Characterization of Fusarium Diversity and Head Microbiota Associated with Rice Spikelet Rot Disease
Abstract
1. Introduction
2. Results
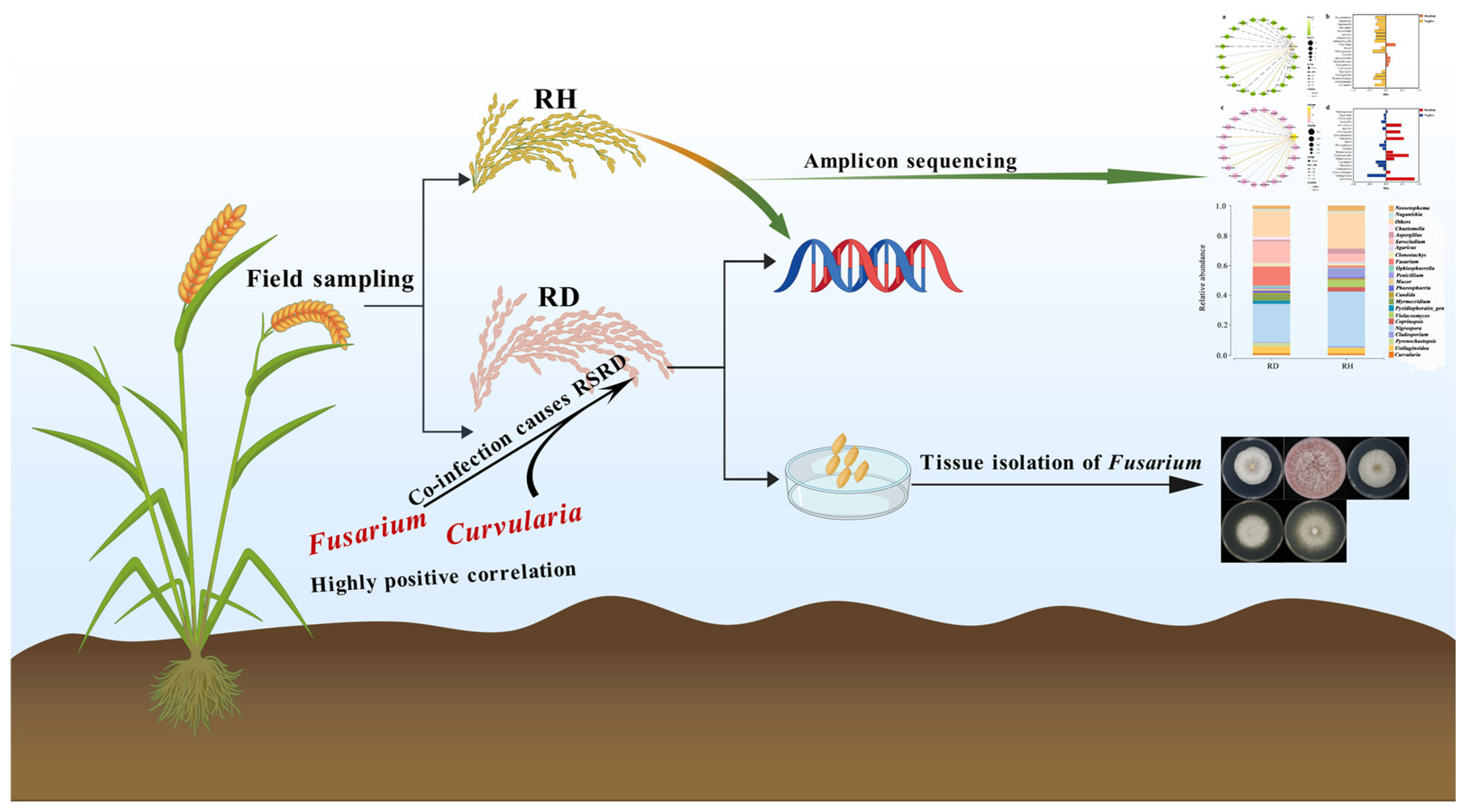
2.1. Composition of Diseased and Relatively Healthy Rice Spikelet Fungal Species
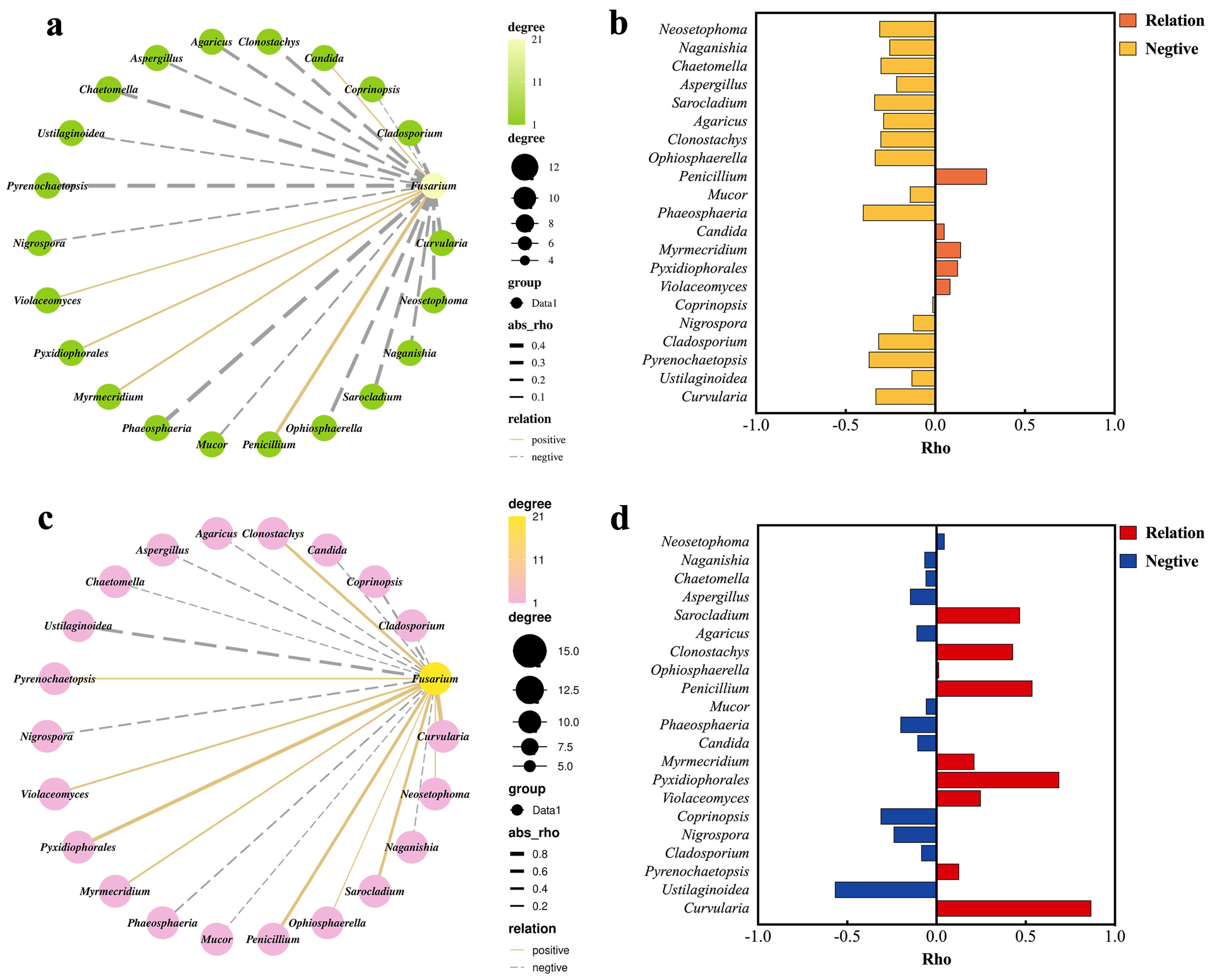
2.2. Fusarium Correlated with Other Fungal Groups
2.3. Fusarium Isolates
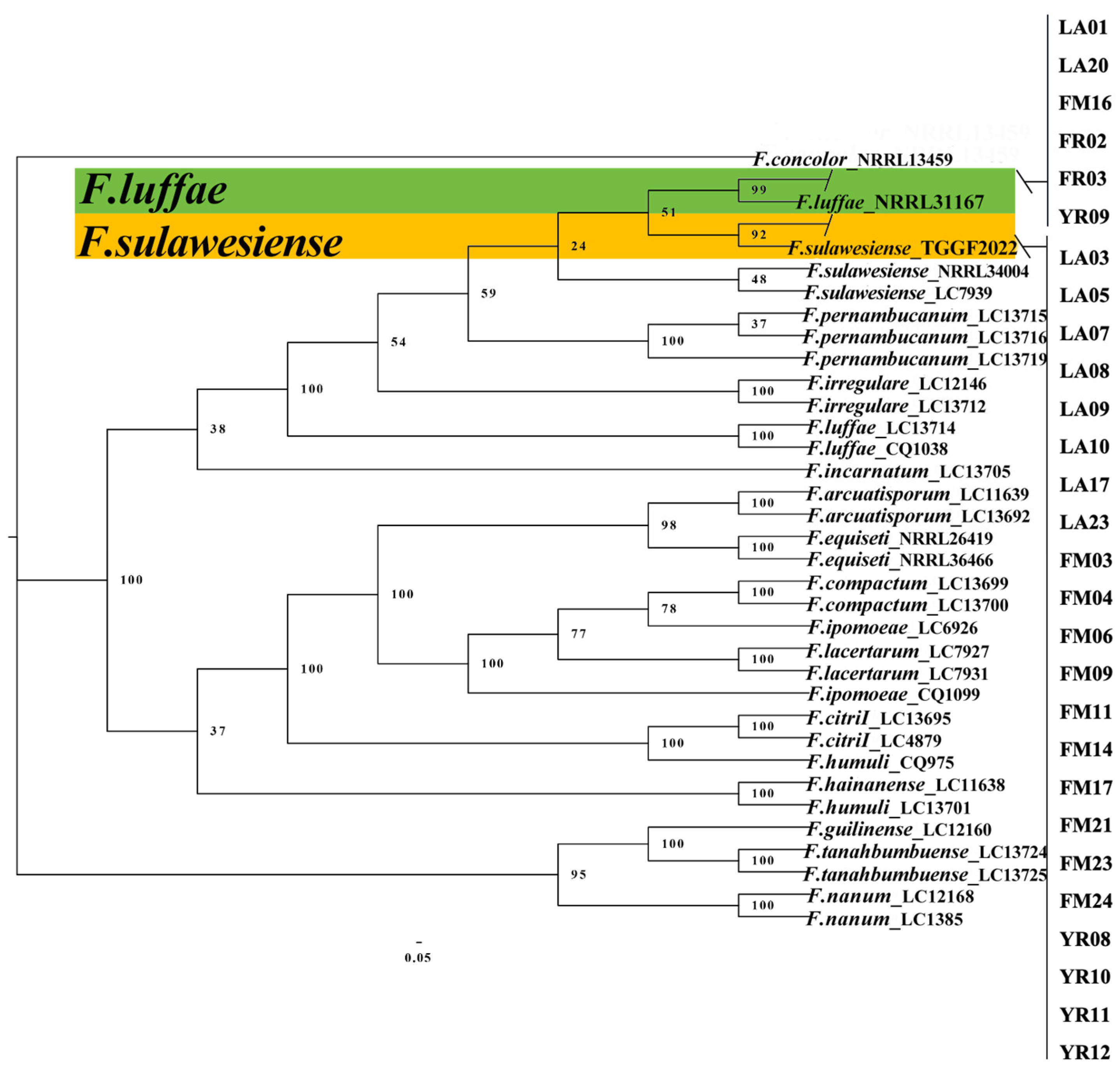
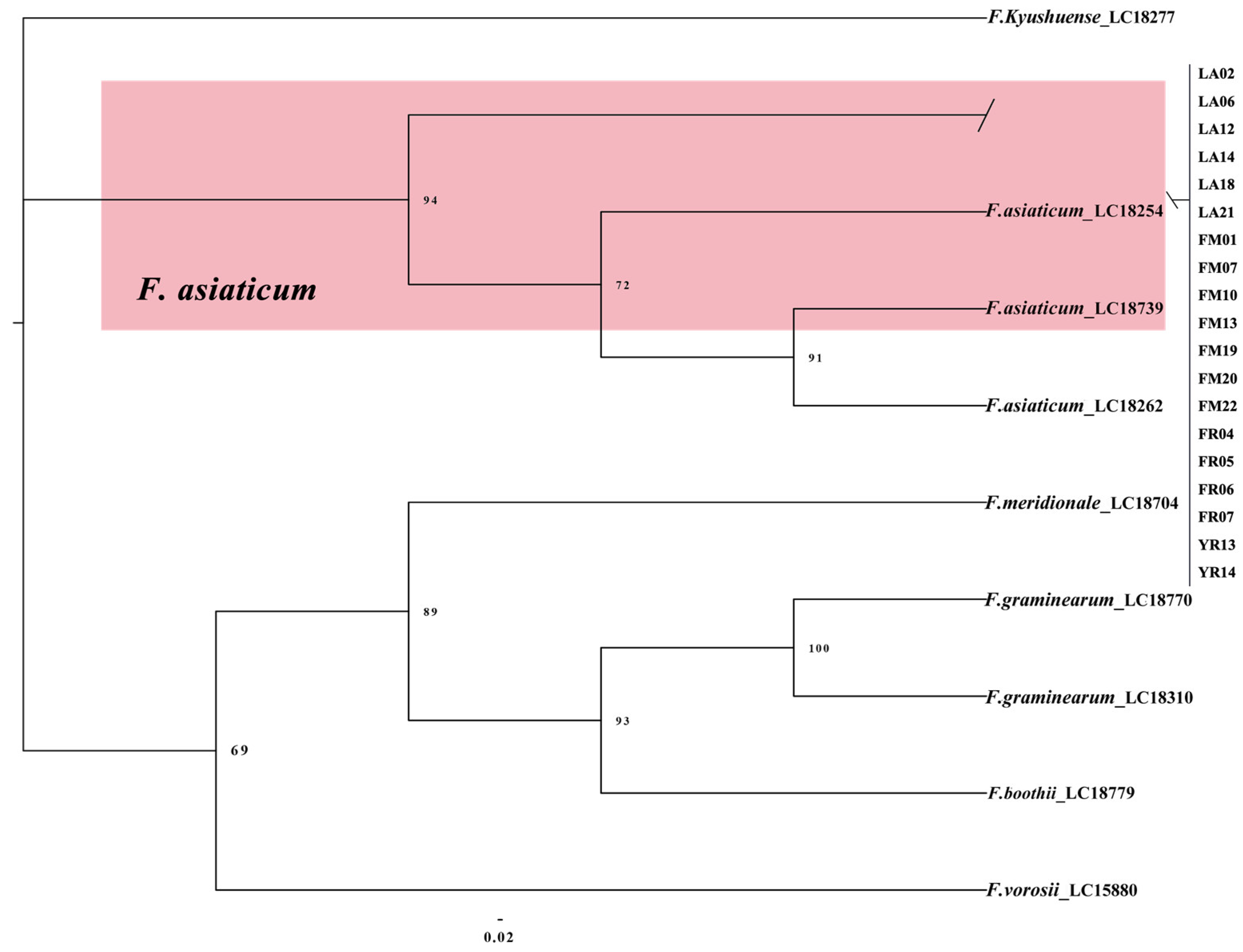
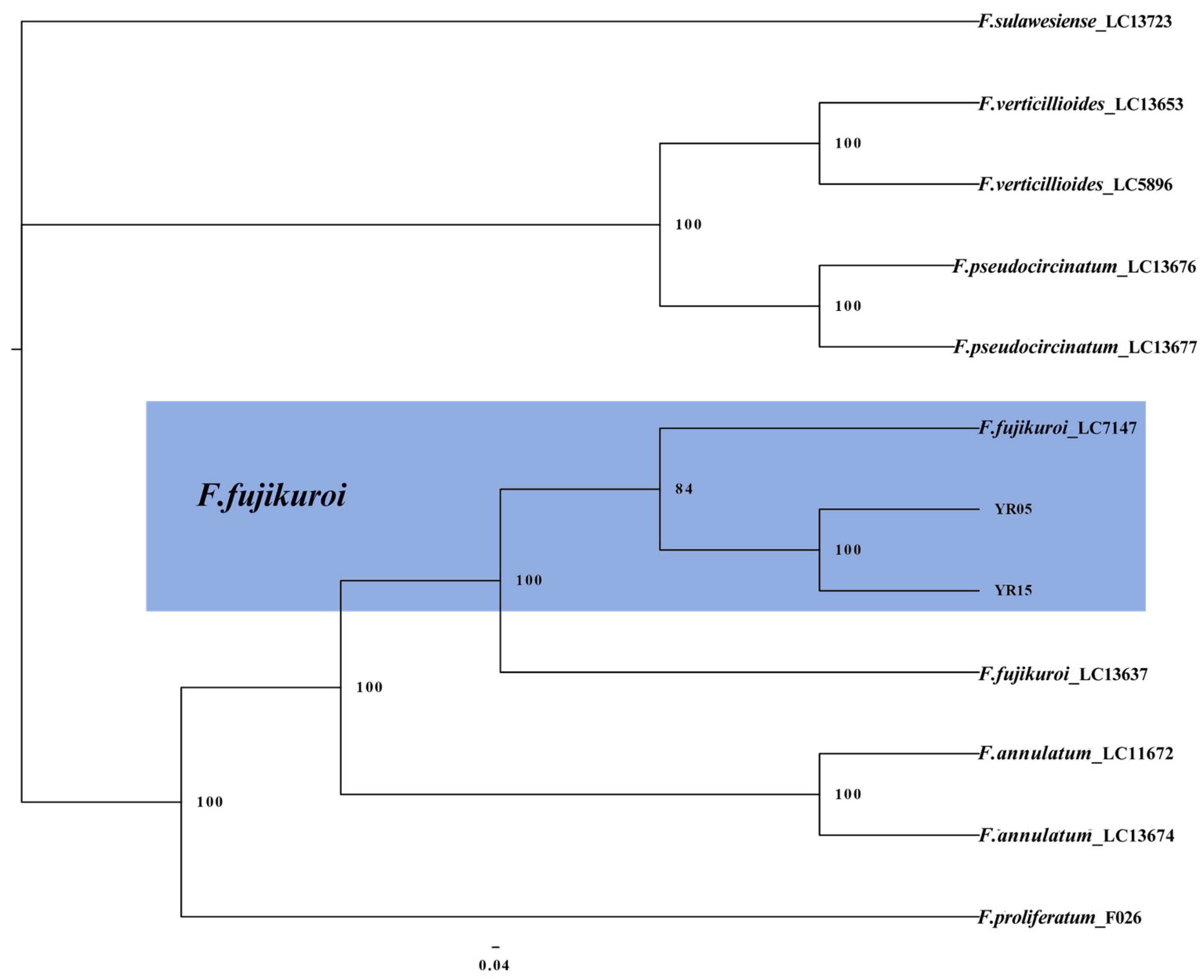
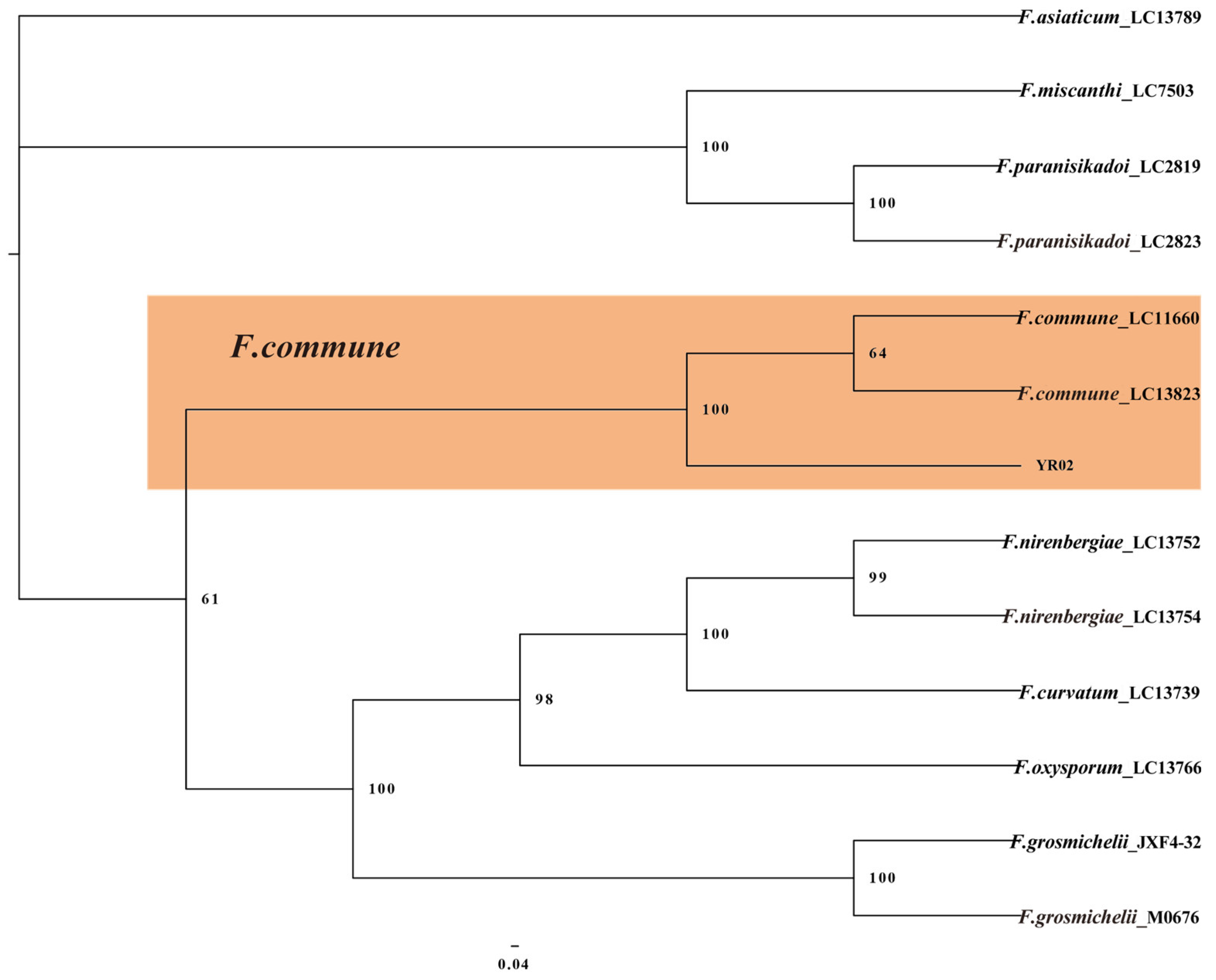
2.4. Multi-Locus Phylogenetic Analysis
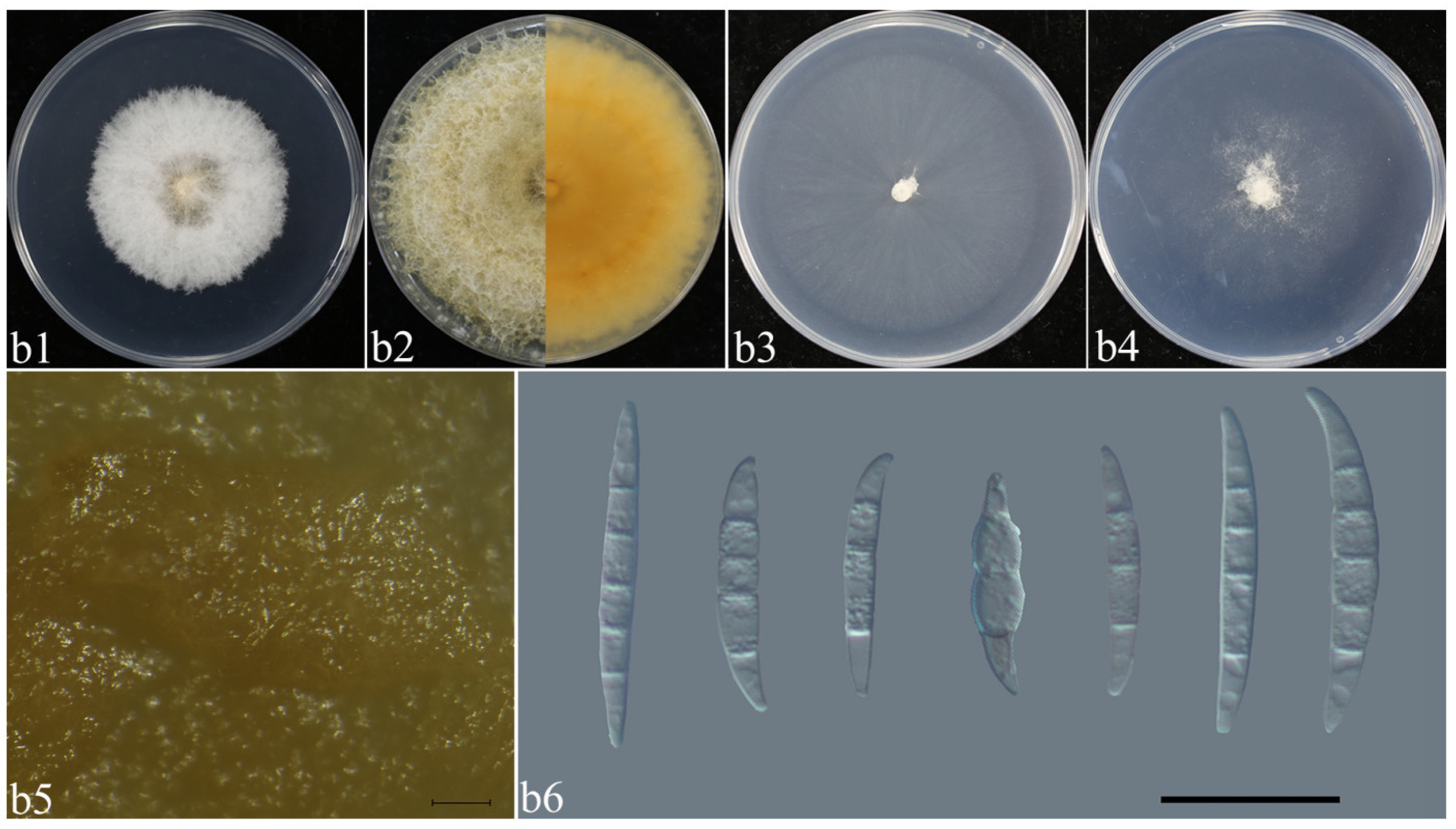
2.5. Morphological Characteristics
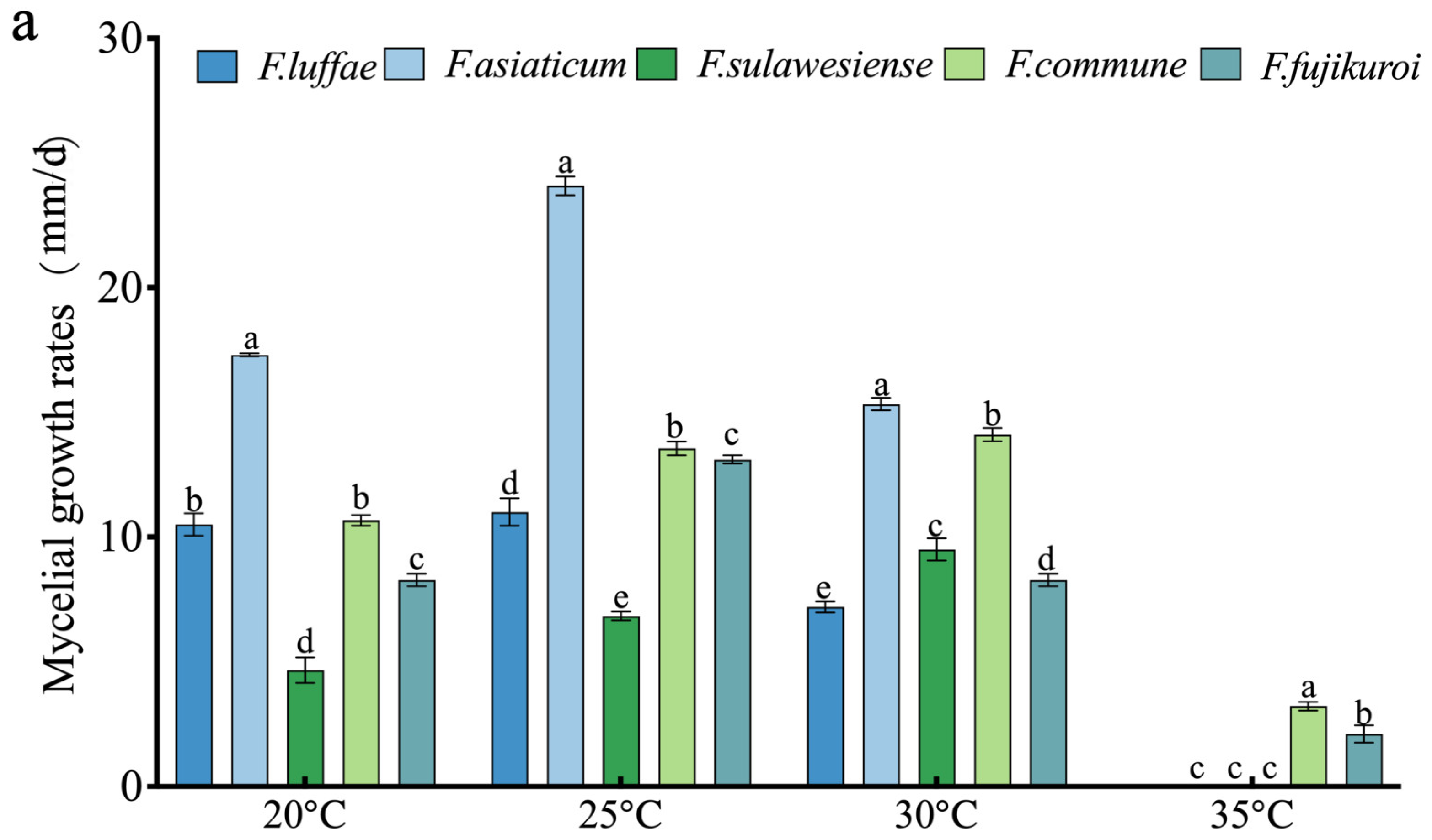
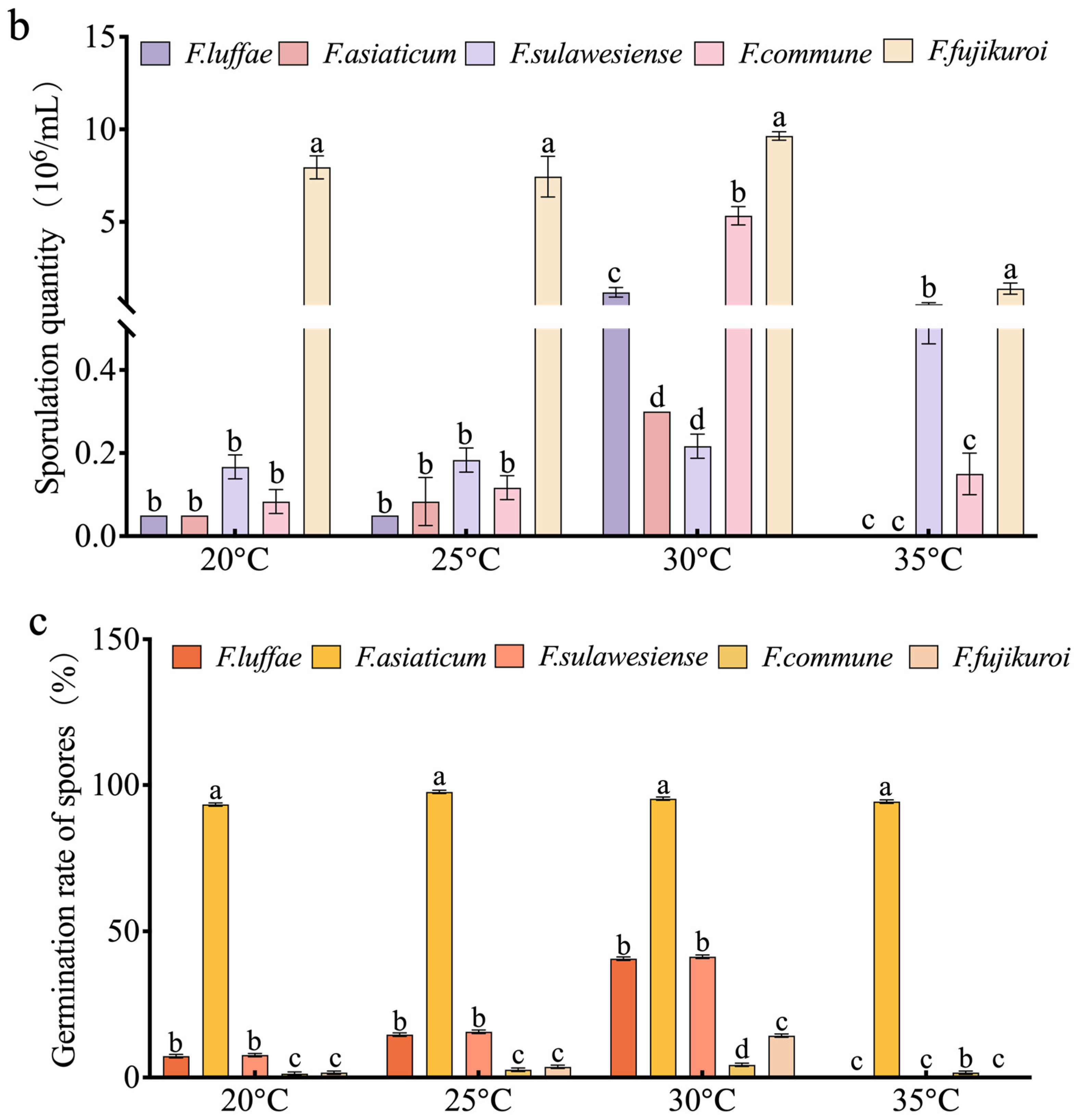
2.6. Effect of Temperature on Growth, Spore Production, and Spore Germination
2.7. Pathogenicity Test
3. Discussion
4. Materials and Methods
4.1. Media
4.2. Sample Collection, Processing, and Preservation
4.3. Extraction of Microbiome DNA
4.4. Meta Amplicon Library Preparation (Illumina)
4.5. Meta Amplicon Analysis
4.6. OTU Taxonomic Annotation and Species Composition and Relative Abundance Analysis
4.7. Correlation Analysis
4.8. Fusarium Isolation and Preservation
4.9. DNA Extraction and Amplification
4.10. Multi-Locus Phylogenetic Analysis of Fusarium Associated with Rice Spikelet Rot
4.11. Morphological Observations
4.12. Assessment of Growth, Spore Production, and Spore Germination on Different Temperatures
4.13. Pathogenicity of Rice Spikelet Rot
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, S.-W.; Wang, L.; Liu, L.-M.; Tang, S.-Q.; Zhu, D.-F.; Savary, S. Rice Spikelet Rot Disease in China—1. Characterization of Fungi Associated with the Disease. Crop Prot. 2011, 30, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Ge, S.; Liang, W.; Liao, W.; Li, W.; Jiao, G.; Wei, X.; Shao, G.; Xie, L.; Sheng, Z.; et al. Genome-Wide Characterization Reveals Variation Potentially Involved in Pathogenicity and Mycotoxins Biosynthesis of Fusarium Proliferatum Causing Spikelet Rot Disease in Rice. Toxins 2022, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, X.; Fu, X.; Zhou, M.; Chen, C.; Song, X.-S. Pathogen Composition and Fungicide Sensitivity in Rice Spikelet Rot Disease. Plant Dis. 2025, PDIS-09. [Google Scholar] [CrossRef] [PubMed]
- Arata, A.F.; Martínez, M.; Castañares, E.; Galizio, R.I.; Fernández, M.D.; Dinolfo, M.I. The Richness of Fusarium Species in Maize Tassels and Their Relationship with Fusarium Stalk Rot. Eur. J. Plant Pathol. 2024, 168, 351–362. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, J.; Wang, S.; Xu, J.; Ma, G.; Shi, J.; Bao, Z. Species Diversity and Toxigenic Potential of Fusarium incarnatum-equiseti Species Complex Isolates from Rice and Soybean in China. Plant Dis. 2021, 105, 2628–2636. [Google Scholar] [CrossRef]
- Burgess, L.; Bryden, W. Fusarium: A Ubiquitous Fungus of Global Significance. Microbiol. Aust. 2012, 33, 22. [Google Scholar] [CrossRef]
- Zhu, Q.; Fei, Y.-J.; Wu, Y.-B.; Luo, D.-L.; Chen, M.; Sun, K.; Zhang, W.; Dai, C.-C. Endophytic Fungus Reshapes Spikelet Microbiome to Reduce Mycotoxin Produced by Fusarium proliferatum through Altering Rice Metabolites. J. Agric. Food Chem. 2023, 71, 11350–11364. [Google Scholar] [CrossRef]
- Chakrawarti, N.; Deo, I.; Verma, R.; Joshi, G.; Kushwaha, K.P.S. First Report of Spikelet Rot Caused by Fusarium Sulawesiense in Rice (Oryza sativa L.) in India. J. Plant Pathol. 2024, 107, 755–756. [Google Scholar] [CrossRef]
- Lei, S.; Wang, L.; Liu, L.; Hou, Y.; Xu, Y.; Liang, M.; Gao, J.; Li, Q.; Huang, S. Infection and Colonization of Pathogenic Fungus Fusarium Proliferatum in Rice Spikelet Rot Disease. Rice Sci. 2019, 26, 60–68. [Google Scholar] [CrossRef]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight From a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Dewey, F.M.; Wong, Y.L.; Seery, R.; Hollins, T.W.; Gurr, S.J. Bacteria Associated with Stagonospora (Septoria) Nodorum Increase Pathogenicity of the Fungus. New Phytol. 1999, 144, 489–497. [Google Scholar] [CrossRef]
- Han, S.L.; Wang, M.M.; Ma, Z.Y.; Raza, M.; Zhao, P.; Liang, J.M.; Gao, M.; Li, Y.J.; Wang, J.W.; Hu, D.M.; et al. Fusarium Diversity Associated with Diseased Cereals in China, with an Updated Phylogenomic Assessment of the Genus. Stud. Mycol. 2023, 104, 87–148. [Google Scholar] [CrossRef]
- He, K.; Zhao, C.; Zhang, M.; Li, J.; Zhang, Q.; Wu, X.; Wei, S.; Wang, Y.; Chen, X.; Li, C. The Chromosome-Scale Genomes of Exserohilum rostratum and Bipolaris zeicola Pathogenic Fungi Causing Rice Spikelet Rot Disease. J. Fungi 2023, 9, 177. [Google Scholar] [CrossRef]
- Pramunadipta, S.; Widiastuti, A.; Wibowo, A.; Suga, H.; Priyatmojo, A. Identification and Pathogenicity of Fusarium Spp. Associated with the Sheath Rot Disease of Rice (Oryza sativa) in Indonesia. J. Plant Pathol. 2022, 104, 251–267. [Google Scholar] [CrossRef]
- Huang, S.-W.; Wang, L.; Liu, L.-M.; Tang, S.-Q.; Zhu, D.-F.; Savary, S. Rice Spikelet Rot Disease in China—2. Pathogenicity Tests, Assessment of the Importance of the Disease, and Preliminary Evaluation of Control Options. Crop Prot. 2011, 30, 10–17. [Google Scholar] [CrossRef]
- Martínez, M.; Arata, A.F.; Fernández, M.D.; Stenglein, S.A.; Dinolfo, M.I. Fusarium Species Richness in Mono- and Dicotyledonous Weeds and Their Ability to Infect Barley and Wheat. Mycol. Prog. 2021, 20, 1203–1216. [Google Scholar] [CrossRef]
- Juraimi, A.; Tasrif, A.; Kadir, J.; Napis, S.; Sastroutomo, S. Phytotoxicity and Field Efficacy of Exserohilum longirostrum JC/M in the Control of Barnyardgrass Ecotypes (Echinochloa crus-galli var. crus-galli (L.) Beauv). Biotropia 2005, 24, 20–29. [Google Scholar] [CrossRef][Green Version]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; De Jonge, R.; Berendsen, R.L. The Soil-Borne Legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two Divergent Intragenomic rDNA ITS2 Types within a Monophyletic Lineage of the Fungus Fusarium Are Nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Best, D.J.; Roberts, D.E. Algorithm AS 89: The Upper Tail Probabilities of Spearman’s Rho. Appl. Stat. 1975, 24, 377. [Google Scholar] [CrossRef]
- Raza, M.; Zhang, Z.-F.; Hyde, K.D.; Diao, Y.-Z.; Cai, L. Culturable Plant Pathogenic Fungi Associated with Sugarcane in Southern China. Fungal Divers. 2019, 99, 1–104. [Google Scholar] [CrossRef]
- Yang, W.; Ma, T.; Liang, D.; Zhang, C. Involvement of the SIX10 Gene in the Pathogenicity of Fusarium Oxysporum Formae Speciales in Strawberries. Int. J. Mol. Sci. 2025, 26, 1123. [Google Scholar] [CrossRef]
- Boutigny, A.-L.; Ward, T.J.; Ballois, N.; Iancu, G.; Ioos, R. Diversity of the Fusarium Graminearum Species Complex on French Cereals. Eur. J. Plant Pathol. 2014, 138, 133–148. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan-Ying, S.; Cai, L. An Optimized Protocol of Single Spore Isolation for Fungi. Cryptogam. Mycol. 2013, 34, 349–356. [Google Scholar] [CrossRef]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and Allied Genera from China: Species Diversity and Distribution. Persoonia-Mol. Phylogeny Evol. Fungi 2022, 48, 1–53. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.J.; Cai, L. Fusarium incarnatum-equiseti Complex from China. Persoonia-Mol. Phylogeny Evol. Fungi 2019, 43, 70–89. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular Systematics and Phylogeography of the Gibberella Fujikuroi Species Complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A Multigene Phylogeny of the Gibberella Fujikuroi Species Complex: Detection of Additional Phylogenetically Distinct Species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-Accessible DNA Sequence Database for Identifying Fusaria from Human and Animal Infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Reeb, V.; Lutzoni, F.; Roux, C. Contribution of RPB2 to Multilocus Phylogenetic Studies of the Euascomycetes (Pezizomycotina, Fungi) with Special Emphasis on the Lichen-Forming Acarosporaceae and Evolution of Polyspory. Mol. Phylogenet. Evol. 2004, 32, 1036–1060. [Google Scholar] [CrossRef]
- Roux, J.; Steenkamp, E.T.; Marasas, W.F.O.; Wingfield, M.J.; Wingfield, B.D. Characterization of Fusarium graminearum from Acacia and Eucalyptus Using β-Tubulin and Histone Gene Sequences. Mycologia 2001, 93, 704–711. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Bianchini, G.; Huelsenbeck, J.P.; Raven, J.A.; Pisani, D.; Knoll, A.H. Model Choice Requires Biological Insight When Studying the Ancestral Habitat of Photosynthetic Eukaryotes. Proc. Natl. Acad. Sci. USA 2017, 114, E10608–E106099. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation Software for the Fast Assembly of Multi-Gene Datasets with Character Set and Codon Information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian Phylogenetic Inference under Mixed Models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a Node or a Foot-Shaped Basal Cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.H.; Kim, H.R.; Chun, Y.; Lee, J.H.; Park, C.; Yoo, H.Y.; Kim, S.W. Rapid and Concise Quantification of Mycelial Growth by Microscopic Image Intensity Model and Application to Mass Cultivation of Fungi. Sci. Rep. 2021, 11, 24157. [Google Scholar] [CrossRef]
- Gale, L.R.; Bryant, J.D.; Calvo, S.; Giese, H.; Katan, T.; O’Donnell, K.; Suga, H.; Taga, M.; Usgaard, T.R.; Ward, T.J.; et al. Chromosome Complement of the Fungal Plant Pathogen Fusarium graminearum Based on Genetic and Physical Mapping and Cytological Observations. Genetics 2005, 171, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Kuo, J.; You, M.P.; Finnegan, P.M.; Barbetti, M.J. Comparative Root Colonisation of Strawberry Cultivars Camarosa and Festival by Fusarium oxysporum f. Sp. Fragariae. Plant Soil 2012, 358, 75–89. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]








| Species | Strain | Location | GenBank No. | |||||
|---|---|---|---|---|---|---|---|---|
| H3 | CAM | TEF1 | RPB1 | RPB2 | TUB2 | |||
| F. sulawesiens (FIESC) | LA03 | Lin’an | - | - | PV208419 | PV208475 | PV208504 | - |
| LA05 | Lin’an | - | - | PV208431 | PV208456 | PV208485 | - | |
| LA07 | Lin’an | - | - | PV208434 | PV208450 | PV208486 | - | |
| LA08 | Lin’an | - | - | PV208420 | PV208451 | PV208483 | - | |
| LA09 | Lin’an | - | - | PV208435 | PV208466 | PV208487 | - | |
| LA10 | Lin’an | - | - | PV208421 | PV208465 | PV208496 | - | |
| LA17 | Lin’an | - | - | PV208422 | PV208457 | PV208488 | - | |
| LA23 | Lin’an | - | - | PV208423 | PV208458 | PV208498 | - | |
| FM03 | Fuyang | - | - | PV208424 | PV208459 | PV208489 | - | |
| FM04 | Fuyang | - | - | PV208436 | PV208467 | PV208499 | - | |
| FM06 | Fuyang | - | - | PV208438 | PV208460 | PV208484 | - | |
| FM09 | Fuyang | - | - | PV208437 | PV208472 | PV208490 | - | |
| FM11 | Fuyang | - | - | PV208430 | PV208461 | PV208500 | - | |
| FM14 | Fuyang | - | - | PV208432 | PV208471 | PV208491 | - | |
| FM17 | Fuyang | - | - | PV208426 | PV208452 | PV208492 | - | |
| FM21 | Fuyang | - | - | PV208433 | PV208453 | PV208493 | - | |
| FM23 | Fuyang | - | - | PV208439 | PV208462 | PV208494 | - | |
| FM24 | Fuyang | - | - | PV208440 | PV208468 | PV208501 | - | |
| FR01 | Fuyang | - | - | PV208441 | PV208463 | PV208495 | - | |
| YR08 | Yuhang | - | - | PV208427 | PV208474 | PV2084503 | - | |
| YR10 | Yuhang | - | - | PV208443 | PV208469 | PV208497 | - | |
| YR11 | Yuhang | - | - | PV208442 | PV208455 | PV2084502 | - | |
| F. luffae (FIESC) | LA01 | Lin’an | - | - | PV208418 | PV208449 | PV208476 | - |
| LA20 | Lin’an | - | - | PV208444 | PV208473 | PV208480 | - | |
| FM16 | Fuyang | - | - | PV208445 | PV208447 | PV208481 | - | |
| FR02 | Fuyang | - | - | PV208446 | PV208470 | PV208482 | - | |
| F. luffae (FIESC) | FR03 | Fuyang | - | - | PV208428 | PV208454 | PV208479 | - |
| YR09 | Yuhang | - | - | PV208425 | PV208448 | PV208477 | - | |
| YR12 | Yuhang | - | - | PV208429 | PV208464 | PV208478 | - | |
| F. asiaticum (FSAMSC) | LA02 | Lin’an | PV053218 | - | PV053200 | PV053237 | PV066010 | - |
| LA06 | Lin’an | PV053219 | - | PV053201 | PV053238 | PV066016 | - | |
| LA12 | Lin’an | PV053220 | - | PV053202 | PV053239 | PV164744 | - | |
| LA14 | Lin’an | PV053221 | - | PV164741 | PV053240 | PV066020 | - | |
| LA18 | Lin’an | PV053222 | - | PV053203 | PV053241 | PV066017 | - | |
| LA21 | Lin’an | PV053223 | - | PV053204 | PV066027 | PV066011 | - | |
| FM01 | Fuyang | PV053224 | - | PV053224 | PV066028 | PV066012 | - | |
| FM07 | Fuyang | PV053225 | - | PV053207 | PV066029 | PV066021 | - | |
| FM10 | Fuyang | PV053231 | - | PV053208 | PV164742 | PV164743 | - | |
| FM13 | Fuyang | PV053232 | - | PV053209 | PV066030 | PV066022 | - | |
| FM19 | Fuyang | PV053233 | - | PV053210 | PV066031 | PV066023 | - | |
| FM20 | Fuyang | PV053226 | - | PV053205 | PV066032 | PV066024 | - | |
| FM22 | Fuyang | PV053234 | - | PV053211 | PV066033 | PV066018 | - | |
| FR04 | Fuyang | PV053227 | - | PV053212 | PV066034 | PV066013 | - | |
| FR05 | Fuyang | PV053228 | - | PV053213 | PV066035 | PV066014 | - | |
| FR06 | Fuyang | PV053229 | - | PV053214 | PV066036 | PV066015 | - | |
| FR07 | Fuyang | PV066015 | - | PV053215 | PV066037 | PV066019 | - | |
| YR13 | Yuhang | PV053230 | - | PV053216 | PV066038 | PV066025 | - | |
| YR14 | Yuhang | PV053236 | - | PV053217 | PV066039 | PV066026 | - | |
| F. fujikuroi (FFSC) | YR05 | Yuhang | - | PQ783814 | PQ783818 | PQ783816 | PQ783815 | PQ783820 |
| YR15 | Yuhang | - | PQ783813 | PQ783819 | PV053199 | PQ783817 | PQ783821 | |
| F. commune (FNSC) | YR02 | Yuhang | - | - | PQ783810 | PQ783811 | PQ783812 | - |
| Species | Growth Rate (mm/d) | Size of Microspore (μm) | |||
|---|---|---|---|---|---|
| PDA | OA | SNA | Length | Width | |
| F. sulawesiense | 6.80 ± 0.81 | 12.07 ± 0.25 | 8.33 ± 0.59 | 29.14 ± 6.96 | 3.96 ± 0.81 |
| F. luffae | 11.00 ± 0.55 | 15.40 ± 0.25 | 8.87 ± 0.49 | 28.89 ± 6.29 | 3.37 ± 0.52 |
| F. asiaticum | 24.20 ± 0.38 | 17.13 ± 0.46 | 10.87 ± 0.46 | 40.40 ± 21.31 | 3.43 ± 0.96 |
| F. fujikuroi | 11.28 ± 0.11 | 11.56 ± 0.08 | 11.04 ± 0.10 | 8.14 ± 1.81 | 2.89 ± 0.70 |
| F. commune | 13.76 ± 0.15 | 11.32 ± 0.10 | 9.60 ± 0.15 | 15.15 ± 8.92 | 3.54 ± 0.99 |
| Medium | Components |
|---|---|
| PDA 1L | 200 g of potato, 20 g of glucose, and 20 g of agar |
| OA 1L | 30 g of oatmeal and 16 g of agar |
| SNA 1L | 1 g of KH2PO4, 1 g of KNO3, 0.5 g of MgSO4·7H2O, 0.5 g of KCl, 0.2 g of glucose, 0.2 g of sucrose, and 20 g of agar |
| 3% Mung bean soup | 30 g of green beans |
| Gene | Length | Sequence (5′-3′) | Temperature | References |
|---|---|---|---|---|
| tef1 | 650 bp | ATGGGTAAGGARGACAAGAC | 48 °C | [30] |
| GGARGTACCAGTSATCATG | ||||
| CaM | 550 bp | GARTWAAGGAGGCCTTCTC | 55 °C | [31] |
| TTTTTGCATCATGAGTTGGAC | ||||
| tub2 | 680 bp | AACATGCGTGAGATTGTAAGT | 55 °C | [19] |
| TAGTGACCCTTGGCCCAGTTG | ||||
| rpb1 | 1600 bp | CAYAARGARTCYATGATGGGWC | 58 °C | [32] |
| GTCATYTGDGTDGCDGYTCDCC | ||||
| rpb2 | 1700 bp | GGGGWGAYCAGAAGAAGGC | 57 °C | [33] |
| GCRTGGATCTTRTCRTCSACC | ||||
| H3 | 500 bp | ACTAAGCAGACCGCCCGCAGG | 60 °C | [34] |
| GCGGGCGAGCTGGATGTCCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Q.; Wu, J.; Ma, T.; Mao, C.; Zhang, C. Characterization of Fusarium Diversity and Head Microbiota Associated with Rice Spikelet Rot Disease. Plants 2025, 14, 1531. https://doi.org/10.3390/plants14101531
Cao Q, Wu J, Ma T, Mao C, Zhang C. Characterization of Fusarium Diversity and Head Microbiota Associated with Rice Spikelet Rot Disease. Plants. 2025; 14(10):1531. https://doi.org/10.3390/plants14101531
Chicago/Turabian StyleCao, Qun, Jianyan Wu, Tianling Ma, Chengxin Mao, and Chuanqing Zhang. 2025. "Characterization of Fusarium Diversity and Head Microbiota Associated with Rice Spikelet Rot Disease" Plants 14, no. 10: 1531. https://doi.org/10.3390/plants14101531
APA StyleCao, Q., Wu, J., Ma, T., Mao, C., & Zhang, C. (2025). Characterization of Fusarium Diversity and Head Microbiota Associated with Rice Spikelet Rot Disease. Plants, 14(10), 1531. https://doi.org/10.3390/plants14101531






