CBD | Cannabidiol |
| CBDA | Cannabidiolic acid |
| CBDV | Cannabidivarin |
| CBDVA | Cannabidivaric acid |
| CBG | Cannabigerol |
| CBGA | Cannabigerolic acid |
| CBN | Cannabinol |
| CBNA | Cannabinolic acid |
| CIELAB | LAB color space defined by the International Commission on Illumination |
| EC | Electrical conductivity |
| LCMS | Liquid chromatography–mass spectrometry |
| LV | Latent variable |
| NIR | Near-infrared |
| PLS | Partial least squares |
| R2cal | Coefficient of determination of calibration |
| R2cv | Coefficient of determination of cross-validation |
| R2pred | Coefficient of determination of prediction |
| RMSEC | Root mean squared error of calibration |
| RMSECV | Root mean squared error of cross-validation |
| RMSEP | Root mean squared error of prediction |
| RPD | Residual prediction deviation |
| TCC | Total cannabinoid concentration |
| TCY | Total cannabinoid yield |
| THC | Tetrahydrocannabinol |
| THCA | Tetrahydrocannabinolic acid |
| THCV | Tetrahydrocannabidivarin |
| THCVA | Tetrahydrocannabidivarinic acid |
| UV-DAD | Ultra-Violet Diode Array Detector |
References
- Institute of Medicine; Mack, A.; Joy, J. Marijuana As Medicine?: The Science Beyond the Controversy; Mack, A., Joy, J., Eds.; The National Academies Press: Washington, DC, USA, 2001; 214p. [Google Scholar] [CrossRef]
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J. Cannabis: The plant, its evolution, and its genetics—With an emphasis on Italy. Rend. Lincei. Sci. Fis. E Naturali 2020, 31, 939–948. [Google Scholar] [CrossRef]
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef]
- Zhang, M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Photoperiodic Flowering Response of Essential Oil, Grain, and Fiber Hemp (Cannabis sativa L.) Cultivars. Front. Plant Sci. 2021, 12, 694153. [Google Scholar] [CrossRef]
- Toth, J.A.; Stack, G.M.; Carlson, C.H.; Smart, L.B. Identification and mapping of major-effect flowering time loci Autoflower1 and Early1 in Cannabis sativa L. Front. Plant Sci. 2022, 13, 991680. [Google Scholar] [CrossRef]
- Devinsky, O.C.; Cross, J.H.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 377, 699–700. [Google Scholar] [CrossRef]
- Johal, H.; Devji, T.; Chang, Y.; Simone, J.; Vannabouathong, C.; Bhandari, M. Cannabinoids in Chronic Non-Cancer Pain: A Systematic Review and Meta-Analysis. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 1179544120906461. [Google Scholar] [CrossRef]
- Rapin, L.; Gamaoun, R.; El Hage, C.; Arboleda, M.F.; Prosk, E. Cannabidiol use and effectiveness: Real-world evidence from a Canadian medical cannabis clinic. J. Cannabis Res. 2021, 3, 19. [Google Scholar] [CrossRef]
- De Faria, S.M.; de Morais Fabricio, D.; Tumas, V.; Castro, P.C.; Ponti, M.A.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.S.; Chagas, M.H.N. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. J. Psychopharmacol. 2020, 34, 189–196. [Google Scholar] [CrossRef]
- Haddad, F.; Dokmak, G.; Karaman, R. The Efficacy of Cannabis on Multiple Sclerosis-Related Symptoms. Life 2022, 12, 682. [Google Scholar] [CrossRef]
- Allan, N.P.; Ashrafioun, L.; Kolnogorova, K.; Raines, A.M.; Hoge, C.W.; Stecker, T. Interactive effects of PTSD and substance use on suicidal ideation and behavior in military personnel: Increased risk from marijuana use. Depress. Anxiety 2019, 36, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Grimison, P.; Mersiades, A.; Kirby, A.; Lintzeris, N.; Morton, R.; Haber, P.; Olver, I.; Walsh, A.; McGregor, I.; Cheung, Y.; et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: A randomised, placebo-controlled, phase II crossover trial. Ann. Oncol. 2020, 31, 1553–1560. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Petrie, G.N.; Williams, L.A.; Mechoulam, R.; Parker, L.A. Effect of prior foot shock stress and Delta(9)-tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety-like responding in the light-dark emergence test in rats. Psychopharmacology 2017, 234, 2207–2217. [Google Scholar] [CrossRef]
- Kim, J.; Choi, P.; Park, Y.T.; Kim, T.; Ham, J.; Kim, J.C. The Cannabinoids, CBDA and THCA, Rescue Memory Deficits and Reduce Amyloid-Beta and Tau Pathology in an Alzheimer’s Disease-like Mouse Model. Int. J. Mol. Sci. 2023, 24, 6827. [Google Scholar] [CrossRef]
- Rock, E.M.; Limebeer, C.L.; Parker, L.A. Effect of cannabidiolic acid and ∆(9)-tetrahydrocannabinol on carrageenan-induced hyperalgesia and edema in a rodent model of inflammatory pain. Psychopharmacology 2018, 235, 3259–3271. [Google Scholar] [CrossRef]
- Rock, E.M.; Kopstick, R.L.; Limebeer, C.L.; Parker, L.A. Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br. J. Pharmacol. 2013, 170, 641–648. [Google Scholar] [CrossRef]
- Bolognini, D.; Rock, E.M.; Cluny, N.L.; Cascio, M.G.; Limebeer, C.L.; Duncan, M.; Stott, C.G.; Javid, F.A.; Parker, L.A.; Pertwee, R.G. Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br. J. Pharmacol. 2013, 168, 1456–1470. [Google Scholar] [CrossRef]
- Stone, N.L.; Murphy, A.J.; England, T.J.; O’Sullivan, S.E. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br. J. Pharmacol. 2020, 177, 4330–4352. [Google Scholar] [CrossRef]
- Shirsath, P. Cannabis Market Report 2025 (Global Edition); CMR315574; Cognitive Market Research: Pune, India, 2025. [Google Scholar]
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of Nitrogen, Phosphorus, and Potassium for Soilless Production of Cannabis sativa in the Flowering Stage Using Response Surface Analysis. Front. Plant Sci. 2021, 12, 764103. [Google Scholar] [CrossRef]
- Crispim Massuela, D.; Hartung, J.; Munz, S.; Erpenbach, F.; Graeff-Honninger, S. Impact of Harvest Time and Pruning Technique on Total CBD Concentration and Yield of Medicinal Cannabis. Plants 2022, 11, 140. [Google Scholar] [CrossRef]
- Linder, E.R.; Young, S.; Li, X.; Henriquez Inoa, S.; Suchoff, D.H. The Effect of Harvest Date on Temporal Cannabinoid and Biomass Production in the Floral Hemp (Cannabis sativa L.) Cultivars BaOx and Cherry Wine. Horticulturae 2022, 8, 959. [Google Scholar] [CrossRef]
- Tran, J.; Vassiliadis, S.; Elkins, A.C.; Cogan, N.O.O.; Rochfort, S.J. Rapid In Situ Near-Infrared Assessment of Tetrahydrocannabinolic Acid in Cannabis Inflorescences Before Harvest Using Machine Learning. Sensors 2024, 24, 5081. [Google Scholar] [CrossRef] [PubMed]
- Deidda, R.; Coppey, F.; Damergi, D.; Schelling, C.; Coic, L.; Veuthey, J.L.; Sacre, P.Y.; De Bleye, C.; Hubert, P.; Esseiva, P.; et al. New perspective for the in-field analysis of cannabis samples using handheld near-infrared spectroscopy: A case study focusing on the determination of Delta(9)-tetrahydrocannabinol. J. Pharm. Biomed. Anal. 2021, 202, 114150. [Google Scholar] [CrossRef] [PubMed]
- Punja, Z.K.; Sutton, D.B.; Kim, T. Glandular trichome development, morphology, and maturation are influenced by plant age and genotype in high THC-containing cannabis (Cannabis sativa L.) inflorescences. J. Cannabis Res. 2023, 5, 12. [Google Scholar] [CrossRef]
- Sutton, D.B.; Punja, Z.K.; Hamarneh, G. Characterization of trichome phenotypes to assess maturation and flower development in Cannabis sativa L. (cannabis) by automatic trichome gland analysis. Smart Agric. Technol. 2023, 3, 100111. [Google Scholar] [CrossRef]
- Naim-Feil, E.; Elkins, A.C.; Malmberg, M.M.; Ram, D.; Tran, J.; Spangenberg, G.C.; Rochfort, S.J.; Cogan, N.O.I. The Cannabis Plant as a Complex System: Interrelationships between Cannabinoid Compositions, Morphological, Physiological and Phenological Traits. Plants 2023, 12, 493. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; Hammond, K.M.; Micheler, M. The inheritance of chemical phenotype in Cannabis sativa L. (III): Variation in cannabichromene proportion. Euphytica 2008, 165, 293–311. [Google Scholar] [CrossRef]
- Tran, J.; Vassiliadis, S.; Elkins, A.C.; Cogan, N.O.I.; Rochfort, S.J. Developing Prediction Models Using Near-Infrared Spectroscopy to Quantify Cannabinoid Content in Cannabis sativa. Sensors 2023, 23, 2607. [Google Scholar] [CrossRef]
- Tran, J.; Elkins, A.C.; Spangenberg, G.C.; Rochfort, S.J. High-Throughput Quantitation of Cannabinoids by Liquid Chromatography Triple-Quadrupole Mass Spectrometry. Molecules 2022, 27, 742. [Google Scholar] [CrossRef]
- Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.-K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2018, 125, 5–20. [Google Scholar] [CrossRef]
- Fahlgren, N.; Feldman, M.; Gehan, M.A.; Wilson, M.S.; Shyu, C.; Bryant, D.W.; Hill, S.T.; McEntee, C.J.; Warnasooriya, S.N.; Kumar, I.; et al. A Versatile Phenotyping System and Analytics Platform Reveals Diverse Temporal Responses to Water Availability in Setaria. Mol. Plant 2015, 8, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Gehan, M.A.; Fahlgren, N.; Abbasi, A.; Berry, J.C.; Callen, S.T.; Chavez, L.; Doust, A.N.; Feldman, M.J.; Gilbert, K.B.; Hodge, J.G.; et al. PlantCV v2: Image analysis software for high-throughput plant phenotyping. PeerJ 2017, 5, e4088. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Funt, B. MaxRGB Reconsidered. J. Imaging Sci. Technol. 2012, 56, 20501-1–20501-10. [Google Scholar] [CrossRef]
- Depetris, M.B.; Dimech, A.M.; Guthridge, K.M. Digitally quantifying growth and verdancy of Lolium plants in vitro. Plants 2025, 14, 1499. [Google Scholar] [CrossRef]
- Elkins, A.C.; Deseo, M.A.; Rochfort, S.; Ezernieks, V.; Spangenberg, G. Development of a validated method for the qualitative and quantitative analysis of cannabinoids in plant biomass and medicinal cannabis resin extracts obtained by super-critical fluid extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1109, 76–83. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]






| RMSEC 1 | R2Cal 2 | RMSECV 3 | R2CV4 | RMSEP 5 | Pred Bias 6 | R2Pred7 | RPD 8 | |
|---|---|---|---|---|---|---|---|---|
| CBDA | 11.91 | 0.61 | 13.53 | 0.50 | 11.65 | 0.55 | 0.69 | 1.66 |
| THCA | 8.11 | 0.73 | 9.63 | 0.63 | 12.05 | −2.44 | 0.55 | 1.46 |
| CBD | 0.45 | 0.64 | 0.52 | 0.53 | 0.42 | −0.04 | 0.67 | 1.68 |
| THC | 0.41 | 0.64 | 0.53 | 0.40 | 0.28 | −0.10 | 0.70 | 1.72 |
| CBC | 0.04 | 0.25 | 0.05 | 0.11 | 0.05 | 0.00 | 0.20 | 1.13 |
| CBN | 0.01 | 0.54 | 0.01 | 0.24 | 0.00 | 0.00 | 0.63 | 1.50 |
| CBDVA | 0.61 | 0.55 | 0.70 | 0.41 | 0.78 | −0.09 | 0.53 | 1.39 |
| CBDV | 0.04 | 0.40 | 0.04 | 0.16 | 0.03 | 0.00 | 0.46 | 1.36 |
| CBGA | 1.14 | 0.20 | 1.24 | 0.07 | 1.74 | −0.50 | 0.02 | 0.91 |
| CBG | 0.09 | 0.51 | 0.10 | 0.33 | 0.09 | −0.02 | 0.39 | 1.27 |
| THCV | 0.09 | 0.35 | 0.12 | 0.02 | 0.04 | −0.01 | 0.18 | 0.78 |
| THCVA | 0.89 | 0.51 | 1.08 | 0.30 | 0.91 | −0.06 | 0.27 | 1.18 |
| CBNA | 0.30 | 0.59 | 0.36 | 0.43 | 0.36 | −0.02 | 0.69 | 1.56 |
| CBCA | 0.66 | 0.43 | 0.73 | 0.32 | 0.73 | −0.08 | 0.16 | 1.10 |
| TCC | 14.35 | 0.54 | 16.12 | 0.42 | 13.17 | 1.67 | 0.32 | 1.19 |
| RMSEC 1 | R2Cal 2 | RMSECV 3 | R2CV 4 | RMSEP 5 | Pred Bias 6 | R2Pred7 | RPD 8 | |
|---|---|---|---|---|---|---|---|---|
| CBDA | 9.87 | 0.74 | 12.31 | 0.60 | 8.25 | −1.43 | 0.84 | 2.26 |
| THCA | 8.22 | 0.76 | 10.00 | 0.64 | 8.06 | −0.96 | 0.69 | 1.82 |
| CBD | 0.38 | 0.78 | 0.49 | 0.63 | 0.26 | 0.09 | 0.74 | 1.86 |
| THC | 0.46 | 0.56 | 0.55 | 0.38 | 0.22 | −0.07 | 0.75 | 1.92 |
| CBC | 0.04 | 0.29 | 0.05 | 0.15 | 0.04 | −0.01 | 0.14 | 1.09 |
| CBN | 0.01 | 0.33 | 0.01 | 0.13 | 0.01 | 0.00 | 0.25 | 1.15 |
| CBDVA | 0.68 | 0.58 | 0.85 | 0.36 | 0.60 | 0.45 | 0.52 | 0.57 |
| CBDV | 0.04 | 0.41 | 0.05 | 0.12 | 0.02 | 0.02 | 0.39 | 0.20 |
| CBGA | 1.25 | 0.11 | 1.44 | 0.00 | 1.49 | −0.26 | 0.02 | 1.02 |
| CBG | 0.08 | 0.49 | 0.09 | 0.35 | 0.11 | −0.03 | 0.57 | 1.34 |
| THCV | 0.10 | 0.22 | 0.13 | 0.01 | 0.03 | 0.01 | 0.36 | 0.79 |
| THCVA | 0.80 | 0.61 | 0.99 | 0.41 | 0.71 | 0.19 | 0.56 | 1.47 |
| CBNA | 0.26 | 0.72 | 0.31 | 0.60 | 0.40 | −0.15 | 0.44 | 1.27 |
| CBCA | 0.70 | 0.31 | 0.82 | 0.11 | 0.74 | −0.06 | 0.38 | 1.25 |
| TCC | 14.54 | 0.50 | 15.95 | 0.40 | 14.54 | 0.22 | 0.37 | 1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, J.; Dimech, A.M.; Vassiliadis, S.; Elkins, A.C.; Cogan, N.O.I.; Naim-Feil, E.; Rochfort, S.J. Determination of Optimal Harvest Time in Cannabis sativa L. Based upon Stigma Color Transition. Plants 2025, 14, 1532. https://doi.org/10.3390/plants14101532
Tran J, Dimech AM, Vassiliadis S, Elkins AC, Cogan NOI, Naim-Feil E, Rochfort SJ. Determination of Optimal Harvest Time in Cannabis sativa L. Based upon Stigma Color Transition. Plants. 2025; 14(10):1532. https://doi.org/10.3390/plants14101532
Chicago/Turabian StyleTran, Jonathan, Adam M. Dimech, Simone Vassiliadis, Aaron C. Elkins, Noel O. I. Cogan, Erez Naim-Feil, and Simone J. Rochfort. 2025. "Determination of Optimal Harvest Time in Cannabis sativa L. Based upon Stigma Color Transition" Plants 14, no. 10: 1532. https://doi.org/10.3390/plants14101532
APA StyleTran, J., Dimech, A. M., Vassiliadis, S., Elkins, A. C., Cogan, N. O. I., Naim-Feil, E., & Rochfort, S. J. (2025). Determination of Optimal Harvest Time in Cannabis sativa L. Based upon Stigma Color Transition. Plants, 14(10), 1532. https://doi.org/10.3390/plants14101532







