Emergent Plants Improve Nitrogen Uptake Rates by Regulating the Activity of Nitrogen Assimilation Enzymes
Abstract
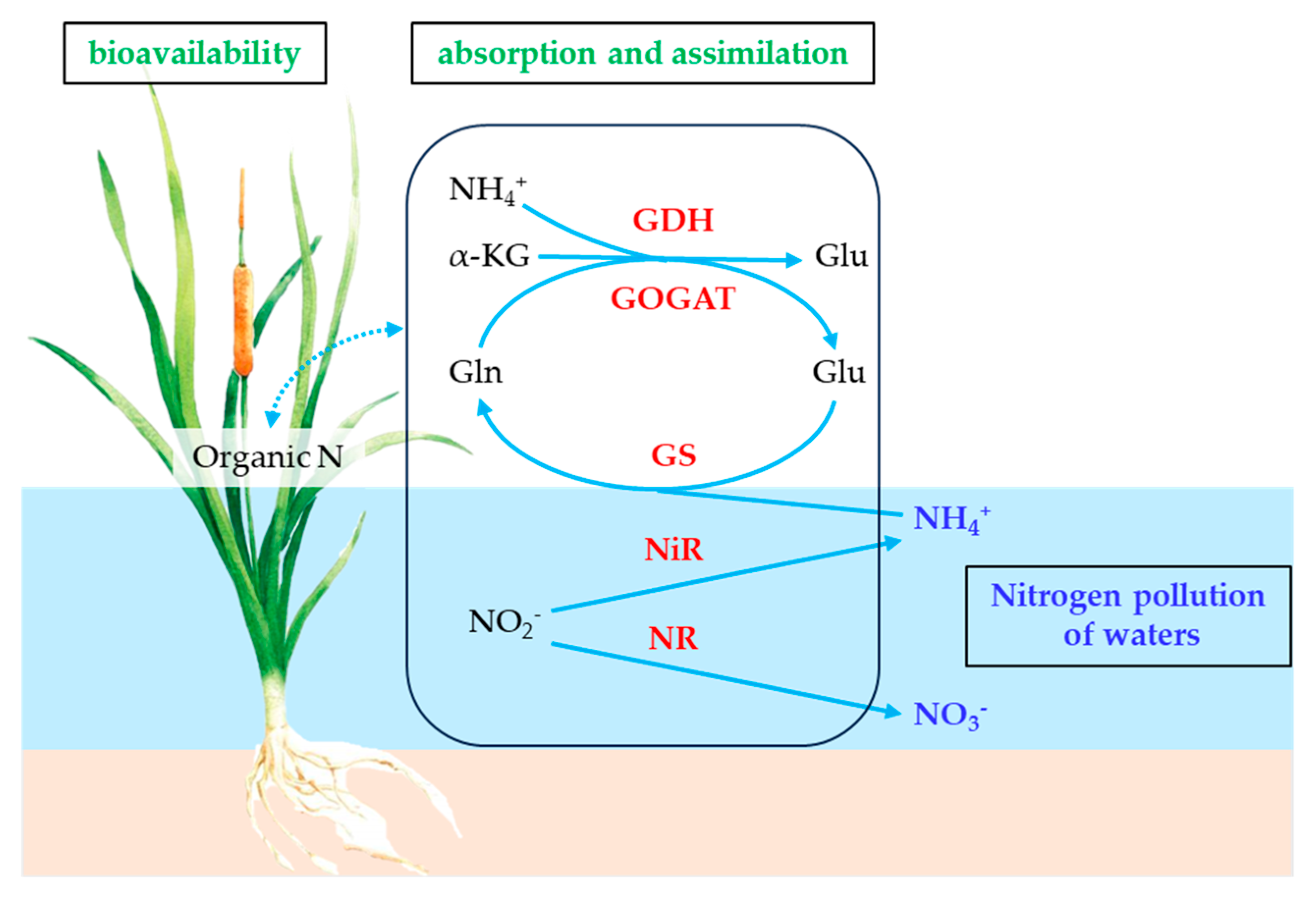
1. Introduction
2. Results
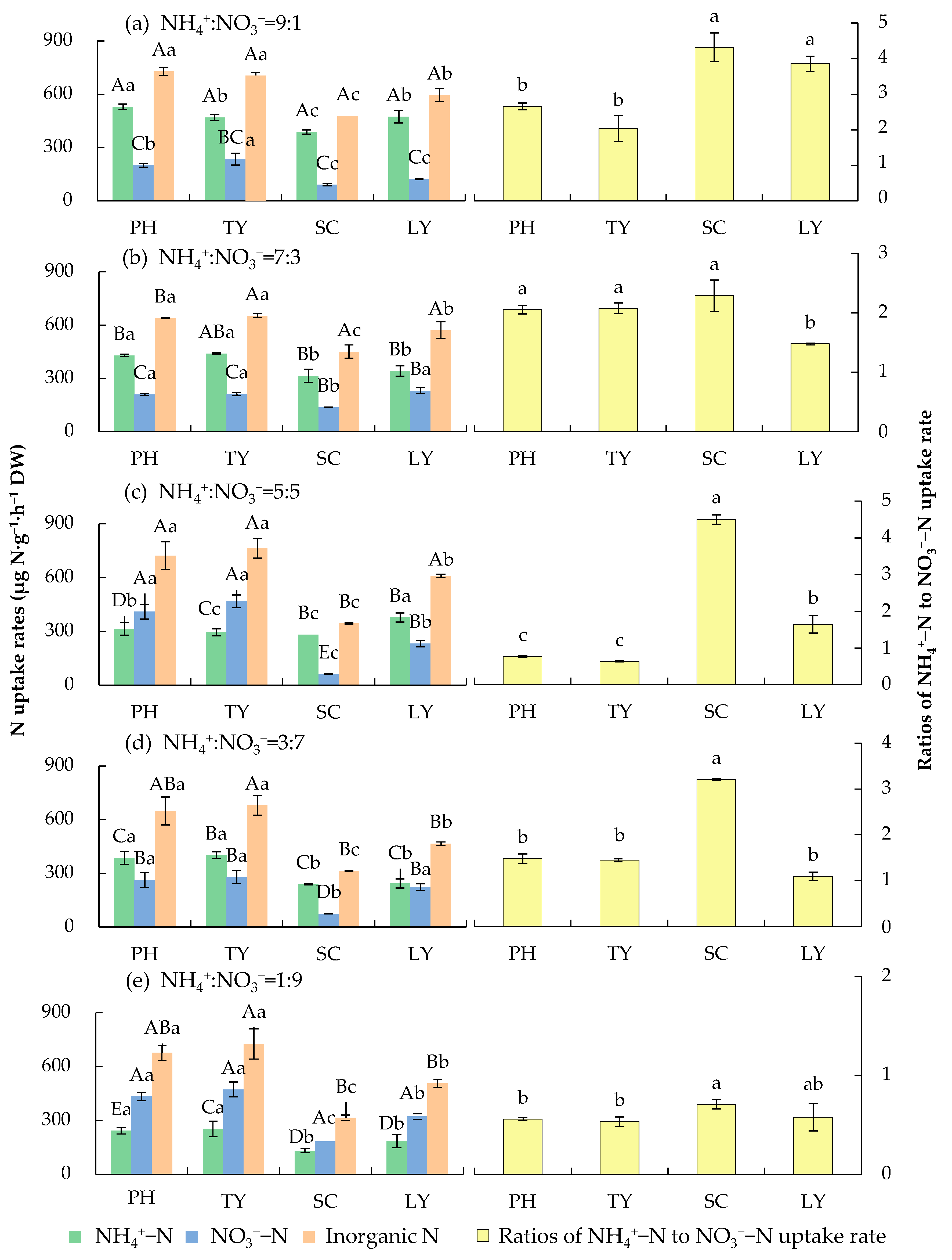
2.1. Nitrogen Uptake Rates of Emergent Plants in Water with Different NH4+/NO3− Ratios
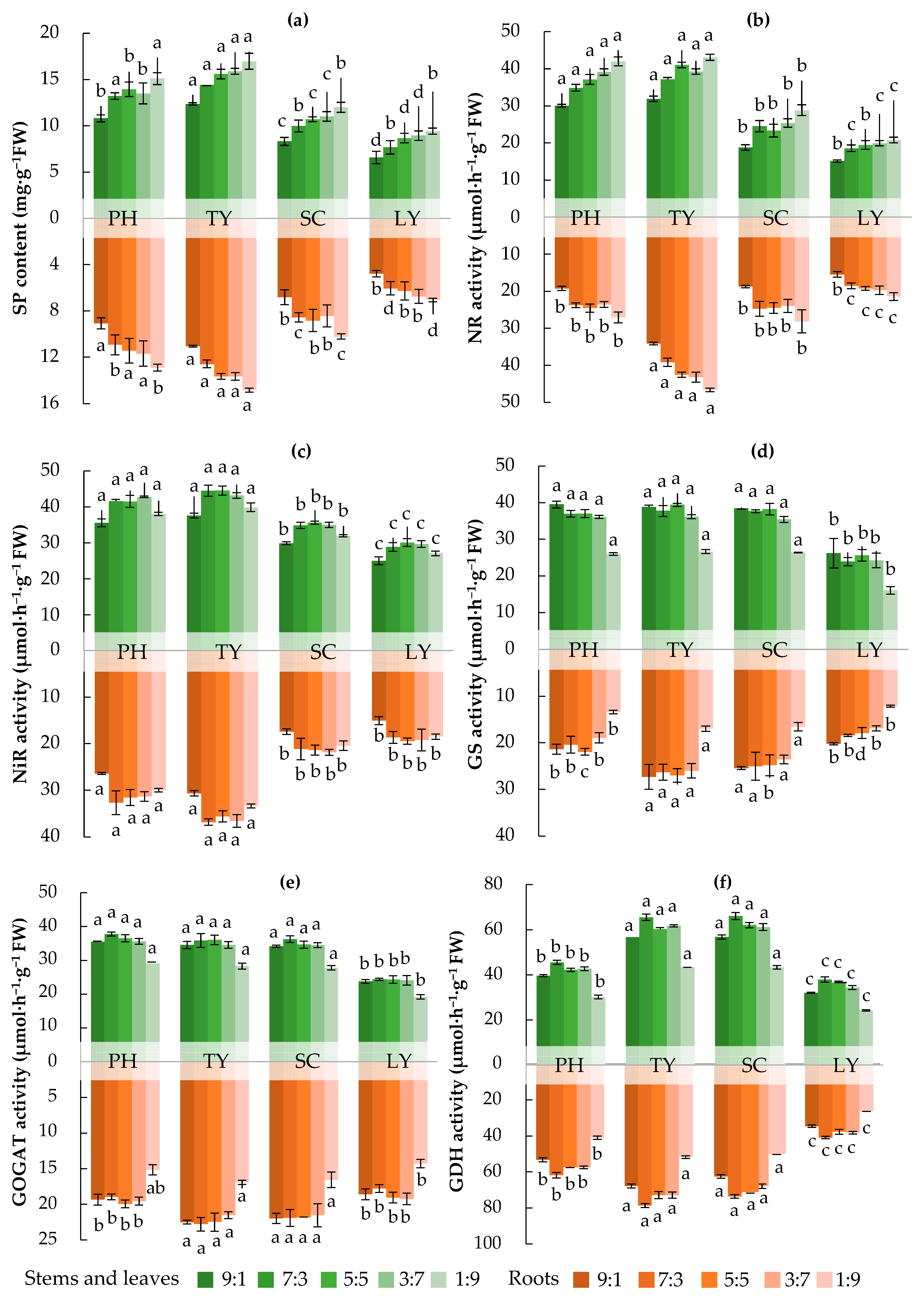
2.2. Nitrogen Assimilation Enzyme Activities of Emergent Plants in Water with Different NH4+/NO3− Ratios
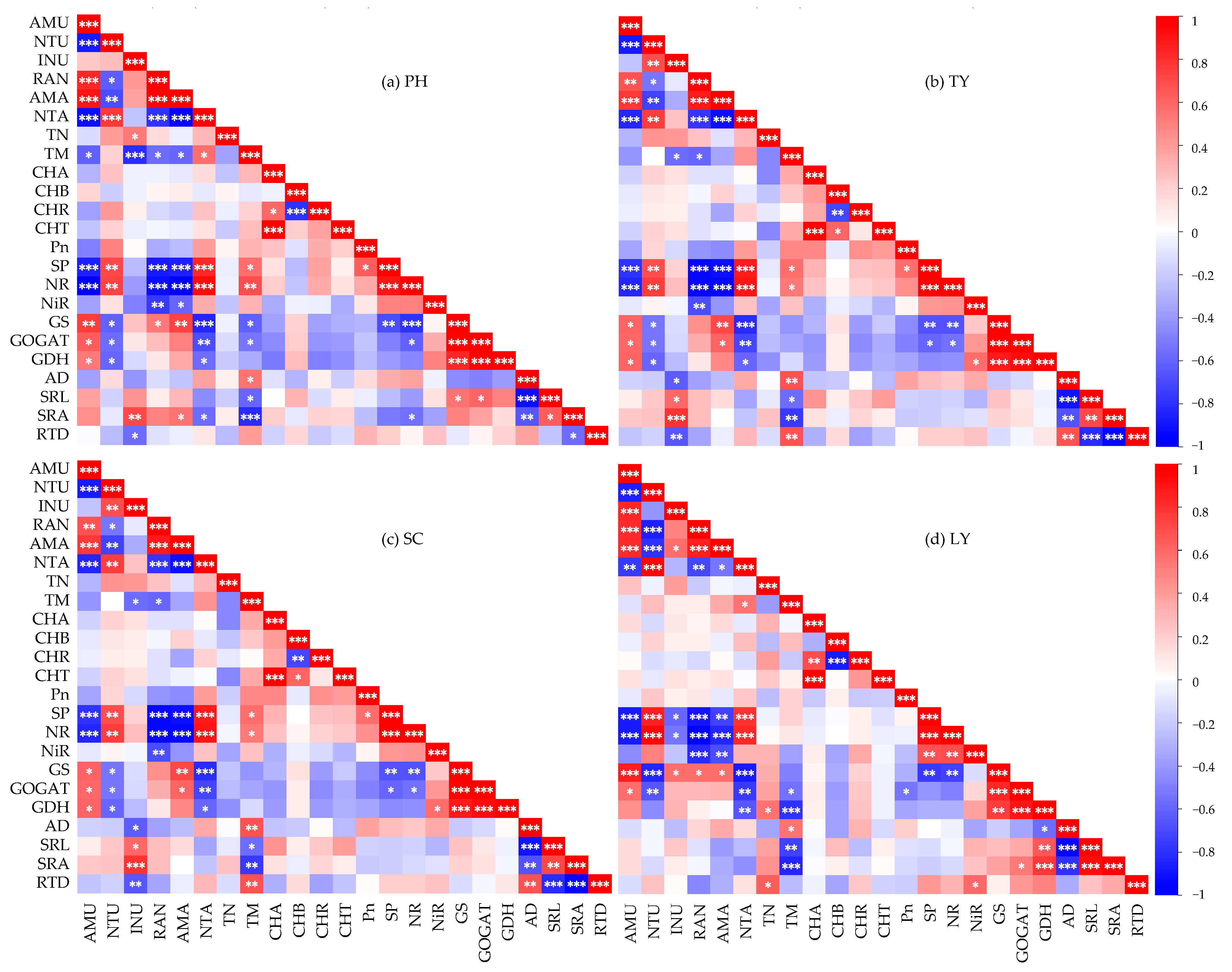
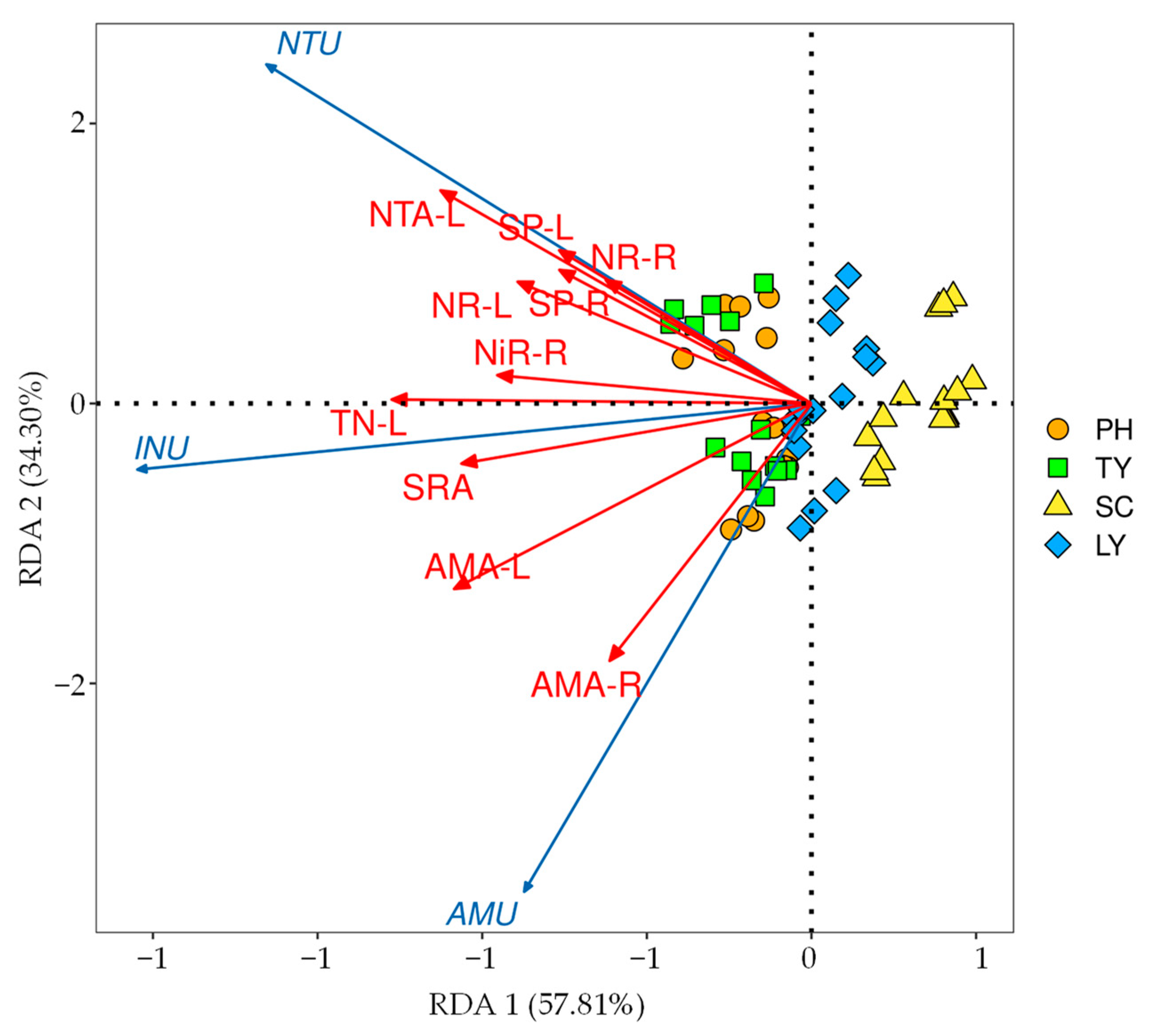
2.3. Influencing Factors on Nitrogen Uptake Rates of Different Emergent Plants
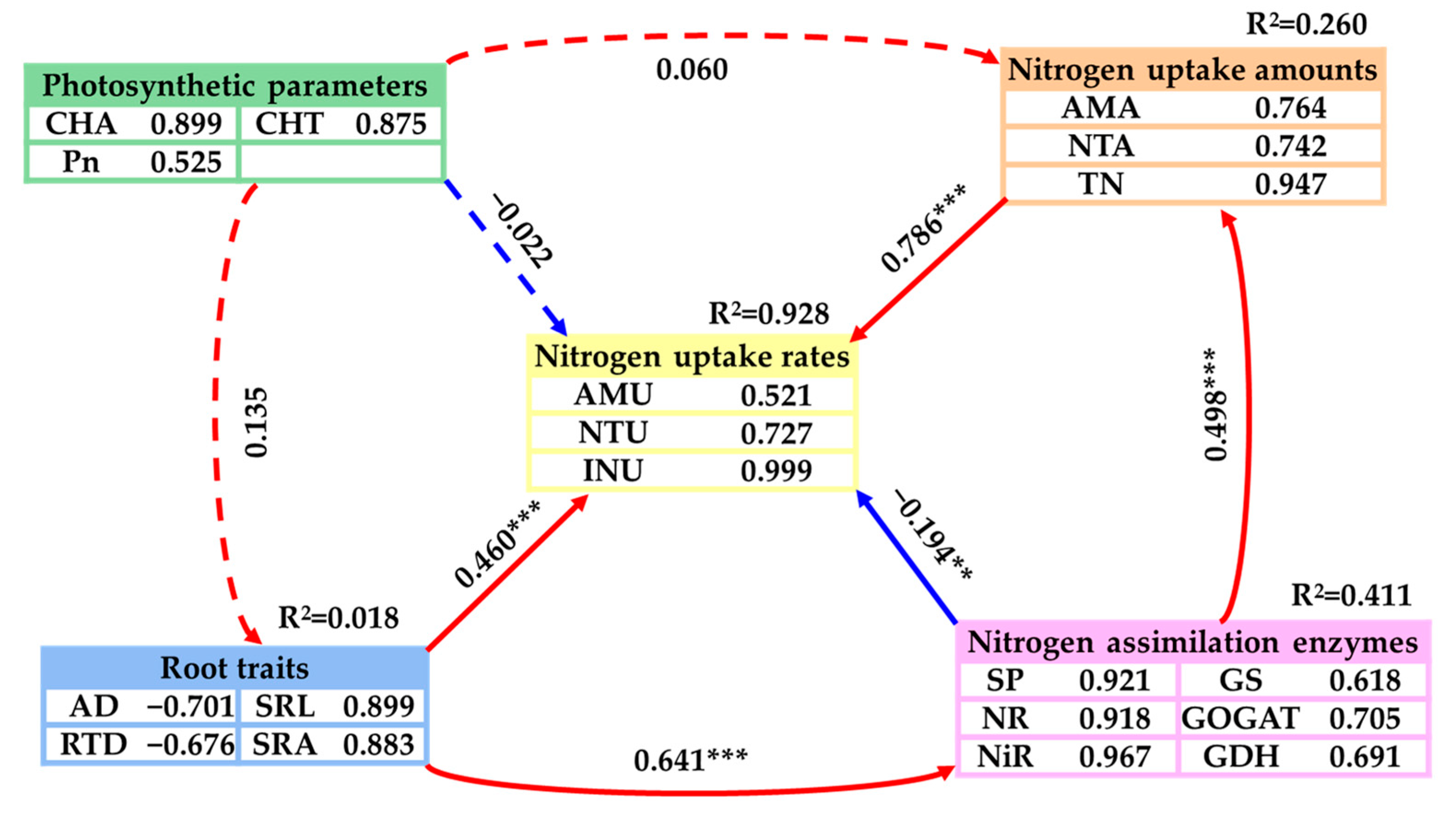
2.4. The Main Driving Factors of the Nitrogen Uptake Rates of Emergent Plants
3. Discussion
3.1. Effects of Different Forms of Nitrogen on Nitrogen Uptake Rate of Emergent Plants
- (1)
- Plants require different levels of energy for the absorption and assimilation of various N forms, and the associated metabolic pathways differ accordingly. NH4+ is directly involved in amino acid synthesis and requires less energy, thus eliminating the additional energy expenditure that is associated with NO3− reduction [22]. While the absorption and assimilation of NO3− demand more energy, its low toxicity reduces the metabolic burden on plants [35]. Plants primarily rely on ammonium transporters for NH4+ uptake, whereas nitrate transporters are essential for NO3− uptake. Consequently, the predominant N form in the water may enhance the uptake of that specific N form by modulating the expression levels of the corresponding transporters [36].
- (2)
- Changes in environmental factors directly or indirectly influence plants’ physiological activities, thereby modulating their N uptake rates. For example, high temperatures significantly reduce the relative growth rate, N uptake rate, NR activity, and photosynthetic parameters of aquatic plants [37]. Factors such as varying light intensities, nutrient concentrations, and hydraulic loads also influence the absorption efficiency of plants [38].
- (3)
- To adapt to varying environmental conditions, aquatic plants modulate their N uptake, assimilation, and transformation via their growth traits (e.g., the root structure and photosynthetic capacity) and interactions with microorganisms. This not only meets their growth demands, but also significantly decreases the N concentrations in water bodies, thereby reflecting a certain degree of environmental adaptability [39,40]. Studies have shown that aquatic plants can increase their direct N uptake and induce changes in N cycling and their microenvironment by altering their root morphology, growth characteristics, leaf biomass, and rhizosphere conditions, thus reducing N pollution in riparian waters [41]. The N assimilation efficiency of aquatic plants is closely linked to their root-to-shoot ratio. Aquatic plants indirectly affect N removal by microorganisms through modifying tailwater quality parameters and can also mediate N transformation by modulating bacterial community structures [42]. In addition, as the N load in sewage increases, plants’ N preference shifts from NH4+-N to NO3−-N. The elevated NR activity that is detected in downstream river plants provides evidence for their enhanced nitrate assimilation capacity and preference [17].
3.2. Effects of Different Forms of Nitrogen on Nitrogen Assimilation Enzyme Activity in Emergent Plants
- (1)
- NR is a key enzyme involved in the assimilation of NO3− by plants, and its activity is influenced by the form of N. Under varying N supply conditions, plants from different ecological groups exhibit significantly different NR activities [44]. When NH4+-N is supplied, the NR activity in plant roots remains low. In contrast, when NO3−-N is supplied, the NR activity in plant roots is significantly higher. This may be attributed to NH4+ competing with NO3− for absorption sites, thereby influencing the uptake of NO3− and the associated NR activity of plants [45]. In addition, NO3− can be assimilated in the roots and subsequently transported to the aerial parts of the plant for further assimilation. This process facilitates the rational and efficient utilization of the carbon skeleton across the entire system. Notably, NR acts as a rate-limiting enzyme, and its activity plays a critical role in regulating N metabolism and protein synthesis [46]. In this study, an increase in the NH4+/NO3− ratio was found to be associated with decreased NR activities and reduced SP contents in the four emergent plants, thereby providing further support for the findings of this study.
- (2)
- GS plays a crucial role in plants’ N metabolism, primarily by mediating the assimilation of NH4+ [47]. Elevated NH4+ levels stimulate GS activity, leading to more efficient conversion of NH4+ into organic N compounds [11]. Studies have demonstrated that plants exhibit significant growth advantages and competitiveness under conditions with a high NH4+/NO3− ratio. This can be attributed to their efficient uptake of NH4+ and the increased activity of enzymes such as GS [4]. In addition, the genes that are most significantly influenced by NO3− and NH4+ treatment were those that are involved in glutamine metabolism, further supporting the link between changes in GS activity and N assimilation in plants [48]. In this study, an increase in the NH4+/NO3− ratio was correlated with enhanced GS activities in the four emergent plants.
- (3)
- GDH plays a crucial role in NH4+ assimilation and glutamate synthesis in plants, thereby modulating the balance of N metabolism and, in turn, significantly contributing to the plants’ growth, development, and stress adaptation [12]. As the NH4+/NO3− ratio increases, plants may mitigate the adverse effects of NH4+ by enhancing their GDH activity; however, when this ratio becomes excessively high, the GDH activity may decline due to impaired energy supply and a disrupted metabolic balance [11].
3.3. Factors Influencing Nitrogen Uptake Rate of Different Emergent Plants
3.4. The Main Drivers of the Nitrogen Uptake Rates of Emergent Plants
4. Materials and Methods
4.1. Experimental Design
4.2. Sampling and Measurements
4.3. Calculations of Various Indicators
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khanna, K.; Kohli, S.K.; Sharma, N.; Kour, J.; Devi, K.; Bhardwaj, T.; Dhiman, S.; Singh, A.D.; Sharma, N.; Sharma, A.; et al. Phytomicrobiome communications: Novel implications for stress resistance in plants. Front. Microbiol. 2022, 13, 912701. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, H.; Zang, S. Purification mechanism of emergent aquatic plants on polluted water: A review. J. Environ. Manag. 2025, 375, 124198. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, R.; Cao, Q.; Dong, J.; Li, C.; Quan, Q.; Huang, M.; Liu, J. Differences of the microbial community structures and predicted metabolic potentials in the lake, river, and wetland sediments in dongping lake basin. Environ. Sci. Pollut. Res. 2020, 27, 19661–19677. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Shi, W.; Kronzucker, H.J.; Hou, L.; Yang, H.; Song, Q.; Liu, J.; Shi, J.; Yang, Q.; et al. Coordination of nitrogen uptake and assimilation favours the growth and competitiveness of moso bamboo over native tree species in high-NH4+ environments. J. Plant Physiol. 2021, 266, 153508. [Google Scholar] [CrossRef]
- Li, L.; Cheng, X.; Kong, X.; Jia, P.; Wang, X.; Zhang, L.; Zhang, X.; Zhang, Y.; Zhang, Z.; Zhang, B. Comparative transcriptomic analysis reveals the negative response mechanism of peanut root morphology and nitrate assimilation to nitrogen deficiency. Plants 2023, 12, 732. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen assimilation in plants: Current status and future prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Huangfu, S.; Zhou, F.; Zheng, X.; Zhang, X.; Hu, L. Removal of ammonia nitrogen from black and odorous water by macrophytes based on laboratory microcosm experiments. RSC Adv. 2023, 13, 3173–3180. [Google Scholar] [CrossRef]
- Sahay, S.; Robledo-Arratia, L.; Glowacka, K.; Gupta, M. Root NRT, NiR, AMT, GS, GOGAT and GDH expression levels reveal NO and ABA mediated drought tolerance in Brassica juncea L. Sci. Rep. 2021, 11, 7992. [Google Scholar] [CrossRef]
- Gu, J.J.; Zhao, H.-W.; Yan, J.; Hu, B.W.; Wang, Z.Q.; Qu, Z.J.; Yu, F.L. Effect of salt stress on nitrogen assimilation of functional leaves and root system of rice in cold region. J. Northeast. Agric. Univ. 2020, 27, 9–16. [Google Scholar]
- Lv, X.; Wang, X.; Pan, J.; Deng, W.; Li, Y. Role of nitrate reductase and nitrite reductase in NaCl tolerance in eelgrass (Zostera marina L.). Ecol. Chem. Eng. S 2022, 29, 111–125. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Xie, J.; Gan, Y.; Coulter, J.A.; Yu, J.; Li, J.; Wang, J.; Zhang, X. Nitrogen source affects the composition of metabolites in pepper (Capsicum annuum L.) And regulates the synthesis of capsaicinoids through the GOGAT–GS pathway. Foods 2020, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Zhang, Y.; Cao, Y.; Wan, T.; Gong, Y.; Dai, C.; Ochieng, W.A.; Nasimiyu, A.T.; Li, W.; Liu, F. Glutamate dehydrogenase plays an important role in ammonium detoxification by submerged macrophytes. Sci. Total Environ. 2020, 722, 137859. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Lu, Y.; Zhao, Y.; Wang, Y.; Han, Z.; Han, Y.; Zhang, J. Analysis of relative expression of key enzyme genes and enzyme activity in nitrogen metabolic pathway of two genotypes of potato (Solanum tuberosum L.) Under different nitrogen supply levels. Horticulturae 2022, 8, 769. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, K.; Xie, J.; Liu, J.; Qiao, Z.; Tan, P.; Peng, F. Ammonium-nitrate mixtures dominated by NH4+-N promote the growth of pecan (Carya illinoinensis) through enhanced n uptake and assimilation. Front. Plant Sci. 2023, 14, 1186818. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Chen, J.; Shang, S.; Zhu, M.; Liang, S.; Zang, Y. Comparative studies on the response of Zostera marina leaves and roots to ammonium stress and effects on nitrogen metabolism. Aquat. Toxicol. 2021, 240, 105965. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Growth, quality, and nitrogen assimilation in response to high ammonium or nitrate supply in cabbage (Brassica campestris L.) And lettuce (Lactuca sativa L.). Agronomy 2021, 11, 2556. [Google Scholar] [CrossRef]
- Guo, H.R.; Wu, Y.; Hu, C.C.; Liu, X.Y. Elevated nitrate preference over ammonium in aquatic plants by nitrogen loadings in a city river. J. Geophys. Res. Biogeosciences 2022, 127, e2021JG006614. [Google Scholar] [CrossRef]
- Zhuang, W.; Wang, M.; Xiao, Y.; Zhou, X.; Wu, N. Differential uptake of nitrogen forms by two herbs in the gurbantunggut desert, central asia. Plant Biol. 2022, 24, 758–765. [Google Scholar] [CrossRef]
- Czaban, W.; Rasmussen, J. Co-occurrence of organic and inorganic n influences asparagine uptake and amino acid profiles in white clover. J. Plant Nutr. Soil Sci. 2021, 184, 162–172. [Google Scholar] [CrossRef]
- Du, Y.; Niu, S.; Jiang, H.; Luo, B.; Wang, Q.; Han, W.; Liu, Y.; Chang, J.; Ge, Y. Comparing the effects of plant diversity on the nitrogen removal and stability in floating and sand-based constructed wetlands under ammonium/nitrate ratio disturbance. Environ. Sci. Pollut. Res. 2021, 28, 69354–69366. [Google Scholar] [CrossRef]
- Meier, M.; Liu, Y.; Lay-Pruitt, K.S.; Takahashi, H.; von Wirén, N. Auxin-mediated root branching is determined by the form of available nitrogen. Nat. Plants 2020, 6, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Liu, M.; Zhang, Q.; Ma, L.; Shi, Y.; Ruan, J. Preferential assimilation of NH4+ over NO3− in tea plant associated with genes involved in nitrogen transportation, utilization and catechins biosynthesis. Plant Sci. 2020, 291, 110369. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Sheng, M.; Wang, L.; Guo, C.; Zhang, S. Effects of short-term n addition on fine root morphological features and nutrient stoichiometric characteristics of Zanthoxylum bungeanum and Medicago sativa seedlings in southwest China karst area. J. Soil Sci. Plant Nutr. 2022, 22, 1805–1817. [Google Scholar] [CrossRef]
- Sun, J.; Jin, L.; Li, R.; Meng, X.; Jin, N.; Wang, S.; Xu, Z.; Liu, Z.; Lyu, J.; Yu, J. Effects of different forms and proportions of nitrogen on the growth, photosynthetic characteristics, and carbon and nitrogen metabolism in tomato. Plants 2023, 12, 4175. [Google Scholar] [CrossRef]
- Aryal, K.; Maraseni, T.; Apan, A. How much do we know about trade-offs in ecosystem services? A systematic review of empirical research observations. Sci. Total Environ. 2022, 806, 151229. [Google Scholar] [CrossRef]
- Zhang, T.; Lei, Q.; Liang, X.; Lindsey, S.; Luo, J.; Pei, W.; Du, X.; Wu, S.; An, M.; Qiu, W.; et al. Optimization of the n footprint model and analysis of nitrogen pollution in irrigation areas: A case study of Ningxia Hui Autonomous Region, China. J. Environ. Manag. 2023, 340, 118002. [Google Scholar] [CrossRef]
- Moore, M.T.; Locke, M.A. Experimental evidence for using vegetated ditches for mitigation of complex contaminant mixtures in agricultural runoff. Water Air Soil Pollut. 2020, 231, 140. [Google Scholar] [CrossRef]
- Pei, W.; Yan, T.; Lei, Q.; Zhang, T.; Fan, B.; Du, X.; Luo, J.; Lindsey, S.; Liu, H. Spatio-temporal variation of net anthropogenic nitrogen inputs (NANI) from 1991 to 2019 and its impacts analysis from parameters in northwest China. J. Environ. Manag. 2022, 321, 115996. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, J.; Liu, L.; Li, Y.; Li, X. A source-sink landscape approach to mitigation of agricultural non-point source pollution: Validation and application. Environ. Pollut. 2022, 314, 120287. [Google Scholar] [CrossRef]
- Lin, X.; Wu, X.; Gao, Z.; Ge, X.; Xiong, J.; Tan, L.; Wei, H. The effects of water depth on the growth of two emergent plants in an in-situ experiment. Sustainability 2022, 14, 11309. [Google Scholar] [CrossRef]
- Qin, J.; Sun, Y.; Qiu, X.; Liu, H.; Zhang, E.; Mao, X. Distribution pattern simulation of multiple emergent plants in river riparian zones. River Res. Appl. 2021, 37, 1180–1190. [Google Scholar] [CrossRef]
- Fang, J.; Dong, J.; Li, C.; Chen, H.; Wang, L.; Lyu, T.; He, H.; Liu, J. Response of microbial community composition and function to emergent plant rhizosphere of a constructed wetland in northern china. Appl. Soil Ecol. 2021, 168, 104141. [Google Scholar] [CrossRef]
- Liu, M.; Xu, X.; Wanek, W.; Sun, J.; Bardgett, R.D.; Tian, Y.; Cui, X.; Jiang, L.; Ma, Z.; Kuzyakov, Y.; et al. Nitrogen availability in soil controls uptake of different nitrogen forms by plants. New Phytol. 2025, 245, 1450–1467. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Gilhooly, W.P.; Jacinthe, P. Nitrogen preference across generations under changing ammonium nitrate ratios. J. Plant Ecol. 2019, 12, 235–244. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Z.; Sun, H.; Liu, X.; Xie, J.; Qiu, Y.; Chai, T.; Chu, C.; Hu, B. Nitrate confers rice adaptation to high ammonium by suppressing its uptake but promoting its assimilation. Mol. Plant 2023, 16, 1871–1874. [Google Scholar] [CrossRef]
- Qian, J.; Jin, W.; Hu, J.; Wang, P.; Wang, C.; Lu, B.; Li, K.; He, X.; Tang, S. Stable isotope analyses of nitrogen source and preference for ammonium versus nitrate of riparian plants during the plant growing season in Taihu lake basin. Sci. Total Environ. 2021, 763, 143029. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, B.; Qian, Z.; Xu, Y.; Liao, X.; Song, P.; Li, X. Effects of increased carbon supply on the growth, nitrogen metabolism and photosynthesis of Vallisneria natans grown at different temperatures. Wetlands 2020, 40, 1459–1467. [Google Scholar] [CrossRef]
- Wang, K.; Hu, Q.; Wei, Y.; Yin, H.; Sun, C.; Liu, G. Uptake kinetics of NH4+, NO3− and H2PO4− by Typha orientalis, Acorus calamus L., Lythrum salicaria L., Sagittaria trifolia L. and Alisma plantago-aquatica Linn. Sustainability 2021, 13, 434. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Q.; Hu, Y.; Zhao, Q.; Li, Q.; Wu, J. Influence of sediment, plants, and microorganisms on nitrogen removal in farmland drainage ditches. Agronomy 2023, 13, 2211. [Google Scholar] [CrossRef]
- Manolaki, P.; Mouridsen, M.B.; Nielsen, E.; Olesen, A.; Jensen, S.M.; Lauridsen, T.L.; Baattrup-Pedersen, A.; Sorrell, B.K.; Riis, T. A comparison of nutrient uptake efficiency and growth rate between different macrophyte growth forms. J. Environ. Manag. 2020, 274, 111181. [Google Scholar] [CrossRef]
- Lu, B.; Qian, J.; Hu, J.; Wang, P.; Jin, W.; Tang, S.; He, Y.; Zhang, C. The role of fine root morphology in nitrogen uptake by riparian plants. Plant Soil 2022, 472, 527–542. [Google Scholar] [CrossRef]
- Wu, H.; Hao, B.; You, Y.; Zou, C.; Cai, X.; Li, J.; Qin, H. Aquatic macrophytes mitigate the conflict between nitrogen removal and nitrous oxide emissions during tailwater treatments. J. Environ. Manag. 2024, 370, 122671. [Google Scholar] [CrossRef]
- Chen, H.; Xiang, Y.; Yao, Z.; Zhang, Q.; Li, H.; Cheng, M. Stability of C:N:P stoichiometry in the plant–soil continuum along age classes in natural pinus tabuliformis carr. Forests of the eastern loess plateau, China. Forests 2023, 14, 44. [Google Scholar] [CrossRef]
- Koyama, L.A.; Terai, M.; Tokuchi, N. Nitrate reductase activities in plants from different ecological and taxonomic groups grown in japan. Ecol. Res. 2020, 35, 708–712. [Google Scholar] [CrossRef]
- Chen, H.; Huang, L. Effect of nitrogen fertilizer application rate on nitrate reductase activity in maize. Appl. Ecol. Environ. Res. 2020, 18, 2879–2894. [Google Scholar] [CrossRef]
- Ali, A. Nitrate assimilation pathway in higher plants: Critical role in nitrogen signalling and utilization. Plant Sci. Today 2020, 7, 182–192. [Google Scholar] [CrossRef]
- Du, J.; Shen, T.; Xiong, Q.; Zhu, C.; Peng, X.; He, X.; Fu, J.; Ouyang, L.; Bian, J.; Hu, L.; et al. Combined proteomics, metabolomics and physiological analyses of rice growth and grain yield with heavy nitrogen application before and after drought. BMC Plant Biol. 2020, 20, 556. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, P.; Yue, C.; Huang, J.; Zhang, Z.; Hua, Y. Transcriptomic dissection of allotetraploid rapeseed (Brassica napus L.) In responses to nitrate and ammonium regimes and functional analysis of BnaA2.gln1;4 in arabidopsis. Plant Cell Physiol. 2022, 63, 755–769. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, B.; Chen, Z.; Xu, K. Grafting enhances the photosynthesis and nitrogen absorption of tomato plants under low-nitrogen stress. J. Plant Growth Regul. 2022, 41, 1714–1725. [Google Scholar] [CrossRef]
- Ma, K.; Zhou, Y.; Ma, Y.; Zhang, T. Increased rice yield by improving the stay-green traits and related physiological metabolism under long-term warming in cool regions. Int. J. Plant Prod. 2024, 18, 175–186. [Google Scholar] [CrossRef]
- Hasan, A.K.; Carrasco-G, F.E.; Carolina Lizana, X.; Calderini, D.F. Low seed rate in square planting arrangement has neutral or positive effect on grain yield and improves grain nitrogen and phosphorus uptake in wheat. Field Crops Res. 2022, 288, 108699. [Google Scholar] [CrossRef]
- Qi, D.; Wu, Q. Physiological characteristics, lint yield, and nitrogen use efficiency of cotton under different nitrogen application rates and waterlogging durations. J. Soil Sci. Plant Nutr. 2023, 23, 5594–5607. [Google Scholar] [CrossRef]
- Wang, M.; Hasegawa, T.; Beier, M.; Hayashi, M.; Ohmori, Y.; Yano, K.; Teramoto, S.; Kamiya, T.; Fujiwara, T. Growth and nitrate reductase activity are impaired in Rice Osnlp4 mutants supplied with nitrate. Plant Cell Physiol. 2021, 62, 1156–1167. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, P.; Tan, L.; Sun, Y.; Zhu, S.; Qiao, F. A review of the influence of habitat on emergent macrophyte growth and its feedback mechanism. Chin. J. Ecol. 2021, 40, 2610. [Google Scholar] [CrossRef]
- Wang, L.; Liao, D.; Rengel, Z.; Shen, J. Soil–plant–microbe interactions in the rhizosphere: Incremental amplification induced by localized fertilization. Front. Agric. Sci. Eng. 2024, 12, 57–68. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; Hartmann, A.; Estevez, J.M.; Bennett, M.J.; von Wirén, N. A spatially concerted epidermal auxin signaling framework steers the root hair foraging response under low nitrogen. Curr. Biol. 2023, 33, 3926–3941.e5. [Google Scholar] [CrossRef]
- Ito, T.; Tanaka-Oda, A.; Masumoto, T.; Akatsuki, M.; Makita, N. Different relationships of fine root traits with root ammonium and nitrate uptake rates in conifer forests. J. For. Res. 2023, 28, 25–32. [Google Scholar] [CrossRef]
- Cun, Z.; Wu, H.M.; Zhang, J.; Shuang, S.; Hong, J.; An, T.; Chen, J.W. High nitrogen inhibits biomass and saponins accumulation in a medicinal plant panax notoginseng. PeerJ 2023, 11, e14933. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, Z.; Jeong, E.; Kim, S.; Seo, J.S.; Um, T.; Shim, J.S. Signaling pathways underlying nitrogen transport and metabolism in plants. BMB Rep. 2023, 56, 56–64. [Google Scholar] [CrossRef]
- Cai, Z.; Li, Q.; Bai, H.; Zhu, C.; Tang, G.; Zhou, H.; Huang, J.; Song, X.; Wang, J. Interactive effects of aquatic nitrogen and plant biomass on nitrous oxide emission from constructed wetlands. Environ. Res. 2022, 213, 113716. [Google Scholar] [CrossRef]
- Qin, W.; Guo, R.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y.; Si, T. Effects of drip irrigation and topdressing on dry matter weight, mineral nutrient absorption and yield of pod-bearing stage in peanut. Acta Agron. Sin. 2020, 47, 520–529. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Z.; Hu, B.; Li, Y.; Teng, A.; Zhong, F. The effects of polypropylene microplastics on the removal of nitrogen and phosphorus from water by Acorus calamus, iris tectorum and functional microorganisms. Chemosphere 2024, 364, 143153. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Cui, Y.; Li, X.; Xiao, K.; Tao, P.; Zhang, Y. Responses of photosynthetic characteristics and enzyme activity of nitrogen metabolism to low nitrogen in maize with different nitrogen tolerance. Int. J. Agric. Biol. Eng. 2020, 13, 133–143. [Google Scholar] [CrossRef]
- Wu, H.; Ding, J. Abiotic and biotic determinants of plant diversity in aquatic communities invaded by water hyacinth [eichhorniacrassipes (mart.) Solms]. Front. Plant Sci. 2020, 11, 555774. [Google Scholar] [CrossRef]
- Hu, S.; He, R.; He, X.; Zeng, J.; Zhao, D. Niche-specific restructuring of bacterial communities associated with submerged macrophyte under ammonium stress. Appl. Environ. Microbiol. 2023, 89, e0071723. [Google Scholar] [CrossRef]
- Wang, R.; Dijkstra, F.A.; Han, X.; Jiang, Y. Root nitrogen reallocation: What makes it matter? Trends Plant Sci. 2024, 29, 1077–1088. [Google Scholar] [CrossRef]
- Shu, X.; Jin, M.; Wang, S.; Xu, X.; Deng, L.; Zhang, Z.; Zhao, X.; Yu, J.; Zhu, Y.; Lu, G.; et al. The effect of nitrogen and potassium interaction on the leaf physiological characteristics, yield, and quality of sweet potato. Agronomy 2024, 14, 2319. [Google Scholar] [CrossRef]
- Jia, X.; Huangfu, C.; Hui, D. Nitrogen uptake by two plants in response to plant competition as regulated by neighbor density. Front. Plant Sci. 2020, 11, 584370. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, M.; Yuan, Y.; Wang, H. Interspecific competition for inorganic nitrogen between canopy trees and underlayer-planted young trees in subtropical pine plantations. For. Ecol. Manag. 2021, 494, 119331. [Google Scholar] [CrossRef]
| Factor | Wald χ2 | Df | p Value | Goodness of Fit | Value | Df | Value/Df |
|---|---|---|---|---|---|---|---|
| Species | 1518.54 | 3 | <0.001 | Deviance | 0.53 | 80 | 0.007 |
| NH4+/NO3− | 91.07 | 4 | <0.001 | Scaled Deviance | 120.09 | 80 | – |
| N form | 609.40 | 1 | <0.001 | Pearson χ2 | 0.52 | 80 | 0.006 |
| Species × NH4+/NO3− | 119.29 | 12 | <0.001 | Scaled Pearson χ2 | 118.07 | 80 | – |
| Species × N form | 174.11 | 3 | <0.001 | Log Likelihood | −511.30 | – | – |
| NH4+/NO3− × N form | 1561.83 | 4 | <0.001 | AIC | 1104.61 | – | – |
| Species × NH4+/NO3− × N form | 392.85 | 12 | <0.001 | AICC | 1148.76 | – | – |
| (Intercept) | 23,275.78 | 1 | <0.001 | BIC | 1218.89 | – | – |
| Omnibus Test (vs. Intercept) | (Likelihood Ratio χ2) 477.45 | 39 | <0.001 | CAIC | 1259.89 | – | – |
| Number | NH4+/NO3− | NH4Cl (mg L−1) | NaNO3 (mg L−1) |
|---|---|---|---|
| 1 | 9:1 | 13.5 | 1.5 |
| 2 | 7:3 | 10.5 | 4.5 |
| 3 | 5:5 | 7.5 | 7.5 |
| 4 | 3:7 | 4.5 | 10.5 |
| 5 | 1:9 | 1.5 | 13.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Liu, R.; Xiang, W.; Lei, P.; Fang, X. Emergent Plants Improve Nitrogen Uptake Rates by Regulating the Activity of Nitrogen Assimilation Enzymes. Plants 2025, 14, 1484. https://doi.org/10.3390/plants14101484
Hong Y, Liu R, Xiang W, Lei P, Fang X. Emergent Plants Improve Nitrogen Uptake Rates by Regulating the Activity of Nitrogen Assimilation Enzymes. Plants. 2025; 14(10):1484. https://doi.org/10.3390/plants14101484
Chicago/Turabian StyleHong, Yu, Ruliang Liu, Wenhua Xiang, Pifeng Lei, and Xi Fang. 2025. "Emergent Plants Improve Nitrogen Uptake Rates by Regulating the Activity of Nitrogen Assimilation Enzymes" Plants 14, no. 10: 1484. https://doi.org/10.3390/plants14101484
APA StyleHong, Y., Liu, R., Xiang, W., Lei, P., & Fang, X. (2025). Emergent Plants Improve Nitrogen Uptake Rates by Regulating the Activity of Nitrogen Assimilation Enzymes. Plants, 14(10), 1484. https://doi.org/10.3390/plants14101484





