2.1. Descriptive Statistics of Soil–Plant Heavy Metal Contents
As can be seen from
Table 1, the average contents of heavy metals Cr, Cu, Zn, Cd, Pb, Hg, and As in the soil were 44.38, 20.89, 55.95, 0.13, 17.5, 0.0093, and 6.64 mg·kg
−1, respectively, and in terms of the average contents of heavy metals, the content of Cd exceeded the background value of Xinjiang soil by 1.08 times. The mean values of Cr, Cu, Zn, Cd, Pb, Hg, and As did not exceed the screening values when compared with the risk screening values of the national standard (GB/15618-2018). The coefficients of variation of each heavy metal in the soil were 35.10%, 47.85%, 22.53%, 45.34%, 11.03%, 41.64%, and 59.13%, which were all of medium variation. From the coefficients of variation, it can be seen that As and Cu have higher degrees of variation and are more affected by external influences.
Table A1 shows the significant comparative differences between the soil contents of each heavy metal and the background values in Xinjiang. Among them, the
p-values of Zn, Pb, Hg, and As were all 1, while the
p-values of Cr, Cu, and Cd were 0.9183, 0.9934, and 0.1994, respectively, which indicated that there was no significant difference between the seven heavy metal elements in the study area and the background values of Xinjiang, whereas the element Cd had a relatively significant difference from the background values.
As can be seen from
Table 2, the average content of seven heavy metals Cr, Cu, Zn, Cd, Pb, Hg, and As in
Anabasis aphylla L. were categorized as 294.24, 6.51, 11.63, 0.17, 1.91, 0.01, and 1.53 mg·kg
−1, of which Cr was 147 times the standard limit value, and Hg, Cu, Zn, Cd, Pb, and As did not exceed the standard limit value; The average content of seven heavy metals—Cr, Cu, Zn, Cd, Pb, Hg, and As—in
Alhagi camelorum Fisch. was 70.10, 3.78, 4.89, 0.06, 0.65, 0.01, and 0.46 mg·kg
−1, of which Cr was 35 times the standard limit value; the average content of seven heavy metals—Cr, Cu, Zn, Cd, Pb, Hg, and As—in
Reaumuria songonica (PalL)Maxim. was 115.46, 5.86, 8.31, 0.12, 3.65, 0.01, and 0.92 mg·kg
−1, of which Cr and Pb were 57.73 and 1.22 times the standard limit values, respectively; and the average contents of seven heavy metals—Cu, Zn, Cd, Pb, Hg, and As—in
Haloxylon ammodendron (C. A. Mey.) Bunge. were 63.79, 3.85, 7.48, 0.08, 0.39, 0.01, and 0.26, of which Cr was 31.89 times the standard limit. From the above, it can be seen that Cr exceeds the standard in the above four plants, which has a greater ecological risk. The coefficients of variation of the heavy metal contents in each plant were large, indicating that the factors affecting the heavy metal contents in plants are complicated.
As shown in
Table A2, the Cr, Cu, Zn, Pb, and As contents were significantly higher than the standard limit (
p = 0.00001), except for Hg (
p = 0.3699).
Table A3 shows that the concentrations of Cr, Cu, Zn, and Cd did not differ significantly among the four plants (
p > 0.05), while the concentrations of Pb, Hg, and As differed significantly (
p < 0.05). Further two-by-two comparisons (in
Table A4) showed that, with regard to the element Pb:
Anabasis aphylla L.—
Haloxylon ammodendron (C. A. Mey.) Bunge. differed significantly (
p = 0.0079), as did
Reaumuria songonica (PalL)Maxim.—
Haloxylon ammodendron (C. A. Mey.) Bunge. (
p = 0.0063). For the element Hg,
Reaumuria songonica (PalL)Maxim.—
Haloxylon ammodendron (C. A. Mey.) Bunge. differed significantly (
p = 0.0098). With regard to the element As,
Anabasis aphylla L.—
Haloxylon ammodendron (C. A. Mey.) Bunge. (
p = 0.0145) and
Reaumuria songonica (PalL)Maxim.—
Haloxylon ammodendron (C. A. Mey.) Bunge. (
p = 0.0096) were the main sources of difference. In summary, the plant heavy metal accumulation characteristics were regulated by species specificity. The interspecific differences in Pb and As were mainly driven by the comparison of
Anabasis aphylla L.,
Reaumuria songonica (PalL)Maxim. and
Haloxylon ammodendron (C. A. Mey.) Bunge., while the differences in Hg were concentrated in
Reaumuria songonica (PalL)Maxim. and
Haloxylon ammodendron (C. A. Mey.) Bunge.
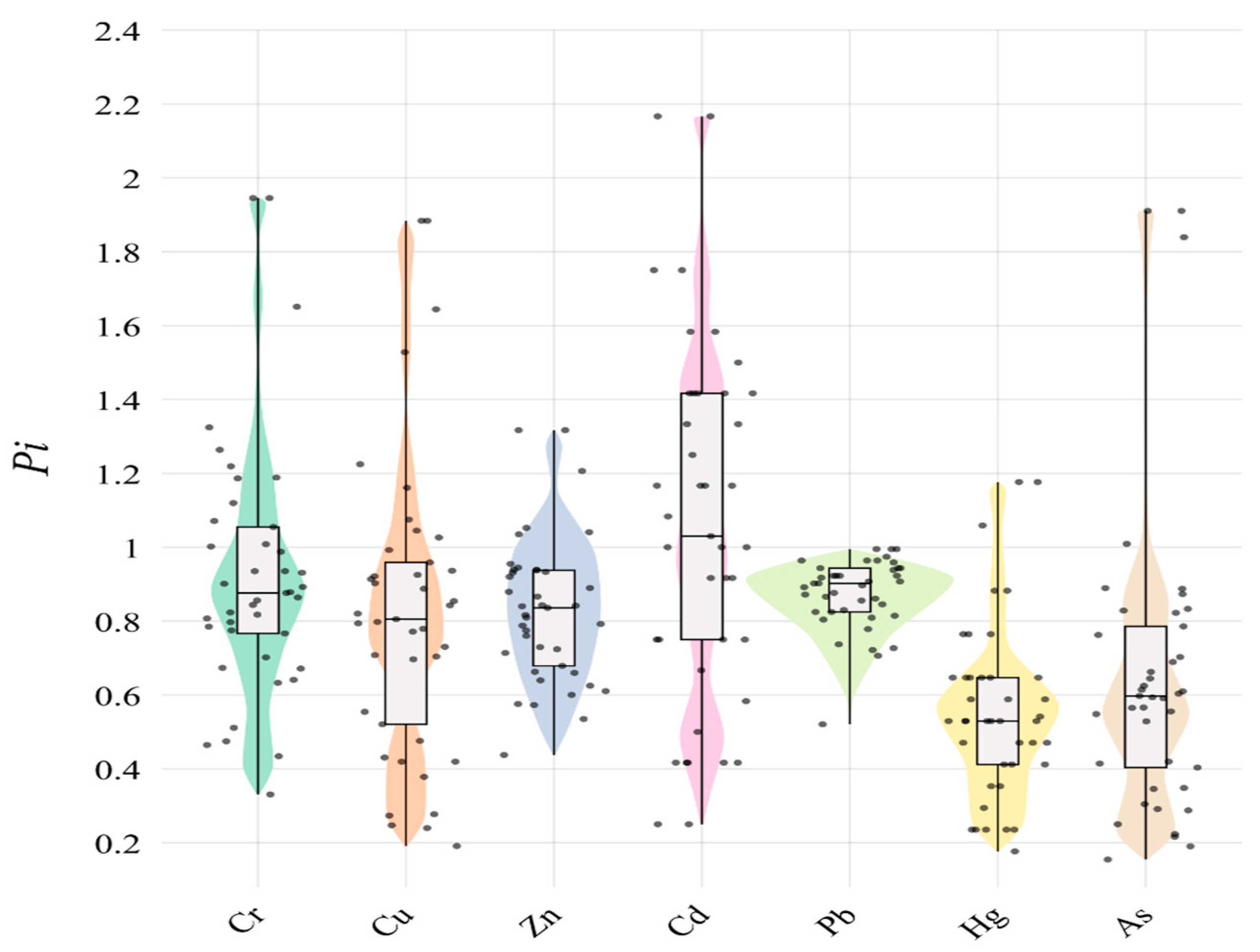
The Nemerow pollution index (
Table 3 and
Figure 1) shows that Cr, Cu, Zn, Cd, and As are in a moderate state of pollution, while Pb and Hg are in a warning limit.
From the composite potential ecological risk index (
Table 4), it can be seen that Cr and Zn in the soil are at the slight ecological hazard level; Cu, Pb, and As are at the medium ecological hazard level; and Cd and Hg are at the very strong ecological hazard level. Combining the NIPI with the RI shows that Cr, Cu, Cd, Hg, and Pb are more seriously polluted.
2.2. Plant–Soil Heavy Metal Correlation Analysis
In order to express the interrelationship between plant heavy metals and soil heavy metals more accurately, the interconnection between plants and soil at the same sampling site will be analyzed. Analyzing the interconnections between soil and plants can help provide a basis for subsequent heavy metal pollution management. The BCF can intuitively respond to the uptake and migration capacity of plants for soil heavy metals, and the uptake capacity of the same plant for different heavy metal elements and the uptake capacity of different plants for the same element are also somewhat different [
50]. As can be seen from
Figure 2, the BCF for Cr were greater than 1 for
Anabasis aphylla L. (7.303),
Alhagi camelorum Fisch. (1.78),
Reaumuria songonica (PalL)Maxim. (2.60), and
Haloxylon ammodendron (C. A. Mey.) Bunge. (1.45), which indicated that Cr could be easily absorbed by these four types of plants ((
) = 3.28). Regarding the BCF of Hg, the BCF of
Anabasis aphylla L. (1.35),
Alhagi camelorum Fisch. (1.46), and
Reaumuria songonica (PalL)Maxim. (1.26) were all greater than 1, and only
Haloxylon ammodendron (C. A. Mey.) Bunge. (0.82 < 1) indicated that Hg was also easily absorbed by the plants and was enriched in the body of the plants ((
) =1.22). The BCF of Cd in
Anabasis aphylla L. (1.424 > 1),
Alhagi camelorum Fisch. (0.71),
Reaumuria songonica (PalL)Maxim. (0.71), and
Haloxylon ammodendron (C. A. Mey.) Bunge. (0.84) indicated that the effect of Cd on plants should not be neglected ((
) =0.92), not being easily enriched in plants and having less effects on plants. The differences in the heavy metal enrichment ability of different plants in the same soil environment indicate that the external environment was not a decisive factor for heavy metal accumulation. Studies have shown that the coexistence of multiple heavy metals in the soil environment of the same region is different from the effect of a single heavy metal in the soil on plants, and the enrichment phenomenon of plants can be antagonistic or synergistic due to the presence of multiple heavy metals [
51,
52,
53].
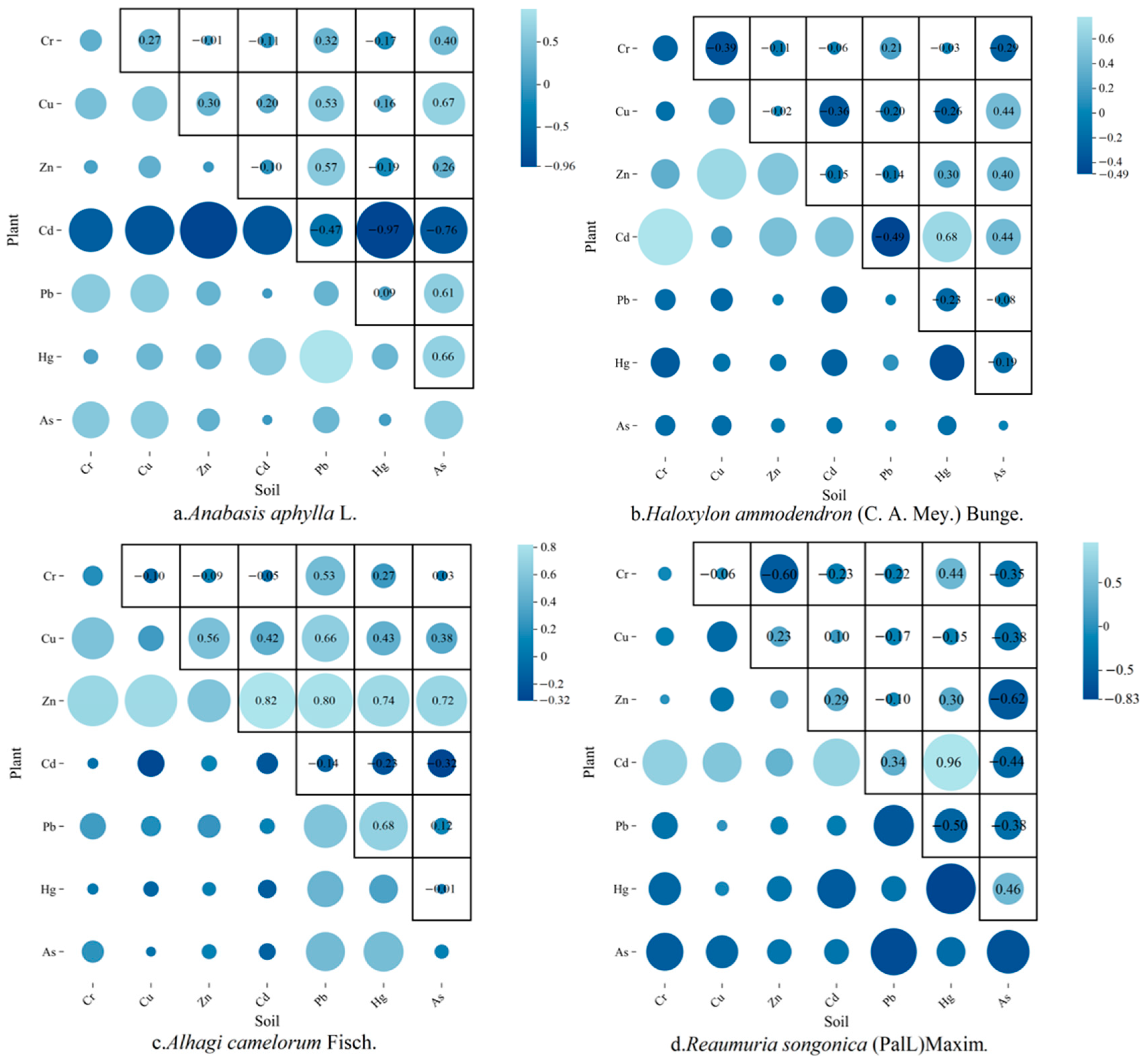
The correlation coefficients can effectively reflect the correlation between soil heavy metals and plant heavy metals, visualize the transfer efficiency of pollutants between environmental media, and explain the main sources of heavy metals in plants. In
Figure 3, the correlations between seven heavy metals in four plant species and in soil are demonstrated. In
Anabasis aphylla L., Cd-plant showed a very strong negative correlation (r = −0.967) with Hg-soil, suggesting that soil Hg contamination may inhibit the phytosorption of Cd or that the two are competitive; Cu-plant showed a significant positive correlation (r = 0.67) with As-soil, reflecting the fact that As can modulate and promote the translocation of Cu, whereas the correlation between Pb-plant and Pb-soil was only weakly correlated (r = 0.313), indicating that its accumulation was dominated by other factors. For
Alhagi camelorum Fisch., Zn-plant showed strong positive correlations with Cd-soil (r = 0.82), Pb-soil (r = 0.795), and Hg-soil (r = 0.739), implying that Zn promotes multi-metal co-transport [
54,
55], and Cd-plant showed no significant correlation with Cd-soil (r = −0.208), indicating that its accumulation is dominated by other factors and thus indicating that its uptake is regulated by non-soil factors (e.g., atmospheric deposition). In
Reaumuria songonica (PalL)Maxim., Cd-plant showed a strong positive correlation (r = 0.96) with Hg-soil, indicating that soil Hg contamination could promote Cd uptake in
Reaumuria songonica (PalL)Maxim., which was diametrically opposed to
Anabasis aphylla L., and demonstrated that the uptake of the same heavy metal varied among different plants, whereas As-plant showed a strong negative correlation (r = −0.761) with Pb-soil, which may be due to Pb As-plant being strongly negatively correlated with Pb-soil (r = −0.761), probably due to the inhibition of As enrichment by Pb; Cr-plant was negatively correlated with Zn-soil (r = −0.602), indicating that excess Zn would lead to the blockage of Cr uptake. In
Haloxylon ammodendron (C. A. Mey.) Bunge., Cd-plant was positively correlated with Cr-soil (r = 0.778), indicating that Cr contamination in the soil may promote Cd accumulation in the plant, while Pb-plant was weakly correlated with soil Hg-soil (r = −0.226), suggesting that the two uptake pathways do not interfere with each other.
Heavy metal accumulation in plants was driven not only by soil concentrations but also by synergistic or antagonistic interactions between elements, such as the “bridging effect” of Zn in
Alhagi camelorum Fisch. and the strong antagonism of Cd-Hg in
Anabasis aphylla L. The low correlation of some elements (e.g., Cd in
Alhagi camelorum Fisch.) with soil concentrations suggests that stomatal uptake, microbial activity, or genetic regulation may dominate the accumulation process [
56].
The correlation coefficients and BCFs between soil heavy metals and plant heavy metals are key indicators for revealing the migration and transformation of pollutants in the soil–plant system, which is of great significance for ecological risk assessment and pollution control. Combining the BCFs and correlation coefficient, it can be found that the Cd in
Haloxylon ammodendron (C. A. Mey.) Bunge. and
Reaumuria songonica (PalL)Maxim. is positively correlated with the Cd content in the soil, with the BCF close to 1, suggesting that Cd is easily enriched and that Cd can have harmful effects on plants; in addition, the Zn in
Haloxylon ammodendron (C. A. Mey.) Bunge., the Cu and As in
Anabasis aphylla L., as well as the Zn and Pb in the
Anabasis aphylla L. are positively correlated with the corresponding elements in the soil, but the enrichment of the above four types of elements in those plants are smaller, indicating that although the appeal of the three species of plants is sensitive to the content of Zn, Cu, As, and Pb in the soil, the accumulation ability is weak. These four species of plants are easily enriched by Cr elements, but the Cr in plants and the Cr in soil show a non-significant correlation, indicating that the four species of plants show extremely strong enrichment of Cr, which is not related to the soil content, which can thus be used for subsequent remediation of contamination [
57].
2.3. Source Identification by APCS-MLR
The APCS-MLR model was used to analyze the sources of heavy metals in the above plants. Using a principal component analysis, the two main factor scores were converted to absolute principal factor scores, and multiple linear regression analyses were carried out, with the content of heavy metal elements in the measured vegetation as the dependent variable and the absolute factor scores as the independent variables, and the results are shown in
Table A5. R
2 is used to measure the correlation between the model and the actual observations, and the closer the value of R
2 is to 1, the better the linear fit and the better the simulation results [
58]. In this study, the R
2 of the regression model for each element was greater than 0.5, and only the R
2 of Pb was slightly lower, at 0.53. The results showed that the model had a better simulation effect.
The APCS-MLR model identified the main factors that had a greater effect on plant heavy metals based on a principal component analysis. The principal component analysis generated a four-dimensional model based on eigenvalues greater than 1, explaining 71.6% of the variance in the dataset. Among them, APCS1 explained 55.24% of the variance and consisted of Cr, Cu, Zn, Cd, and As, with a loading factor ≥ 0.64 (
Table 5), which is a strong loading [
59]. All of the above elements were positively correlated, and the significant correlation between heavy metal concentrations indicates that these elements have a common source [
60]. From
Figure 4, it can be seen that factor 1 has a large contribution to the elements Cd, Zn, and Cr with 63.49%, 55.66%, and 45.51%, respectively, as well as a large effect on Cu and As. Some studies have shown that As enters the environment with metal mining and smelting activities [
61,
62], and in China, As is mainly associated with Cu, Cu, and Sn deposits [
63], and mining activities and smelting likewise cause rapid changes in the element Cd [
64,
65]. Therefore, factor 1 was initially determined to be mineral mining and smelting activities.
APCS2 explained 16.35% of the variance, including Cu, Pb, Hg, and As, of which the Hg and Pb loading coefficients reached 0.77 versus 0.74, and combined with
Figure 4, it was concluded that factor 2 was closely related to the source of the element Pb, with a contribution of 72.92%. This study shows that gasoline combustion leads to an increase in Pb content [
66], which determines that factor 2 is a transportation source.
When the APCS-MLR model was utilized to resolve the pollution sources, a class of unknown sources was resolved. Combined with
Figure 4, it was concluded that unknown source 3 had a relatively high degree of association with elemental Hg, with a contribution rate of 56.18%. The enrichment of elemental Hg is usually considered to be dominated by mining pollution sources [
67], and the Hg in the roots of woody trees and shrubs is mainly absorbed from the soil, while that in the aboveground part is mainly absorbed from the atmosphere [
68,
69], from which it can be deduced that unknown source 3 is a mixed source of complex origins.
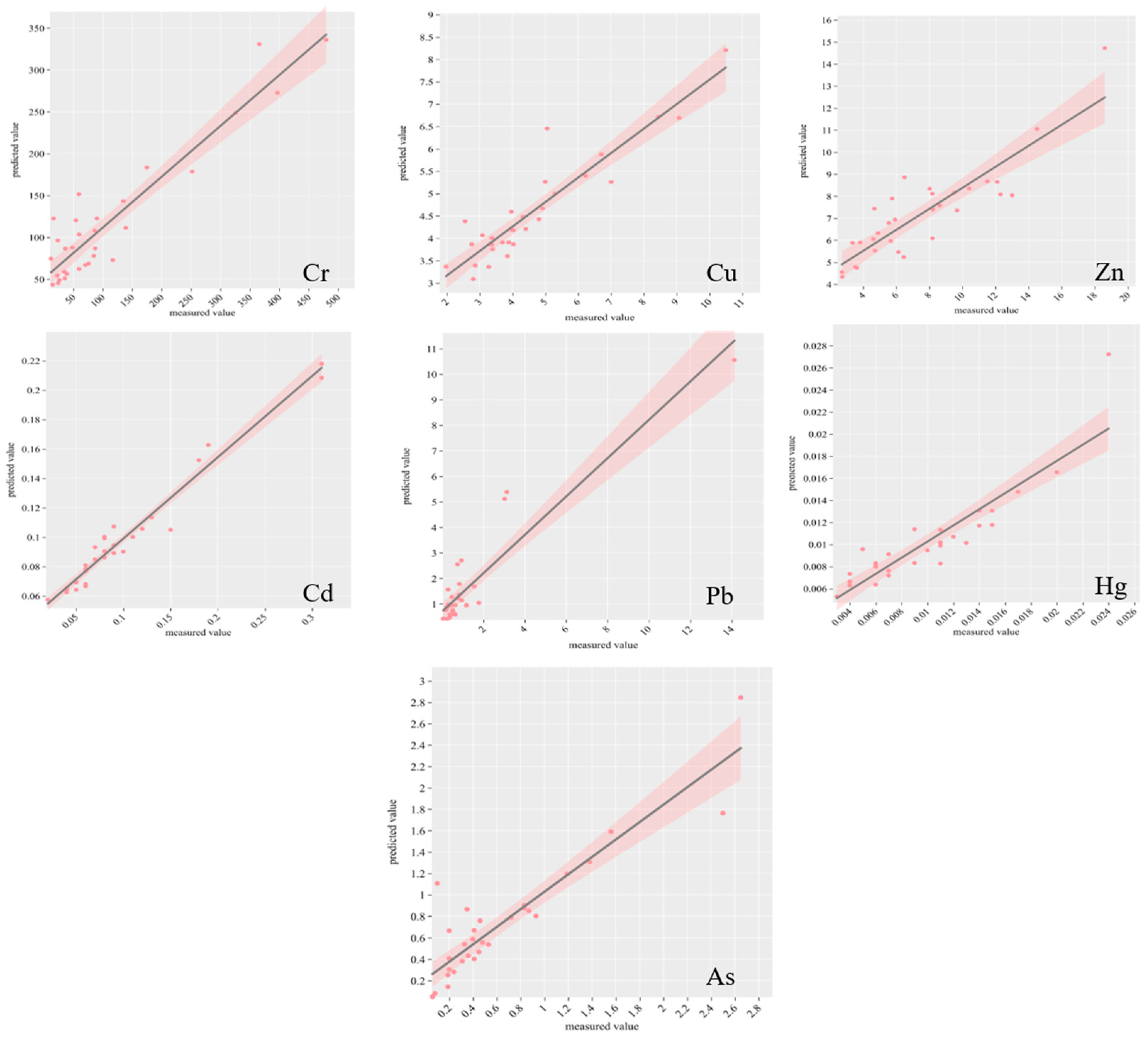
2.4. Random Forest Modeling to Predict Pollution Sources
The RF model prediction performance will be evaluated by R
2, RMSE, MAE, and RPD, with R
2 > 0.5 meaning that the model simulation performance is good [
70]; the model fit is shown in
Figure A1. According to
Table 6, which assesses the predictive effectiveness of the RF model, the model showed good statistical significance for most of the heavy metal elements. In terms of goodness of fit (R
2), the R
2 values of the training set range from 0.72 (Zn) to 0.85 (Pb), indicating the model’s ability to explain data variability. In the test set, the R
2 values of Cd (0.86), Cu (0.82), and As (0.81) are higher than or close to those of the training set. This demonstrates the model’s excellent generalization ability. However, the R
2 value for Zn in the test set drops significantly to 0.54. This suggests that the model’s predictive ability may be limited by feature selection or differences in the data distribution. Regarding error analysis, the RMSE and MAE of the test set are generally lower than those of the training set (e.g., the RMSE of Cu decreases from 0.94 to 0.45, and the MAE of Pb decreases from 0.46 to 0.16). This indicates that the model has high prediction accuracy and stability for most elements. However, the RMSE of the Cr test set (58.88) is slightly lower than that of the training set, and the absolute error remains high. This may reflect data noise or a large concentration span. Regarding model consistency (RPD), the high RPD values of Cd (2.66), Cu (2.35), and As (2.27) suggest robust and reliable predictions, while the low RPD values of Zn (1.48) and Cr (1.78) imply unstable predictions. Taken together, the model’s predictions for elements such as Cd, Cu, Pb, and As are statistically significant (R
2 ≥ 0.75 for the test set) and suitable for practical scenarios, such as pollution a source analysis or risk assessment. However, Zn predictions need further optimization; introducing relevant environmental factors or adjusting the model structure may be necessary. Overall, the model is valuable in environmental science but needs improvement.
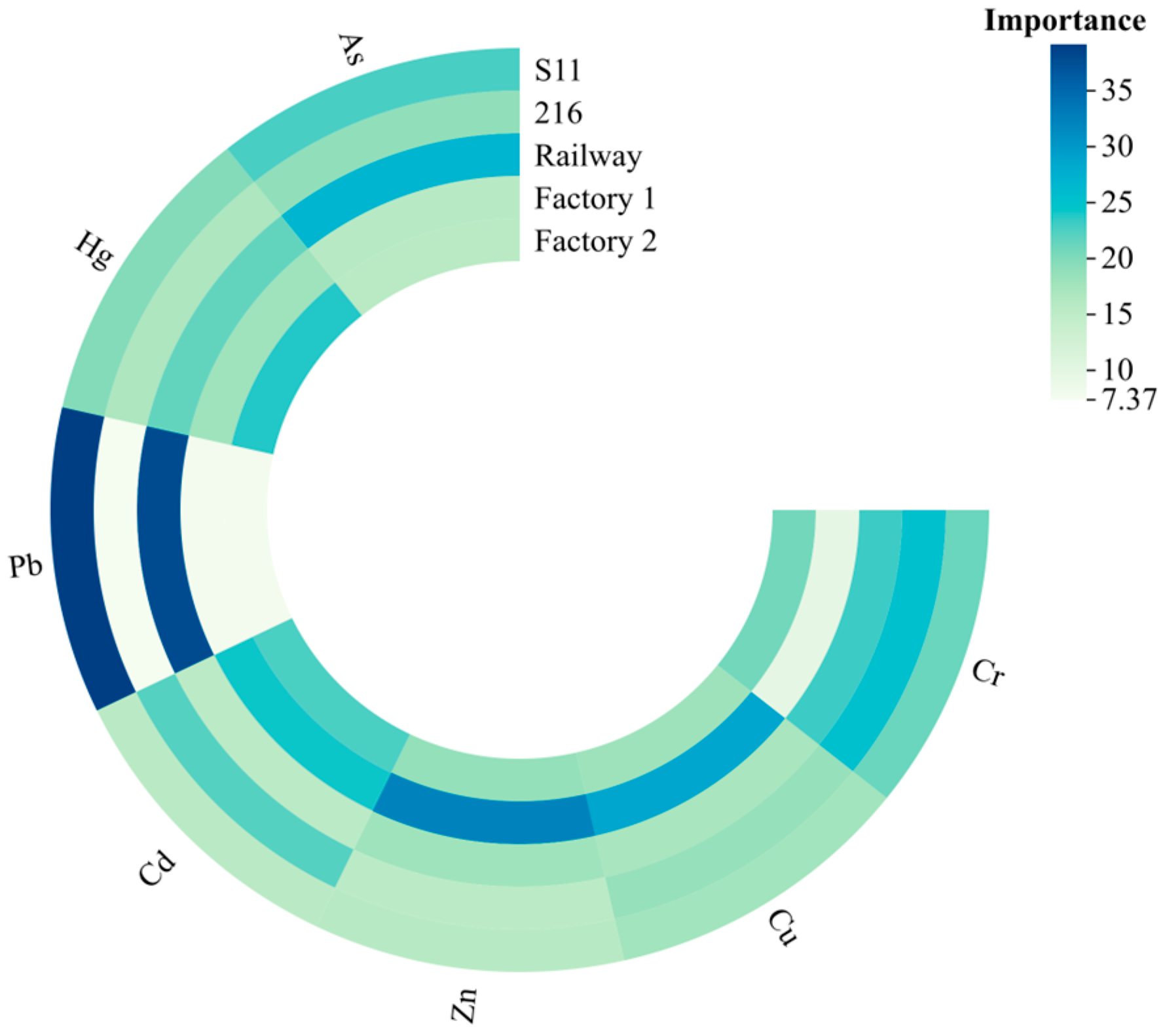
The results of the RF model pollution source analysis are shown in
Figure 5. As a whole, the two roads, railroad and S11, contributed the largest percentage. For Cr elemental pollution sources, 216 provincial road contributes, the most with 25.32%; factory 1 contributes the least, with 9.55%; and the remaining three sources account for 20.78 to 23.14. For Cu, factory 1 contributes the most, with 28.70%, and the other four sources account for 17.13 to 18.53. For Zn, factory 1 contributes the most, with 32.28%, and the other four sources account for the range of 17.13 to 18.53. For Zn, the largest contribution is from factory 1, with 32.28%, and the other 4 sources range from 15.36 to 18.76. The sources of Cd are more complex, with 24.24%, 22.68%, and 22.16% from factory 1, factory 2, and Provincial Highway 216, respectively, and 15.40% and 15.52% from railroads and S11, respectively, for the rest. For Pb, the greater contribution is made by railroads and S11, with 37.99% and 39.15%, both of which account for the majority of the pollution sources. The greater contribution of elemental Hg is made by plant 2 and railroads, with 23.91% and 21.54%, respectively, while the remaining three sources range from 16.81 to 19.82. For As, the greatest contribution is made by Railroad and S11, with 26.82% and 22.77%, and the remaining 3 sources range from 15.79 to 18.97.
Overall, the synergistic increase in the contributions of Cr, Zn, and Cd in the factory 1 and factory 2 areas indicates that all three elements are influenced by industrial activities, whereas the close association of the elements Pb and As with S11 suggests that the transportation activities have a greater impact on them. The remaining elements, Hg and Cu, are influenced by a combination of industrial production and transportation.