Comparative Study on the Effects of Silicon Nanoparticles and Cellulose Nanocrystals on Drought Tolerance in Tall Fescue (Festuca arundinacea Schreb.)
Abstract
1. Introduction
2. Results
2.1. Characteristics of Nanomaterials
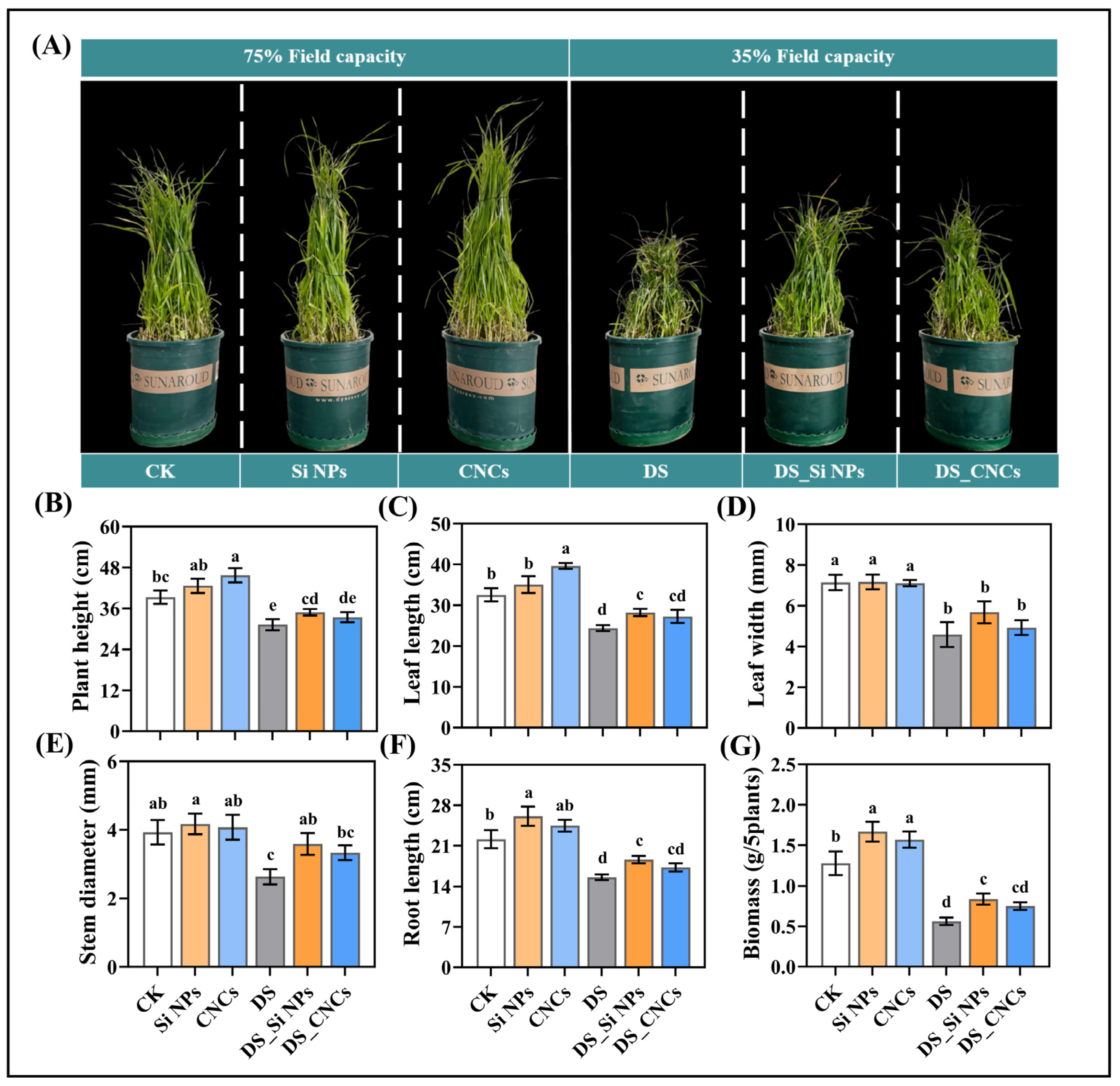
2.2. Effects on Plant Growth
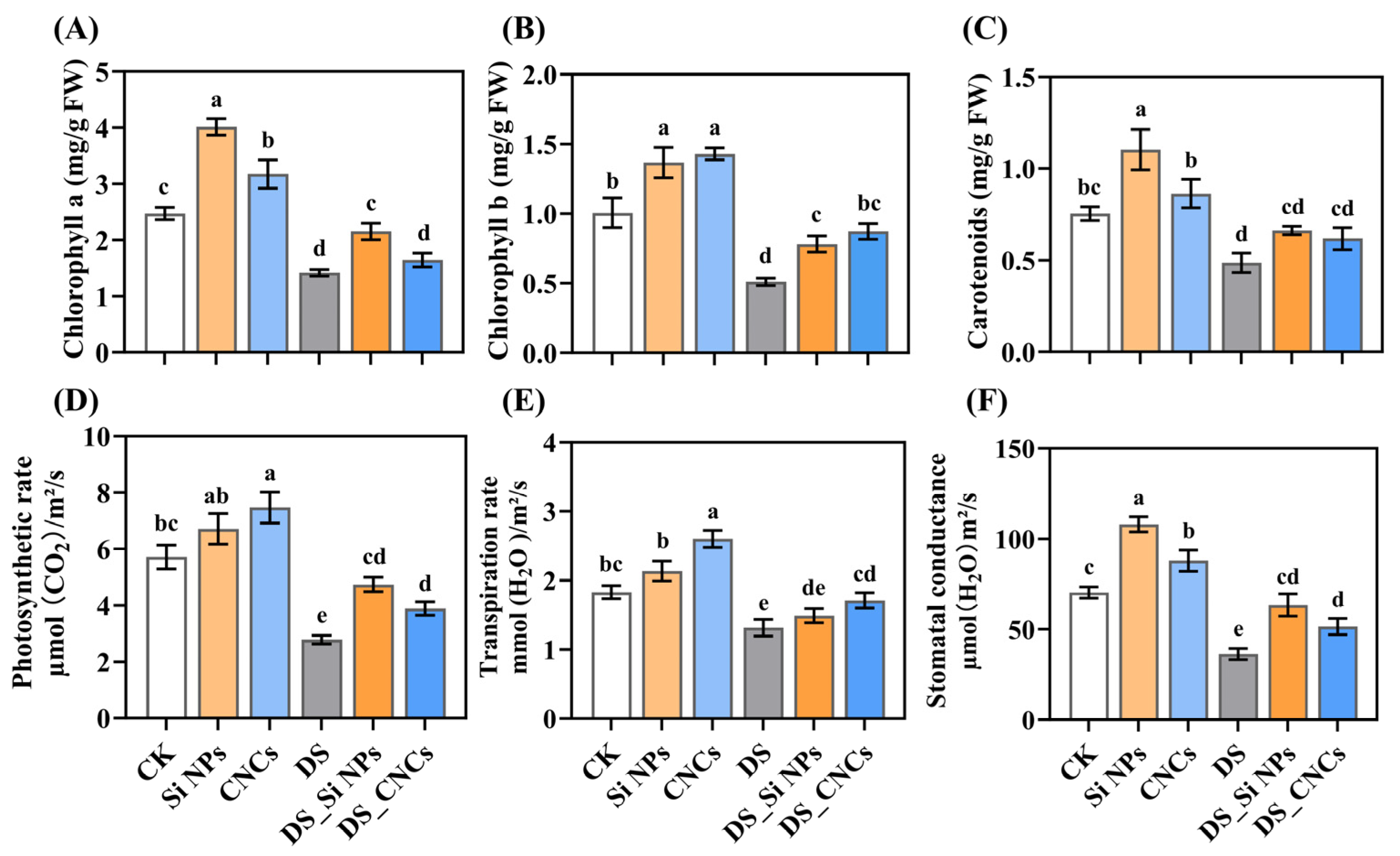
2.3. Effects on Photosynthesis
2.4. Effects on Leaf Anatomical Structure
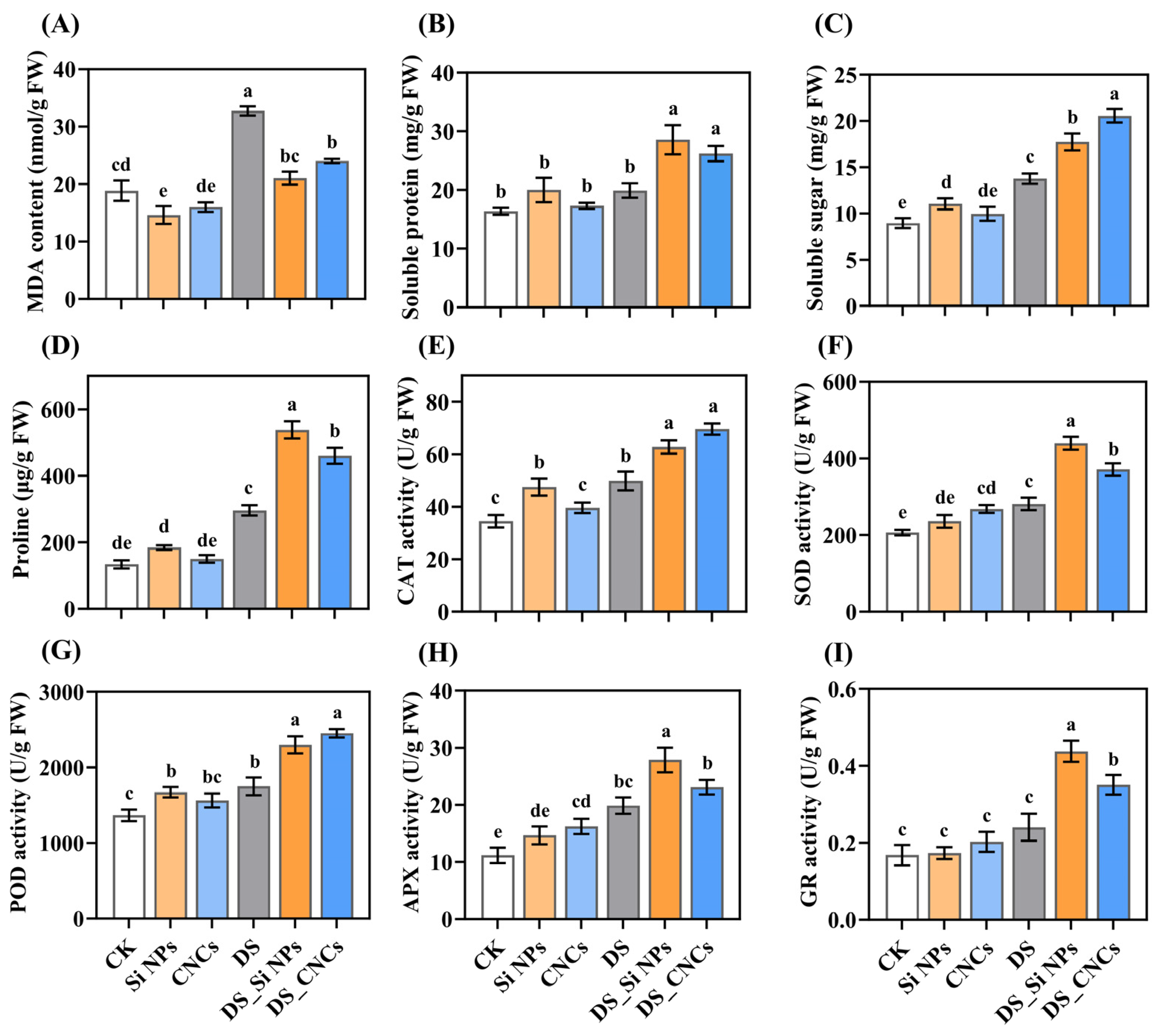
2.5. Effects on MDA, Osmotic Adjustment Substances, and Antioxidant Enzyme Activity
2.6. Effects on Nitrogen, Phosphorus, Potassium, and Silicon Accumulation
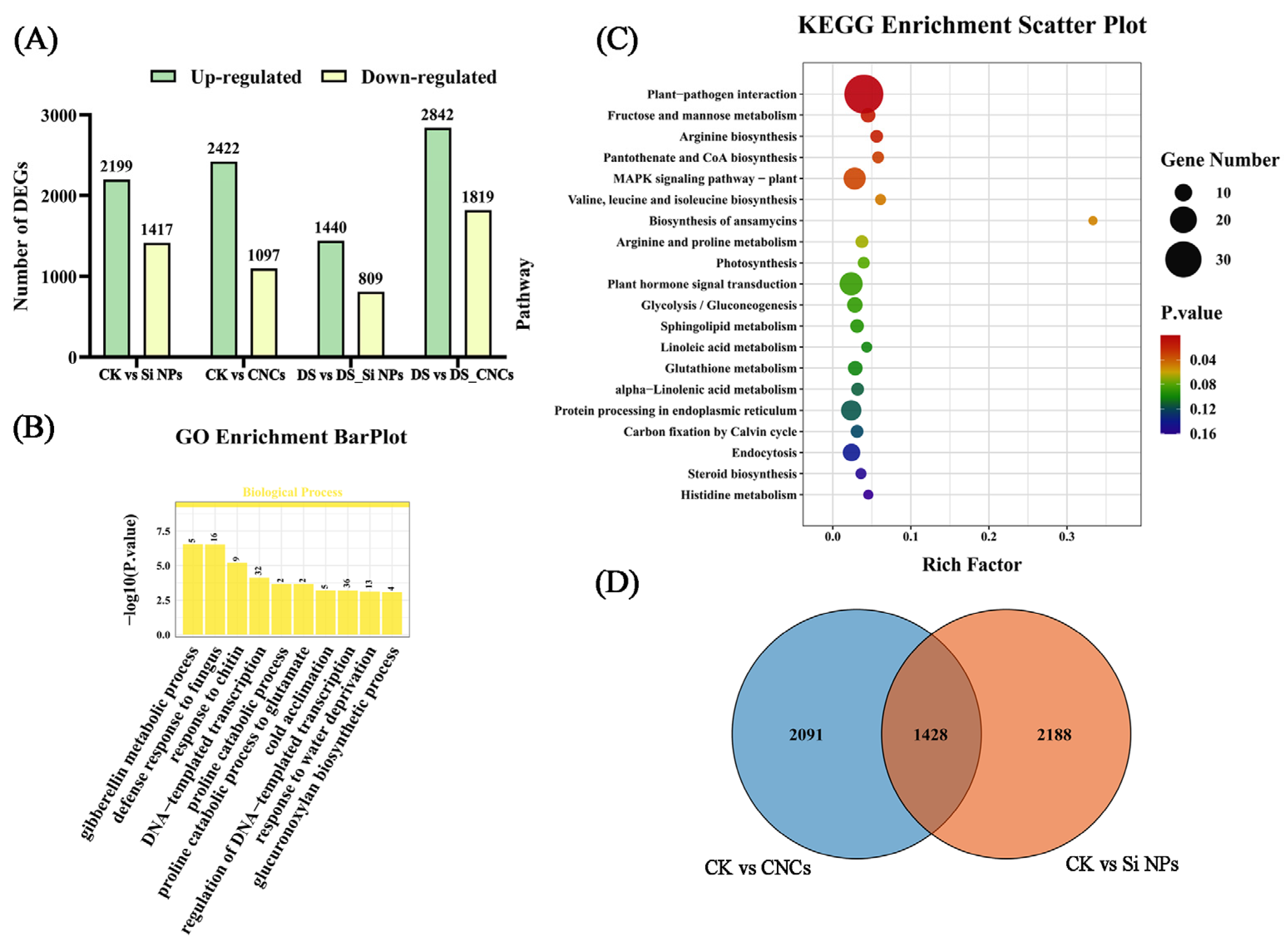
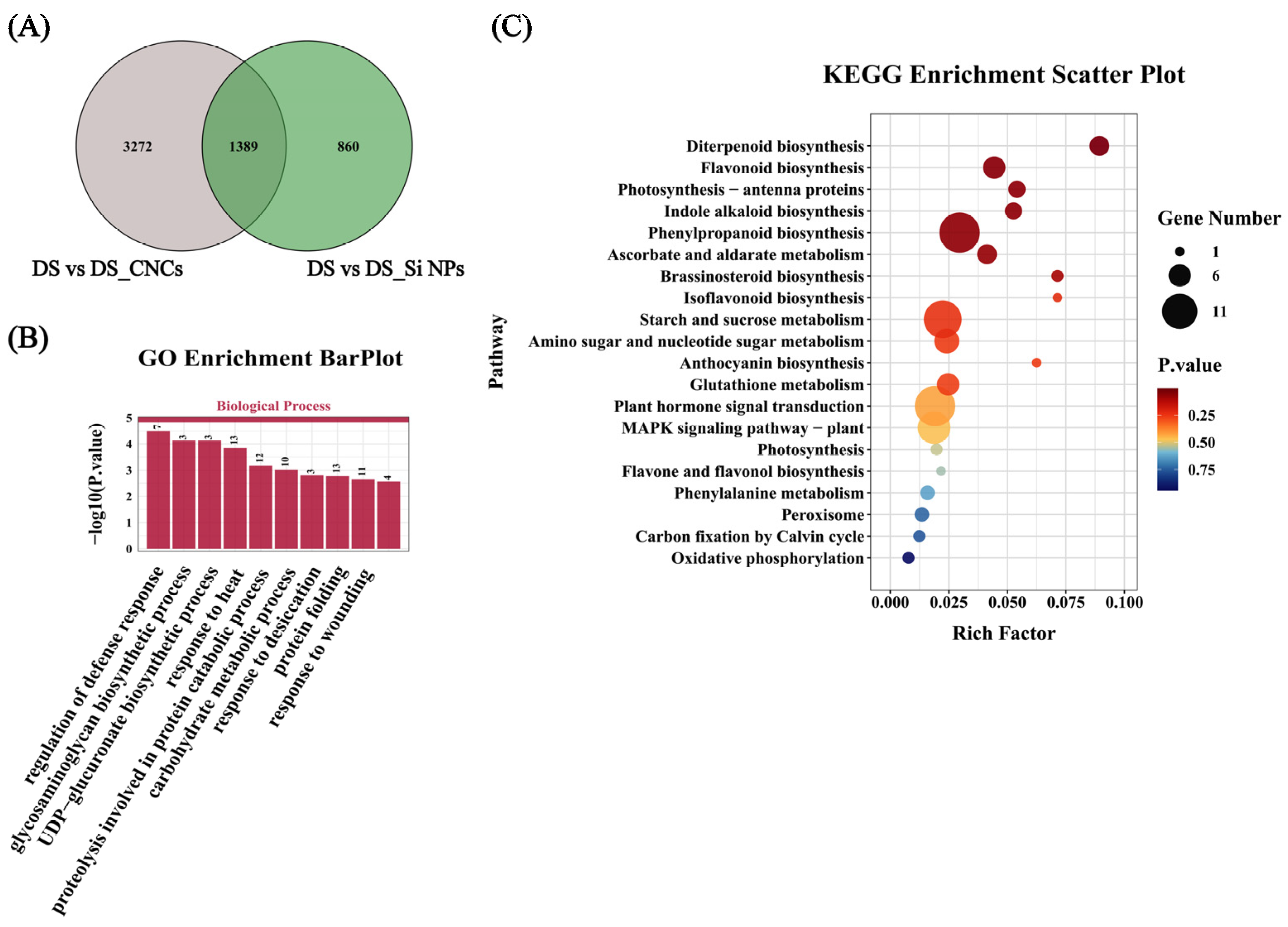
2.7. Transcriptome Analysis of Leaves Under Different Treatments
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Growth Parameters and Biomass Measurement
4.4. Chlorophyll and Gas Exchange Parameter Measurement
4.5. Leaf Anatomical Structure Analysis
4.6. MDA, Osmotic Regulators, and Antioxidant Enzyme Activity Measurement
4.7. Determination of Nitrogen, Phosphorus, Potassium, and Silicon Content
4.8. RNA Extraction, Library Construction, and Sequencing
4.9. Transcriptomic Data Analysis
4.10. Quantitative Real-Time PCR Validation
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Si NPs | Silicon nanoparticles |
| CNCs | Cellulose nanocrystals |
| TEM | Transmission electron microscope |
| FTIR | Fourier-transform infrared spectrometer |
| MDA | Malondialdehyde |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| APX | Ascorbate peroxidase |
| GR | Glutathione reductase |
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| Si | Silicon |
| ROS | Reactive oxygen species |
| DHAR | Dehydroascorbate reductase |
| DDW | Double distilled water |
| FAA | Formalin-acetic acid-alcohol |
| TBA | Thiobarbituric acid |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| XRD | X-ray diffraction |
References
- Andres, P.; Zapater, V.; Pamplona, M. Stabilization of motorway slopes with herbaceous cover, Catalonia, Spain. Restor. Ecol. 1996, 4, 51–60. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, R.R.; Ai, X.Y.; Jiao, C.; Xu, W.N.; Wei, L.; Ai, Y.W. Enzyme activity and microbial biomass availability in artificial soils on rock-cut slopes restored with outside soil spray seeding (OSSS): Influence of topography and season. J. Environ. Manag. 2018, 211, 287–295. [Google Scholar] [CrossRef]
- Baggio, T.; Martini, M.; Bettella, F.; D’Agostino, V. Debris flow and debris flood hazard assessment in mountain catchments. Catena 2024, 245, 108338. [Google Scholar] [CrossRef]
- Faiz, H.; Ng, S.; Rahman, M. A state-of-the-art review on the advancement of sustainable vegetation concrete in slope stability. Constr. Build. Mater. 2022, 326, 126502. [Google Scholar] [CrossRef]
- Lai, H.Q.; Du, J.X.; Zhou, C.Y.; Liu, Z. Experimental study on ecological performance improvement of sprayed planting concrete based on the addition of polymer composite material. Int. J. Environ. Res. Public Health 2022, 19, 12121. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Lin, C.; Ma, X.F.; Song, Z.Z.; Chen, Z.H.; Jiang, C.H.; Qi, C.Q. Polyvinyl acetate-based soil stabilization for rock slope ecological restoration. J. Environ. Manag. 2022, 324, 116209. [Google Scholar] [CrossRef]
- Gao, G.J.; Yuan, J.G.; Han, R.H.; Xin, G.R.; Yang, Z.Y. Characteristics of the optimum combination of synthetic soils by plant and soil properties used for rock slope restoration. Ecol. Eng. 2007, 30, 303–311. [Google Scholar] [CrossRef]
- Chiang, F.; Mazdiyasni, O.; AghaKouchak, A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity. Nat. Commun. 2021, 12, 2754. [Google Scholar] [CrossRef]
- Kang, R.J.; Li, M.Y.; Guo, S.W.; Xia, D.; Liu, L.M.; Dong, W.H.; Xu, W.N.; Lv, Y.C. Effect of brassinolide on stoichiometric stability characteristics of tall fescue under dought stress in ecological restoration. Sustainability 2024, 16, 5942. [Google Scholar] [CrossRef]
- Shu, Q.; Xia, D.; Ma, Y.Y.; Zhang, Y.; Luo, T.; Ma, J.X.; Liu, F.; Yan, S.X.; Liu, D.X. Response of physiological characteristics of ecological restoration plants to substrate cement content under exogenous arbuscular mycorrhizal fungal inoculation. Front. Plant Sci. 2022, 13, 1028553. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 17672. [Google Scholar] [CrossRef]
- Boaretto, L.F.; Carvalho, G.; Borgo, L.; Creste, S.; Landell, M.G.A.; Mazzafera, P.; Azevedo, R.A. Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol. Biochem. 2014, 74, 165–175. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and Plants: Current Knowledge and Technological Perspectives. Front. Plant Sci. 2016, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Ilyas, N.; Mashwani, Z.U.R.; Hayat, R.; Yasmin, H.; Noureldeen, A.; Ahmad, P. Synergistic effects of plant growth promoting rhizobacteria and silicon dioxide nano-particles for amelioration of drought stress in wheat. Plant Physiol. Biochem. 2021, 166, 160–176. [Google Scholar] [CrossRef]
- Sulaiman; Ahmad, A.; Hassim, M.F.N. Effects of silica nanoparticles on morpho-histological and antioxidant activities of rice seedlings under drought stress. S. Afr. J. Bot. 2024, 168, 497–508. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Rajan, B.S.; Basha, K.S.; Madhu, P.; Raghav, G.R. A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Channab, B.E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, modification, biodegradation and applications in agriculture as slow/controlled release fertilizer, superabsorbent, and crop protection: A review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef]
- Jiang, S.X.; Li, P.; Li, L.; Amiralian, N.; Rajah, D.; Xu, Z.P. Effectively enhancing topical delivery of agrochemicals onto plant leaves with nanocelluloses. Green Chem. 2023, 25, 8253–8265. [Google Scholar] [CrossRef]
- Batool, T.; Ali, S.; Seleiman, M.F.; Naveed, N.H.; Ali, A.; Ahmed, K.; Abid, M.; Rizwan, M.; Shahid, M.R.; Alotaibi, M.; et al. Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci. Rep. 2020, 10, 16975. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, K.F.; Górnik, K.; Awad, R.M.; Ayoub, A.; Abada, H.S.; Mosa, W.F.A. Influence of selenium, titanium, and silicon nanoparticles on the growth, yield, and fruit quality of mango under drought conditions. Horticulturae 2023, 9, 1231. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Mohammadi, A.S.; Nasab, S.B.; Ansari, N.A.; Juárez-Maldonado, A. Evaluation the impact of silicon nanoparticle on growth and water use efficiency of greenhouse tomato in drought stress condition. Appl. Water Sci. 2024, 14, 196. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Singla-Pareek, S.L.; Pareek, A.; Singh, A.K. Shaping the root system architecture in plants for adaptation to drought stress. Physiol. Plant. 2022, 174, e13651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Q.; Zhang, W.X.; Ding, C.J.; Hu, Z.M.; Fan, C.M.; Zhang, J.; Li, Z.H.; Diao, S.F.; Shen, L.; Zhang, B.Y.; et al. A breeding strategy for improving drought and salt tolerance of poplar based on CRISPR/Cas9. Plant Biotechnol. J. 2022, 21, 2160–2162. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.M.; Bai, Q.X.; Lv, J.J.; Tian, W.J.; Mao, K.L.; Wei, Q.Q.; Zheng, Y.M.; Yang, H.H.; Gao, C.Y.; Wan, D.S. The fasciclin-like arabinogalactan protein FLA11 of Ostrya rehderiana impacts wood formation and salt stress in Populus. Environ. Exp. Bot. 2024, 219, 105651. [Google Scholar] [CrossRef]
- Beckers, B.; De Beeck, M.O.; Weyens, N.; Van Acker, R.; Van Montagu, M.; Boerjan, W.; Vangronsveld, J. Lignin engineering in field-grown poplar trees affects the endosphere bacterial microbiome. Proc. Natl. Acad. Sci. USA 2016, 113, 2312–2317. [Google Scholar] [CrossRef]
- Begovic, L.; Abicic, I.; Lalic, A.; Lepedus, H.; Cesar, V.; Leljak-Levanic, D. Lignin synthesis and accumulation in barley cultivars differing in their resistance to lodging. Plant Physiol. Biochem. 2018, 133, 142–148. [Google Scholar] [CrossRef]
- Fang, S.; Yang, H.Y.; Duan, L.C.; Shi, J.; Guo, L. Potassium fertilizer improves drought stress alleviation potential in sesame by enhancing photosynthesis and hormonal regulation. Plant Physiol. Biochem. 2023, 200, 107744. [Google Scholar] [CrossRef]
- Liang, Y.P.; Liu, H.; Fu, Y.Y.; Li, P.H.; Li, S.; Gao, Y. Regulatory effects of silicon nanoparticles on the growth and photosynthesis of cotton seedlings under salt and low-temperature dual stress. BMC Plant Biol. 2023, 23, 504. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Mansour, E.; El-Sobky, E.; Abdul-Hamid, M.I.; Taha, T.F.; Elakkad, H.A.; Arnaout, S.; Eid, R.S.M.; El-Tarabily, K.A.; Yasin, M.A.T. Physio-biochemical and agronomic responses of faba beans to exogenously applied nano-silicon under drought stress conditions. Front. Plant Sci. 2021, 12, 637783. [Google Scholar] [CrossRef] [PubMed]
- Bozic, M.; Micic, D.I.; Andelkovic, V.; Delic, N.; Nikolic, A. Maize transcriptome profiling reveals low temperatures affect photosynthesis during the emergence stage. Front. Plant Sci. 2025, 16, 1527447. [Google Scholar] [CrossRef]
- Joshi, P.S.; Agarwal, P.; Agarwal, P.K. Overexpression of AlNAC1 from recretohalophyte Aeluropus lagopoides alleviates drought stress in transgenic tobacco. Environ. Exp. Bot. 2021, 181, 104277. [Google Scholar] [CrossRef]
- Zou, J.C.; Zhang, Q.N.; Amoako, F.K.; Ackah, M.; Li, H.N.; Shi, Y.S.; Li, J.B.; Jiang, Z.J.; Zhao, W.G. Genome-wide transcriptome profiling of mulberry (Morus alba) response to boron deficiency and toxicity reveal candidate genes associated with boron tolerance in leaves. Plant Physiol. Biochem. 2024, 207, 108316. [Google Scholar] [CrossRef]
- Fei, L.W.; Chu, J.P.; Zhang, X.; Dong, S.X.; Dai, X.L.; He, M.R. Physiological and proteomic analyses indicate delayed sowing improves photosynthetic capacity in wheat flag leaves under heat stress. Front. Plant Sci. 2022, 13, 848464. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B-Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Wang, F.F.; Liu, P.; Li, J.J.; Xu, S.T.; Chen, H.X.; Xie, L.T. Effects of four antibiotics on the photosynthetic light reactions in the green alga Chlorella pyrenoidosa. Comp. Biochem. Phys. C 2024, 281, 109927. [Google Scholar] [CrossRef]
- Zu, X.F.; Lu, Y.K.; Wang, Q.Q.; La, Y.M.; Hong, X.Y.; Tan, F.; Niu, J.Y.; Xia, H.H.; Wu, Y.F. Increased drought resistance 1 mutation increases drought tolerance of upland rice by altering physiological and morphological traits and limiting ROS levels. Plant Cell Physiol. 2021, 62, 1168–1184. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Barker, A. Effects of nanosilicon dioxide on leaf anatomy, chlorophyll fluorescence, and mineral element composition of strawberry under salinity stress. J. Plant Nutr. 2021, 44, 3005–3019. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef]
- Hameed, A.; Farooq, T.; Hameed, A.; Sheikh, M.A. Silicon-mediated priming induces acclimation to mild water-deficit stress by altering physio-biochemical attributes in wheat plants. Front. Plant Sci. 2021, 12, 625541. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Umm-e, H.; Ashraf, M.; Ashraf, M.; Sadiq, M. Exogenous application of L-methionine mitigates the drought-induced oddities in biochemical and anatomical responses of bitter gourd (Momordica charantia L.). Sci. Hortic. 2020, 267, 109333. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Ditta, A.; Suleman, M.; Ullah, M. Zinc-induced anti-oxidative defense and osmotic adjustments to enhance drought stress tolerance in sunflower (Helianthus annuus L.). Environ. Exp. Bot. 2022, 193, 104682. [Google Scholar] [CrossRef]
- Zhou, H.L.; Xu, P.J.; Zhang, L.J.; Huang, R.M.; Yang, M.F.; Wang, K.Y.; Fan, H. Nitrogen application promotes drought-stressed sugar beet growth by improving photosynthesis, osmoregulation, and antioxidant defense. J. Soil Sci. Plant Nutr. 2023, 23, 1272–1285. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; Rehman, M.Z.U.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Kadkhodaei, S.; Vaculik, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Hortic. 2023, 309, 111686. [Google Scholar] [CrossRef]
- Bao, L.F.; Liu, J.H.; Mao, T.Y.; Zhao, L.B.; Wang, D.S.; Zhai, Y.L. Nanobiotechnology-mediated regulation of reactive oxygen species homeostasis under heat and drought stress in plants. Front. Plant Sci. 2024, 15, 1418515. [Google Scholar] [CrossRef]
- Kusvuran, S. Microalgae (Chlorella vulgaris Beijerinck) alleviates drought stress of broccoli plants by improving nutrient uptake, secondary metabolites, and antioxidative defense system. Hortic. Plant J. 2021, 7, 221–231. [Google Scholar] [CrossRef]
- Wahi, D.; Bisht, K.; Gautam, S.; Salvi, P.; Lohani, P. Green synthesized nano silica: Foliar and soil application provides drought endurance in Eleucine coracana. Environ. Sci. Nano 2024, 11, 3412–3429. [Google Scholar] [CrossRef]
- Roach, T.; Neuner, G.; Kranner, I.; Buchner, O. Heat acclimation under drought stress induces antioxidant enzyme activity in the alpine plant Primula minima. Antioxidants 2023, 12, 1093. [Google Scholar] [CrossRef]
- Liu, C.; Sun, H.G.; Xu, Y.Z.; Wu, C. Effects of SiO2 nanoparticles on root structures, gas exchange, and antioxidant activities of Cunninghamia lanceolata seedlings under drought stress. J. Plant Nutr. 2023, 46, 3771–3793. [Google Scholar] [CrossRef]
- Li, S.C. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M. Arbuscular mycorrhizal fungi mediated enhanced biomass, root morphological traits and nutrient uptake under drought stress: A meta-analysis. J. Fungi 2022, 8, 660. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S.; et al. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.L.; Gao, Z.Y.; Zhang, Y.; Zhu, L.; Hu, J.; Ren, D.Y.; Xu, G.H.; Qian, Q. Driving the expression of RAA1 with a drought-responsive promoter enhances root growth in rice, its accumulation of potassium and its tolerance to moisture stress. Environ. Exp. Bot. 2018, 147, 147–156. [Google Scholar] [CrossRef]
- Karimian, N.; Nazari, F.; Samadi, S. Morphological and biochemical properties, leaf nutrient content, and vase life of tuberose (Polianthes tuberosa L.) affected by root or foliar applications of silicon (Si) and silicon nanoparticles (Si NPs). J. Plant Growth Regul. 2021, 40, 2221–2235. [Google Scholar] [CrossRef]
- Ahire, M.L.; Mundada, P.S.; Nikam, T.D.; Bapat, V.A.; Penna, S. Multifaceted roles of silicon in mitigating environmental stresses in plants. Plant Physiol. Biochem. 2021, 169, 291–310. [Google Scholar] [CrossRef]
- Vatansever, R.; Ozyigit, I.I.; Filiz, E.; Gozukara, N. Genome-wide exploration of silicon (Si) transporter genes, Lsi1 and Lsi2 in plants; insights into Si-accumulation status/capacity of plants. Biometals 2017, 30, 185–200. [Google Scholar] [CrossRef]
- Chen, H.; Wu, W.Q.; Du, K.; Yang, J.; Kang, X.Y. CCT39 transcription factor promotes chlorophyll biosynthesis and photosynthesis in poplar. Plant Cell Environ. 2024, 48, 136–3150. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, A.N.; Zhang, Y.R.; Zhao, J.; Wang, Y.Y.; Zhang, L.L.; Zhang, S. Ectopic expression of HaPEPC1 from the desert shrub Haloxylon ammodendron confers drought stress tolerance in Arabidopsis thaliana. Plant Physiol. Biochem. 2024, 208, 108536. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.H.; Pan, Z.J.; Dai, P.F.; Shen, Y.B.; Lu, Y.Z.; Han, B. Effect of waterlogging stress on leaf anatomical structure and ultrastructure of Phoebe sheareri seedlings. Forests 2023, 14, 1294. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Hayat, S. Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the performance of different components influencing the photosynthetic machinery in Brassica juncea L. Plant Physiol. Biochem. 2018, 129, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Tasdighi, H.; Gholamhoseini, M. Evaluation of proline, chlorophyll, soluble sugar content and uptake of nutrients in the German chamomile (Matricaria chamomilla L.) under drought stress and organic fertilizer treatments. Asian Pac. J. Trop. 2016, 6, 886–891. [Google Scholar] [CrossRef]
- Wu, X.; Li, Z.H.; Gong, L.; Li, R.X.; Zhang, X.; Zheng, Z. Ecological adaptation strategies of plant functional groups in the upper reaches of the Tarim River based on leaf functional traits. Environ. Exp. Bot. 2023, 215, 105490. [Google Scholar] [CrossRef]
- Rahman, M.; Mostofa, M.G.; Keya, S.S.; Rahman, A.; Das, A.K.; Islam, R.; Abdelrahman, M.; Bhuiyan, S.U.; Naznin, T.; Ansary, M.U.; et al. Acetic acid improves drought acclimation in soybean: An integrative response of photosynthesis, osmoregulation, mineral uptake and antioxidant defense. Physiol. Plant. 2021, 172, 334–350. [Google Scholar] [CrossRef]
- Muñoz, V.; France, A.; Uribe, H.; Hirzel, J. Nitrogen and irrigation rates affected leaf phosphorus and potassium concentrations in different cultivars of pot-grown blueberry. J. Soil Sci. Plant Nutr. 2023, 23, 965–973. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]


| Treatments | Leaf Thickness (μm) | Ue Thickness (μm) | Le Thickness (μm) | Uec Thickness (μm) | Lec Thickness (μm) | VB Area (μm2) |
|---|---|---|---|---|---|---|
| CK | 335.04 ± 11.3 cd | 14.28 ± 1.54 d | 23.81 ± 2.39 c | 1.43 ± 0.21 c | 2.18 ± 0.38 c | 3892.21 ± 919.86 c |
| Si NPs | 462.65 ± 11.16 a | 18.34 ± 4 bcd | 37.14 ± 3.81 abc | 2.68 ± 0.54 ab | 4.05 ± 0.45 ab | 6126.82 ± 783.16 abc |
| CNCs | 382.37 ± 27.09 bc | 15.12 ± 3.01 cd | 35.98 ± 8.92 abc | 1.85 ± 0.18 bc | 3.22 ± 0.58 bc | 7700.13 ± 1330.01 ab |
| DS | 263.32 ± 17.61 e | 24.82 ± 4.64 abc | 32.23 ± 5.74 bc | 2.18 ± 0.27 bc | 2.73 ± 0.43 bc | 4779.6 ± 692.31 c |
| DS_Si NPs | 418.7 ± 30.39 ab | 30.31 ± 2.89 a | 52 ± 7.59 a | 3.28 ± 0.63 a | 5.1 ± 0.76 a | 8200.35 ± 917.88 a |
| DS_CNCs | 289.12 ± 23.56 de | 26.81 ± 4.63 ab | 42.45 ± 4.08 ab | 2.34 ± 0.34 abc | 4.04 ± 0.44 ab | 5496.32 ± 805.68 bc |
| Treatments | N Concentration (g/kg DW) | P Concentration (g/kg DW) | K Concentration (g/kg DW) | Si Concentration (g/kg DW) |
|---|---|---|---|---|
| CK | 20.48 ± 1.31 ab | 4.81 ± 0.36 bc | 5.01 ± 0.42 b | 3.83 ± 0.26 bc |
| Si NPs | 23.22 ± 1.35 a | 5.45 ± 0.56 ab | 6.07 ± 0.26 a | 7.24 ± 0.27 a |
| CNCs | 21.42 ± 0.73 a | 6.39 ± 0.53 a | 5.17 ± 0.35 b | 4.67 ± 0.46 b |
| DS | 13.77 ± 0.93 d | 3.44 ± 0.13 d | 3.99 ± 0.2 c | 3.25 ± 0.12 c |
| DS_Si NPs | 18.35 ± 1.05 bc | 4.73 ± 0.25 bc | 5.22 ± 0.16 b | 6.72 ± 0.49 a |
| DS_CNCs | 15.98 ± 0.29 cd | 4.25 ± 0.39 cd | 4.86 ± 0.29 b | 3.53 ± 0.39 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Hu, S.; Bai, X.; Ren, J.; Tian, K.; Zhang, H.; Zhang, Z.; Nguyen, V. Comparative Study on the Effects of Silicon Nanoparticles and Cellulose Nanocrystals on Drought Tolerance in Tall Fescue (Festuca arundinacea Schreb.). Plants 2025, 14, 1461. https://doi.org/10.3390/plants14101461
Li M, Hu S, Bai X, Ren J, Tian K, Zhang H, Zhang Z, Nguyen V. Comparative Study on the Effects of Silicon Nanoparticles and Cellulose Nanocrystals on Drought Tolerance in Tall Fescue (Festuca arundinacea Schreb.). Plants. 2025; 14(10):1461. https://doi.org/10.3390/plants14101461
Chicago/Turabian StyleLi, Meng, Sile Hu, Xulong Bai, Jie Ren, Kanliang Tian, Huili Zhang, Zhilong Zhang, and Vanquy Nguyen. 2025. "Comparative Study on the Effects of Silicon Nanoparticles and Cellulose Nanocrystals on Drought Tolerance in Tall Fescue (Festuca arundinacea Schreb.)" Plants 14, no. 10: 1461. https://doi.org/10.3390/plants14101461
APA StyleLi, M., Hu, S., Bai, X., Ren, J., Tian, K., Zhang, H., Zhang, Z., & Nguyen, V. (2025). Comparative Study on the Effects of Silicon Nanoparticles and Cellulose Nanocrystals on Drought Tolerance in Tall Fescue (Festuca arundinacea Schreb.). Plants, 14(10), 1461. https://doi.org/10.3390/plants14101461









