Functional Characterization of Anthocyanin Biosynthesis-Related Dihydroflavonol 4-reductase (DFR) Genes in Blueberries (Vaccinium corymbosum)
Abstract
1. Introduction
2. Results
2.1. Identification and Physiochemical Properties Analyses Results of VcDFRs
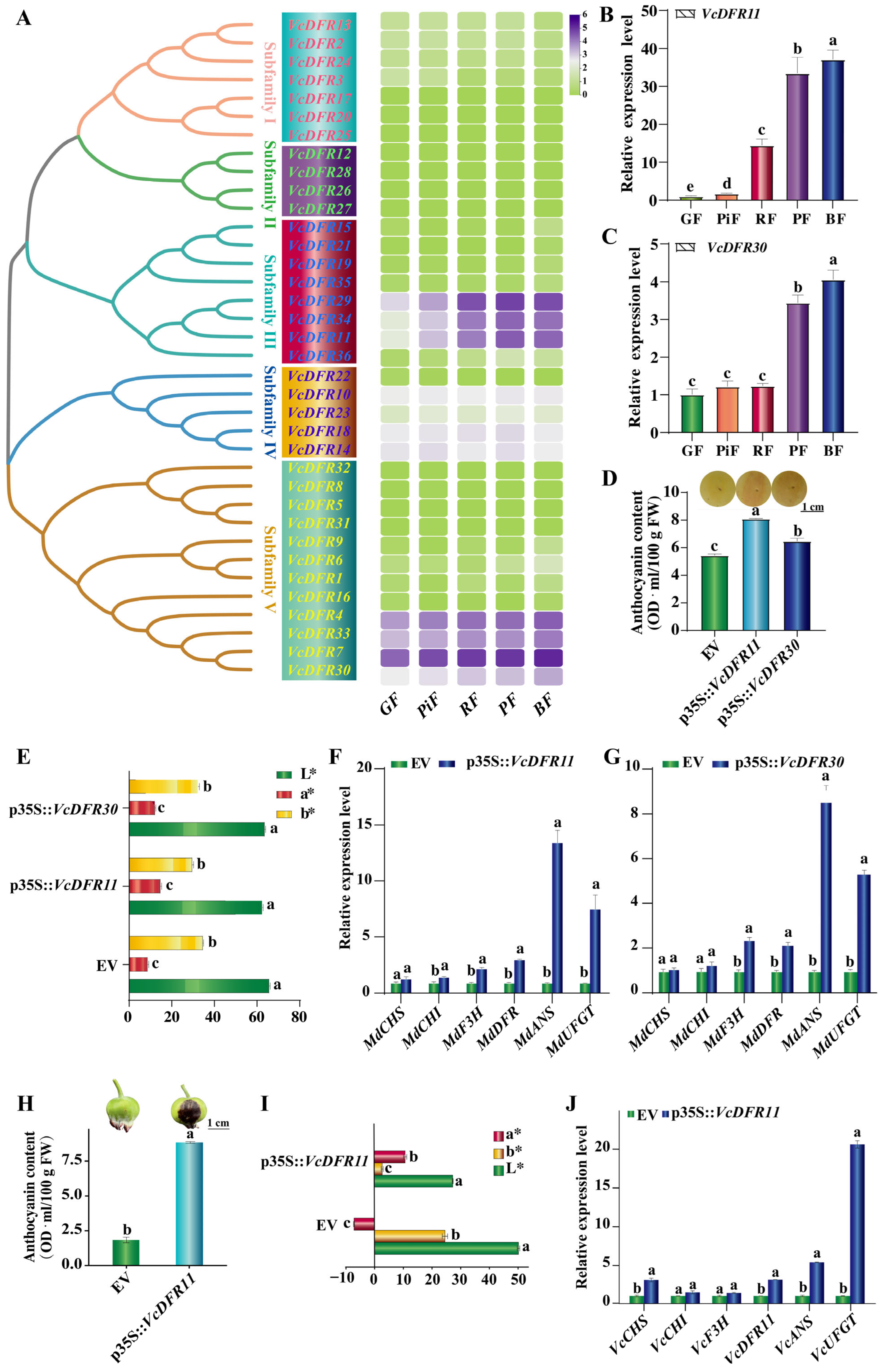
2.2. Phylogenetic and Sequence Similarity Analyses Results of VcDFRs
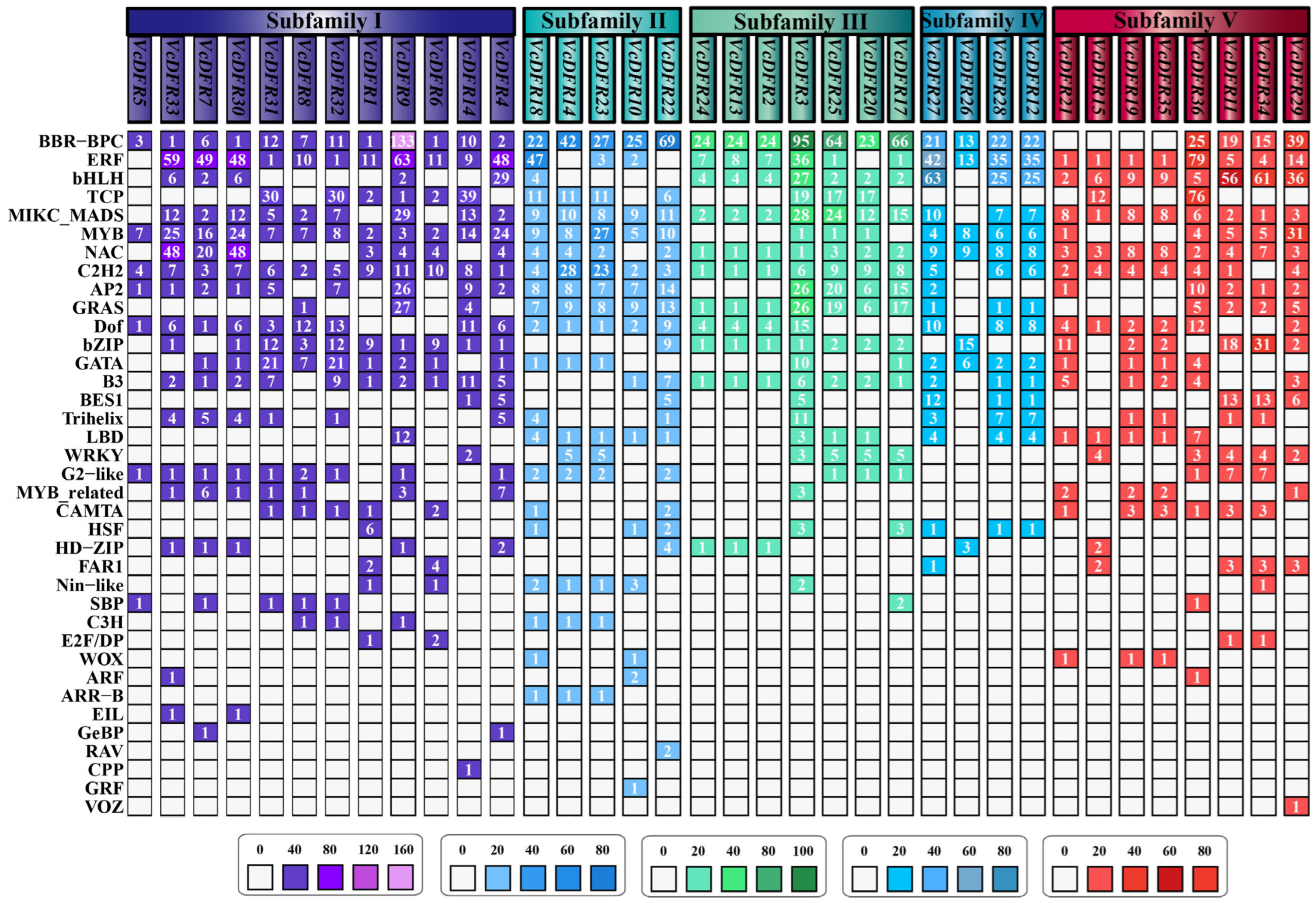
2.3. Synteny, Conserved Motif, and Gene Structure Analyses Results of VcDFRs
2.4. Promoter Analysis Results
2.5. Gene Expression Analysis of VcDFRs
2.6. Effects of Transient Overexpression of VcDFR11 and VcDFR30 on Anthocyanin Accumulations
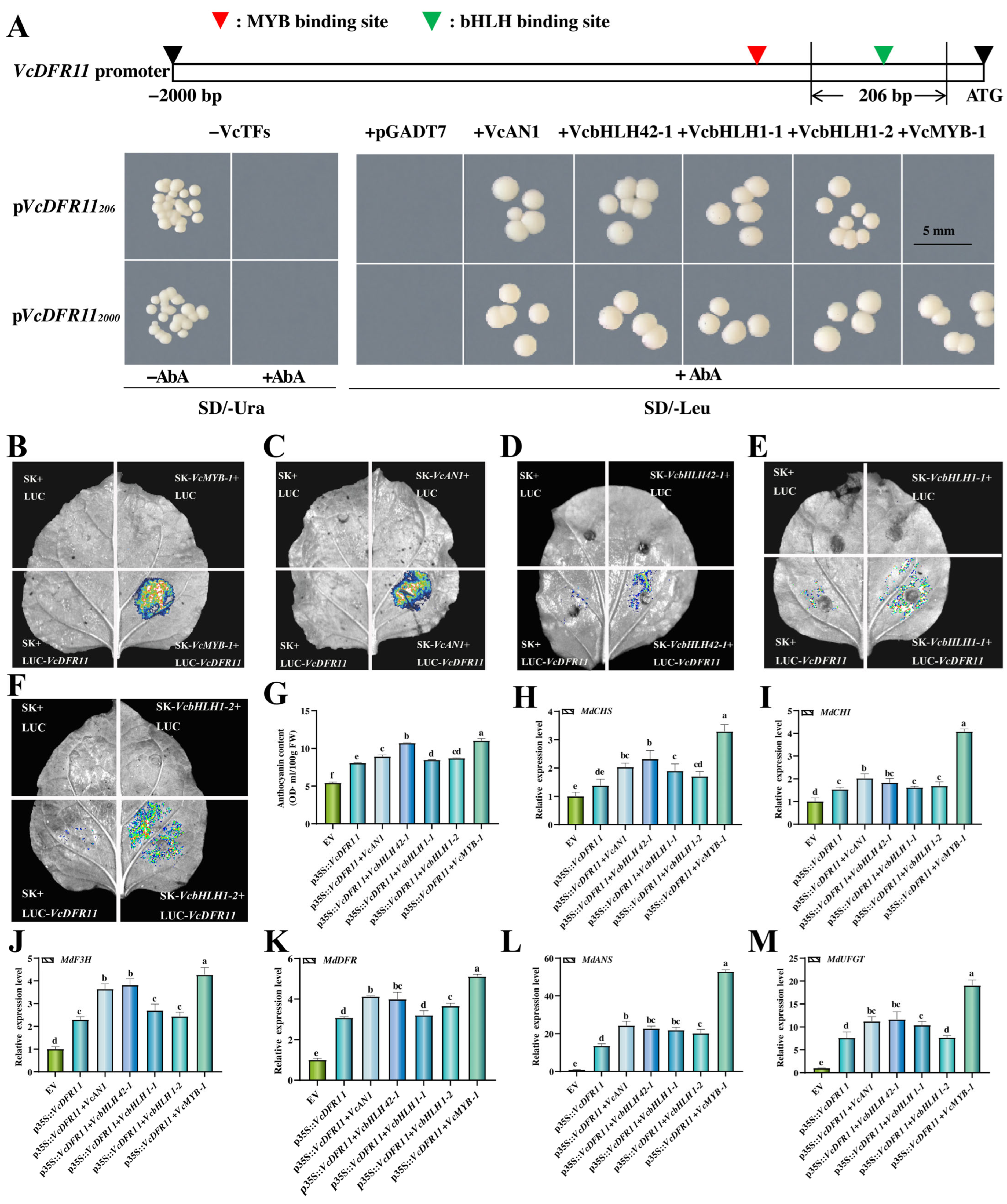
2.7. Anthocyanin-Related VcbHLHs and VcMYB-1 Can Bind to and Active the VcDFR11 Promoter
2.8. Co-Overexpression of Anthocyanin-Related TFs with VcDFR11 Exhibited Better Anthocyanin Accumulaiton-Promoting Effects than Overexpressing VcDFR11 Alone
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of Blueberry DFR Proteins
4.3. Physiochemical Property, Subcellular Localization, and Phylogenetic Analyses of VcDFRs
4.4. Synteny, Gene Structure and Conserved Motif Analyses
4.5. Promoter Analysis
4.6. RNA Isolation and Gene Cloning
4.7. Gene Expression Analysis
4.8. Vector Construction and Transient Overexpression Assays in Apple and Blueberry Fruits
4.9. DNA Isolation, VcDFR11 Promoter Cloning, and Yeast One Hybrid (Y1H)
4.10. Dual-Luciferase Assay (LUC)
4.11. Firefly Luciferase Complementation Imaging (LCI)
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino acid |
| ABA | Abscisic acid |
| ANS | Anthocyanindin synthase |
| BF | Blue fruit |
| bp | Base pair |
| CDS | Coding sequence |
| DFR | Dihydroflavonol 4-reductase |
| ET | Ethylene |
| GA | Gibberellin |
| GF | Green fruit |
| LCI | Firefly luciferase complementation imaging |
| LUC | Dual-luciferase assay |
| MeJA | Methyl jasmonate |
| PF | Purple fruit |
| PiF | Pink fruit |
| qRT-PCR | Quantitative real-time PCR |
| RF | Red fruit |
| RT-PCR | Reverse transcription PCR |
| SA | Salicylic acid |
| TF | Transcription factor |
| UFGT | UDP-glucose: flavonoid-3-O-glucosyltransferase |
| UTR | Untranslated region |
| Y1H | Yeast one hybrid |
References
- Johnson, E.T.; Ryu, S.; Yi, H.; Shin, B.; Cheong, H.; Choi, G. Alteration of a single amino acid changes the substrate specificity of dihydroflavonol 4-reductase. Plant J. 2001, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-Y.; Krishnakumar, V.; Chan, A.P.; Thibaud-Nissen, F.; Schobel, S.; Town, C.D. Araport11: A complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 2017, 89, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shi, G.; Ye, W.; Behera, J.R.; Kilaru, A.; Wang, L. Functional role of DFR genes in various blue Iris for the regulation of delphinidin synthesis. Plant Physiol. Biochem. 2025, 219, 109355. [Google Scholar] [CrossRef]
- Sepideh, R.; Bahman, Z.; Farhad, N.-F.; Ali, S. Expression analysis of anthocyanin biosynthesis key regulatory genes involved in pomegranate (Punica granatum L.). Sci. Hortic. 2015, 186, 84–88. [Google Scholar]
- Tian, J.; Chen, M.-c.; Zhang, J.; Li, K.-t.; Song, T.-t.; Zhang, X.; Yao, Y.-c. Characteristics of dihydroflavonol 4-reductase gene promoters from different leaf colored Malus crabapple cultivars. Hortic. Res. 2017, 4, 17070. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Wang, H.; Tian, Z.; Xin, S.; Zhu, P. The dihydroflavonol 4-reductase BoDFR1 drives anthocyanin accumulation in pink-leaved ornamental kale. Theor. Appl. Genet. 2021, 134, 159–169. [Google Scholar] [CrossRef]
- Shirley, B.W.; Hanley, S.; Goodman, H.M. Effects of ionizing radiation on a plant genome: Analysis of two Arabidopsis transparent testa mutations. Plant Cell 1992, 4, 333–347. [Google Scholar]
- Wang, X.; Chen, X.; Luo, S.; Ma, W.; Li, N.; Zhang, W.; Tikunov, Y.; Xuan, S.; Zhao, J.; Wang, Y.; et al. Discovery of a DFR gene that controls anthocyanin accumulation in the spiny Solanum group: Roles of a natural promoter variant and alternative splicing. Plant J. 2022, 111, 1096–1109. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Liu, Y.; Zhou, F.; Zhu, P. A single-base insertion in BoDFR1 results in loss of anthocyanins in green-leaved ornamental kale. Theor. Appl. Genet. 2022, 135, 1855–1865. [Google Scholar] [CrossRef]
- Jihye, K.; Won Je, L.; Tien Thanh, V.; Chan Young, J.; Suk-Whan, H.; Hojoung, L. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep. 2017, 36, 1215–1224. [Google Scholar]
- Ruan, H.; Shi, X.; Gao, L.; Rashid, A.; Li, Y.; Lei, T.; Dai, X.; Xia, T.; Wang, Y. Functional analysis of the dihydroflavonol 4-reductase family of Camellia sinensis: Exploiting key amino acids to reconstruct reduction activity. Hortic. Res. 2022, 9, uhac098. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Xiong, C.; Lin, A.; Zhang, C.; Sun, W.; Zhang, J.; Yang, C.; Lu, Y.; Li, H.; Ye, Z.; et al. SlBBX20 interacts with the COP9 signalosome subunit SlCSN5-2 to regulate anthocyanin biosynthesis by activating SlDFR expression in tomato. Hortic. Res. 2021, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, Y.; Guo, R.; Liu, J.; Jing, K.; Zuo, J.; Yuan, Y.; Jiang, F.; Dong, N. Integrated genomic and transcriptomic analyses reveal the genetic and molecular mechanisms underlying hawthorn peel color and seed hardness diversity. J. Genet. Genom. 2025. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Song, M.; Wang, Z.; Zhai, Y.; Hu, C.; Perl, A.; Ma, H. Independent flavonoid and anthocyanin biosynthesis in the flesh of a red-fleshed table grape revealed by metabolome and transcriptome co-analysis. BMC Plant Biol. 2023, 23, 361. [Google Scholar] [CrossRef]
- Yang, K.; Hou, Y.; Wu, M.; Pan, Q.; Xie, Y.; Zhang, Y.; Sun, F.; Zhang, Z.; Wu, J. DoMYB5 and DobHLH24, transcription factors involved in regulating anthocyanin accumulation in Dendrobium officinale. Int. J. Mol. Sci. 2023, 24, 7552. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffe, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- An, J.-P.; Zhang, X.-W.; Bi, S.-Q.; You, C.-X.; Wang, X.-F.; Hao, Y.-J. The ERF transcription factor MdERF38 promotes drought stress-induced anthocyanin biosynthesis in apple. Plant J. 2020, 101, 573–589. [Google Scholar] [CrossRef]
- Khan, I.A.; Cao, K.; Guo, J.; Li, Y.; Wang, Q.; Yang, X.; Wu, J.; Fang, W.; Wang, L. Identification of key gene networks controlling anthocyanin biosynthesis in peach flower. Plant Sci. 2022, 316, 111151. [Google Scholar] [CrossRef]
- Xu, C.; Xue, X.; Li, Z.; Chen, M.; Yang, Y.; Wang, S.; Shang, M.; Qiu, L.; Zhao, X.; Hu, W. The PpMYB75-PpDFR module reveals the difference between ‘SR’ and its bud variant ‘RMHC’ in peach red flesh. J. Plant Res. 2024, 137, 241–254. [Google Scholar] [CrossRef]
- Liu, Y.; Lin-Wang, K.; Espley, R.V.; Wang, L.; Li, Y.; Liu, Z.; Zhou, P.; Zeng, L.; Zhang, X.; Zhang, J.; et al. StMYB44 negatively regulates anthocyanin biosynthesis at high temperatures in tuber flesh of potato. J. Exp. Bot. 2019, 70, 3809–3824. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bian, R.; Zhang, Z.; Li, L.; Zhang, J. Transcription factors repressing anthocyanin biosynthesis in horticultural crops. Fruit Res. 2025, 5, e007. [Google Scholar] [CrossRef]
- Krishnanand, P.K.; Nicholi, V.; Purushothaman, N.; Sathya, E.; Massimo, I.; Umesh, K.R.; Kalpalatha, M. Admixture analysis using genotyping-by-sequencing reveals genetic relatedness and parental lineage distribution in highbush blueberry genotypes and cross derivatives. Int. J. Mol. Sci. 2021, 22, 163. [Google Scholar]
- Wu, Y.; Han, T.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Known and potential health benefits and mechanisms of blueberry anthocyanins: A review. Food Biosci. 2023, 55, 103050. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Guo, S.; Qu, P.; Liu, J.; Cheng, C. Characterization of blueberry glutathione S-transferase (GST) genes and functional analysis of VcGSTF8 reveal the role of ‘MYB/bHLH-GSTF’ module in anthocyanin accumulation. Ind. Crops Prod. 2024, 218, 119006. [Google Scholar] [CrossRef]
- Zifkin, M.; Jin, A.; Ozga, J.A.; Zaharia, L.I.; Schernthaner, J.P.; Gesell, A.; Abrams, S.R.; Kennedy, J.A.; Constabel, C.P. Gene Expression and metabolite profiling of developing highbush blueberry fruit indicates transcriptional regulation of flavonoid metabolism and activation of abscisic acid metabolism. Plant Physiol. 2012, 158, 200–224. [Google Scholar] [CrossRef]
- Yang, J.; Li, B.; Shi, W.; Gong, Z.; Chen, L.; Hou, Z. Transcriptional activation of anthocyanin biosynthesis in developing fruit of blueberries (Vaccinium corymbosum L.) by preharvest and postharvest UV irradiation. J. Agric. Food Chem. 2018, 66, 10931–10942. [Google Scholar] [CrossRef]
- Tang, Q.; Chi, F.-M.; Liu, H.-D.; Zhang, H.-J.; Song, Y. Single-molecule real-time and Illumina sequencing to analyze transcriptional regulation of flavonoid synthesis in blueberry. Front. Plant Sci. 2021, 12, 754325. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Q.; Chi, F.; Liu, H.; Zhang, H.; Song, Y. Sucrose non-fermenting1-related protein kinase VcSnRK2.3 promotes anthocyanin biosynthesis in association with VcMYB1 in blueberry. Front. Plant Sci. 2023, 14, 1018874. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Liu, H.; Wang, Q.; Feng, X.; Wang, C.; Sun, Y.; Zhang, X.; Zhu, S. Genome-wide identification of putative dihydroflavonol 4-reductase (DFR) gene family in eight Solanaceae species and expression analysis in Solanum lycopersicum. PeerJ 2023, 11, e16124. [Google Scholar] [CrossRef]
- Gupta, V.; Estrada, A.D.; Blakley, I.; Reid, R.; Patel, K.; Meyer, M.D.; Andersen, S.U.; Brown, A.F.; Lila, M.A.; Loraine, A.E. RNA-Seq analysis and annotation of a draft blueberry genome assembly identifies candidate genes involved in fruit ripening, biosynthesis of bioactive compounds, and stage-specific alternative splicing. Gigascience 2015, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, D.; Wang, B.; Yang, X.; Wu, H.; Qu, P.; Yan, L.; Li, T.; Cheng, C.; Qiu, D. Characterization of highbush blueberry (Vaccinium corymbosum L.) anthocyanin biosynthesis related MYBs and functional analysis of VcMYB gene. Curr. Issues Mol. Biol. 2023, 45, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, F.; Wang, B.; Wu, H.; Wu, J.; Liu, J.; Sun, Y.; Cheng, C.; Qiu, D. Identification, characterization and expression analysis of anthocyanin biosynthesis-related bHLH genes in blueberry (Vaccinium corymbosum L.). Int. J. Mol. Sci. 2021, 22, 13274. [Google Scholar] [CrossRef]
- Qian, X.; Zheng, W.; Hu, J.; Ma, J.; Sun, M.; Li, Y.; Liu, N.; Chen, T.; Wang, M.; Wang, L.; et al. Identification and expression analysis of DFR gene family in Brassica napus L. Plants 2023, 12, 2583. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Cai, X.; Shan, X.; Gao, R.; Yang, S.; Han, T.; Wang, S.; Wang, L.; Gao, X. Dihydroflavonol 4-reductase genes from Freesia hybrida play important and partially overlapping roles in the biosynthesis of flavonoids. Front. Plant Sci. 2017, 8, 428. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, H.; Gao, Q.; Li, Y.; Xiang, H.; Zeng, W.; Xu, L.; Liu, X.; Li, J.; Mi, Q.; et al. Effects of editing DFR genes on flowers, leaves, and roots of tobacco. BMC Plant Biol. 2023, 23, 349. [Google Scholar] [CrossRef]
- Lei, T.; Huang, J.; Ruan, H.; Qian, W.; Fang, Z.; Gu, C.; Zhang, N.; Liang, Y.; Wang, Z.; Gao, L.; et al. Competition between FLS and DFR regulates the distribution of flavonols and proanthocyanidins in Rubus chingii Hu. Front. Plant Sci. 2023, 14, 1134993. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Wu, Y.; Zheng, L.; Zhang, G. Regulatory mechanisms of anthocyanin biosynthesis in apple and pear. Int. J. Mol. Sci. 2021, 22, 8441. [Google Scholar] [CrossRef]
- Gao, H.-N.; Jiang, H.; Cui, J.-Y.; You, C.-X.; Li, Y.-Y. Review: The effects of hormones and environmental factors on anthocyanin biosynthesis in apple. Plant Sci. 2021, 312, 111024. [Google Scholar] [CrossRef]
- Ahmed, N.U.; Park, J.-I.; Jung, H.-J.; Yang, T.-J.; Hur, Y.; Nou, I.-S. Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene 2014, 550, 46–55. [Google Scholar] [CrossRef]
- Feyissa, B.A.; Arshad, M.; Gruber, M.Y.; Kohalmi, S.E.; Hannoufa, A. The interplay between miR156/SPL13 and DFR/WD40-1 regulate drought tolerance in alfalfa. BMC Plant Biol. 2019, 19, 434. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Le Gourrierec, J.; Baudry, A.; Huep, G.; Lanet, E.; Debeaujon, I.; Routaboul, J.-M.; Alboresi, A.; Weisshaar, B.; Lepiniec, L. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana. Plant J. 2008, 55, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Schaart, J.G.; Dubos, C.; De La Fuente, I.R.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.-M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, H.; Zhang, Y.; Feng, X.; Dai, Y.; Zhu, P. A novel three-layer module BoMYB1R1-BoMYB4b/BoMIEL1-BoDFR1 regulates anthocyanin accumulation in kale. Plant J. 2024, 119, 1737–1750. [Google Scholar] [CrossRef]
- Colle, M.; Leisner, C.P.; Wai, C.M.; Ou, S.; Bird, K.A.; Wang, J.; Wisecaver, J.H.; Yocca, A.E.; Alger, E.I.; Tang, H.; et al. Haplotype-phased genome and evolution of phytonutrient pathways of tetraploid blueberry. Gigascience 2019, 8, giz012. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Jaakola, L.; Maatta, K.; Pirttila, A.M.; Torronen, R.; Karenlampi, S.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, P.; Hao, S.; Li, R.; Zhang, Y.; Zhao, Q.; Wen, P.; Cheng, C. Characterization and functional analysis of chalcone synthase genes in highbush blueberry (Vaccinium corymbosum). Int. J. Mol. Sci. 2023, 24, 13882. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, J.; Ma, H.; Pei, L.; Liu, X.; Wang, H.; Chen, R.; Zhao, Z.; Guo, H. Changes in the VOC of fruits at different refrigeration stages of Ruixue and the participation of carboxylesterase MdCXE20 in the catabolism of volatile esters. Foods 2023, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, Y.; Hu, Y.; Yin, L.; Qu, P.; Wang, P.; Mu, X.; Zhang, S.; Xie, P.; Cheng, C.; et al. Comparison of bioactive compounds and antioxidant activities in differentially pigmented Cerasus humilis fruits. Molecules 2023, 28, 6272. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Guo, Z.; Li, H.; Mu, X.; Wang, P.; Zhang, S.; Yang, T.; Cai, H.; Wang, Q.; Lü, P.; et al. Integrated metabolic, transcriptomic and chromatin accessibility analyses provide novel insights into the competition for anthocyanins and flavonols biosynthesis during fruit ripening in red apple. Front. Plant Sci. 2022, 13, 975356. [Google Scholar] [CrossRef]
- Yan, P.; Tuo, D.; Shen, W.; Deng, H.; Zhou, P.; Gao, X. A Nimble cloning-compatible vector system for high-throughput gene functional analysis in plants. Plant Commun. 2023, 4, 100471. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Guo, S.; Zhang, Z.; Li, R.; Du, S.; Hao, S.; Cheng, C. Functional Characterization of Anthocyanin Biosynthesis-Related Dihydroflavonol 4-reductase (DFR) Genes in Blueberries (Vaccinium corymbosum). Plants 2025, 14, 1449. https://doi.org/10.3390/plants14101449
Zhang Y, Guo S, Zhang Z, Li R, Du S, Hao S, Cheng C. Functional Characterization of Anthocyanin Biosynthesis-Related Dihydroflavonol 4-reductase (DFR) Genes in Blueberries (Vaccinium corymbosum). Plants. 2025; 14(10):1449. https://doi.org/10.3390/plants14101449
Chicago/Turabian StyleZhang, Yongyan, Sijian Guo, Zening Zhang, Ruide Li, Shitao Du, Siyi Hao, and Chunzhen Cheng. 2025. "Functional Characterization of Anthocyanin Biosynthesis-Related Dihydroflavonol 4-reductase (DFR) Genes in Blueberries (Vaccinium corymbosum)" Plants 14, no. 10: 1449. https://doi.org/10.3390/plants14101449
APA StyleZhang, Y., Guo, S., Zhang, Z., Li, R., Du, S., Hao, S., & Cheng, C. (2025). Functional Characterization of Anthocyanin Biosynthesis-Related Dihydroflavonol 4-reductase (DFR) Genes in Blueberries (Vaccinium corymbosum). Plants, 14(10), 1449. https://doi.org/10.3390/plants14101449







