Insight into the Influence of Ecological Factors on Shaping Distribution Patterns of Camptotheca acuminata for Conservation and Management
Abstract
1. Introduction
2. Results
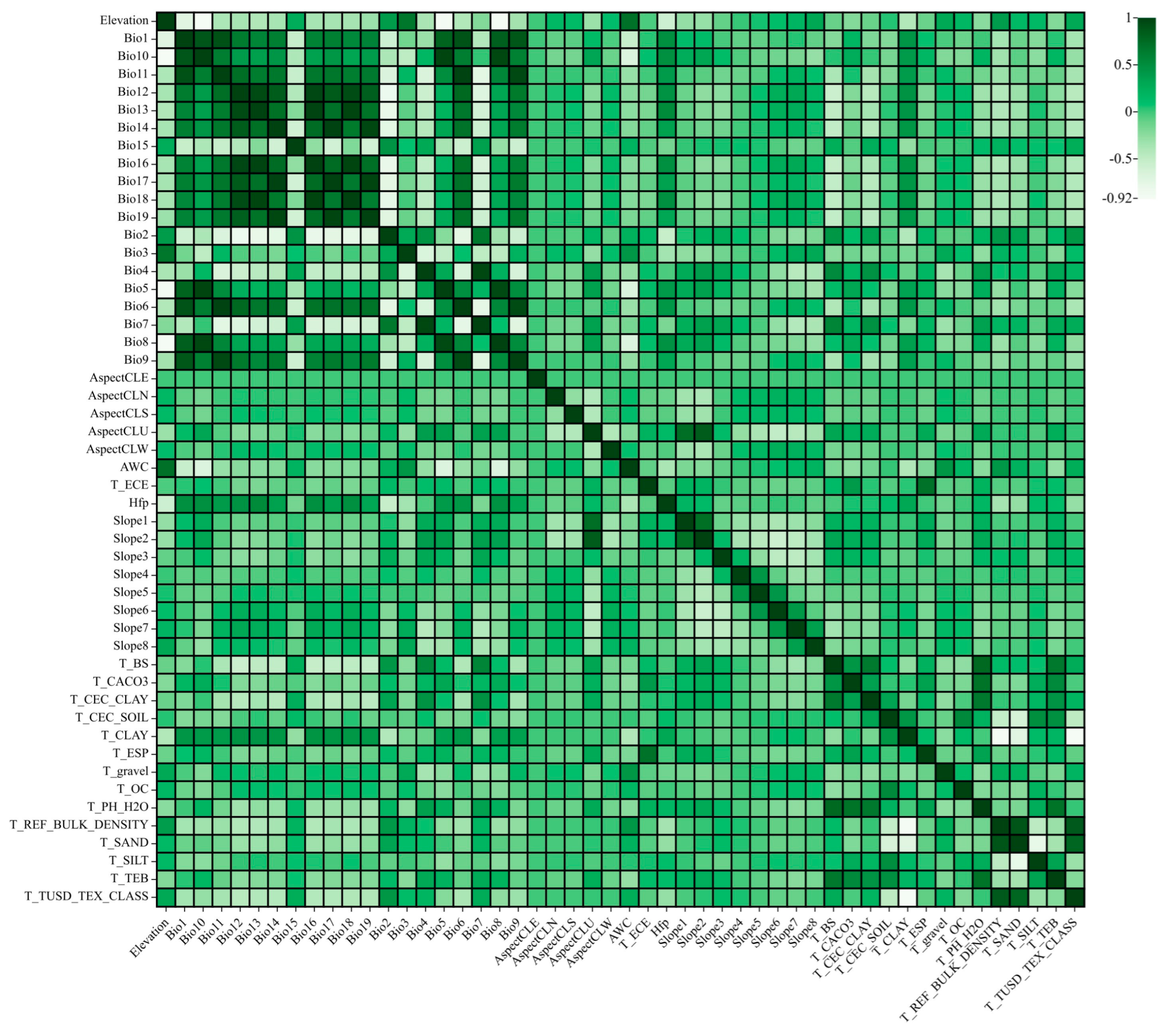
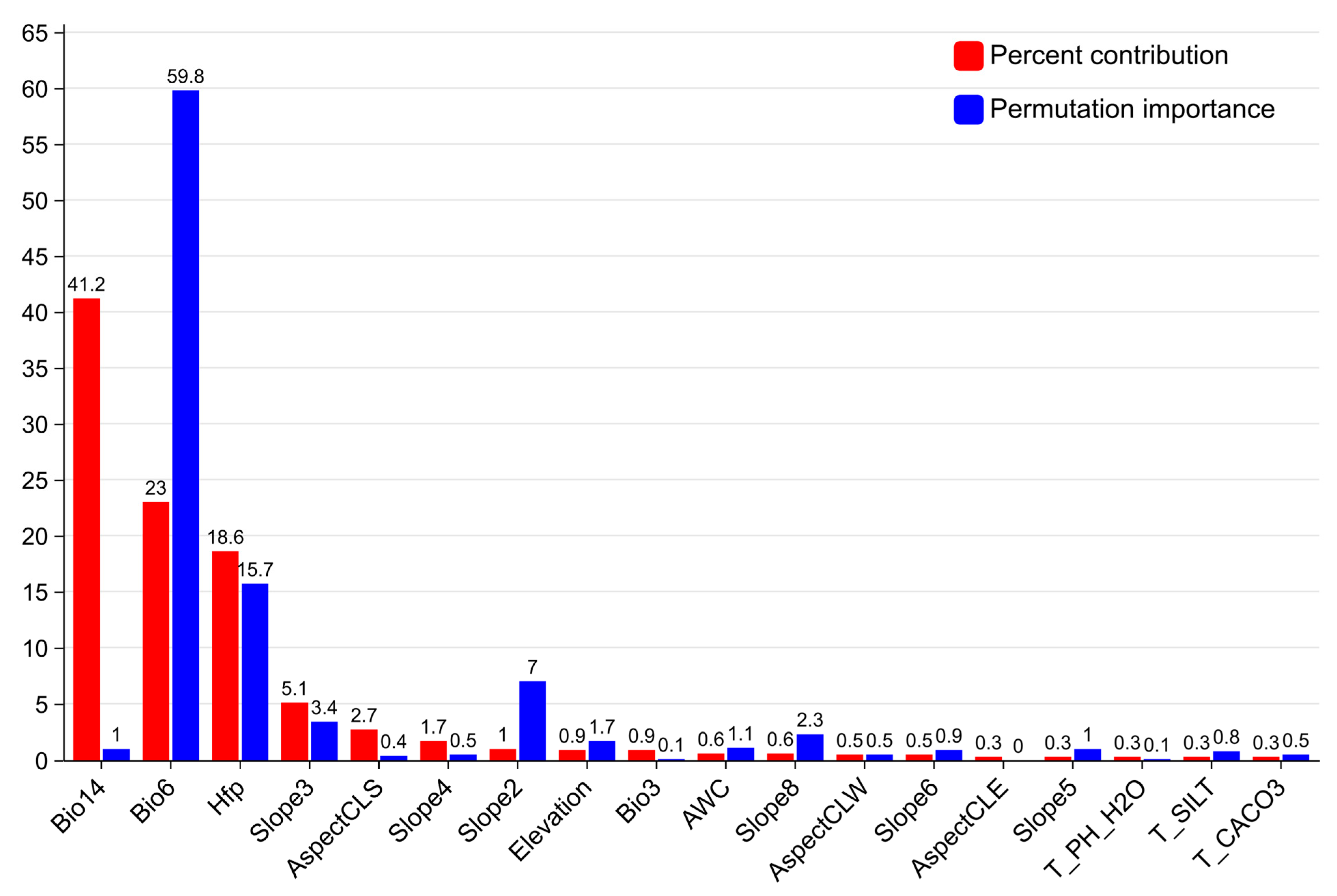
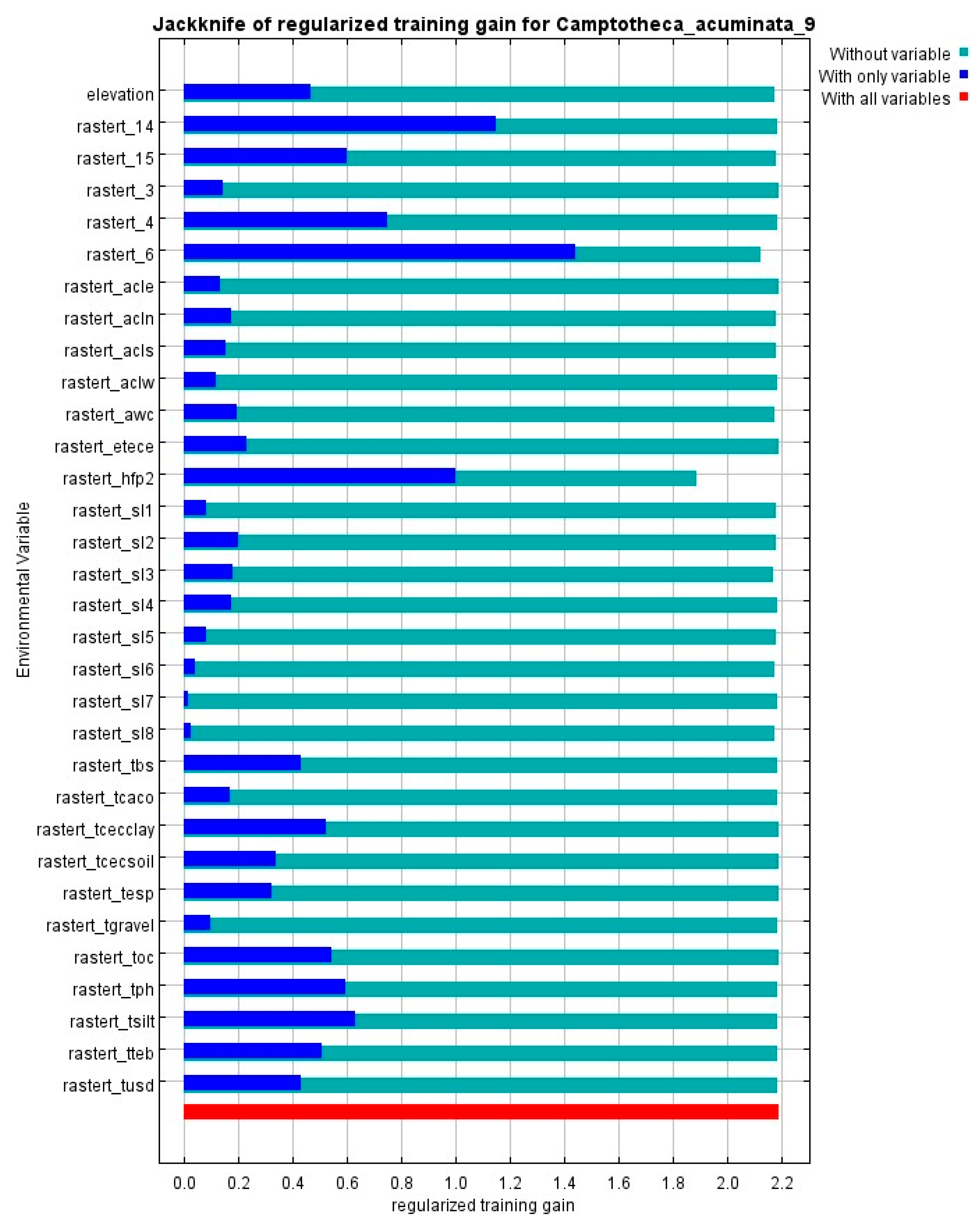
2.1. Environmental Factor Selection Results Affecting the Distribution of C. acuminata
2.2. MaxEnt Model Accuracy Evaluation for Predicting the Distribution of C. acuminata
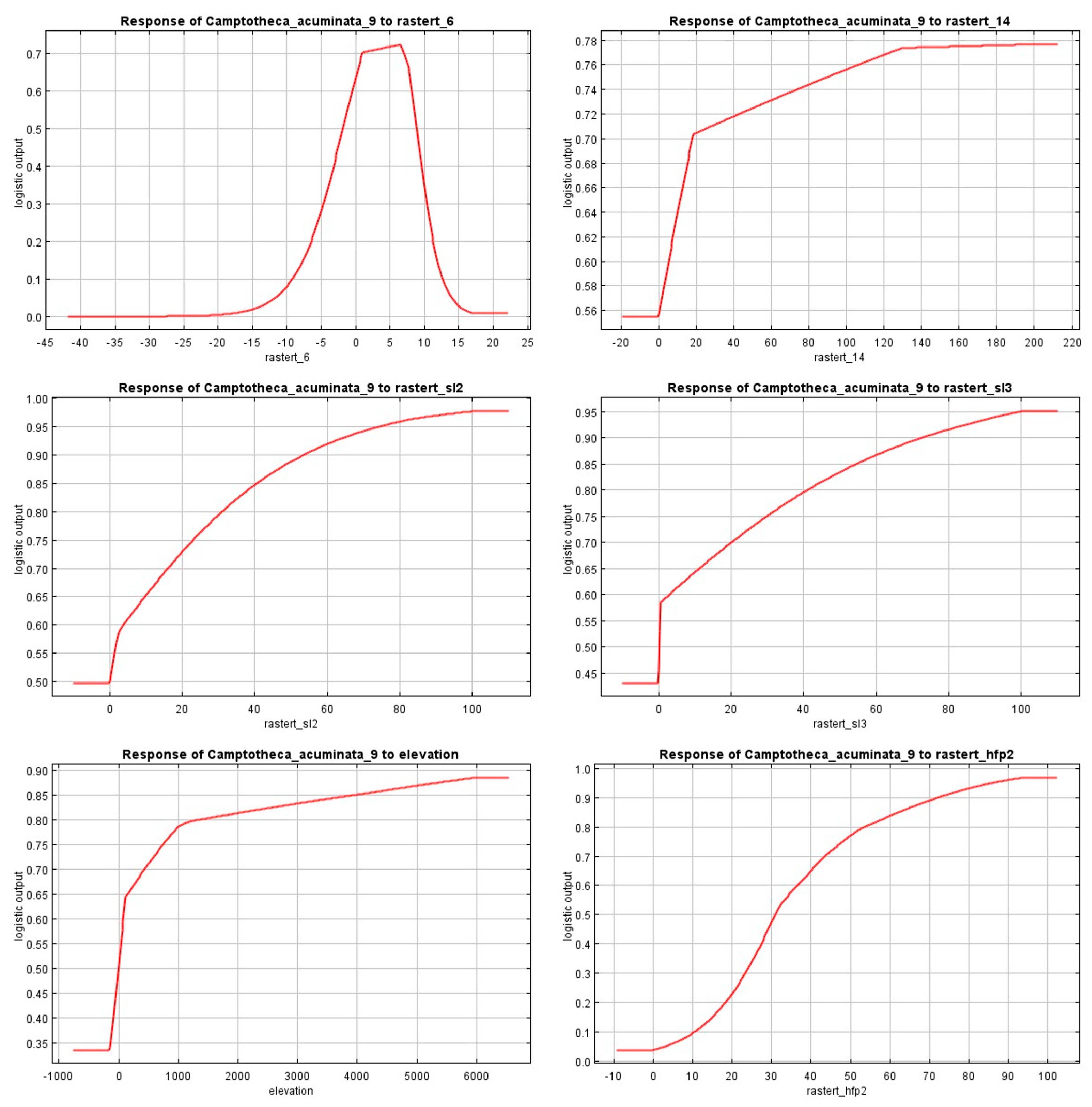
2.3. Response of Dominant Environmental Factors Based on MaxEnt Model Prediction Results
2.4. Prediction of the Potential Distribution Area of C. acuminata Under Different Climate Scenarios
2.4.1. Current Geographical Distribution
2.4.2. Potential Geographical Distribution in Future Periods
2.4.3. Dynamic Changes in Suitable Habitats of C. acuminata Under Different Future Climate Scenarios
2.4.4. Migration Map of the Geometric Centre of Suitable Habitats in Different Periods
3. Discussion
3.1. Errors in Distribution Point Data and Environmental Variable Selection
3.2. Changes in the Suitable Distribution Area of C. acuminata in Different Periods
3.3. Ecological Characteristics Affecting the Distribution Area of C. acuminata
4. Materials and Methods
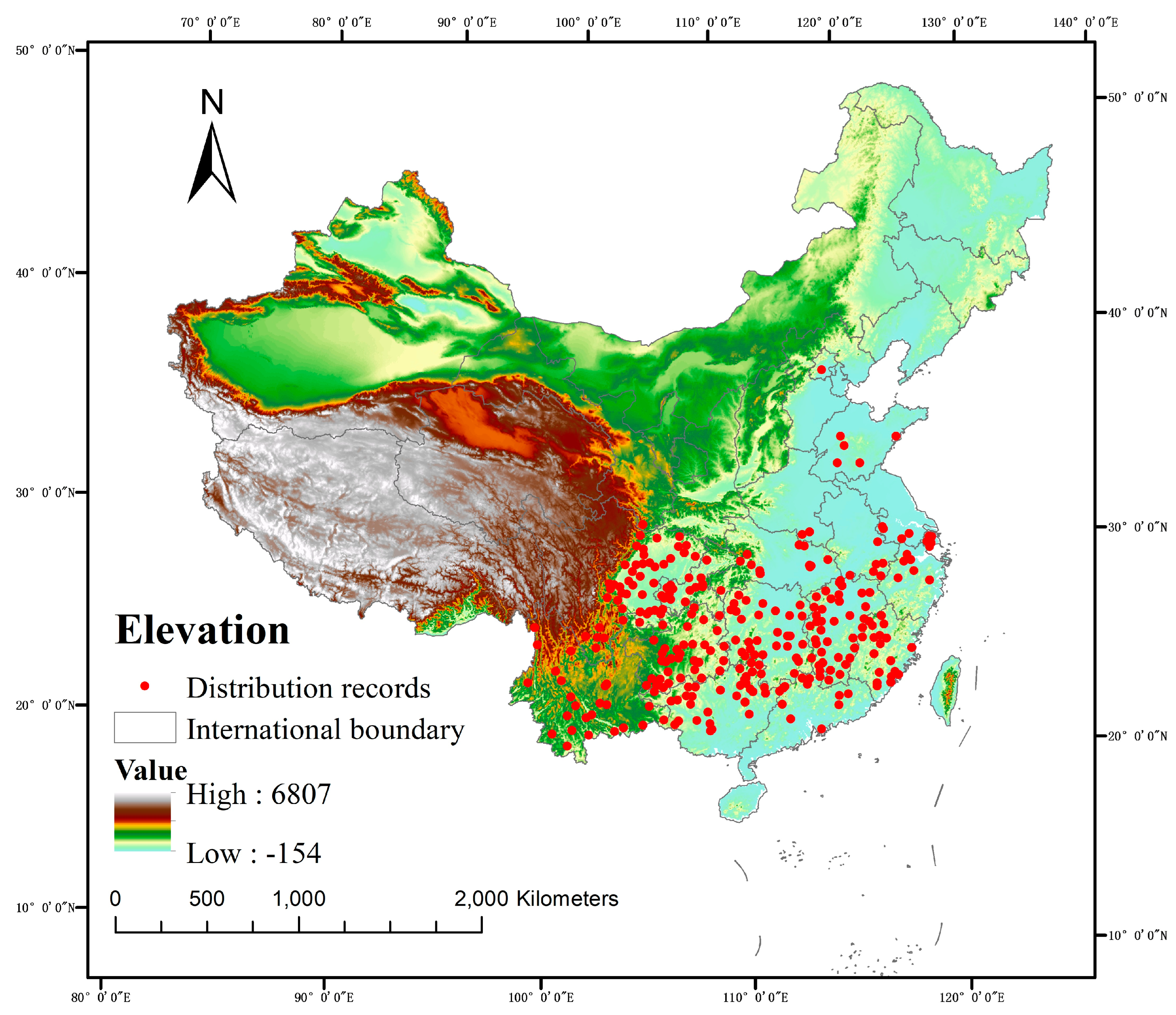
4.1. Collection and Processing of Species Distribution Data
4.2. Environmental Factors Data
4.3. Model Pre-Run and Evaluation Metrics
4.4. Environmental Factor Correlation Analysis
4.5. Model Computation
4.6. Habitat Suitability Classification
4.7. Dynamic Changes in the Distribution Area of C. acuminata Under Different Future Climate Scenarios
4.8. Centroid Migration of Suitable Habitats in Different Periods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.H. Research on the Ecology of C. acuminata Resources. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2001. [Google Scholar]
- Ye, S.Y. A review of the development and utilization of camptothecin. Mod. Agric. Sci. Technol. 2021, 3, 212–213. [Google Scholar]
- Sun, W.B.; Yang, J.; Dao, Z.L. Study and Conservation of Plant Species with Extremely Small Populations (PSESP) in Yunnan Province, China; Science Press: Beijing, China, 2019. [Google Scholar]
- Isinkaralar, K.; Isinkaralar, O.; Özel, H.B.; Şevik, H. A Comparative Study About Physical Properties of Copper Oxide and Zinc Oxide Nanoparticles on Fagus orientalis L. as Bioindicator. Water Air Soil Pollut. 2024, 235, 738. [Google Scholar] [CrossRef]
- Cantürk, U.; Koç, İ.; Özel, H.B.; Şevik, H. Possible changes of Pinus nigra distribution regions in Türkiye with the impacts of global climate change. BioResources 2024, 19, 6190–6214. [Google Scholar] [CrossRef]
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, 301–307. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Lavergne, S.; Roquet, C.; Boulangeat, I.; Lafourcade, B.; Araujo, M.B. Consequences of climate change on the tree of life in Europe. Nature 2011, 470, 531–534. [Google Scholar] [CrossRef]
- Ariel, A.F.; Gabriella, L.S. Ecoregional vulnerability assessment for the functional richness of South American carnivorans (Mammalia: Carnivora). J. Mammalian Evol. 2014, 21, 289–300. [Google Scholar]
- Rodrigues, E.; Cohen, M.C.; Liu, K.B.; Pessenda, L.C.; Yao, Q.; Ryu, J.; Rossetti, D.; de Souza, A.; Dietz, M. The effect of global warming on the establishment of mangroves in coastal Louisiana during the Holocene. Geomorphology 2021, 381, 107533. [Google Scholar] [CrossRef]
- Chen, Z.; Li, L.; Wang, Y.Z. Spatial and temporal distribution characteristics of Paris polyphylla var. yunnanensis and the prediction of steroidal saponins content. Ind. Crops Prod. 2025, 227, 120840. [Google Scholar] [CrossRef]
- Zhang, M.G.; Zhou, Z.K.; Chen, W.Y.; Cannon, C.H.; Raes, N.; Slik, J.F. Major declines of woody plant species ranges under climate change in Yunnan, China. Divers. Distrib. 2014, 20, 405–415. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Liu, T.; Yan, F. Impact of recent vegetation greening on temperature and precipitation over China. Agric. For. Meteorol. 2020, 295, 108200. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Tian, Z.; Lu, Z.; Huo, D.; Zhang, Z.; Sun, Q.; Wu, H.; Wang, J.; Kang, Y.; Xie, Y.; Tian, Y.; et al. Strategic analysis of avian conservation within key areas and key species—A case study of Baiyangdian, China. J. Environ. Manag. 2025, 373, 123821. [Google Scholar] [CrossRef]
- Adhikari, P.; Lee, Y.H.; Poudel, A.; Hong, S.H.; Park, Y.S. Global spatial distribution of Chromolaena odorata habitat under climate change: Random forest modeling of one of the 100 worst invasive alien species. Sci. Rep. 2023, 13, 9745. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; He, B.; Wu, Y.; Xue, Y.; Deng, H.; Li, S.; Dong, X.; Lu, L. Rethinking conservation and restoration strategies of endangered and key medicinal Clavicarpa plants in Yunnan-Kweichow Plateau’s Karst Areas under climate change. Ecol. Evol. 2025, 15, e70790. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.; Feng, L.; Li, H.; Dai, Z.; Cheng, T.; Liu, S.; Ma, L.; Luo, X.; Wang, Y.; et al. The combined effects of climate, soil, and rhizospheric microorganisms determine the quality and suitable production zones of Stellaria dichotoma L. var. lanceolata Bge. in China. Chem. Biol. Technol. Agric. 2024, 11, 185. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, B.; Wan, F.; Xiao, Q.; Dai, L. Application of ROC curve analysis in evaluating the performance of alien species’ potential distribution models. Biodivers. Sci. 2007, 15, 365–372. [Google Scholar]
- Zhang, S.; Liu, X.; Li, R.; Wang, X.; Cheng, J.; Yang, Q.; Kong, H. AHP-GIS and MaxEnt for delineation of potential distribution of Coffea arabica plantation under future climate in Yunnan, China. Ecol. Indic. 2021, 132, 108327. [Google Scholar] [CrossRef]
- Vaughan, I.P.; Ormerod, S.J. The continuing challenges of testing species distribution models: Testing distribution models. J. Appl. Ecol. 2005, 42, 720–730. [Google Scholar] [CrossRef]
- Liu, J.J.; Wang, J.; Zhai, T.L.; Li, Z.H.; Huang, L.Y.; Yuan, S.H. Gradient characteristics of China’s land use patterns and identification of the East–West Natural-Socio-Economic Transitional Zone for national spatial planning. Land Use Policy 2021, 109, 105671. [Google Scholar] [CrossRef]
- Ying, J.L. Predicting the Impact of Climate Change on the Potential Distribution of Endemic Viburnum in China Based on the MaxEnt Model. Master’s Thesis, University of Chinese Academy of Sciences (Wuhan Botanical Garden, CAS), Wuhan, China, 2021. [Google Scholar]
- Lv, Z.; Jiu, S.; Wang, L.; Xu, Y.; Wang, J.; Liu, X.; Xu, J.; Wang, Y.; Haider, M.S.; Liu, R.; et al. Climate change affects the suitability of Chinese cherry (Prunus pseudocerasus Lindl.) in China. Mol. Hortic. 2025, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Zhang, G.; Sun, X.; Chen, S.; Huang, L. Assessing the quality ecology of endemic tree species in China based on machine learning models and UPLC methods: The example of Eucommia ulmoides Oliv. J. Clean. Prod. 2024, 452, 142021. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Guo, Z.; Dai, L.; Wu, Y.; Liu, H.; Li, Y.; Chen, H.; Zhang, Y.; Zhao, Y.; et al. Integrating MaxEnt model and landscape ecology theory for studying spatiotemporal dynamics of habitat: Suggestions for conservation of endangered Red-crowned crane. Ecol. Indic. 2020, 116, 106472. [Google Scholar] [CrossRef]
- Wang, G.Z.; Geng, Q.F.; Xiao, M.Y.; Zhang, M.Y.; Zhang, Y.Y.; Wang, Z.S. Predicting Pseudolarix amabilis potential habitat based on four niche models. Sheng Tai Xue Bao 2020, 40, 6096–6104. [Google Scholar]
- Feng, L.; Sun, J.; Shi, Y.; Wang, G.; Wang, T. Predicting suitable habitats of Camptotheca acuminata considering both climatic and soil variables. Forests 2020, 11, 891. [Google Scholar] [CrossRef]
- Wang, J.J.; Cao, B.; Bai, C.K.; Zhang, L.L.; Che, L. Potential distribution prediction and suitability evaluation of Fritillaria cirrhosa D.Don based on MaxEnt modeling and GIS. Bull. Bot. Res. 2014, 34, 642–649. [Google Scholar]
- Cao, X.F.; Qian, G.L.; Hu, B.S.; Liu, F.Q. Prediction of potential suitable distribution area of Flaveria bidentis in China based on niche models. Chin. J. Appl. Ecol. 2010, 21, 3063–3069. [Google Scholar]
- Duan, J.Q.; Zhou, G.S. Climatic suitability of double rice planting regions in China. Sci. Agric. Sin. 2012, 45, 218–227. [Google Scholar]
- Zhu, G.P.; Qiao, H.J. Effect of the MaxEnt model’s complexity on the prediction of species’ potential distributions. Biodivers. Sci. 2016, 24, 1189–1196. [Google Scholar] [CrossRef]
- Li, X. Present distribution, medical value and development foreground of Camptotheca Decne. J. Shaanxi Norm. Univ. (Nat. Sci. Ed.) 2004, S2, 169–173. [Google Scholar]
- Varol, T.; Cetin, M.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. The Effects of Climate Change Scenarios on Carpinus betulus and Carpinus orientalis in Europe. Water Air Soil Pollut. 2022, 233, 45. [Google Scholar] [CrossRef]
- Tekin, O.; Cetin, M.; Varol, T.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. Altitudinal Migration of Species of Fir (Abies spp.) in Adaptation to Climate Change. Water Air Soil Pollut. 2022, 233, 385. [Google Scholar] [CrossRef]
- Varol, T.; Canturk, U.; Cetin, M.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. Identifying the suitable habitats for Anatolian boxwood (Buxus sempervirens L.) for the future regarding climate change. Theor. Appl. Climatol. 2022, 150, 637–647. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, S.B.; Lyu, Y.Q.; Zhao, Y.; Lyu, S.H. Characteristics and future projections of summer extreme precipitation in Sichuan Province, China. J. Mountain Sci. 2020, 17, 1696–1711. [Google Scholar] [CrossRef]
- Xu, C.C.; Wu, W.X.; Ge, Q.S. Impact assessment of climate change on rice yields using the ORYZA model in the Sichuan Basin, China. Int. J. Climatol. 2018, 38, 2922–2939. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Zhu, F.; Yuan, C. Risk evaluation of Tithonia diversifolia dispersal in Yunnan Province, China. Guihaia 2021, 41, 789–798. [Google Scholar]
- Liu, J.; Jia, L.; Wang, L.; Sun, C.; Wang, X.; Zheng, Y.; Chen, Z.; Weng, X. Potential distribution and ecological characteristics of genus Sapindus in China based on MaxEnt model. Sci. Silvae Sin. 2021, 57, 1–12. [Google Scholar]
- Wei, Y.; Zhang, L.; Wang, J.; Wang, W.; Niyati, N.; Guo, Y.; Wang, X. Chinese caterpillar fungus (Ophiocordyceps sinensis) in China: Current distribution, trading, and futures under climate change and overexploitation. Sci. Total Environ. 2021, 755, 142548. [Google Scholar] [CrossRef]
- Zhao, G.H. Analysis of Spatial Distribution Pattern Evolution and Influencing Factors of Ziziphus jujuba Under Climate Change in China. Master’s Thesis, Shanxi Normal University, Linfen, China, 2020. [Google Scholar]
- Guo, J.; Liu, X.P.; Zhang, Q.; Zhang, D.F.; Xie, C.X.; Liu, X. Prediction for the potential distribution area of Codonopsis pilosula at global scale based on MaxEnt model. Chin. J. Appl. Ecol. 2017, 28, 992–1000. [Google Scholar]
- Yi, Y.J.; Cheng, X.; Yang, Z.F.; Zhang, S.H. MaxEnt modeling for predicting the potential distribution of endangered medicinal plant (H. riparia Lour.) in Yunnan, China. Ecol. Eng. 2016, 92, 260–269. [Google Scholar] [CrossRef]
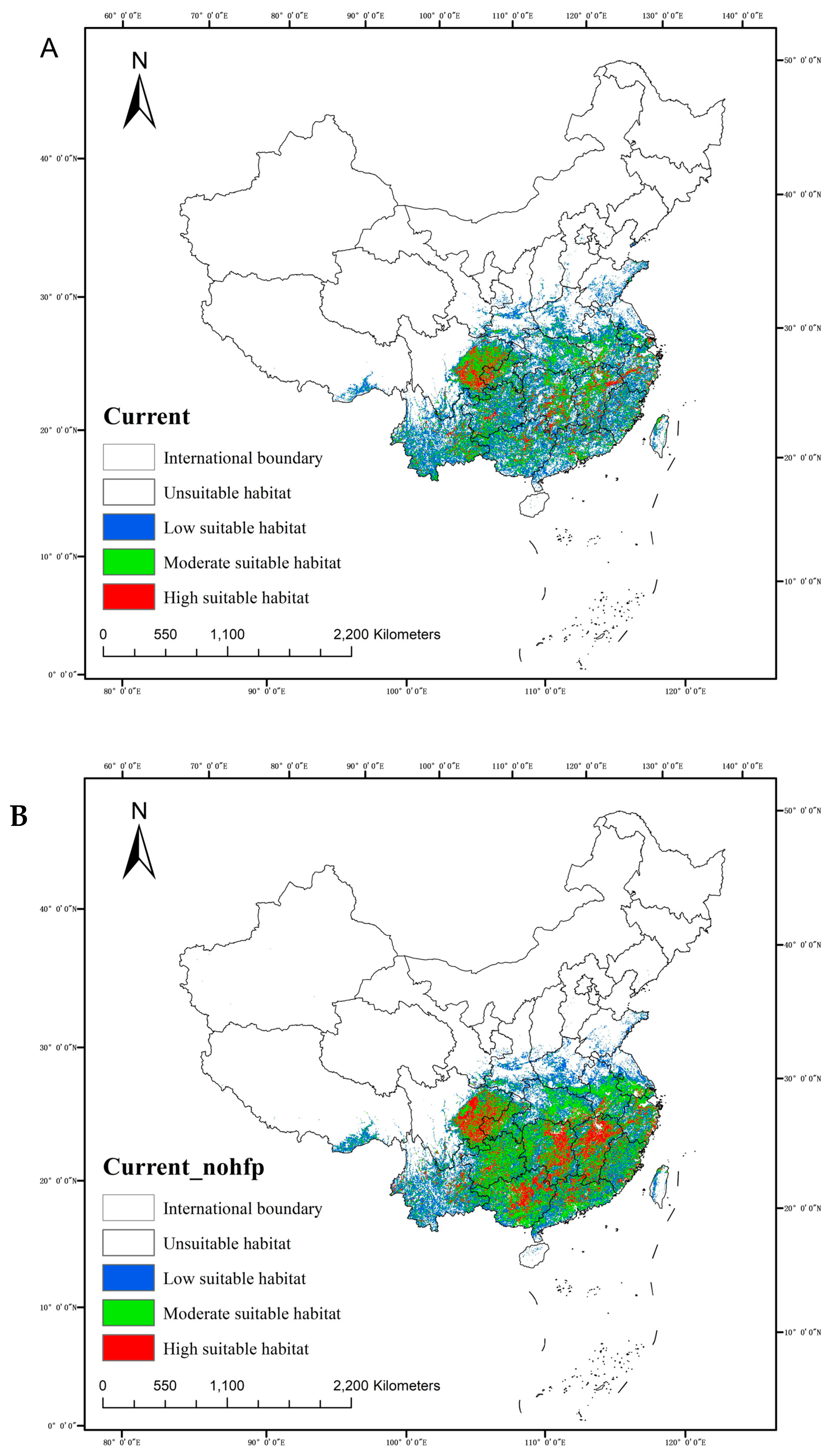
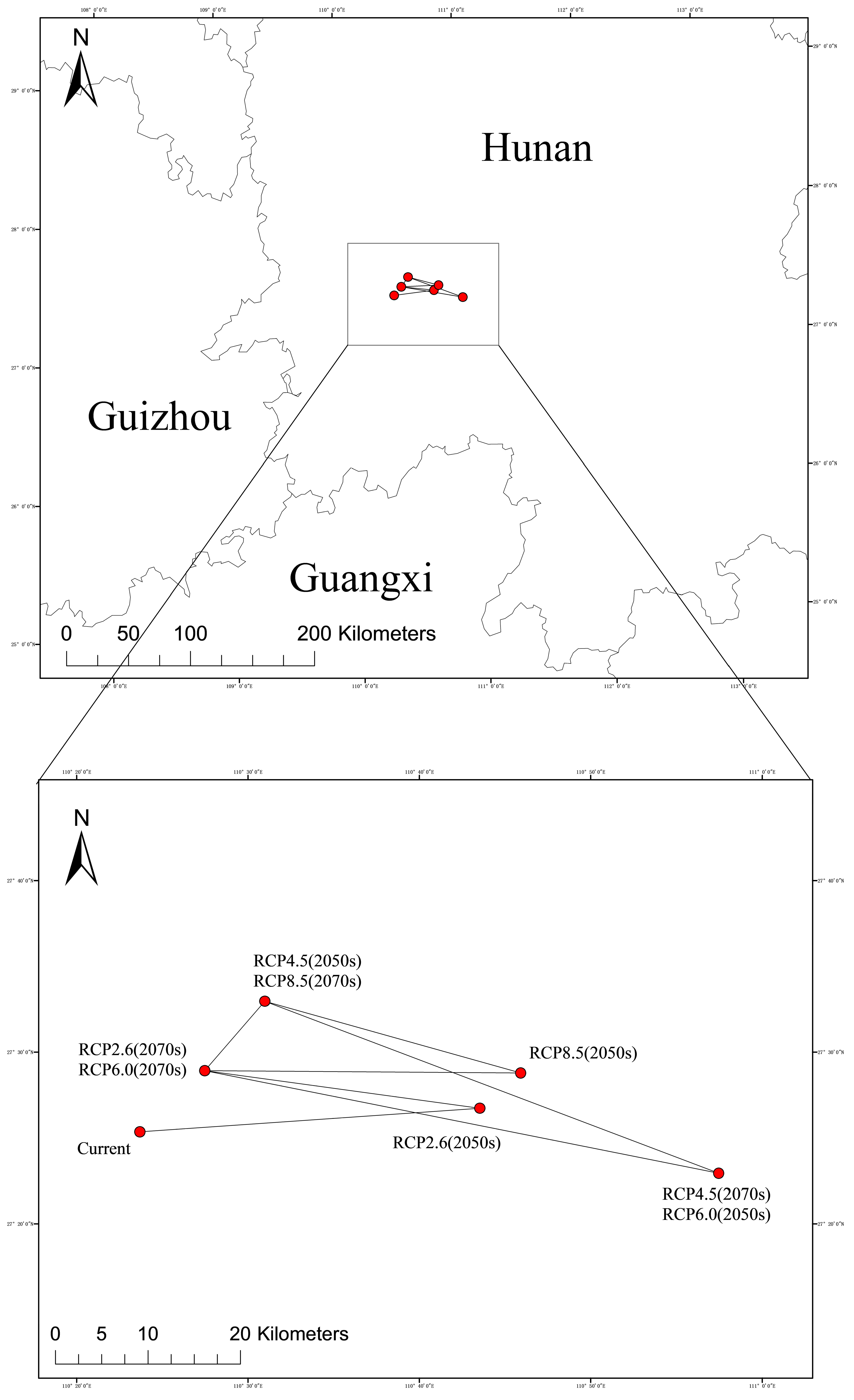
| Period | Climate Scenarios | Low-Level Suitable Area /×104 km2 (%) | Moderately Suitable Area /×104 km2 (%) | Highly Suitable Area /×104 km2 (%) | Total Suitable Area /×104 km2 | |
|---|---|---|---|---|---|---|
| With-Hfp | Current | / | 82.91146 (54.72%) | 58.11458 (38.36%) | 16.93403 (11.18%) | 151.51563 |
| 2050s | RCP2.6 | 80.63368 (51.05%) | 57.74826 (36.56%) | 17.79514 (11.27%) | 157.96007 | |
| RCP4.5 | 78.09028 (50.00%) | 57.41319 (36.76%) | 16.34722 (10.47%) | 156.17708 | ||
| RCP6.0 | 79.32986 (52.24%) | 53.71007 (35.37%) | 15.12153 (9.96%) | 151.85069 | ||
| RCP8.5 | 72.82813 (49.15%) | 55.86285 (37.70%) | 19.07465 (12.87%) | 148.16146 | ||
| 2070s | RCP2.6 | 79.88889 (54.06%) | 55.80556 (37.77%) | 16.03819 (10.85%) | 147.76563 | |
| RCP4.5 | 72.63889 (47.87%) | 55.18403 (36.37%) | 18.06076 (11.90%) | 151.73264 | ||
| RCP6.0 | 82.10069 (56.28%) | 60.67014 (41.59%) | 17.67361 (12.11%) | 145.88368 | ||
| RCP8.5 | 79.56076 (49.59%) | 59.42188 (37.04%) | 17.68576 (11.02%) | 160.44444 | ||
| No-Hfp | Current | / | 67.90451 (43.34%) | 85.16666 (54.36%) | 31.87674 (20.35%) | 156.66840 |
| 2050s | RCP2.6 | 72.60069 (39.25%) | 82.33507 (44.52%) | 33.04514 (17.87%) | 184.94791 | |
| RCP4.5 | 72.20660 (38.41%) | 82.22049 (43.74%) | 31.87500 (16.96%) | 187.98090 | ||
| RCP6.0 | 68.02084 (36.51%) | 86.92188 (46.66%) | 36.66493 (19.68%) | 186.30209 | ||
| RCP8.5 | 72.09722 (37.63%) | 81.07639 (42.31%) | 35.51910 (18.54%) | 191.60765 | ||
| 2070s | RCP2.6 | 70.75347 (37.50%) | 93.54340 (49.57%) | 30.99306 (16.43%) | 188.69271 | |
| RCP4.5 | 69.59375 (35.64%) | 84.28125 (43.16%) | 30.61285 (15.68%) | 195.28993 | ||
| RCP6.0 | 75.45313 (40.90%) | 83.92361 (45.49%) | 29.36806 (15.92%) | 184.48785 | ||
| RCP8.5 | 80.06771 (42.42%) | 85.97049 (45.55%) | 32.50000 (17.22%) | 188.74480 |
| Data Type | Ecological Factor | Units |
|---|---|---|
| Climate factor | Mean annual temperature (bio1) | °C |
| Mean monthly temperature range (bio2) | °C | |
| Isothermality (bio3) | / | |
| Temperature seasonality (bio4) | / | |
| Max temperature of the warmest month (bio5) | °C | |
| Min temperature of the coldest month (bio6) | °C | |
| Temperature annual range (bio7) | °C | |
| Mean temperature of the wettest quarter (bio8) | °C | |
| Mean temperature of the driest quarter (bio9) | °C | |
| Mean temperature of warmest quarter (bio10) | °C | |
| Mean temperature of coldest quarter (bio11) | °C | |
| Annual precipitation (bio12) | mm | |
| Precipitation of wettest month (bio13) | mm | |
| Precipitation of driest month (bio14) | mm | |
| Precipitation seasonality (bio15) | mm | |
| Precipitation of wettest quarter (bio16) | mm | |
| Precipitation of driest quarter (bio17) | mm | |
| Precipitation of warmest quarter (bio18) | mm | |
| Precipitation of coldest quarter (bio19) | mm | |
| Soil factor | Volume percentage of gravel in the topsoil (T_gravel) | %vol. |
| Percentage of sand in the topsoil (T_SAND) | % wt. | |
| Percentage of silt in the topsoil (T_SILT) | % wt. | |
| Percentage of clay in the topsoil (T_CLAY) | % wt. | |
| Texture class name and code (T_USDA_TEX_ CLASS) | name | |
| Cation exchange capacity (T_REF_BULK_DENSITY) | kg/dm3 | |
| Percentage of organic carbon in the topsoil (T_OC) | % weight | |
| Soil reaction of topsoil (T_PH_H2O) | log(H+) | |
| Cation exchange capacity of the clay fraction in the topsoil (T_CEC_CLAY) | cmol/kg | |
| Cation exchange capacity in the topsoil (T_CEC_SOIL) | cmol/kg | |
| Base saturation in the topsoil (T_BS) | % | |
| Calcium carbonate (lime) content in the topsoil (T_CACO3) | % | |
| Exchangeable sodium percentage in the topsoil (T_ESP) | % | |
| Electrical conductivity of topsoil (T_ECE) | dS/m | |
| Total exchangeable bases in the topsoil (T_TEB) | cmol/kg | |
| Available water storage capacity in mm/m of the soil unit (AWC) | % | |
| Landform factor | Elevation | m |
| Slope | % | |
| Aspect | ° | |
| Human activity factors | Global Human Footprint | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Li, Y.; Manda, T.; Lin, J.; Huang, T.; Zhang, Y.; Yang, L. Insight into the Influence of Ecological Factors on Shaping Distribution Patterns of Camptotheca acuminata for Conservation and Management. Plants 2025, 14, 1466. https://doi.org/10.3390/plants14101466
Wang T, Li Y, Manda T, Lin J, Huang T, Zhang Y, Yang L. Insight into the Influence of Ecological Factors on Shaping Distribution Patterns of Camptotheca acuminata for Conservation and Management. Plants. 2025; 14(10):1466. https://doi.org/10.3390/plants14101466
Chicago/Turabian StyleWang, Taojing, Yuchen Li, Teja Manda, Jie Lin, Tianyu Huang, Yao Zhang, and Liming Yang. 2025. "Insight into the Influence of Ecological Factors on Shaping Distribution Patterns of Camptotheca acuminata for Conservation and Management" Plants 14, no. 10: 1466. https://doi.org/10.3390/plants14101466
APA StyleWang, T., Li, Y., Manda, T., Lin, J., Huang, T., Zhang, Y., & Yang, L. (2025). Insight into the Influence of Ecological Factors on Shaping Distribution Patterns of Camptotheca acuminata for Conservation and Management. Plants, 14(10), 1466. https://doi.org/10.3390/plants14101466






