Combination of Nanobioproduct and Chemical Ethylene Synthesis Inhibitor with Entomopathogenic Fungi: A Novel Management Strategy for Coffee Berry Borer in Arabica Coffee
Abstract
:1. Introduction
2. Results
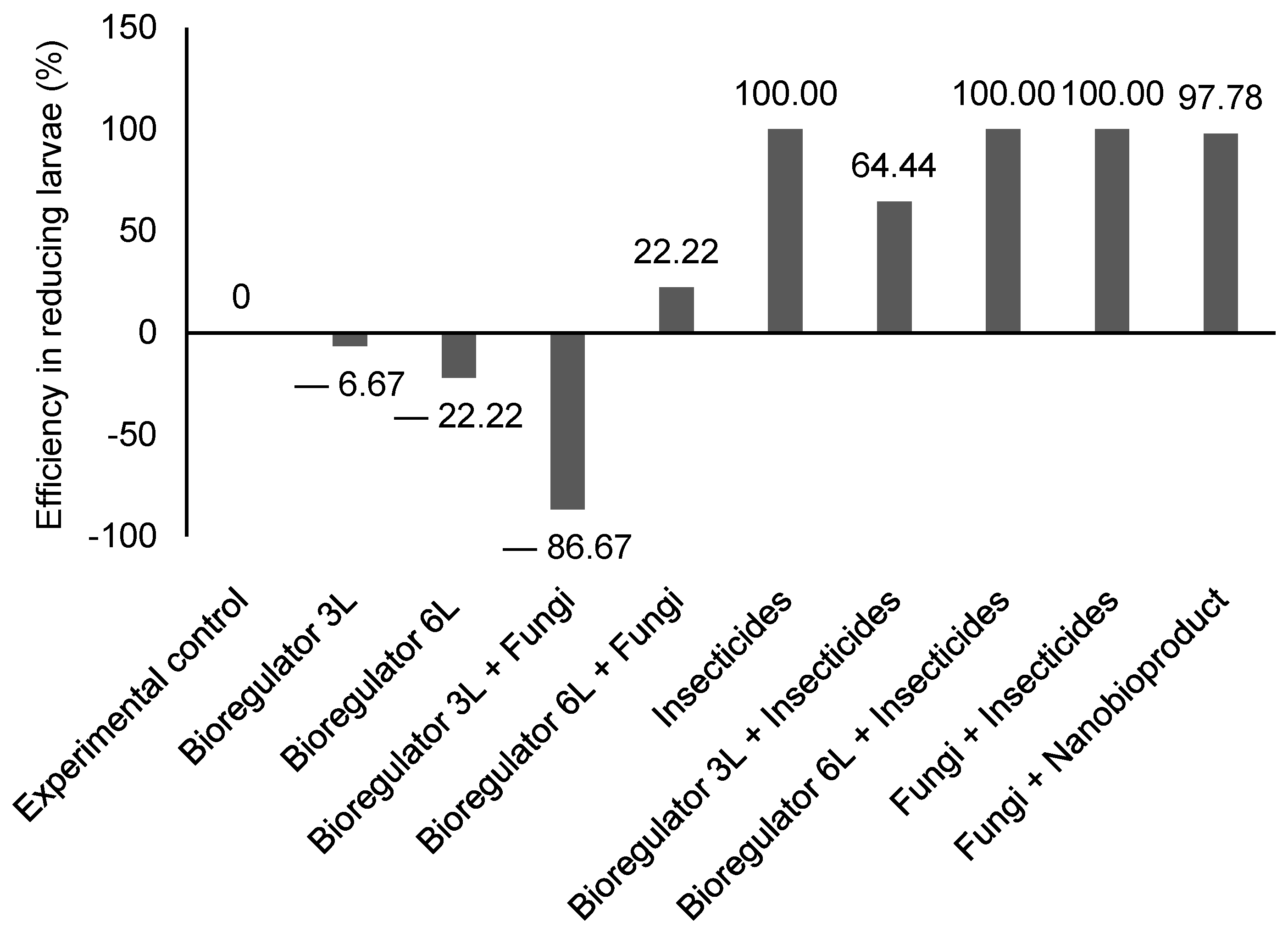
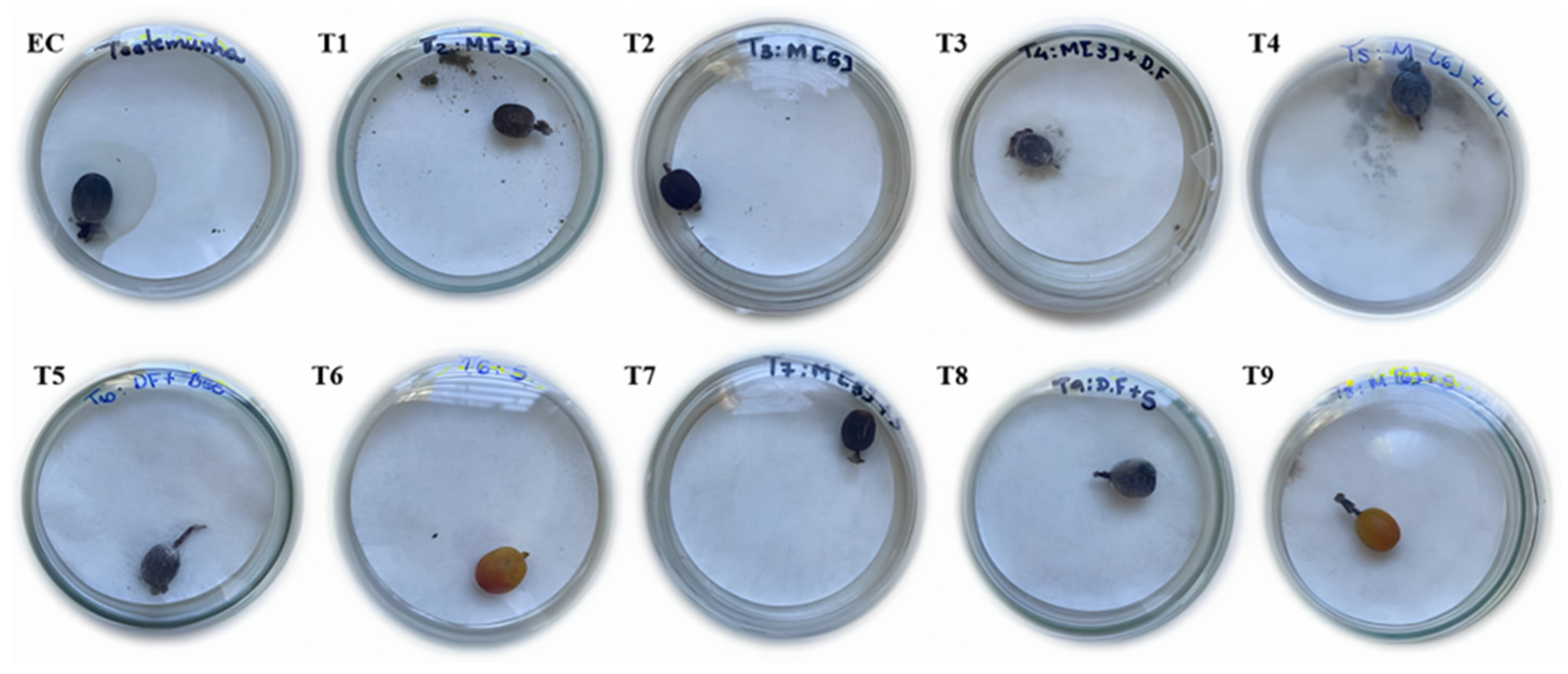
2.1. Biological Development Bioassay with Hypothenemus Hampei
2.2. Field Experiment
2.2.1. Physical and Sensory Analysis of Coffee Beans
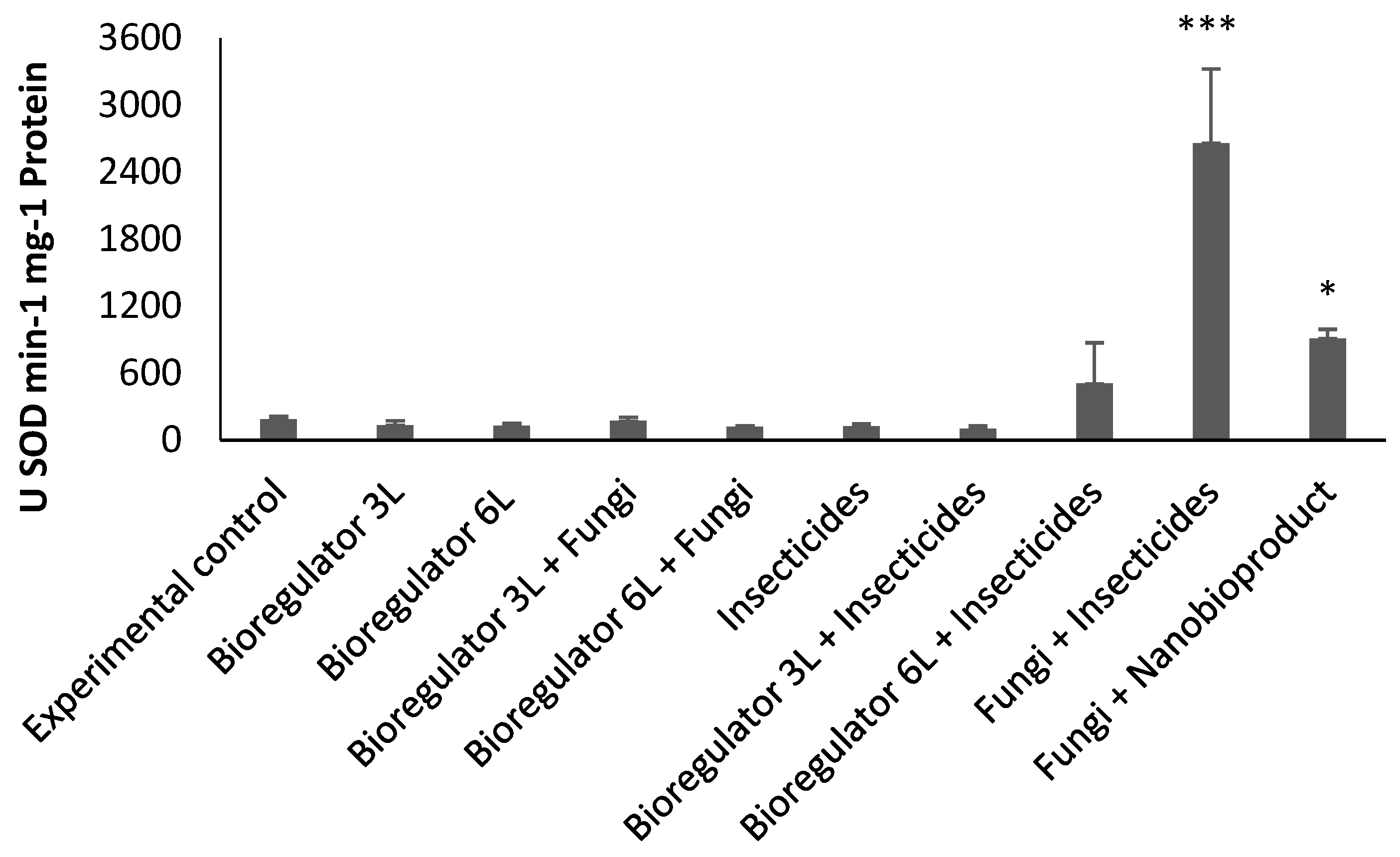
2.2.2. Enzymatic Analysis
3. Discussion
4. Materials and Methods
4.1. Treatments and Experimental Conditions
4.2. Preparation of Ethylene Synthesis Inhibitor Nanobioproduct
4.3. Biological Development Bioassay with Hypothenemus Hampei
4.4. Field Experiment
4.4.1. Physical and Sensory Analysis of Coffee Beans
4.4.2. Enzymatic Analysis
4.5. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cure, J.R.; Rodríguez, D.; Gutierrez, A.P.; Ponti, L. The coffee agroecosystem: Bio-economic analysis of coffee berry borer control (Hypothenemus hampei). Sci. Rep. 2020, 10, 12262. [Google Scholar] [CrossRef] [PubMed]
- CONAB. Relatório 2024. 2024. Available online: https://www.conab.gov.br/ultimas-noticias/5740-producao-de-cafe-de-2024-e-estimada-em-54-79-milhoes-de-sacas-influenciada-por-clima (accessed on 14 March 2025).
- Waller, J.M.; Bigger, M.; Hillocks, R.J. Coffee Pests, Diseases and Their Management; CABI: Wallingford, UK, 2007. [Google Scholar]
- Infante, F.; Pérez, J.; Vega, F.E. The coffee berry borer: The centenary of a biological invasion in Brazil. Braz. J. Biol. 2014, 74 (Suppl. S1), S125–S126. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.O.J.; Souza, B.H.S.; Costa, D.C.M.; Carneiro, F.S.; Dias, D.M.; Silva Júnior, M.B. Application of ethylene synthesis inhibitor in arabica coffee on field infestation, preference, and performance of Hypothenemus hampei. Arthropod-Plant Interact. 2023, 17, 777–786. [Google Scholar] [CrossRef]
- Moreno-Ramirez, N.; Bianchi, F.J.; Manzano, M.R.; Dicke, M. Ecology and management of the coffee berry borer (Hypothenemus hampei): The potential of biological control. BioControl 2024, 69, 199–214. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Caarls, L.; Vos, I.A.; Pieterse, C.M.J.; Van Wees, S.C.M. Ethylene: Traffic controller on hormonal crossroads to defense. Plant Physiol. 2015, 169, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Does, D.V.; Zamioudis, C.; Leon-Reyes, A.; Wees, S.C.M.V. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 8, 489–521. [Google Scholar] [CrossRef]
- Bungala, L.T.D.C.; Park, C.; Dique, J.E.L.; Sathasivam, R.; Shin, S.Y.; Park, S.U. Ethylene: A modulator of the phytohormone-mediated insect herbivory network in plants. Insects 2024, 15, 404. [Google Scholar] [CrossRef]
- Dias, R.E.B.A.; Silva, F.D.; Cunha, J.P.B.; Avelar, R.C.; Fernandes, F.C. Influence of the use of biosynthesis inhibitor ethylene on the efficiency of combine harvesting. Coffee Sci. 2014, 9, 527–536. [Google Scholar]
- Bettiol, W.; Lessa, C.M.S.; Oliveira, L.M. Biocontrole de Doenças de Plantas: Uso e Perspectivas; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2009. [Google Scholar]
- Alınç, T.; Cusumano, A.; Peri, E.; Torta, L.; Colazza, S. Trichoderma harzianum strain T22 modulates direct defense of tomato plants in response to Nezara viridula feeding activity. J. Chem. Ecol. 2021, 47, 455–462. [Google Scholar] [CrossRef]
- Rotaru, V.I.; Risnoveanu, L. Interactive effects of plant growth-promoting rhizobacteria and phosphate sources on growth and phosphorus nutrition of soybean under moderate drought. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 872–880. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Cardoso e Bufalo, T.; Bazzana, M.J.F.; Souza, B.H.S.; Dias, E.S.; Rodrigues, J.D.; Antunes, A.P.A.; Molina, Y.C.; Sepulveda, A.M.G.; Ladislao, M.S.; Sousa, L.F. Sistema de Liberação Lenta de Nanopartículas por Microrganismos e/ou Consórcio de Microrganismos e/ou Suas Estruturas de Virulência Para a Aplicação em Biotização e/ou Formulações de Biopesticidas, Biofertilizantes e Biofortificantes. Patente BR1020230164439. Instituto Nacional de Propriedade Industrial. 2023. Available online: https://busca.inpi.gov.br/pePI/servlet/PatenteServletController (accessed on 19 March 2025).
- Phogat, A.; Singh, J.; Kumar, V.; Malik, V. Toxicity of the acetamiprid insecticide for mammals: A review. Environ. Chem. Lett. 2022, 20, 1453–1478. [Google Scholar] [CrossRef]
- Aznar-Alemany, Ò.; Eljarrat, E.J.P.I. Introduction to pyrethroid insecticides: Chemical structures, properties, mode of action and use. In Pyrethroid Insecticides; Springer: Cham, Switzerland, 2020; Volume 92, pp. 1–16. [Google Scholar] [CrossRef]
- Rix, R.R.; Cutler, G.C. Review of molecular and biochemical responses during stress-induced stimulation and hormesis in insects. Sci. Total Environ. 2022, 827, 154085. [Google Scholar] [CrossRef] [PubMed]
- Vimal, N.; Soni, P.; Bansal, N.; Pal, R.; Kumar, A. Radiation hormesis to improve the quality of adult Spodoptera litura (Fabr.). Insects 2022, 13, 933. [Google Scholar] [CrossRef] [PubMed]
- Jalal, A.; Oliveira Júnior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Cutler, G.C. Insecticide-induced hormesis and arthropod pest management. Pest Manag. Sci. 2014, 70, 690–697. [Google Scholar] [CrossRef]
- Campos, S.O.; Santana, I.V.; Silva, C.; Santos-Amaya, O.F.; Guedes, R.N.C.; Pereira, E.J.G. Bt-induced hormesis in Bt-resistant insects: Theoretical possibility or factual concern? Ecotoxicol. Environ. Saf. 2019, 183, 10957. [Google Scholar] [CrossRef]
- Kumar, P.; Dubey, R.C. Plant growth promoting rhizobacteria for biocontrol of phytopathogens and yield enhancement of Phaseolus vulgaris. J. Curr. Perspect. Appl. Microbiol. 2012, 1, 38. [Google Scholar]
- Rashid, M.H.O.; Chung, Y.R. Induction of systemic resistance against insect herbivores in plants by beneficial soil microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, R.A.S.; Silva, E.A.; Oliveira, T.M.; Pereira, D.L. Efeito do Mathury® na maturação de frutos de café variedade Catuaí Vermelho. Rev. Cultiv. Saber 2013, 6, 66–74. [Google Scholar]
- War, A.R.; Paulraj, M.G.; Ignacimuthu, S.; Sharma, H.C. Induced resistance to Helicoverpa armigera through exogenous application of jasmonic acid and salicylic acid in groundnut, Arachis hypogaea. Pest Manag. Sci. 2015, 71, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.G.; Souza, B.H.S.; Marchiori, P.E.R.; Bôas, L.V.V. Characterization of priming, induced resistance, and tolerance to Spodoptera frugiperda by silicon fertilization in maize genotypes. J. Pest Sci. 2022, 95, 1387–1400. [Google Scholar] [CrossRef]
- Jakupi, M.; Demirbas, S. Production of secondary metabolites through elicitors: Their application in agriculture. J. Balkan Sci. Technol. 2022, 1, 165–172. [Google Scholar] [CrossRef]
- Malook, S.U.; Tan, X.; Ali, A.; Ameen, M.; Mehmood, A.; Khan, M.I. Molecular and biochemical mechanisms of elicitors in pest resistance. Life 2022, 12, 844. [Google Scholar] [CrossRef]
- Ngugia, H.K.; Dedej, S.; Delaplane, K.S.; Savelle, A.T.; Scherma, H. Effect of flower-applied Serenade biofungicide (Bacillus subtilis) on pollination-related variables in rabbiteye blueberry. Biol. Control 2005, 33, 32–38. [Google Scholar] [CrossRef]
- Yao, A.; Bochow, H.; Karimov, S.; Boturov, U.; Sanginboy, S.; Sharipov, A. Effect of FZB 24® Bacillus subtilis as a biofertilizer on cotton yields in field tests. Arch. Phytopathol. Plant Prot. 2006, 39, 323–328. [Google Scholar] [CrossRef]
- Gupta, V.P.; Bochow, H.; Dolej, S.; Fischer, I. Plant growth-promoting Bacillus subtilis strain as potential inducer of systemic resistance in tomato against Fusarium wilt. Z. Pflanzenkrankh. Pflanzenschutz 2000, 107, 145–154. [Google Scholar]
- Ongena, M.; Duby, F.; Jourdan, E.; Beaudry, T.; Jadin, V.; Dommes, J.; Thonart, P. Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl. Microbiol. Biotechnol. 2005, 67, 692–698. [Google Scholar] [CrossRef]
- Lanna Filho, R.; Ferro, H.M.; Pinho, R.S.C. Controle biológico mediado por Bacillus subtilis. Rev. Trópica Ciências Agrárias Biológicas 2010, 4, 12–20. [Google Scholar]
- Aristizábal, L.F.; Bustillo, A.M.; Garcia, F.; Garcia, A.; Lopez, A.; Giraldo, J.P. Establishing an integrated pest management program for coffee berry borer (Hypothenemus hampei) in Hawaii and Puerto Rico coffee agroecosystems: Achievements and challenges. Insects 2023, 14, 603. [Google Scholar] [CrossRef]
- Escobar-Ramírez, S.; Ríos-Morales, O.; Díaz-Castillo, L.; Hernández, L.; Rivas, J. Biological control of the coffee berry borer: Main natural enemies, control success, and landscape influence. Biol. Control 2019, 136, 103992. [Google Scholar] [CrossRef]
- Dantas, A.A.A.; Carvalho, L.G.; Ferreira, E. Classificação e tendências climáticas em Lavras, MG. Ciência Agrotecnol. 2007, 31, 1862–1866. [Google Scholar] [CrossRef]
- Aristizábal, L.F.; Bustillo, A.E.; Arthurs, S.P. Integrated pest management of coffee berry borer: Strategies from Latin America that could be useful for coffee farmers in Hawaii. Insects 2016, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- SCA Standard 103-2024; Coffee Value Assessment: Descriptive Assessment. Specialty Coffee Association (SCA): Irvine, CA, USA, 2024. Available online: https://sca.coffee/research/coffee-standards (accessed on 12 November 2024).
- de Fátima Pereira Silva, P.; de Resende, M.L.V.; Reichel, T.; de Almeida, R.F.; dos Santos, P.H.D.; Soares, M.A. Potassium phosphite activates components associated with constitutive defense responses in Coffea arabica cultivars. Mol. Biotechnol. 2023, 65, 1777–1795. [Google Scholar] [CrossRef]
- Biemelt, S.; Keetman, U.; Albrecht, G. Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings. Plant Physiol. 1998, 116, 651–658. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1997, 59, 309–314. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]




| Treatment | Note | Acid | Body | Sweetness | Finish | Defect | Type |
|---|---|---|---|---|---|---|---|
| EC | 83.50 | 5.89 | 6.22 | 6.11 | 5.78 | 104.83 a | 5.57 |
| T1 | 85.33 | 6.89 | 7.11 | 7.44 | 7.11 | 99.17 ab | 5.67 |
| T2 | 84.56 | 6.44 | 6.67 | 6.56 | 6.33 | 59.50 b | 4.67 |
| T3 | 83.11 | 5.89 | 5.89 | 5.89 | 5.44 | 87.83 ab | 5.67 |
| T4 | 81.50 | 5.33 | 5.78 | 5.44 | 4.56 | 102.00 a | 6.00 |
| T5 | 85.22 | 6.78 | 7.22 | 7.22 | 6.67 | 85.00 ab | 5.67 |
| T6 | 83.33 | 6.11 | 6.33 | 6.22 | 5.67 | 87.83 ab | 6.00 |
| T7 | 83.50 | 6.00 | 6.22 | 6.22 | 5.56 | 70.83 ab | 5.00 |
| T8 | 83.11 | 5.89 | 5.89 | 5.44 | 5.44 | 76.50 ab | 5.33 |
| T9 | 83.11 | 5.89 | 6.00 | 5.78 | 5.33 | 99.17 ab | 5.67 |
| Treatments | Composition |
|---|---|
| EC | Experimental control |
| T1 | Bioregulator 3 L ha−1 |
| T2 | Bioregulator 6 L ha−1 |
| T3 | Bioregulator 3 L ha−1 + Fungi |
| T4 | Bioregulator 6 L ha−1 + Fungi |
| T5 | Insecticides |
| T6 | Bioregulator 3 L ha−1 + Insecticides |
| T7 | Bioregulator 6 L ha−1 + Insecticides |
| T8 | Insecticides + Fungi |
| T9 | Fungi + Nanobioproduct |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, L.F.; Antunes, A.P.A.; Moreira, M.M.; Arantes, É.H.; Souza, E.G.; Souza, B.H.S.; Cardoso e Bufalo, T.; Freitas, C.G.; Dambroz, C.; Dória, J. Combination of Nanobioproduct and Chemical Ethylene Synthesis Inhibitor with Entomopathogenic Fungi: A Novel Management Strategy for Coffee Berry Borer in Arabica Coffee. Plants 2025, 14, 1495. https://doi.org/10.3390/plants14101495
Sousa LF, Antunes APA, Moreira MM, Arantes ÉH, Souza EG, Souza BHS, Cardoso e Bufalo T, Freitas CG, Dambroz C, Dória J. Combination of Nanobioproduct and Chemical Ethylene Synthesis Inhibitor with Entomopathogenic Fungi: A Novel Management Strategy for Coffee Berry Borer in Arabica Coffee. Plants. 2025; 14(10):1495. https://doi.org/10.3390/plants14101495
Chicago/Turabian StyleSousa, Lilian F., Ana P. A. Antunes, Maísa M. Moreira, Érika H. Arantes, Ezequiel G. Souza, Bruno H. S. Souza, Tatiana Cardoso e Bufalo, Camila G. Freitas, Caroline Dambroz, and Joyce Dória. 2025. "Combination of Nanobioproduct and Chemical Ethylene Synthesis Inhibitor with Entomopathogenic Fungi: A Novel Management Strategy for Coffee Berry Borer in Arabica Coffee" Plants 14, no. 10: 1495. https://doi.org/10.3390/plants14101495
APA StyleSousa, L. F., Antunes, A. P. A., Moreira, M. M., Arantes, É. H., Souza, E. G., Souza, B. H. S., Cardoso e Bufalo, T., Freitas, C. G., Dambroz, C., & Dória, J. (2025). Combination of Nanobioproduct and Chemical Ethylene Synthesis Inhibitor with Entomopathogenic Fungi: A Novel Management Strategy for Coffee Berry Borer in Arabica Coffee. Plants, 14(10), 1495. https://doi.org/10.3390/plants14101495










