A Deep Dive into the Botanical and Medicinal Heritage of Taxus
Abstract
1. Introduction
1.1. Historical and Cultural Significance of Taxus
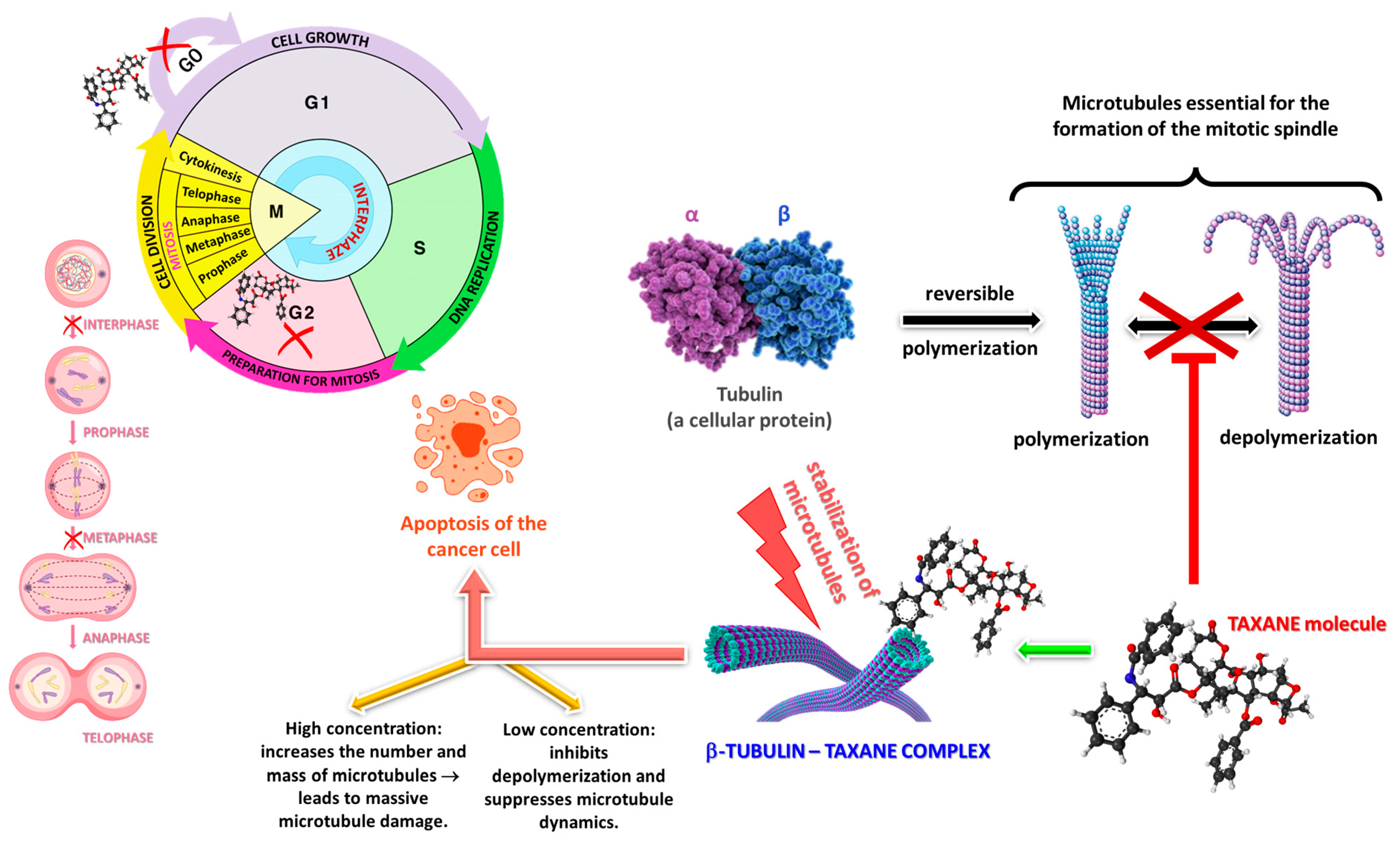
1.2. Pharmacological Importance and Discovery of Taxanes
1.3. Sustainability Concerns and Research Trends
1.4. Scope of This Review
2. Botanical Overview of Taxus Species
3. Traditional Uses in Folk Medicine
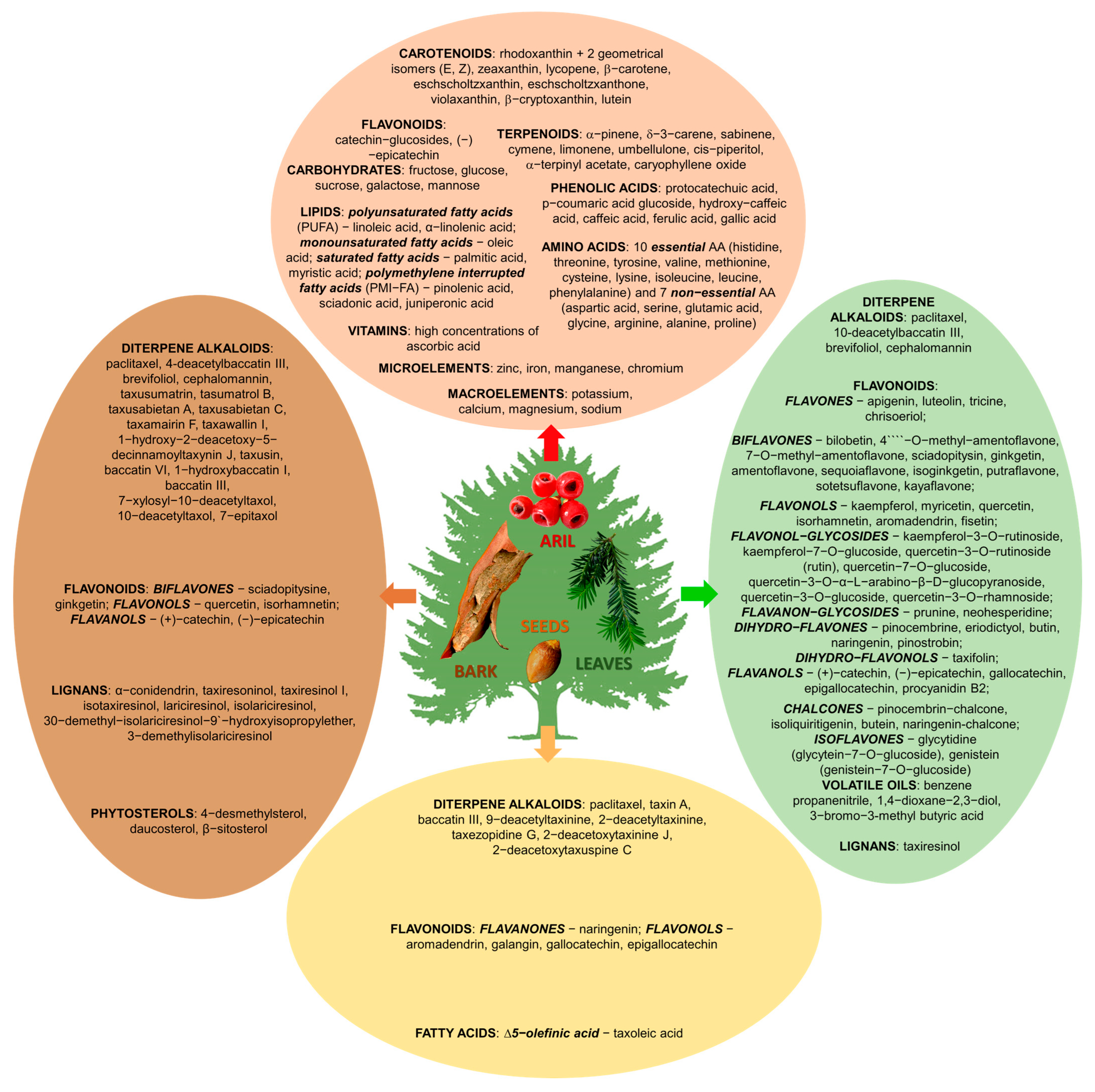
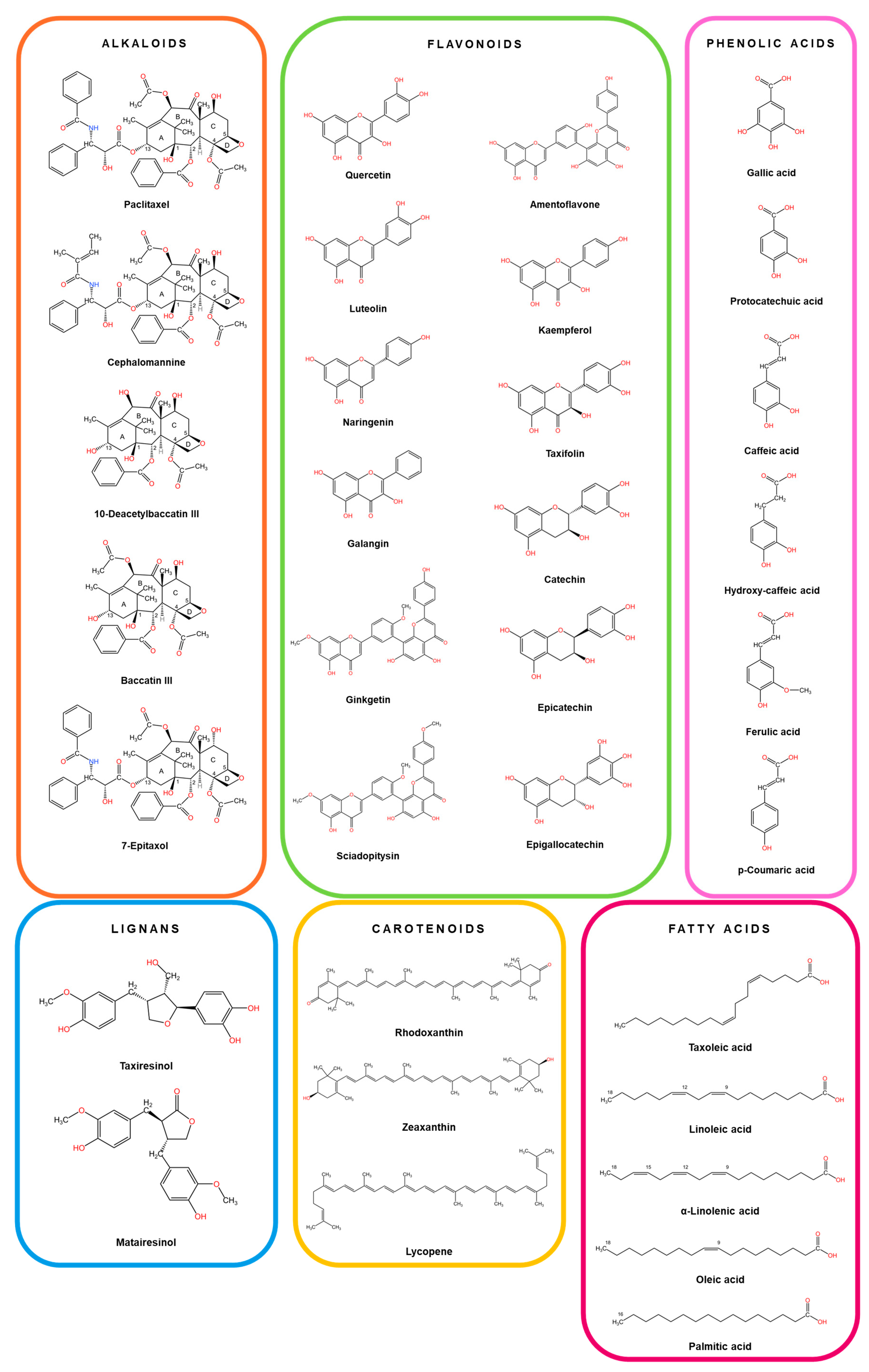
4. Phytochemistry of Taxus Species
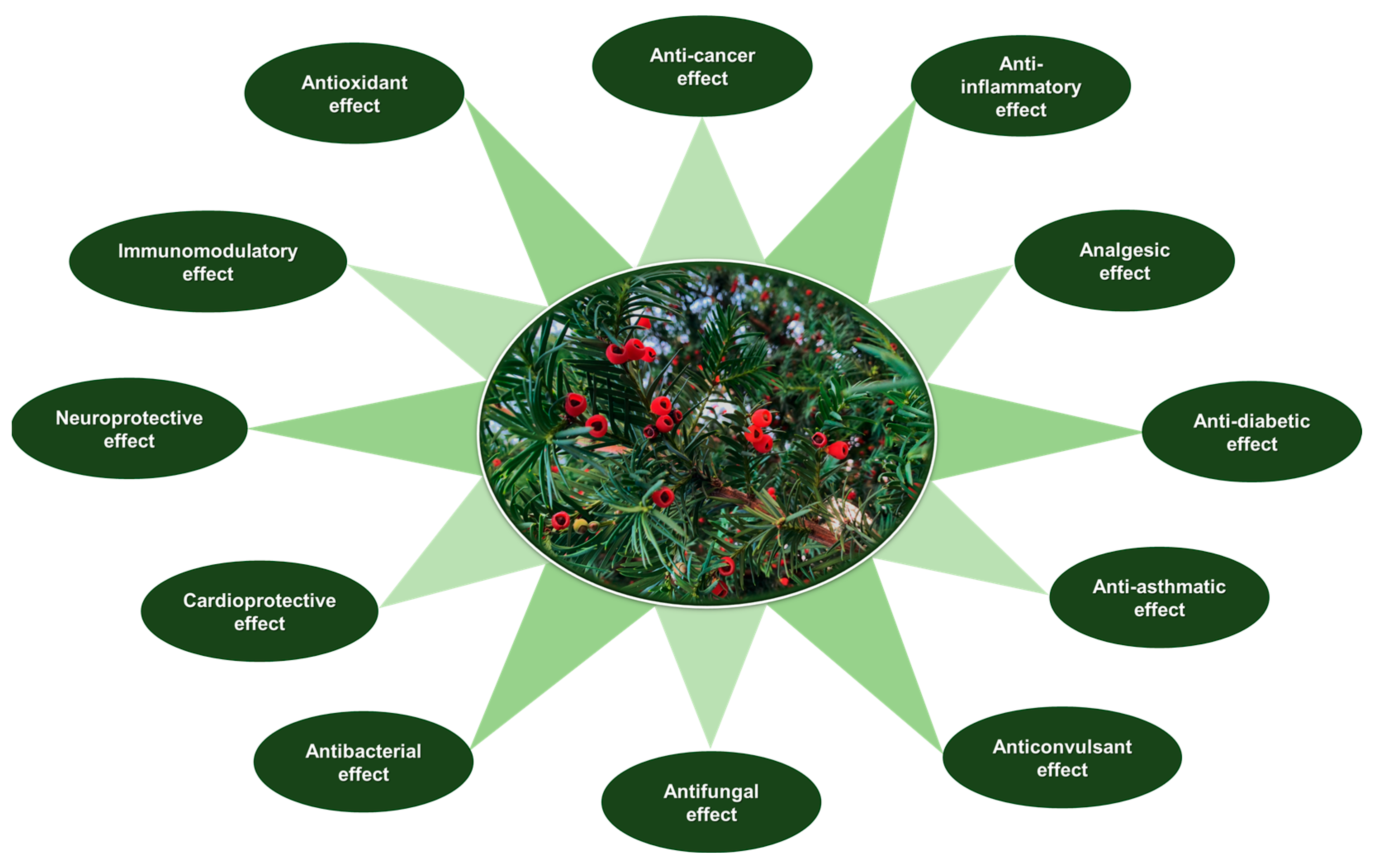
5. Pharmacological and Therapeutic Applications
5.1. Medicinal Benefits
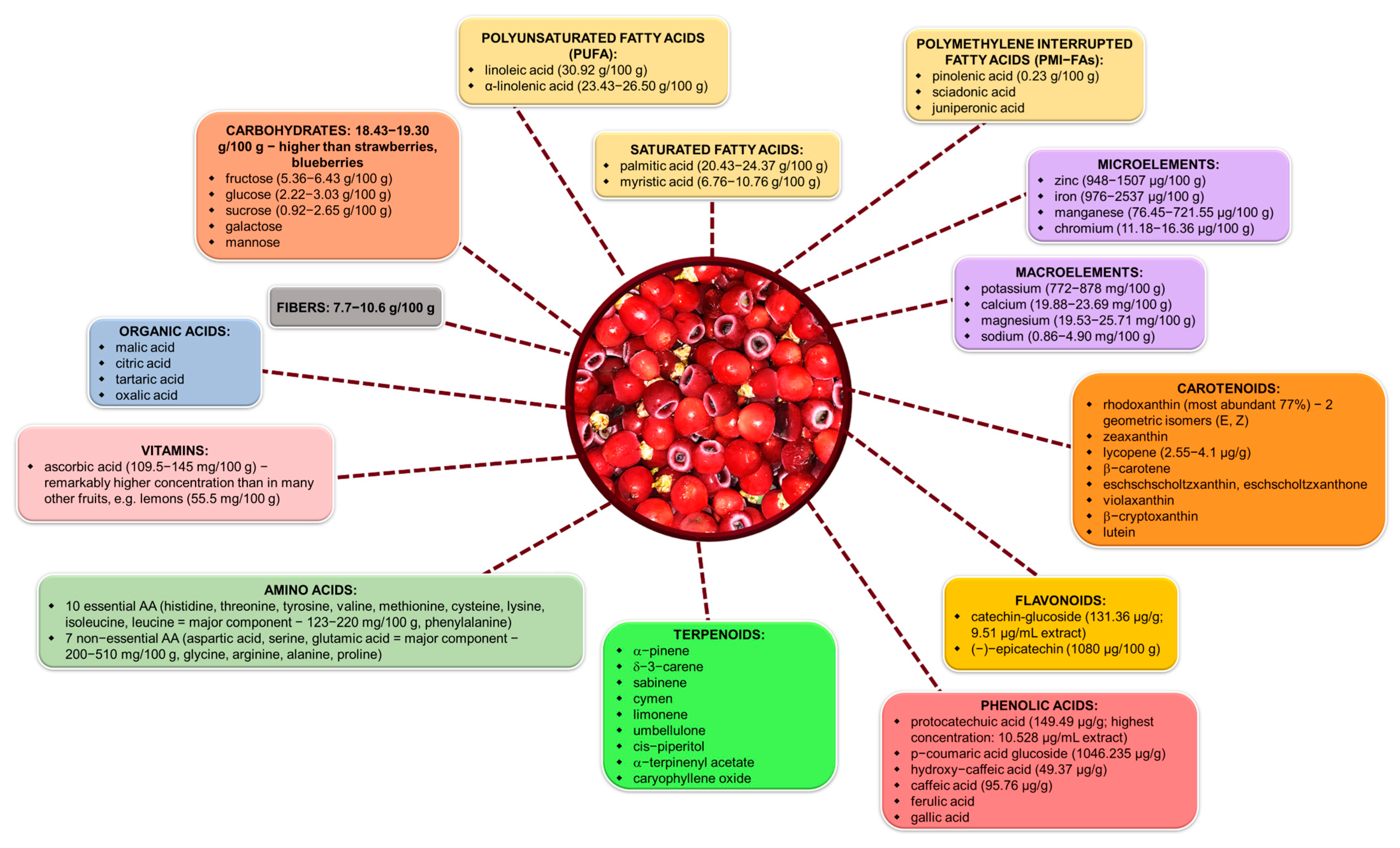
5.2. Nutritional Value and Food Potential of Taxus Arils
- α-Linolenic acid (ALA), a key omega-3 fatty acid with cardioprotective and anti-inflammatory effects, was identified at ~1800 mg/100 g in Taxus arils. This level, while lower than in chia (17,500 mg/100 g) or flaxseed (22,800 mg/100 g) [150,151], greatly exceeds ALA levels found in conventional nuts such as walnuts (900 mg/100 g) or almonds (~1.5 mg/100 g) [152], and is almost absent in most fruits and vegetables.
- Sciadonic acid, a rare polymethylene-interrupted fatty acid (PMI-FA) with emerging anti-inflammatory potential, is present at ~276 mg/100 g in the aril, a feature not shared by typical food plants [32].
- Total protein content in the aril (~9.5%) surpasses that of most fruits (e.g., avocado: 2%, banana: 1.1%) and aligns with levels seen in legumes like cooked quinoa (~8%) or green peas (5.4%) [153,154,155,156]. More importantly, the essential amino acid (EAA) to total amino acid ratio of 40.5% is on par with high-quality proteins such as egg (43%) or soy (36–38%) [32,157].
- Mineral content is also notable. The potassium concentration (~500 mg/100 g) exceeds that of banana (358 mg/100 g) [154] and compares favorably with avocado (485 mg/100 g) [153] and sweet potato (337 mg/100 g) [158]. The iron content (3.0 mg/100 g) surpasses that of spinach (1.05 mg/100 g) [159], but is lower than that in lentils (7.1 mg/100 g) (https://fdc.nal.usda.gov/food-details/2644283/nutrients (accessed on 4 May 2025)).
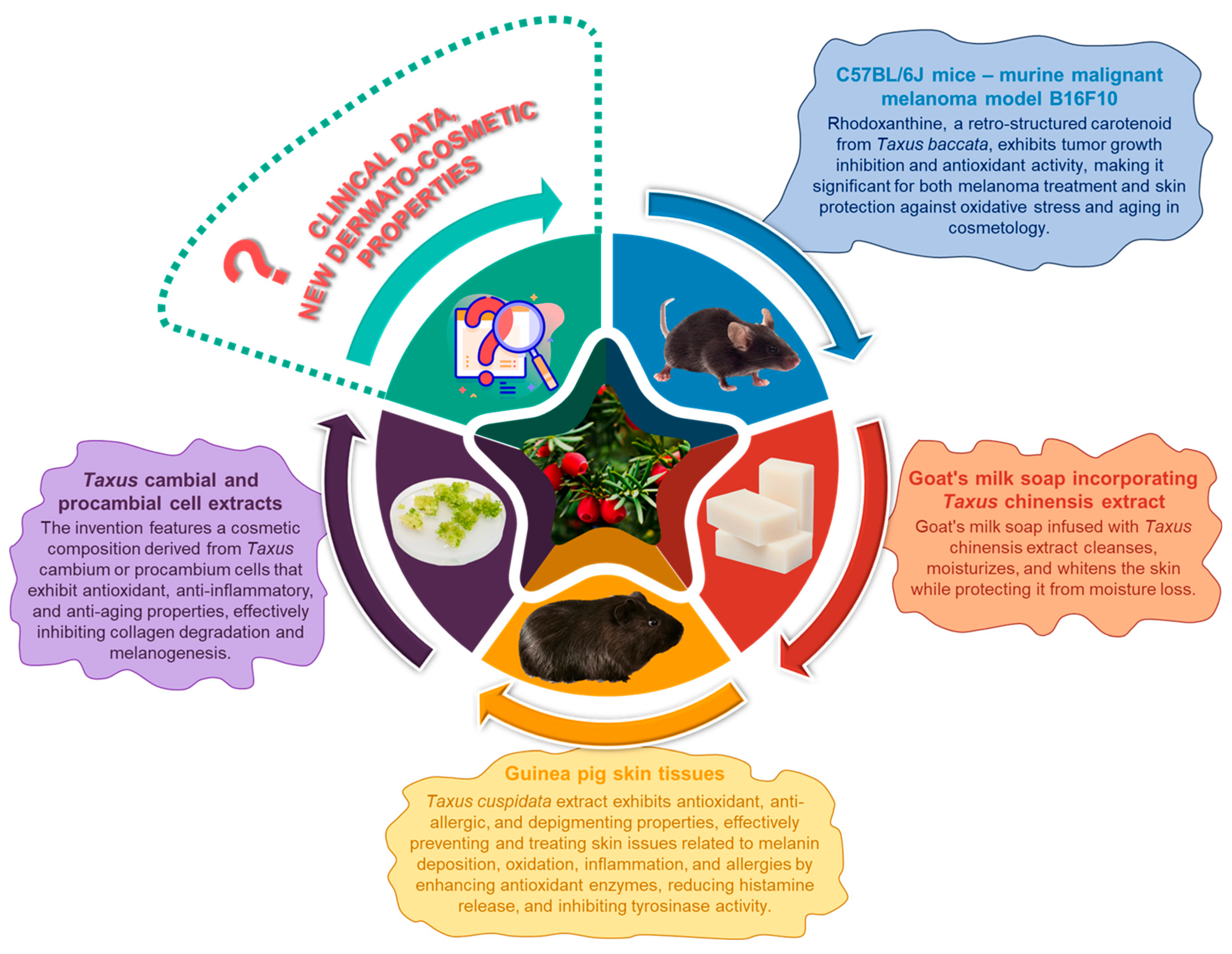
5.3. Dermatological Applications
- A cosmetic composition incorporating cultured cells from the cambial or procambium regions of Taxus stems: This invention pertains to a formulation exhibiting antioxidant, anti-inflammatory, and anti-aging properties. It comprises one or more cell lines derived from the cambial or procambium tissues of Taxus stems, alongside their respective extracts, lysates, and culture media. The authors of this invention have endeavored to formulate compositions grounded in natural compounds that demonstrate significant antioxidant and anti-inflammatory efficacy. The inventors have established that cell lines derived from Taxus cambium and Taxus procambium, as well as their extracts, possess remarkable capabilities to mitigate inflammation and impede the aging process of the skin, which constitutes the core premise of this invention. The extract and cell line culture medium derived from the cell lines described in the present invention demonstrate a significant capacity to inhibit the synthesis of matrix metalloproteinase 1 (MMP-1), exhibiting effects comparable to those of retinoic acid, which is widely recognized for its potent anti-aging properties. This finding implies that the invention effectively mitigates collagen degradation, thereby contributing to the prevention of skin aging and the reduction of wrinkles, positioning it as a valuable candidate for anti-aging applications. Furthermore, the invention has been shown to inhibit melanogenesis in mouse melanoma cells, indicating its potential as a depigmenting agent [161]. The outcomes presented in this patent hold considerable significance, with the inventors having successfully developed functional formulations based on these findings.
- 2.
- Goat’s milk soap incorporating Taxus chinensis extract: This invention pertains to a daily cleansing product, specifically a goat’s milk soap infused with Taxus chinensis extract. The formulation of the soap is as follows: 3–5 g of Taxus chinensis extract, 20–28 g of fresh goat’s milk, 0.8–1.2 g of virgin olive oil, 0.8–1.2 g of palm oil, 0.8–1.2 g of coconut oil, 0.8–1.2 g of mustard oil, and 45–50 g of soap base. The extract of Taxus chinensis is known to contain various skin-protective nutrients, while fresh goat’s milk is recognized for its skin-whitening properties. This soap formulation is designed to effectively cleanse the skin, promote whitening and moisturization, and retain moisture, thereby mitigating the risk of intracellular water loss [167].
6. Conclusions and Future Directions
- Exploration of underutilized plant parts, such as arils, to uncover novel bioactives with pharmacological potential.
- Development of green synthesis methods for taxanes, including metabolic engineering in microbial systems and tissue culture optimization.
- Investigation into combinatorial therapies, leveraging taxane synergy with other plant-derived compounds or modern drug delivery platforms, especially nanocarriers.
- Clinical translation of lesser-known compounds, such as biflavonoids and polymethylated fatty acids, for use in oncology, neurology, and immunology.
- Integrative omics approaches to map species-specific metabolic pathways and understand interspecies variability in phytochemical content.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | Adenosine Deaminase |
| Al | Aluminum |
| Cd | Cadmium |
| DNA | Deoxyribonucleic Acid |
| ED₅₀ | Median Effective Dose |
| FDA | Food and Drug Administration |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GLUT4 | Glucose Transporter Type 4 |
| GPX4 | Glutathione Peroxidase 4 |
| HDL | High-Density Lipoprotein |
| HIV | Human Immunodeficiency Virus |
| HPLC-MS/MS | High-Performance Liquid Chromatography–Tandem Mass Spectrometry |
| IC₅₀ | Half-Maximal Inhibitory Concentration |
| IL-1β | Interleukin 1-Beta |
| IUCN | International Union for Conservation of Nature |
| LDL | Low-Density Lipoprotein |
| MMP-1 | Matrix Metalloproteinase 1 |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| NCI | National Cancer Institute |
| Ni | Nickel |
| NSCLC | Non-Small Cell Lung Cancer |
| P-gp | P-Glycoprotein |
| PMI-FAs | Polymethylated Fatty Acids |
| RDA | Recommended Dietary Allowance |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SOD | Superoxide Dismutase |
| TNF-α | Tumor Necrosis Factor-Alpha |
| UPLC-ESI-MS/MS | Ultra-Performance Liquid Chromatography Coupled with Electrospray Ionization Tandem Mass Spectrometry |
| UV | Ultraviolet |
| WHO | World Health Organization |
References
- Benham, S.E.; Houston Durrant, T.; Caudullo, G.; de Rigo, D. Taxus baccata in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 183–184. [Google Scholar]
- Jia, X.; Feng, S.; Zhang, H.; Liu, X. Plastome Phylogenomics Provide Insight into the Evolution of Taxus. Forests 2022, 13, 1590. [Google Scholar] [CrossRef]
- Hageneder, F. Ancient Trees and Their Social Recognition: Taxus baccata L. In Yew and Ancient Kingship Rituals—European Traditions and Their Anatolian Roots. In Proceedings of the 1st International Yew Workshop of Turkey in Duzce, Düzce, Turkey, 28 September–4 October 2015. [Google Scholar]
- Cywa, K.; Kula, K. Problem of yew Taxus baccata L. wood toxicity. Xylological studies of medieval everyday objects from Poland. J. Archaeol. Sci. Rep. 2023, 49, 103921. [Google Scholar] [CrossRef]
- Uzquiano, P.; Allué, E.; Antolín, F.; Burjachs, F.; Picornel, L.; Piqué, R.; Zapata, L. All about yew: On the trail of Taxus baccata in southwest Europe by means of integrated palaeobotanical and archaeobotanical studies. Veg. Hist. Archaeobot. 2015, 24, 229–247. [Google Scholar] [CrossRef]
- Delahunty, J.L. The Ethnobotanical History and Holocene Extent of Yew (Taxus baccata L.) on the Irish Landscape. J. Ethnobiol. 2007, 27, 204–229. [Google Scholar] [CrossRef]
- Cusack, C.M. Scotland’s Sacred Tree: The Fortingall Yew. J. Syd. Soc. Sacred Hist. 2013, 3, 1–12. [Google Scholar]
- LIFE BACCATA. The Yew Tree, a Tree of Great Cultural and Ethnographic Value. LIFE BACCATA Project Website. 2021. Available online: https://www.life-baccata.eu/en/news/yew-tree-tree-great-cultural-and-ethnographic-value (accessed on 31 March 2025).
- Thomas, R. Leaves of the Tree: Scientific and Cultural Insights into Scotland’s Yew Trees. Scotland’s Yew Trees Website. Available online: https://scotlands-yew-trees.org/science-research/leaves-of-the-tree/ (accessed on 31 March 2025).
- Asturias.com. The Yew, an Ancient Tree. Available online: https://en.asturias.com/the-yew-an-ancient-tree/ (accessed on 31 March 2025).
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Quinn, M.; Plebanski, M. Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer. BioMed Res. Int. 2015, 2015, 413076. [Google Scholar] [CrossRef]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updates 2021, 54, 100742. [Google Scholar] [CrossRef]
- Swamy, M.K.; Pullaiah, T.; Chen, Z.-S. Paclitaxel: Sources, Chemistry, Anticancer Actions, and Current Biotechnology; Elsevier: Waltham, MA, USA, 2022. [Google Scholar]
- World Health Organization. WHO Model List of Essential Medicines, 23rd List; WHO: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02 (accessed on 30 March 2025).
- Fauzee, N.J.; Dong, Z.; Wang, Y.L. Taxanes: Promising Anti-Cancer Drugs. Asian Pac. J. Cancer Prev. 2011, 12, 2215–2224. [Google Scholar]
- Sousa-Pimenta, M.; Estevinho, L.M.; Szopa, A.; Basit, M.; Khan, K.; Armaghan, M.; Ibrayeva, M.; Sönmez Gürer, E.; Calina, D.; Hano, C.; et al. Chemotherapeutic Properties and Side-Effects Associated with the Clinical Practice of Terpene Alkaloids: Paclitaxel, Docetaxel, and Cabazitaxel. Front. Pharmacol. 2023, 14, 1157306. [Google Scholar] [CrossRef]
- de Oliveira, R.; Zhao, P.; Li, N.; de Santa Maria, L.C.; Vergnaud, J.; Ruiz, J.; Astruc, D.; Barratt, G. Synthesis and In Vitro Studies of Gold Nanoparticles Loaded with Docetaxel. Int. J. Pharm. 2013, 454, 703–711. [Google Scholar] [CrossRef]
- Sun, B.; Lovell, J.F.; Zhang, Y. Current Development of Cabazitaxel Drug Delivery Systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1854. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A. Cabazitaxel: A Novel Taxane for Metastatic Castration-Resistant Prostate Cancer—Current Implications and Future Prospects. J. Pharmacol. Pharmacother. 2013, 4, 230–237. [Google Scholar] [CrossRef]
- Katekar, R.; Singh, P.; Garg, R.; Verma, S.; Gayen, J.R. Emerging Nanotechnology-Based Combination Therapies of Taxanes for Multiple Drug-Resistant Cancers. Pharm. Dev. Technol. 2022, 27, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxidative Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef]
- Frisvold, G.B. Bioprospecting and Incentives for Biodiversity Conservation: Lessons from the History of Paclitaxel. In Sustainable Resource Development in the 21st Century: Essays in Memory of Peter Berck; Springer International Publishing: Cham, Switzerland, 2023; pp. 179–206. [Google Scholar]
- Yin, J.Y.; Lai, M.; Yu, X.Y.; Su, D.D.; Xiong, X.Y.; Li, Y.L. Comprehensive Strategies for Paclitaxel Production: Insights from Plant Cell Culture, Endophytic Microorganisms, and Synthetic Biology. Hortic. Res. 2024, 12, uhae346. [Google Scholar] [CrossRef]
- Data Bridge Market Research. Global Taxane Market—By Type, Application, Participants, and Countries: Forecast to 2029; DBMR: Pune, India, 2024; Available online: https://www.databridgemarketresearch.com/reports/global-taxane-market (accessed on 30 March 2025).
- Wahab, A.; Khera, R.A.; Rehman, R.; Mushtaq, A.; Blama, A.; Merzaia, M.W. A Review on Phytochemistry and Medicinal Uses of Taxus wallichiana L. (Himalayan Yew). Int. J. Chem. Biochem. Sci. 2016, 9, 116–120. [Google Scholar]
- Belwal, T.; Bhatt, I.D.; Devkota, H.P. (Eds.) Himalayan Fruits and Berries: Bioactive Compounds, Uses and Nutraceutical Potential; Academic Press: Cambridge, MA, USA, 2022; Chapter 39; pp. 419–429. [Google Scholar]
- Barrales-Cureño, H.J.; Ramos Valdivia, A.C.; Soto Hernández, M. Increased Production of Taxoids in Suspension Cultures of Taxus globosa after Elicitation. Future Pharmacol. 2022, 2, 45–54. [Google Scholar] [CrossRef]
- Bhuju, S.; Gauchan, D.P. Taxus wallichiana (Zucc.), an Endangered Anti-Cancerous Plant: A Review. Int. J. Res. 2018, 5, 10–21. [Google Scholar]
- Teibo, J.O.; Irozuru, C.E.; Teibo, T.K.; Omotoso, O.E.; Babalghith, A.O.; Batiha, G.E. Perspective Chapter: Appraisal of Paclitaxel (Taxol) Pros and Cons in the Management of Cancer–Prospects in Drug Repurposing. In Drug Repurposing—Advances, Scopes and Opportunities in Drug Discovery; IntechOpen: London, UK, 2023. [Google Scholar]
- Li, N.; Pan, Z.; Zhang, D.; Wang, H.X.; Yu, B.; Zhao, S.P.; Guo, J.J.; Wang, J.W.; Yao, L.; Cao, W.G. Chemical Components, Biological Activities, and Toxicological Evaluation of the Fruit (Aril) of Two Precious Plant Species from Genus Taxus. Chem. Biodivers. 2017, 14, e1700305. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Antoniewska, A.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Skoczeń-Słupska, R. Bioactive Components, Volatile Profile and In Vitro Antioxidative Properties of Taxus baccata L. Red Arils. Molecules 2021, 26, 4474. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Antoniewska, A.; Smoleń, S.; Łukasiewicz, M.; Baranowski, D.; Duda, I.; Pietsch, J. Red Arils of Taxus baccata L.—A New Source of Valuable Fatty Acids and Nutrients. Molecules 2021, 26, 723. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, E.; González-García, V.; Martín-Gil, J.; Lorenzo-Vidal, B.; Palacio-Bielsa, A.; Martín-Ramos, P. Phytochemical Screening and Antibacterial Activity of Taxus baccata L. against Pectobacterium spp. and Dickeya chrysanthemi. Horticulturae 2023, 9, 201. [Google Scholar] [CrossRef]
- Dumitraş, D.A.; Bunea, A.; Vodnar, D.C.; Hanganu, D.; Pall, E.; Cenariu, M.; Gal, A.F.; Andrei, S. Phytochemical Characterization of Taxus baccata L. Aril with Emphasis on Evaluation of the Antiproliferative and Pro-Apoptotic Activity of Rhodoxanthin. Antioxidants 2022, 11, 1039. [Google Scholar] [CrossRef]
- Poudel, R.C.; Gao, L.M.; Möller, M.; Baral, S.R.; Uprety, Y.; Liu, J.; Li, D.Z. Yews (Taxus) along the Hindu Kush-Himalayan Region: Exploring the Ethnopharmacological Relevance among Communities of Mongol and Caucasian Origins. J. Ethnopharmacol. 2013, 147, 190–203. [Google Scholar] [CrossRef]
- Nisar, M.; Khan, I.; Simjee, S.U.; Gilani, A.H.; Perveen, H. Anticonvulsant, Analgesic and Antipyretic Activities of Taxus wallichiana Zucc. J. Ethnopharmacol. 2008, 116, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D. Ethnobotanical and Pharmacological Importance of Taxus wallichiana Zucc. Plant Sci. Today 2020, 7, 122–134. [Google Scholar] [CrossRef]
- Adhikari, P.; Joshi, K.; Pandey, A. Taxus-Associated Fungal Endophytes: Anticancerous to Other Biological Activities. Fungal Biol. Rev. 2023, 45, 100308. [Google Scholar] [CrossRef]
- Akhalkatsi, M. Chapter 9.4.4. Code of Georgia: Yew Forest (Taxus baccata). In Plant Species in Natura 2000 Habitats in Georgia; Publisher of Plant Genetic Resources Division of Botany Institute: Tbilisi, Georgia, 2019; pp. 258–262. [Google Scholar]
- Poudel, R.C.; Kunwar, R.M.; Sher, H.; Ur-Rahman, I.; Bussmann, R.W.; Paniagua-Zambrana, N.Y. Taxus baccata (L.) Borkh., Taxus contorta Griff., Taxus mairei (Lemée & H. Lév.) S.Y. Hu, Taxus wallichiana—Taxaceae. In Ethnobotany of the Himalayas; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–14. [Google Scholar]
- Li, W.; Li, J.; Wei, J.; Niu, C.; Yang, D.; Jiang, B. Response of Photosynthesis, the Xanthophyll Cycle, and Wax in Japanese Yew (Taxus cuspidata L.) Seedlings and Saplings under High Light Conditions. PeerJ 2023, 11, e14757. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Feng, J.; Chen, C.; Chen, J.; Long, T.; Li, J.; Zang, R.; Li, J. Differential Responses to Climate and Land-Use Changes in Threatened Chinese Taxus Species. Forests 2019, 10, 766. [Google Scholar] [CrossRef]
- Novriyanti, E.; Susilo, A. Conservation Strategy for Taxus sumatrana, a Species with Limited Geographical Distribution yet Limitless Benefit and Economic Value. IOP Conf. Ser. Earth Environ. Sci. 2020, 533, 012006. [Google Scholar] [CrossRef]
- Ruprecht, H.; Dhar, A.; Aigner, B.; Oitzinger, G.; Klumpp, R.; Vacik, H. Structural Diversity of English Yew (Taxus baccata L.) Populations. Eur. J. For. Res. 2010, 129, 189–198. [Google Scholar] [CrossRef]
- Wang, T.; Li, L.; Qin, Y.; Lu, B.; Xu, D.; Zhuang, W.; Shu, X.; Zhang, F.; Wang, N.; Wang, Z. Effects of Seasonal Changes on Chlorophyll Fluorescence and Physiological Characteristics in the Two Taxus Species. Plants 2023, 12, 2636. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species. Search Results for: Taxus baccata; International Union for Conservation of Nature: Gland, Switzerland, 2024; Available online: https://www.iucnredlist.org/search?query=Taxus%20baccata (accessed on 30 March 2025).
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/search/list?taxonomies=120119&searchType=species (accessed on 3 May 2025).
- Cai, Q.; Song, Q.; Jiang, K.; Lin, Y.; Zhang, Y.; Zhang, J.; Lin, S.; Huang, L.; Xue, Q.; Huang, Z.; et al. Quality Evaluation of Compounds in Leaves of Six Taxus Species Based on UPLC-MS/MS and Chemometrics. Front. Chem. 2023, 11, 1193188. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Gao, L.-M.; Mill, R.R.; Liu, J.; Zhang, D.-Q.; Poudel, R.C.; Li, D.-Z. A Multidisciplinary Approach Reveals Hidden Taxonomic Diversity in the Morphologically Challenging Taxus wallichiana Complex. Taxon 2013, 62, 1161–1177. [Google Scholar] [CrossRef]
- Kýpeťová, M.; Walas, Ł.; Jaloviar, P.; Iszkuło, G. Influence of Herbivory Pressure on the Growth Rate and Needle Morphology of Taxus baccata L. Juveniles. Dendrobiol. 2017, 79, 10–19. [Google Scholar] [CrossRef]
- Juyal, D.; Thawani, V.; Thaledi, S.; Joshi, M. Ethnomedical Properties of Taxus wallichiana Zucc. (Himalayan Yew). J. Tradit. Complement. Med. 2014, 4, 159–161. [Google Scholar] [CrossRef]
- Sinha, D. A Review on Taxanes: An Important Group of Anticancer Compounds Obtained from Taxus sp. Int. J. Pharm. Sci. Res. 2020, 11, 1969–1985. [Google Scholar]
- Ya, Y.-H.; Ma, J.-W.; Ta, X.-L. Research Progress on the Source, Production, and Anti-Cancer Mechanisms of Paclitaxel. Chin. J. Nat. Med. 2020, 18, 890–897. [Google Scholar]
- Xiao, L.; Lao, W.G.; Tan, Y.; Qu, X. In Vitro Investigation of Anti-Diabetic Effect of Taxus cuspidata Extracts by Ultrasound Assisted Method. Am. J. Chin. Med. 2012, 40, 1205–1215. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Y.; Chen, K.; Wang, H.; Xie, W. Study on the Chemical Constituents, Pharmacological Activities, and Clinical Application of Taxus. Am. J. Chin. Med. 2024, 52, 1329–1357. [Google Scholar] [CrossRef]
- Binwal, M.; Babu, V.; Israr, K.M.; Kashyap, P.K.; Maurya, A.K.; Padalia, R.C.; Tandon, S.; Bawankule, D.U. Taxoids-Rich Extract from Taxus wallichiana Alleviates High-Fat Diet-Induced Insulin Resistance in C57BL/6 Mice through Inhibition of Low-Grade Inflammation. Inflammopharmacology 2023, 31, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Yu, W.; Xu, Q.; Liu, R.; Ying, S.; Dong, J.; Bao, Y.; Lyu, Q.; Shi, C.; Xia, J.; et al. Detoxification and underlying mechanisms towards toxic alkaloids by Traditional Chinese Medicine processing: A comprehensive review. Phytomedicine 2024, 129, 155623. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-L.; Zhang, Q.; Liu, J.; He, L.-Y.; Huang, Q.-W.; Peng, W.; Wu, C.-J. Processing methods and mechanisms for alkaloid-rich Chinese herbal medicines: A review. J. Integr. Med. 2021, 19, 89–103. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Q.; Zhao, Y.; Xiong, J.; Wang, F.; Zhang, T.; Zhang, C. Extraction, Purification, and Biological Activities of Polysaccharides from Branches and Leaves of Taxus cuspidata S. et Z. Molecules 2019, 16, 2926. [Google Scholar] [CrossRef]
- Khajuria, A.K.; Manhas, R.K.; Kumar, H.; Bisht, N.S. Ethnobotanical Study of Traditionally Used Medicinal Plants of Pauri District of Uttarakhand, India. J. Ethnopharmacol. 2021, 276, 114204. [Google Scholar] [CrossRef]
- Gahtori, R.; Tripathi, A.H.; Kumari, A.; Negi, N.; Paliwal, A.; Tripathi, P.; Joshi, P.; Rai, R.C.; Upadhyay, S.K. Anticancer Plant-Derivatives: Deciphering Their Oncopreventive and Therapeutic Potential in Molecular Terms. Future J. Pharm. Sci. 2023, 9, 14. [Google Scholar] [CrossRef]
- Stefanović, M.; Ristić, M.; Popović, Z.; Matić, R.; Nikolić, B.; Vidaković, V.; Obratov-Petković, D.; Bojović, S. Chemical Composition and Interpopulation Variability of Essential Oils of Taxus baccata L. from Serbia. Chem. Biodivers. 2016, 13, 943–953. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Q.; Dong, M.; Kiyota, H.; Gu, Y.; Cong, B. Natural Taxanes: Developments Since 1828. Chem. Rev. 2011, 111, 7652–7709. [Google Scholar] [CrossRef]
- Wei, Q.; Li, Q.; Wang, R. Flavonoid Components, Distribution, and Biological Activities in Taxus: A review. Molecules 2023, 28, 1713. [Google Scholar] [CrossRef]
- Aslam, M.; Handoo, S.A.; Bazaz, M.A.; Sharma, O.P.; Reshi, Z.A.; Khuraijam, J.S.; Siddiqi, T.O. Ethnomedicinal properties, phytoconstituents and biological utilization of Taxus wallichiana Zucc.: An overview. NeBIO Int. J. Environ. Biodivers. 2017, 8, 67–77. [Google Scholar]
- Wang, T.; Zhang, F.; Zhuang, W.; Shu, X.; Wang, Z. Metabolic Variations of Flavonoids in Leaves of T. media and T. mairei Obtained by UPLC-ESI-MS/MS. Molecules 2019, 24, 3323. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Conner, C.F. Taxanes and Taxoids of the Genus Taxus—A Comprehensive Inventory of Chemical Diversity. Phytochemistry 2021, 190, 112829. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, A.; Thakur, S.; Mutreja, V.; Bhardwaj, G. A brief review on phytochemistry and pharmacology of Taxus baccata L. Mater. Today Proc. 2022, 48, 1569–1574. [Google Scholar] [CrossRef]
- Kuo, W.; Chen, F.; Chen, K.; Chen, J. Taxusumatrin, a New Taxoid from the Stem Bark of Taxus sumatrana. Chem. Nat. Compd. 2015, 51, 427–430. [Google Scholar] [CrossRef]
- Hafezi, K.; Hemmati, A.A.; Abbaszadeh, H.; Valizadeh, A.; Makvandi, M. Anticancer activity and molecular mechanisms of α-conidendrin, a polyphenolic compound present in Taxus yunnanensis, on human breast cancer cell lines. Phytother. Res. 2020, 34, 1397–1408. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, N.; Xie, W. Research on the Medicinal Chemistry and Pharmacology of Taxus × media. Int. J. Mol. Sci. 2024, 25, 5756. [Google Scholar] [CrossRef]
- Frunzete, M.; Rodideal, T.; Grigore, M.; Ion, V.A.; Bădulescu, L.; Ciocan, R.M.; Zamfirache, M.M. Investigations on the chemical composition of volatile oils extracted from the leaves of spontaneous and cultivated Taxus baccata L. trees. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13383. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, K.; Jin, Y.C. Comparison of Chemical Composition and Antimicrobial Activities of the Essential Oil of Taxus media and Taxus chinensis Var. Mairei Leaves. Adv. Mater. Res. 2011, 343–344, 1092–1097. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Hosseinihashemi, S.K.; Jalaligoldeh, A.; Efhamisisi, D.; Mousavinezhad, S.H. Chemical Composition of Taxus baccata L. Leaves and Male Cones Water:Methanol Extracts. Celal Bayar Üniv. Bilim. Derg. 2020, 16, 251–255. [Google Scholar] [CrossRef]
- Yousaf, A.; Waseem, M.; Javed, A.; Baig, S.; Ismail, B.; Baig, A.; Ismail, B.; Baig, A.; Shahzadi, I.; Nawazish, S.; et al. Augmented anticancer effect and antibacterial activity of silver nanoparticles synthesized by using Taxus wallichiana leaf extract. PeerJ 2022, 10, e14391. [Google Scholar] [CrossRef]
- Wolff, R.L.; Pédrono, F.; Marpeau, A.M.; Christie, W.W.; Gunstone, F.D. The seed fatty acid composition and the distribution of Δ5-olefinic acids in the triacylglycerols of some Taxaceae (Taxus and Torreya). J. Am. Oil Chem. Soc. 1998, 75, 1637–1641. [Google Scholar] [CrossRef]
- Dumitraş, D.; Dreanca, A.I.; Pall, E.; Gal, A.F.; Rus, V.; Morohoschi, A.G.; Cotul, M.; Nan, M.; Andrei, S. Inhibition of Tumor Growth and Modulation of Antioxidant Activity of Rhodoxanthin Isolated from Taxus baccata Aril against B16F10 Murine Malignant Melanoma. Antioxidants 2022, 11, 2264. [Google Scholar] [CrossRef]
- Schex, R.; Lieb, V.M.; Schäfer, C.; Schweiggert, R.; Steingass, C.B. Carotenoid profiles of red- and yellow-colored arils of cultivars of Taxus baccata L. and Taxus × media Rehder. Phytochemistry 2021, 186, 112741. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Geng, W.; Wang, Z.; Wang, Q.; Pang, L.; Wang, B.; Wang, P.Q.; Qu, F.F.; Zhang, X.F. Analysis of the utilization value of different tissues of Taxus×Media based on metabolomics and antioxidant activity. BMC Plant Biol. 2023, 23, 285. [Google Scholar] [CrossRef]
- Mahmutović-Dizdarević, I.; Žilić, D.; Dukić, B. New insights into the antifungal activity of Taxus baccata L. Genet. Appl. 2019, 3, 65–70. [Google Scholar] [CrossRef]
- Gai, Q.; Jiao, J.; Wang, X.; Liu, J.; Fu, Y.; Lu, Y.; Wang, Z.; Xu, X. Simultaneous determination of taxoids and flavonoids in twigs and leaves of three Taxus species by UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 189, 113456. [Google Scholar] [CrossRef]
- Gu, Q.; Li, Y.; Chen, Y.; Yao, P.; Ou, T. Sciadopitysin: Active component from Taxus chinensis for anti-Alzheimer’s disease. Nat. Prod. Res. 2013, 27, 2157–2160. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Vaidya, A. Comprehensive review on pharmacological effects and mechanism of actions of taxifolin: A bioactive flavonoid. Pharmacol. Res.-Mod. Chin. Med. 2023, 7, 100240. [Google Scholar] [CrossRef]
- Sati, B.; Purohit, V.; Bhatt, S.; Andola, C. Isolation of bioactive compounds of Taxus baccata and Swertia chirata plants of Uttarakhand region by GC-MS. Int. J. Sci. Dev. Res. 2021, 6, 108–110. [Google Scholar]
- Kajani, A.A.; Zarkesh-Esfahani, S.H.; Bordbar, A.; Khosropour, A.R.; Razmjou, A.; Kardi, M. Anticancer effects of silver nanoparticles encapsulated by Taxus baccata extracts. J. Mol. Liq. 2016, 223, 549–556. [Google Scholar] [CrossRef]
- Xia, Q.; Ma, Y.; Wang, J. Biosynthesis of Silver Nanoparticles Using Taxus yunnanensis Callus and Their Antibacterial Activity and Cytotoxicity in Human Cancer Cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Meng, H.; Yang, H. Antidiabetic activity of Taxus cuspidata polysaccharides in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2012, 50, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Meimei, C.; Fengzhen, W.; Huangwei, L.; Candong, L.; Zhaoyang, Y. Discovery of Taxus chinensis fruit wine as potentially functional food against Alzheimer’s disease by UHPLC-QE-MS/MS, network pharmacology and molecular docking. J. Food Biochem. 2022, 46, 14502. [Google Scholar] [CrossRef] [PubMed]
- Barranco-Palma, C.I.; González-Trujano, M.E.; Martínez-Vargas, D.; Narváez-González, H.F.; Conde-Martínez, V.; Vibrans, H.; López-Upton, J.; Soto-Hernández, M. Phytochemical Profile of Taxus globosa Schltdl. and Its Anxiolytic, Antinociceptive, and Toxicological Evaluation in Mice. J. Ethnopharmacol. 2025, 342, 119383. [Google Scholar] [CrossRef]
- Lim, P.T.; Goh, B.H.; Lee, W.L. Taxol: Mechanisms of Action Against Cancer, an Update with Current Research. In Paclitaxel; Academic Press: Cambridge, MA, USA, 2022; pp. 47–71. [Google Scholar]
- Shao, F.; Wilson, I.W.; Qiu, D. The Research Progress of Taxol in Taxus. Curr. Pharm. Biotechnol. 2021, 22, 360–366. [Google Scholar] [CrossRef]
- Zhu, M.; Horbinski, C.; Garzotto, M.; Qian, D.; Beer, T.; Kyprianou, N. Tubulin-Targeting Chemotherapy Impairs Androgen Receptor Activity in Prostate Cancer. Cancer Res. 2010, 70, 7992–8002. [Google Scholar] [CrossRef]
- Taplin, M.; Balk, S. Has the Time Arrived for Biomarker-Directed Therapy in Castration-Resistant Prostate Cancer? JAMA Oncol. 2015, 1, 577. [Google Scholar] [CrossRef]
- Barbuti, A.; Chen, Z. Paclitaxel through the Ages of Anticancer Therapy: Exploring Its Role in Chemoresistance and Radiation Therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef]
- Thadani-Mulero, M.; Nanus, D.; Giannakakou, P. Androgen Receptor on the Move: Boarding the Microtubule Expressway to the Nucleus. Cancer Res. 2012, 72, 4611–4615. [Google Scholar] [CrossRef]
- Xiao, J.; Ma, Q.; Cai, R.; Miao, J.; Yan, Z.; Yang, X.; Chen, Y. Acute Anti-Cancer Activity of Crude Extracts from two Endophytic Fungi Chaetomium cochliodes and Penicillium Sp. in Cancer Cell Lines and Mice. Int. J. Pharmacol. 2022, 18, 1583–1592. [Google Scholar] [CrossRef]
- Durak, I.; Buber, S.; Durak, Z.; Devrim, E.; Kocaoglu, H. Aqueous extract from Taxus baccata inhibits adenosine deaminase activity significantly in cancerous and noncancerous human gastric and colon tissues. Pharmacogn. Mag. 2014, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, F.S.; Putri, D.E.; Putra, Y.U.; Hamidi, D. Cytotoxic activity of Taxus sumatrana (MIQ.) de Laub. bark, leaves, and shoots on HELA, T47D, and MCF-7/HER2 cell lines. Int. J. Appl. Pharm. 2024, 16, 93–98. [Google Scholar] [CrossRef]
- Bekhouche, M.; Benyammi, R.; Slaoui, M.K.; Krimat, S.; Paris, C.; Khelifi, L.; Morsli, A. Flavonoid Profile and Antioxidant Properties of Algerian Common Yew (Taxus baccata L.). Clin. Phytosci. 2022, 8, 17. [Google Scholar] [CrossRef]
- Ahmad, M.; Yaseen, M.; Bhat, A.; Ganai, B.A.; Zargar, M.A.; Ganie, S.A.; Qureshi, R. Taxus wallichiana as a potential in vitro antioxidant with good lethal effect on pathogenic bacterial strains. Am. J. Phytomed. Clin. Ther. 2015, 3, 209–221. [Google Scholar]
- Benlembarek, K.; Lograd, T.; Ramdani, M.; Figueredo, G.; Chalard, P. Chemical composition, antibacterial, antifungal and antioxidant activities of Taxus baccata essential oil from Algeria. Biodiversitas 2021, 22, 5475–5483. [Google Scholar] [CrossRef]
- Khan, D.M.; Bernaitis, L.; Shobha, K.L.; Ashok, M.; Shenoy, R.P. Antifungal activity of Taxus baccata, Phyllanthus debilis, Plectranthus amboinicus against Candida species of clinical origin. Int. J. Biol. Pharm. Res. 2013, 4, 386–389. [Google Scholar]
- Patel, P.; Patel, K.; Gandhi, T. Evaluation of Effect of Taxus baccata Leaves Extract on Bronchoconstriction and Bronchial Hyperreactivity in Experimental Animals. J. Young Pharm. 2011, 3, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, R.; Wang, S.; Dong, Z. Paclitaxel: New Uses for an Old Drug. Drug Des. Dev. Ther. 2014, 8, 279–284. [Google Scholar]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Af. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Jang, C.H.; Moon, N.; Lee, J.; Kwon, M.J.; Oh, J.; Kim, J.S. Luteolin synergistically enhances antitumor activity of Oxaliplatin in clorectal carcinoma via AMPK inhibition. Antioxidants 2022, 11, 626. [Google Scholar] [CrossRef]
- Al-Yamani, M.J.; Asdaq, S.M.B.; Alamri, A.S.; Alsanie, W.F.; Alhomrani, M.; Alsalman, A.J.; Al Hawaj, M.A.; Alanazi, A.A.; Alanzi, K.D.; Imran, M. The role of serotonergic and catecholaminergic systems for possible antidepressant activity of apigenin. Saudi J. Biol. Sci. 2022, 29, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kong, S.; Song, F.; Li, L.; Jiang, H. Pharmacokinetic study of luteolin, apigenin, chrysoeriol and diosmetin after oral administration of Flos Chrysanthemi extract in rats. Fitoterapia 2012, 83, 1616–1622. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Yang, Q.; Gong, X.; Ma, H.; Dang, K.; Chen, G.; Gao, X.; Feng, B. Analysis of Flavonoid Metabolites in Buckwheat Leaves Using UPLC-ESI-MS/MS. Molecules 2019, 24, 1310. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Wiwart, M. Antifungal activity of biflavones from Taxus Baccata and Ginkgo Biloba. Z. Naturforsch. C-A J. Biosci. 2003, 58, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.H.; Shieh, Y.D.; Hsu, L.C.; Kuo, L.M.Y.; Lin, J.H.; Liaw, C.C.; Kuo, Y.H. Naturally occurring cytotoxic [3′→ 8″-biflavonoids from Podocarpus nakaii. J. Tradit. Complement. Med. 2012, 2, 220–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suh, K.S.; Chon, S.; Jung, W.W.; Choi, E.M. Protective effects of sciadopitysin against methylglyoxal-induced degeneration in neuronal SK-N-MC cells. J. Appl. Toxicol. 2022, 42, 274–284. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, L.; Yin, W.; Nie, Y.; Zeng, P.; Yang, X. Anti-tumor effect of ginkgetin on human hepatocellular carcinoma cell lines by inducing cell cycle arrest and promoting cell apoptosis. Cell Cycle 2022, 21, 74–85. [Google Scholar] [CrossRef]
- Rizk, Y.S.; de Jesus Hardoim, D.; Santos, K.B.A.; Zaverucha-do-Valle, T.; Taniwaki, N.N.; Almeida-Souza, F.; Carollo, C.A.; Vannier-Santos, M.A.; de Arruda, C.C.P.; da Silva Calabrese, K. Amentoflavone isolated from Selaginella sellowii Hieron induces mitochondrial dysfunction in Leishmania amazonensis promastigotes. Parasitol. Int. 2022, 86, 102458. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Rabeh, M.A.; Raey, M.A.E.; El-Kadder, E.M.A.; Sobeh, M.; Abdelmohsen, U.R.; Albohy, A.; Andrianov, A.M.; Bosko, I.P.; Al-Sanea, M.M.; et al. Metabolomic profiling of three Araucaria species, and their possible potential role against COVID-19. J. Biomol. Struct. Dyn. 2022, 40, 6426–6438. [Google Scholar] [CrossRef]
- Shao, N.; Feng, Z.; Li, N. Isoginkgetin inhibits inflammatory response in the fibroblast-like synoviocytes of rheumatoid arthritis by suppressing matrix metallopeptidase 9 expression. Chem. Biol. Drug Des. 2022, 99, 923–929. [Google Scholar] [CrossRef]
- Martínez, G.; Mijares, M.R.; De Sanctis, J.B. Effects of flavonoids and its derivatives on immune cell responses. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, Y.; Cheng, Z.; Hu, Y.; Liu, T. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-α/NF-κB and PI3K/AKT signaling pathway. Cell Death Discov. 2018, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, M.; Sayedipour, S.; Pourazar, A.; Shanehsazzadeh, M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res. Pharm. Sci. 2014, 9, 463. [Google Scholar] [PubMed]
- Peng, S.; Fang, C.; He, H.; Song, X.; Zhao, X.; Zou, Y.; Li, L.; Jia, R.; Yin, Z. Myricetin exerts its antiviral activity against infectious bronchitis virus by inhibiting the deubiquitinating activity of papain-like protease. Poult. Sci. 2022, 101, 101626. [Google Scholar] [CrossRef]
- Escribano-Ferrer, E.; Queralt Regue, J.; Garcia-Sala, X.; Boix Montanes, A.; Lamuela-Raventos, R.M. In vivo anti-inflammatory and antiallergic activity of pure naringenin, naringenin chalcone, and quercetin in mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Zhan, Y.; Ta, W.; Tang, W.; Hua, R.; Wang, J.; Wang, C.; Lu, W. Potential antiviral activity of isorhamnetin against SARS-CoV-2 spike pseudotyped virus in vitro. Drug Dev. Res. 2021, 82, 1124–1130. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, E.N.; Jeong, G.S. Aromadendrin protects neuronal cells from methamphetamine-induced neurotoxicity by regulating endoplasmic reticulum stress and PI3K/Akt/mTOR signaling pathway. Int. J. Mol. Sci. 2021, 22, 2274. [Google Scholar] [CrossRef]
- Liana, L.; Rizal, R.; Widowati, W.; Fioni, F.; Akbar, K.; Fachrial, E.; Lister, I.N.E. Antioxidant and anti-hyaluronidase activities of dragon fruit peel extract and kaempferol-3-o-rutinoside. J. Kedokt. Brawijaya 2019, 30, 247–252. [Google Scholar] [CrossRef]
- Sharma, S.; Dahiya, A.; Kumar, S.; Verma, Y.K.; Dutta, A. Quercetin 3-O-rutinoside prevents radiation induced oxidative damage and inflammation by coordinated regulation of Nrf2/NF-κB/NLRP3-inflammasome signaling in gastrointestine. Phytomed. Plus 2023, 3, 100385. [Google Scholar] [CrossRef]
- Gansukh, E.; Kazibwe, Z.; Pandurangan, M.; Judy, G.; Kim, D.H. Probing the impact of quercetin-7-O-glucoside on influenza virus replication influence. Phytomedicine 2016, 23, 958–967. [Google Scholar] [CrossRef]
- Tang, P.; Tang, Y.; Liu, Y.; He, B.; Shen, X.; Zhang, Z.J.; Qin, D.L.; Tian, J. Quercetin-3-O-α-L-arabinopyranosyl-(1→2)-β-D-glucopyra-noside isolated from Eucommia ulmoides oliver leaf relieves insulin resistance in HepG2 cells via the IRS-1/PI3K/Akt/GSK-3β pathway. Biol. Pharm. Bull. 2022, b22, 00597. [Google Scholar]
- Wu, D.; Duan, R.; Tang, L.; Hu, X.; Geng, F.; Sun, Q.; Zhang, Y.; Li, H. Binding mechanism and functional evaluation of quercetin 3-rhamnoside on lipase. Food Chem. 2021, 15, 129960. [Google Scholar] [CrossRef] [PubMed]
- Na, E.J.; Ryu, J.Y. Anti-inflammatory effects of prunin on UVB-irradiated human keratinocytes. Biomed. Dermatol. 2018, 2, 14. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, S.; Ma, P.; Che, D.; Liu, R.; Zhang, Y.; Wang, J.; Li, C.; Ding, Y.; Fu, J. Neohesperidin suppresses IgE-mediated anaphylactic reactions and mast cell activation via Lyn-PLC-Ca2+ pathway. Phytother. Res. 2019, 33, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Le Lee, J.; Loe, M.W.; Lee, R.C.; Chu, J.J. Antiviral activity of pinocembrin against Zika virus replication. Antivir. Res. 2019, 167, 13–24. [Google Scholar] [CrossRef]
- Omer, A.B.; Dalhat, M.H.; Khan, M.K.; Afzal, O.; Altamimi, A.S.; Alzarea, S.I.; Almalki, W.H.; Kazmi, I. Butin mitigates memory impairment in Streptozotocin-Induced diabetic rats by inhibiting oxidative stress and inflammatory responses. Metabolites 2022, 12, 1050. [Google Scholar] [CrossRef]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-inflammatory and anticancer activities of Naringenin-loaded liquid crystalline nanoparticles in vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef]
- Yoon, J.H.; Youn, K.; Jun, M. Discovery of pinostrobin as a melanogenic agent in cAMP/PKA and p38 MAPK signaling pathway. Nutrients 2022, 14, 3713. [Google Scholar] [CrossRef]
- Rehman, K.; Chohan, T.A.; Waheed, I.; Gilani, Z.; Akash, M.S.H. Taxifolin prevents postprandial hyperglycemia by regulating the activity of α-amylase: Evidence from an in vivo and in silico studies. J. Cell. Biochem. 2019, 120, 425–438. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, Y.; Xiong, J.; Wang, F.; Xiao, L.; Bao, S.; Yu, X. Extraction, purification, and biological activities of flavonoids from branches and leaves of Taxus cuspidata S. et Z. BioResources 2021, 16, 2655–2682. [Google Scholar] [CrossRef]
- Vue, B.; Zhang, S.; Chen, Q.H. Flavonoids with therapeutic potential in prostate cancer. Anticancer Agents Med. Chem. 2016, 16, 1205–1229. [Google Scholar] [CrossRef] [PubMed]
- Veselova, M.V.; Fedoreev, S.A.; Vasilevskaya, N.A.; Denisenko, V.A.; Gerasimenko, A.V. Antioxidant activity of polyphenols from the far-east plant Taxus cuspidata. Pharm. Chem. J. 2007, 41, 88–93. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Shen, Z.; Zhou, Q.; Wang, M.F. Identification of the antiglycative components of Hong Dou Shan (Taxus chinensis) leaf tea. Food Chem. 2019, 297, 124942. [Google Scholar] [CrossRef]
- Elbaz, H.A.; Lee, I.; Antwih, D.A.; Liu, J.; Hüttemann, M.; Zielske, S.P. Epicatechin stimulates mitochondrial activity and selectively sensitizes cancer cells to radiation. PLoS ONE 2014, 9, e88322. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.A.; Shin, Y.S.; Kang, S.U.; Kim, J.H.; Oh, Y.T.; Park, K.H.; Lee, B.H.; Kim, C.H. Radioprotective effect of epicatechin in cultured human fibroblasts and zebrafish. J. Radiat. Res. 2014, 55, 32–40. [Google Scholar] [CrossRef]
- Xiao, T.; Cui, M.; Zheng, C.; Zhang, P.; Ren, S.; Bao, J.; Gao, D.; Sun, R.; Wang, M.; Lin, J.; et al. Both baicalein and gallocatechin gallate effectively inhibit SARS-CoV-2 replication by targeting M pro and sepsis in mice. Inflammation 2022, 45, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, H.; Wang, J.; Zeng, J.; Tan, Y.; Wang, Y.; Yu, X.; Li, W.; Wang, P.; Yang, Z.; et al. Procyanidin B2 improves endothelial progenitor cell function and promotes wound healing in diabetic mice via activating Nrf2. J. J. Cell. Mol. Med. 2021, 25, 652–665. [Google Scholar] [CrossRef]
- Ruddock, P.S.; Charland, M.; Ramirez, S.; López, A.; Towers, G.N.; Arnason, J.T.; Liao, M.; Dillon, J.A.R. Antimicrobial activity of flavonoids from Piper lanceaefolium and other Colombian medicinal plants against antibiotic susceptible and resistant strains of Neisseria gonorrhoeae. Sex. Transm. Dis. 2011, 38, 82–88. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Alshammari, G.M.; Ajarem, J.S.; Al-Zahrani, A.Y.; Alzuwaydi, A.; Eid, R.; Yahya, M.A. Isoliquiritigenin prevents Doxorubicin-induced hepatic damage in rats by upregulating and activating SIRT1. Biomed. Pharmacother. 2022, 146, 112594. [Google Scholar] [CrossRef]
- Gao, L.; Cui, S.; Huang, Z.; Cui, H.; Alahmadi, T.A.; Manikandan, V. Antinociceptive and anti-inflammatory activities of butein in different nociceptive and inflammatory mice models. Saudi J. Biol. Sci. 2021, 28, 7090–7097. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, S.; Jiang, K.; Wang, Y.; Yang, M.; Guo, M. Glycitin alleviates lipopolysaccharide-induced acute lung injury via inhibiting NF-κB and MAPKs pathway activation in mice. Int. Immunopharmacol. 2019, 75, 105749. [Google Scholar] [CrossRef]
- Tokalov, S.V.; Abramyuk, A.M.; Abolmaali, N.D. Protection of p53 wild type cells from taxol by genistein in the combined treatment of lung cancer. Nutr. Cancer 2010, 62, 795–801. [Google Scholar] [CrossRef]
- Chen, M.; Wang, F.; Lei, H.; Yang, Z.; Li, C. In Silico Insights into Micro-Mechanism Understanding of Extracts of Taxus chinensis Fruits Against Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 97, 727–740. [Google Scholar] [CrossRef]
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/2262075/nutrients (accessed on 4 May 2025).
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/2710819/nutrients (accessed on 4 May 2025).
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef] [PubMed]
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/2710824/nutrients (accessed on 4 May 2025).
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/1105314/nutrients (accessed on 4 May 2025).
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/2512372/nutrients (accessed on 4 May 2025).
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/2644291/nutrients (accessed on 4 May 2025).
- Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation, Geneva, Switzerland, 2002; WHO Technical Report Series No. 935; World Health Organization: Geneva, Switzerland, 2007; Available online: https://iris.who.int/handle/10665/43411 (accessed on 4 May 2025).
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/2346404/nutrients (accessed on 4 May 2025).
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/1999633/nutrients (accessed on 4 May 2025).
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, D.K.; So, Y.M.; Li, Y.K.; Dzin, J.V. Cosmetic Composition Containing Culture Cells of Yew (Taxus) Stem Cambium or Procambium. RU2520606C2, 2009. Available online: https://patents.google.com/patent/RU2520606C2/en (accessed on 30 March 2025).
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Ramawat, K.G. Nutraceuticals and Antioxidants in Prevention of Diseases. Nat. Prod. 2013, 2559–2580. [Google Scholar]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of plant extracts cosmetics in the field of anti-aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Ng, C.; Yen, H.; Hsiao, H.; Su, S. Phytochemicals in Skin Cancer Prevention and Treatment: An Updated Review. Int. J. Mol. Sci. 2018, 19, 941. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, L.; Sun, H.; Chen, C.; Feng, J.; Chen, Y.; Lin, Y.; Kopylov, P.; Wang, Q.; Zhang, Y. Pharmacodynamics of frigid zone plant Taxus cuspidata S. et Z. against skin melanin deposition, oxidation, inflammation and allergy. Frigid Zone Med. 2023, 3, 42–52. [Google Scholar] [CrossRef]
- Chen, F. Taxus chinensis Goat Milk Soap, and Preparation Method Thereof. CN105602766A, 2015. Hangzhou Kaixiai Biotechnology Co. Ltd. Available online: https://patents.google.com/patent/CN105602766A/en (accessed on 30 March 2025).

| Species | IUCN Status | Geographic Distribution |
|---|---|---|
| Taxus wallichiana | Endangered | Himalayas: Afghanistan, India, Nepal, Bhutan, southern China |
| Taxus chinensis | Endangered | Southern China (Yunnan, Guizhou, Sichuan), northern Vietnam |
| Taxus calcicola | Vulnerable | China (Yunnan, Guizhou) |
| Taxus baccata | Least concern | Europe, North Africa, Western Asia |
| Taxus brevifolia | Near threatened | Pacific Northwest (USA: California to Alaska), British Columbia |
| Taxus canadensis | Least concern | Eastern Canada, Northeastern USA (Appalachians, Great Lakes region) |
| Taxus mairei | Vulnerable | Southern and eastern China, Vietnam, Taiwan |
| Taxus contorta | Endangered | Western Himalayas (Pakistan, India, Nepal) |
| Taxus floridana | Critically endangered | Northern Florida (Gadsden and Liberty Counties) |
| Taxus globosa | Endangered | Mexico and Central America (Sierra Madre Oriental) |
| Taxus cuspidata | Least concern | Northeast China, Korea, Japan, Russian Far East |
| Species and Region: | Plant Part Used: | Administration: | Traditional Uses: | References: |
|---|---|---|---|---|
| Taxus wallichiana (India—Bhotiya tribe) | Bark | Tea |
| [26] |
| Decoction |
| |||
| Decoction with jaggery |
| |||
| Paste |
| |||
| Young branches | Tincture |
| ||
| Decoction |
| |||
| Leaves | Decoction/juice |
| ||
| Powder |
| |||
| Extract/juice |
| |||
| Taxus wallichiana (India) | Bark, seeds | Extract (oral) |
| [37] |
| Bark | Tea |
| ||
| Tea |
| |||
| Paste |
| |||
| Tea mixed with salt and ghee |
| |||
| Paste mixed with egg yolk |
| |||
| Decoction |
| |||
| Leaves | Juice from the leaves |
| ||
| Decoction |
| |||
| Decoction of leaves with honey |
| |||
| Tea |
| |||
| Bark, leaves | Tea |
| ||
| Young branches | Tincture |
| ||
| Stem | Decoction |
| ||
| The entire plant | — |
| ||
| Taxus baccata (India—Pauri district, Uttarakhand) | Bark, leaves | — |
| [60] |
| Taxus contorta, Taxus mairei, Taxus wallichiana (Hindu Kush-Himalayan region) | Arils | Consumed as is |
| [35] |
| Taxus contorta, Taxus mairei, Taxus wallichiana (Hindu Kush-Himalayan region) | Leaves | Juice from leaves with honey |
| |
| Taxus contorta, Taxus mairei, Taxus wallichiana (Hindu Kush-Himalayan region) | Leaves | Juice or decoction (oral) |
| |
| Aril | Consumed as is |
| ||
| Leaves, bark | Juice |
| ||
| Bark | Tea |
| ||
| Taxus wallichiana (Hindu Kush-Himalayan region) | Leaves, bark | Paste with honey |
| |
| Taxus contorta, Taxus wallichiana (Hindu Kush-Himalayan region) | Leaves | Leaf juice (oral) |
| |
| Bark | Paste (external use) |
| ||
| Decoction |
| |||
| Taxus wallichiana | — | Decoction, tea, juice |
| [51,61] |
| — | Poultice |
| ||
| Bark | Paste |
| ||
| Bark, leaves | Steam baths |
| ||
| Stem | Decoction—in Pakistan |
| ||
| Bark, leaves | Unani Medicine |
| ||
| Young branches | Ayurvedic tincture |
| ||
| Taxus baccata | — | Asturias, León |
| [5] |
| Aril | Aril pulp—syrup (Northern Spain) |
| ||
| Taxus wallichiana (Indian Ayurvedic Pharmacopoeia) | Leaves | Powder |
| [26] |
| Taxus baccata (Ukraine, Bukovina) | Bark | Bark decoction |
| [4] |
| Taxus baccata (Narew River, Northeast Poland) | Bark | Bark powder |
| |
| Taxus baccata (Central Balkan Peninsula) | Bark | Crushed bark |
| |
| Taxus baccata (Poland—Wólka Jagielczynska village, Częstochowa) | Bark | Infusion with milk or fumigation of the wood |
|
| Plant Part | Chemical Compound | Pharmacological Properties | References | ||
|---|---|---|---|---|---|
| ALKALOIDS | |||||
| Bark, leaves, roots | Paclitaxel (Taxol A) 0.007–0.01% | Anticancer effect: widely used to treat various types of cancer (lung, breast, blood, liver, brain, kidney, prostate, colon, cervical, gastric, pancreatic, Kaposi’s sarcoma). Antiviral effect (HIV, SARS-CoV-2). Low doses: treatment of liver, lung, and kidney fibrosis (0.3 mg/kg, 2×/week), coronary artery restenosis (1.3–10 µg/mm2), Alzheimer’s disease. | [25,27,28,29,104] | ||
| Leaves | 10-Deacetylbaccatin III (1.76%) | Ethyl acetate extract: benefits against insulin resistance associated with inflammation and high-fat diet in C57BL/6 mice; significantly improves glucose uptake in skeletal muscle cells with inflammation-induced insulin resistance; dose-dependent reduction in lipid accumulation; decreased LDL, triglycerides, and total cholesterol, increased HDL; reduction of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β); enhanced GLUT4 expression and distribution; no cytotoxicity at effective dose. | [56,81] | ||
| Leaves | Brevifoliol (7.59%) | [56] | |||
| Bark | 4-Deacetylbaccatin III | Anti-inflammatory, analgesic effect (in vitro). | [51] | ||
| Bark | Tasumatrol B | Pronounced anti-inflammatory effect, remarkable analgesic effect. | [25,51,65] | ||
| Bark | Taxusabietane A | Significant anti-inflammatory effect (in vivo, 5–10 mg/kg), via 5-lipoxygenase inhibition at IC50 = 57 ± 0.31 μmol/L. | [25,51,65] | ||
| Bark | Taxusabietane C | Significant anti-inflammatory effect (IC50 = 69 ± 0.31 μmol/L). | [65] | ||
| Bark | Taxamairin F | Significant anti-inflammatory effect (IC50 = 73 ± 0.14 μmol/L). | [65] | ||
| Bark | Taxawallin I | Methanolic extract: strong cytotoxic activity in vitro against various cancer cell lines (A498, MDR 2780AD, NCI-H226, HepG2). | [25,65] | ||
| Bark | 1-Hydroxy-2-deacetoxy-5-decinnamoyltaxinine J | Significant, dose-dependent anticancer effect against Colo 320DM, MCF-7, KB PA1, WRL-68 cell lines; immunomodulatory activity (1 µg/mL), enhances concanavalin A effects. | [25,65] | ||
| Bark | Taxusine | Ethanolic extract: anti-inflammatory, antinociceptive properties. | [68] | ||
| Bark | Baccatin VI | ||||
| Bark | 1-Hydroxybaccatin I | ||||
| FLAVONOIDS | |||||
| Branches, leaves | Flavone | Apigenin | Antioxidant (0.5–32 µg/mL—strong inhibition of reactive oxygen species); anticancer (synergistic with paclitaxel in HeLa cervical carcinoma cells, reducing cell viability by 29% and increasing apoptosis by 24%); antidepressant (in mice at 50 mg/kg—increased immobility, swimming, and climbing time); anti-inflammatory (50–200 µmol/L—inhibits nitric oxide production and phagocytosis); potential protective effect in UV-induced cutaneous tumors. | [64,66,105,106,107,108] | |
| Branches | Luteolin | Antioxidant (0.5–32 µg/mL); anticancer (synergistic effect with paclitaxel in human colorectal carcinoma cells); anti-inflammatory. | [64,105,106,108] | ||
| Leaves | Tricin | High potential as a functional agent in glycemic control; anti-inflammatory and anticancer effects. | [66,109] | ||
| Leaves | Chrysoeriol | Exhibits anti-inflammatory, anticancer, and anti-osteoporotic activity. | [66,108] | ||
| Leaves, branches | Biflavone | Bilobetin | Antibacterial and significant antifungal activity (against Alternaria alternata, Cladosporium oxysporum, Fusarium culmorum—median effective doses ED₅₀ = 14, 11, and 17 mmol/L, respectively; inhibited growth of C. oxysporum and F. culmorum at 100 mmol/L); acts as an efflux transporter inhibitor of P-glycoprotein (P-gp) and CYP3A4, enhancing the oral absorption of paclitaxel by limiting P-gp activity at a concentration of 50 mg/mL; concurrently, it reduced both the expression and activity of CYP3A4 at 100 µg/mL. | [64,110] | |
| Leaves | 4‴-O-methyl amentoflavone | Anticancer effect (human breast carcinoma MCF-7 cells were dramatically suppressed at ED₅₀ = 4.56–16.24 µg/mL → cellular apoptosis). | [64,111] | ||
| Leaves | 7-O-methyl amentoflavone | Shows significant antifungal activity (especially against Alternaria alternata at 100 µmol/L). | [64] | ||
| Leaves, branches, bark | Sciadopitysin | Antibacterial effect; anti-Alzheimer’s activity (95% ethanolic extract: inhibits β-amyloid fibril aggregation); neuroprotective action at concentrations ranging from 0.4 to 50 μM—neuronal cell viability (SK-N-MC cells) increased at 400 μM, and cellular apoptosis was inhibited at 0.1–1 μM → this suggests potential as a novel therapeutic compound for Alzheimer’s disease; significant antifungal activity (potent inhibitory effect particularly against Cladosporium oxysporum, with ED₅₀ = 9 μM); as a P-glycoprotein (P-gp) and CYP3A4 inhibitor, it enhances the oral absorption of paclitaxel by limiting P-gp activity at a concentration of 50 mg/mL, while simultaneously reducing both expression and activity of CYP3A4 at 100 μg/mL. | [64,82,110,112] | ||
| Leaves, bark, branches, aril | Ginkgetin | Antibacterial and anticancer effects (inhibitory activity against HepG2 hepatocellular carcinoma cell line at 50 µmol/mL, resulting in reduced cell viability and decreased number of cancer cells); significant antifungal activity (particularly against Alternaria alternata at 100 µmol/L); acts as an efflux transporter inhibitor of P-glycoprotein (P-gp) and CYP3A4, enhancing the oral absorption of paclitaxel by limiting P-gp activity at a concentration of 50 mg/mL; simultaneously, it reduced both the expression and activity of CYP3A4 at a concentration of 100 µg/mL. | [64,110,113] | ||
| Leaves, branches | Amentoflavone | Antibacterial and anti-leishmanial effects (IC₅₀ = 28.5 ± 2 µmol/L, inducing mitochondrial disruption in Leishmania amazonensis); antiviral activity (inhibitory effect against SARS-CoV-2); significant antifungal activity. Acts as an efflux transporter inhibitor of P-glycoprotein (P-gp) and CYP3A4, enhancing the oral absorption of paclitaxel by limiting P-gp activity at a concentration of 50 mg/mL; simultaneously, it reduced both the expression and activity of CYP3A4 at a concentration of 100 µg/mL. | [64,110,114,115] | ||
| Leaves, branches | Sequoiaflavone | Antibacterial activity; functions as an efflux transporter inhibitor of P-glycoprotein (P-gp) and CYP3A4, enhancing the oral absorption of paclitaxel by limiting P-gp activity at a concentration of 50 mg/mL; simultaneously, it reduced both the expression and activity of CYP3A4 at a concentration of 100 µg/mL. | [64,110] | ||
| Leaves, branches | Isoginkgetin | Exhibits anti-inflammatory and anticancer activity (apparent inhibitory effect on A549 lung cancer cells at concentrations ranging from 2.5 to 20 µmol/L). | [64,116] | ||
| Leaves, branches | Putraflavone | Anti-inflammatory activity (inhibits reactive oxygen species production and CD69 expression). | [64,117] | ||
| Leaves | Sotetsuflavone | Anticancer activity (apparent inhibitory effect on A549 lung cancer cells at 200 mmol/L, associated with increased E-cadherin expression and decreased N-cadherin expression). | [64,118] | ||
| Leaves, branches | Kayaflavone | Antiviral activity (inhibitory effect against SARS-CoV-2). | [64,115] | ||
| Leaves, branches | Flavonols | Kaempferol | Antioxidant activity (0.5–32 µg/mL); antiviral effects (strong antiviral activity through inhibition of HIV-1 reverse transcriptase at 100 µg/mL); anti-inflammatory properties. | [64,105,119] | |
| Leaves | Myricetin | Antiviral activity (limited effect against infectious bronchitis virus at 100 µmol/mL, with approximately 50% viral activity reduction observed at 10 µmol/mL). | [64,120] | ||
| Leaves, bark, branches | Quercetin | Antioxidant, anti-inflammatory, and antiallergic activity (intravenous administration: inhibits mast cell degranulation); hepatoprotective effect. | [64,83,105,121] | ||
| Leaves, bark | Isorhamnetin | Antiviral activity (suppressed the growth and invasion of SARS-CoV-2 in the human body). | [64,122] | ||
| Leaves | Aromadendrin | Neuroprotective effect (enhanced cell viability at 20 µmol/L and increased confluency at 2 mmol/L in SH-SY5y neuronal cells). | [64,123] | ||
| Leaves | Fisetin | Significantly reduces renal hypertrophy and albuminuria in diabetic mouse models, primarily by inhibiting the progression of glycation; shows beneficial effects in diabetes mellitus. | [64] | ||
| Leaves, branches | Flavonol glycosides | Kaempferol-3-O-rutinoside | Antioxidant effect (hydroxyl radical scavenging activity, IC₅₀ = 351.46 ± 2.30 µg/mL); potent anti-aging effect (hyaluronidase inhibition at IC₅₀ = 84.07 ± 10.46 µg/mL). | [64,124] | |
| Leaves, branches | Kaempferol-7-O-glucoside | Antiviral activity (strong antiviral effects through inhibition of HIV-1 reverse transcriptase at 100 µg/mL). | [64,119] | ||
| Leaves, branches | Quercetin-3-O-rutinoside (rutin) | Anticancer activity (radioprotective effect on intestinal cancer by modulating ROS levels and antioxidant proteins, and inhibiting inflammasome activation at a dose of 10.25 mg/kg). | [64,125] | ||
| Leaves | Quercetin-7-O-glucoside | Antiviral activity (strong inhibitory effect against influenza virus strains at IC₅₀ = 3.1–8.19 µg/mL, associated with reduced ROS levels and suppression of virus-induced autophagy). | [64,126] | ||
| Leaves | Quercetin-3-O-α-L-arabinopyranosyl-β-D-glucopyranoside | Antidiabetic effect (enhances glucose uptake and glycogen synthesis at 20 µmol/L). | [64,127] | ||
| Leaves, branches | Quercetin-3-O-glucoside | Anticancer activity (significant antitumor effects against MCF-7 and HeLa cancer cell lines with IC₅₀ values of 36.4 µmol/L and 52.5 µmol/L, respectively). | [57,64] | ||
| Leaves, branches | Quercetin-3-rhamnoside | Antilipase activity (inhibits lipase activity at concentrations ranging from 0 to 3 × 10⁻⁵ mol/L). | [64,128] | ||
| Leaves | Flavanone glycosides | Prunin | Strong ROS-inhibitory activity; shows medicinal potential against UV-induced cutaneous tumorigenesis. | [66,129] | |
| Leaves | Neohesperidin | Exhibits antioxidant, anti-inflammatory, and antiallergic activities, especifically effective in preventing immediate and delayed allergic diseases caused by mast cell degranulation. | [66,130] | ||
| Branches | Dihydroflavones | Pinocembrin | Antiviral activity (significant suppression of Zika virus invasion at IC₅₀ = 17.4 µmol/L, by reducing viral RNA and protein expression). | [64,131] | |
| Branches | Eriodictyol | Antidiabetic effect (enhances cell viability and superoxide dismutase activity, while reducing ROS generation at concentrations of 5, 10, and 20 µmol/L in diabetic mice). | [64,132] | ||
| Branches | Butin | Antidiabetic effect (at 10 and 20 mg/kg: significantly reduces blood glucose levels, oxidative stress, and neuroinflammation; enhances neurobehavioral parameters and metabolic levels). | [64,132] | ||
| Branches | Naringenin | Anticancer activity (as nanoparticles: reduces proliferation and migration of A549 lung cancer cells); anti-inflammatory effect (nanoparticles: attenuates pro-inflammatory cytokines and their expression levels); antiallergic activity (intravenous administration: inhibits mast cell degranulation). | [64,121,133] | ||
| Branches | Pinostrobin | Promotes melanogenesis (inhibits tyrosinase activity with an IC₅₀ value of 700 µmol/L). | [64,134] | ||
| Leaves | Dihydroflavonols | Taxifolin | Antidiabetic activity (IC₅₀ = 0.038–0.647 mg/mL: inhibits α-amylase and α-glucosidase; regulates postprandial hyperglycemia); significant antioxidant and anti-inflammatory activity (40 mg/kg); chemopreventive, hepatoprotective, and cardioprotective properties (100 µg/kg/day: inhibits angiotensin II-converting enzyme and suppresses ROS formation; 3.3 mg/kg: lowers elevated blood pressure). Anti-Alzheimer’s potential (prevents and/or treats cognitive dysfunction related to β-amyloid fibril aggregation); antiviral activity (against hepatitis A virus at 59 µg/mL); antibacterial action (Propionibacterium acnes; Toxoplasma gondii in combination with pyrimethamine, IC₅₀ = 1.39 µg/mL); antilipase and antityrosinase effects; inhibits ROS production. Enhances the efficacy of ceftazidime and levofloxacin in treating methicillin-resistant Staphylococcus aureus (MRSA) infections; suppresses osteoclast activity and mitigates ovariectomy-induced bone loss (potential alternative to estrogen therapy). Demonstrates therapeutic potential in treating bacterial infections (including acne and toxoplasmosis), liver disorders (including autoimmune hepatitis), cardiovascular diseases, inflammation, cancer, viral infections, Alzheimer’s disease, diabetes, allergic reactions, and immune deficiencies. | [64,83,135] | |
| Leaves, bark, branches, roots | Flavanols | (+)-Catechin | Antioxidant activity (inhibits autooxidation at 0.01 mol/L; IC₅₀ = 16.88 µg/mL compared to ascorbic acid at 14.48 µg/mL); antidiabetic effect (significantly inhibits α-amylase at IC₅₀ = 0.752 mg/mL and α-glucosidase); anticancer activity (significant antitumor effects against MCF-7, HeLa, and HepG2 cancer cell lines); hepatoprotective properties. | [57,64,83,136,137,138,139] | |
| Leaves, bark, branches, roots | (−)-Epicatechin | Antioxidant activity (inhibits autooxidation at 0.01 mol/L; IC₅₀ = 20.20 µg/mL compared to ascorbic acid at 14.48 µg/mL); antidiabetic effect (significantly inhibits α-amylase at IC₅₀ = 0.655 mg/mL and α-glucosidase). Anticancer activity (significant antitumor effects against MCF-7, HeLa, and HepG2 cancer cell lines; reduces radiation resistance and enhances therapeutic effects by activating cyclooxygenase in pancreatic cancer cells at 200 µmol/L); also demonstrates radioprotective effects in human fibroblasts at 20 µmol/L. | [57,64,136,138,139,140,141] | ||
| Leaves | Gallocatechin | Antidiabetic and antiviral activity (suppresses SARS-CoV-2 replication with an IC₅₀ = 13.14 ± 2.081 µmol/L). | [64,139,142] | ||
| Leaves | Epigallocatechin | Antidiabetic activity. | [64,139] | ||
| Leaves | Procyanidin B2 | Antidiabetic activity (protected endothelial progenitor cell function, reduced oxidative damage, and promoted wound healing and angiogenesis in diabetic mice). | [64,136,143] | ||
| Branches | Chalcones | Pinocembrin chalcone | Antibacterial activity (moderate inhibitory effect against Neisseria gonorrhoeae at 128 µg/mL). | [64,144] | |
| Branches | Isoliquiritigenin | Significant hepatoprotective activity (at 10 µmol/L, reduced transaminase and inflammatory cytokine levels while enhancing catalase activity). | [64,145] | ||
| Branches | Butein | Significant anti-inflammatory and antinociceptive effects, demonstrated by reduced nociception in thermal and paw edema tests in mice at 10–20 mg/kg, accompanied by decreased levels of inflammatory cytokines. | [64,146] | ||
| Branches | Naringenin chalcone | Anti-inflammatory and antiallergic activity (intravenous administration: inhibits mast cell degranulation). | [64,121] | ||
| Leaves | Isoflavones | Glycitin (glycitein-7-O-glucoside) | Protective effect against UV-induced skin photoaging in primary human dermal fibroblasts and against lipopolysaccharide-induced acute lung injury. | Exhibits beneficial effects in the fight against cancer, in the treatment of cardiovascular diseases, as well as in the prevention and management of Alzheimer’s disease. | [66,147] |
| Leaves | Genistin (genistein-7-O-glucoside) | Protective effect of genistin on wild-type p53 cells against taxol-induced cytotoxicity in the combined treatment of lung cancer. | [66,148] | ||
| LIGNANS | |||||
| Bark, leaves | Taxiresinol | Anticancer activity (methanolic extracts). | Anticancer, antiulcer activities. | [25,26,65,80] | |
| Roots | (−)-7-O-methyltanegool | α-glucosidase inhibitory effects. | |||
| Roots | 13α-Conidendrin | ||||
| Roots | 4-Formosanol | ||||
| Roots | 14(+)-Tsugacetal | ||||
| Roots | 14α-Intermedianol | ||||
| Roots | 14-Oxabicyclooctalignan | ||||
| Roots | Lanceolatanin C | ||||
| Roots | Lanceolatanin D | ||||
| Roots | Matairesinol | ||||
| Roots | 147-Methoxymatairesinol | ||||
| Roots | 17-Oxomatairesinol | ||||
| Bark | Taxiresinol I | Ethanolic extract exhibits anticancer activity (in vitro) against ovarian, colon, breast, and liver cancer cells; also shows anti-inflammatory and antinociceptive properties; antiulcer effects; and antifungal activity against Nigrospora oryzae, Epidermophyton floccosum, Curvularia lunata, and Pleurotus ostreatus. | |||
| Bark | Isotaxiresinol | Exhibits therapeutic potential in postmenopausal osteoporosis by promoting bone formation and inhibiting bone resorption. | |||
| Bark | Lariciresinol | Exhibits anti-inflammatory and antinociceptive properties, as well as antifungal activity against Nigrospora oryzae, Epidermophyton floccosum, Curvularia lunata, and Pleurotus ostreatus. | [38,68,80] | ||
| Bark | Isolariciresinol | ||||
| Bark | 30-demethyl-isolariciresinol-9′-hydroxyisopropyl ether | ||||
| Bark | 3-demethyl-isolariciresinol | ||||
| PHYTOSTEROLS | |||||
| Bark | Daucosterol | Exhibits cytotoxic activity against various cancer cell lines, including HepG2 (liver cancer), MCF-7 (breast cancer), HeLa (cervical cancer), and A549 (lung cancer). | [26,37,65] | ||
| Bark, seeds | β-Sitosterol | Exhibits antiviral, anti-inflammatory, antipyretic, and uterotrophic effects. | |||
| Body Lotion Based on Taxus Cambium and Procambium | Cream Based on Taxus Cambium and Procambium |
|
|
| The formulations described in the present invention demonstrate efficacy in the prevention and delay of skin aging. Furthermore, these formulations exhibit notable depigmenting and anti-inflammatory properties. | |
| Type of Extract/Chemical Compound | Therapeutic Action | References |
|---|---|---|
| Hydro-methanolic extract | Exhibits antibacterial activity against Pectobacterium sp. and Dickeya chrysanthemi, with a minimum inhibitory concentration (MIC) ranging from 1000 to 1500 µg/mL. | [33] |
| Ethanolic Extract (Rhodoxanthin) | Demonstrated significant antiproliferative and pro-apoptotic effects on murine melanoma B16F10 cells in vitro. Oral administration of 7 mg rodoxanthin/kg body weight for 21 days significantly reduced tumor growth (by 42.18%) and tumor weight (by 15.74%) in CD57BL/6J mice bearing B16F10 melanoma; also significantly increased erythrocyte count, hemoglobin, and hematocrit levels in treated tumor-bearing mice. Showed dose-dependent in vitro cytotoxic activity on metastatic murine melanoma B16F10 cell line—the highest tested concentration (C1 = 0.18 µmol/mL) reduced cell viability to 32.29%, while the lowest concentration (C5 = 0.025 µmol/mL) reduced viability to 48.58%, indicating an inverse correlation between rodoxanthin concentration and melanoma cell viability. Exhibited cytoprotective effects against hydrogen peroxide-induced oxidative stress in human HaCaT epidermal keratinocytes and human retinal epithelial cells, improving cell viability by 12.55%, 13%, and 9.66%, respectively. | [34,80] |
| Ethanolic Extract (Phenolic Compounds) | Exhibits moderate antioxidant activity (IC₅₀ = 68.46 mg/mL), with an antioxidant capacity (10.7 µmol/100 mL) comparable to that of blackcurrant fruits (Ribes nigrum) (10 µmol/100 mL). | [77] |
| Methanolic Extract | Exhibits antifungal activity against Candida albicans and Aspergillus brasiliensis. | [80] |
| Methanolic, Acetone, and Distilled Water Extracts | Antioxidant activity (the methanolic extract showed the highest potency, followed by the distilled water extract; the acetone extract was the least effective). Hypoglycemic activity (inhibition of α-amylase and α-glucosidase)—the methanolic extract was the most potent, followed by the distilled water extract, with the acetone extract showing the weakest effect. | [30] |
| Carotenoids | Reduce the harmful effects of reactive oxygen species (ROS), contributing to the prevention and mitigation of degenerative diseases, macular degeneration, cardiovascular conditions, and several types of cancer, including lung, gastric, pancreatic, breast, and prostate cancers. | [77] |
| Terpenoids | Exhibit antimicrobial activity (limonene, p-cymene, α/β-pinene), anti-inflammatory and chemopreventive effects (limonene), antioxidant and hepatoprotective properties, as well as antiulcer activity (α-pinene, limonene) and antispasmodic action (α-pinene). | [31,84] |
| Ferulic acid, p-Coumaric acid, Caffeic acid | Exhibit immunomodulatory, cytoprotective, and antioxidant effects. | [31,32] |
| Ascorbic Acid | The aril of Taxus species provides the full recommended dietary allowance (RDA) of vitamin C for healthy adults. | [31,32] |
| Carbohydrates | A total of 100 g of aril yields approximately 106 kilocalories, making it suitable as a low-calorie snack option. | [31,32] |
| Amino Acids | The aril can serve as a high-quality protein source in human nutrition, containing 43% essential amino acids (according to WHO, foods with over 40% essential amino acids are considered ideal protein sources). The content of branched-chain amino acids (leucine, isoleucine, valine) in the aril (18.4%) is comparable to that found in animal-derived proteins (20%). | [31,32] |
| Macroelements and Microelements | The potassium content of the aril is comparable to that of bananas, which are widely considered a typical dietary source of potassium. A 100 g serving of aril provides 11–15% of the recommended daily intake of zinc, 9–15% of iron, and 30–50% of chromium. | [31,32] |
| Aril Juice | It alleviates Alzheimer’s disease by modulating several biological processes, including oxidative stress, inflammatory responses, neuronal apoptosis, insulin secretion, amyloid fibril formation, and T-cell co-stimulation. | [88,149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jîjie, A.-R.; Iliescu, D.; Sbârcea, L.; Boru, C.; Pătrașcu, D.; Iftode, O.A.; Minda, I.-D.; Avram, Ș.; Trandafirescu, C.-M.; Dehelean, C.A.; et al. A Deep Dive into the Botanical and Medicinal Heritage of Taxus. Plants 2025, 14, 1439. https://doi.org/10.3390/plants14101439
Jîjie A-R, Iliescu D, Sbârcea L, Boru C, Pătrașcu D, Iftode OA, Minda I-D, Avram Ș, Trandafirescu C-M, Dehelean CA, et al. A Deep Dive into the Botanical and Medicinal Heritage of Taxus. Plants. 2025; 14(10):1439. https://doi.org/10.3390/plants14101439
Chicago/Turabian StyleJîjie, Alex-Robert, Dan Iliescu, Laura Sbârcea, Casiana Boru, Dalia Pătrașcu, Oana Andrada Iftode, Ionela-Daliana Minda, Ștefana Avram, Cristina-Maria Trandafirescu, Cristina Adriana Dehelean, and et al. 2025. "A Deep Dive into the Botanical and Medicinal Heritage of Taxus" Plants 14, no. 10: 1439. https://doi.org/10.3390/plants14101439
APA StyleJîjie, A.-R., Iliescu, D., Sbârcea, L., Boru, C., Pătrașcu, D., Iftode, O. A., Minda, I.-D., Avram, Ș., Trandafirescu, C.-M., Dehelean, C. A., & Moacă, E.-A. (2025). A Deep Dive into the Botanical and Medicinal Heritage of Taxus. Plants, 14(10), 1439. https://doi.org/10.3390/plants14101439









