Divergent Climate Sensitivity and Spatiotemporal Instability in Radial Growth of Natural and Planted Pinus tabulaeformis Forests Across a Latitudinal Gradient
Abstract
1. Introduction
2. Results
2.1. Chronology Statistical Characteristics
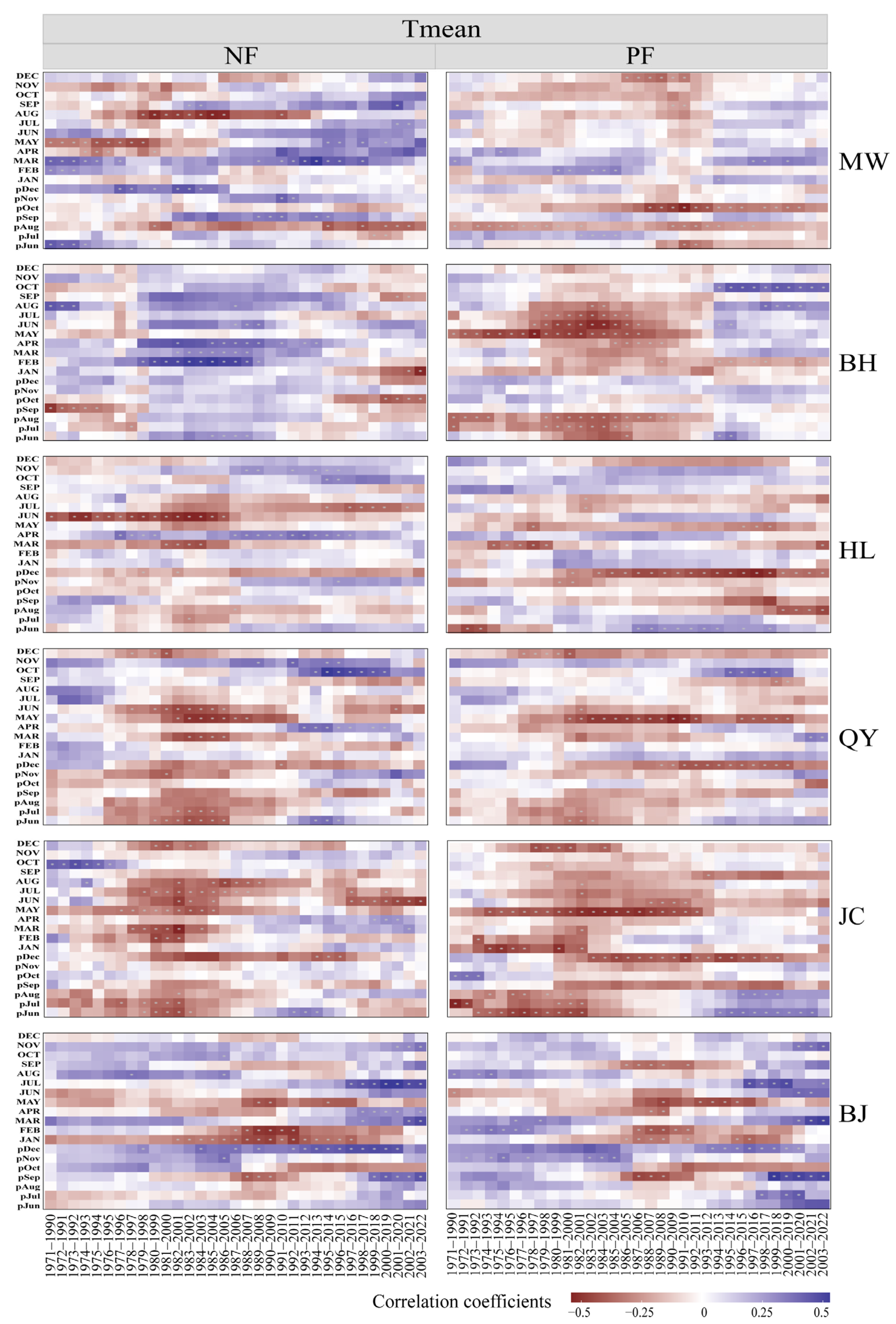
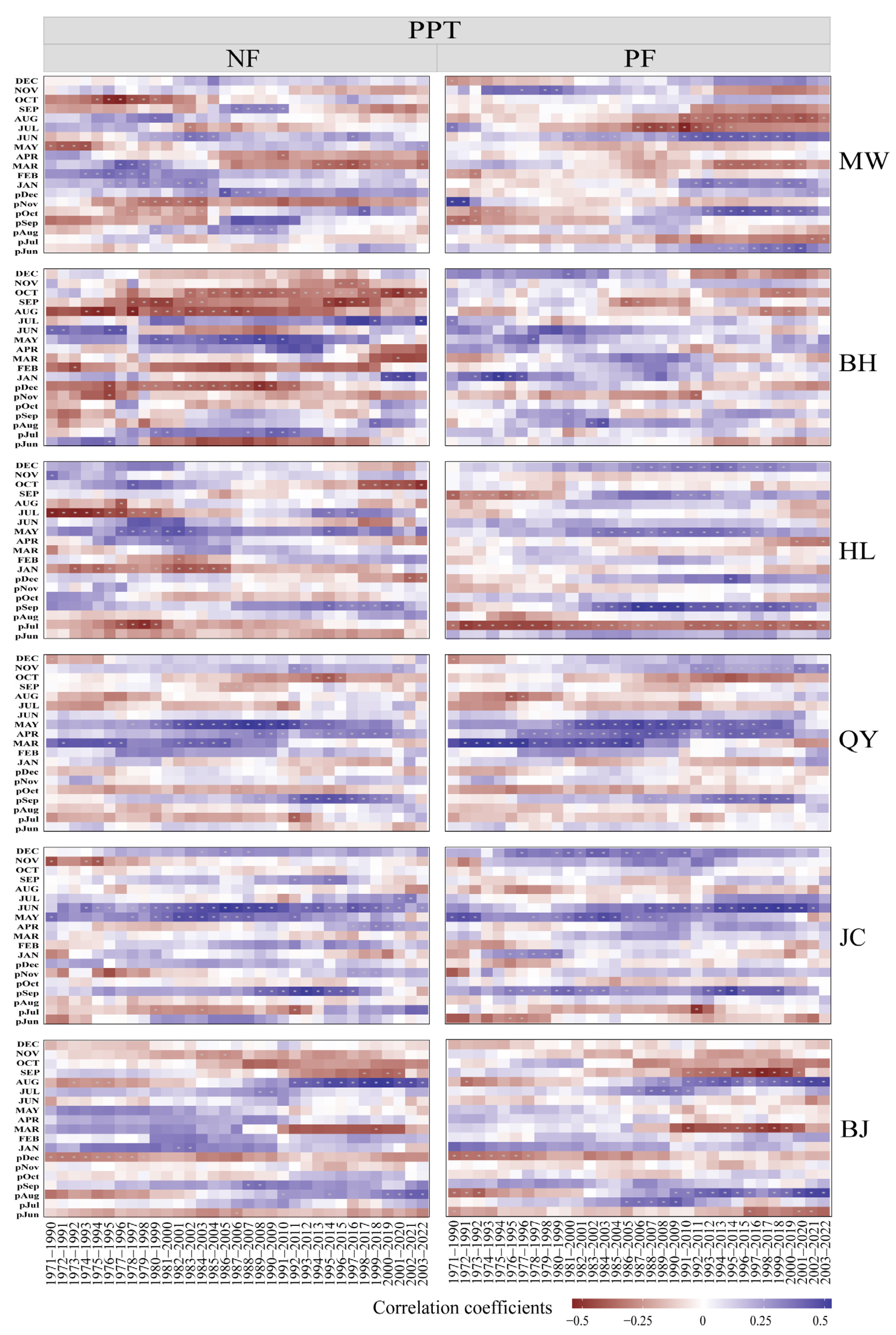
2.2. Growth–Climate Correlations
2.3. Temporal Stability of Growth–Climate Relationships
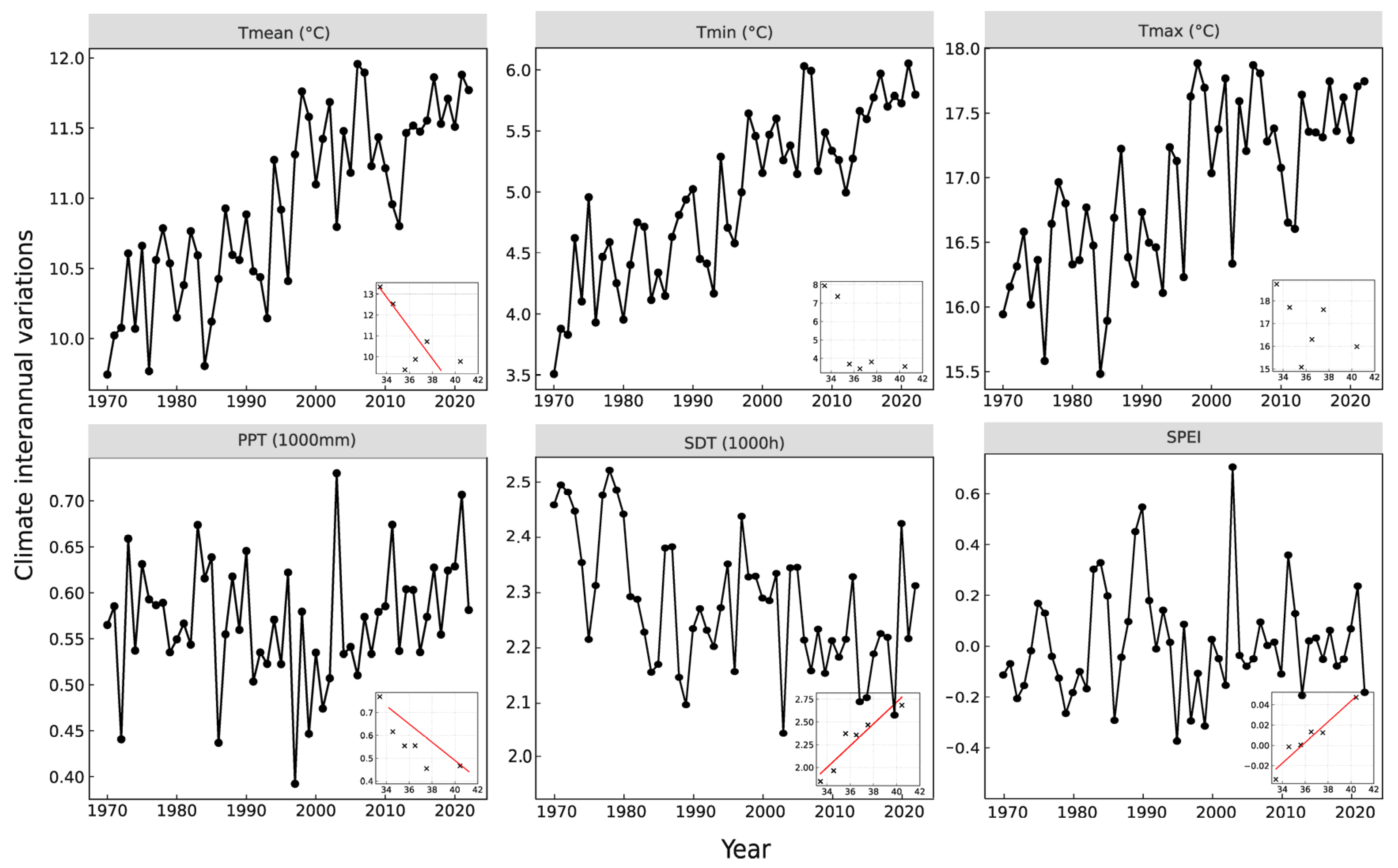
2.4. Spatial Gradient Analysis of Growing-Season Variables
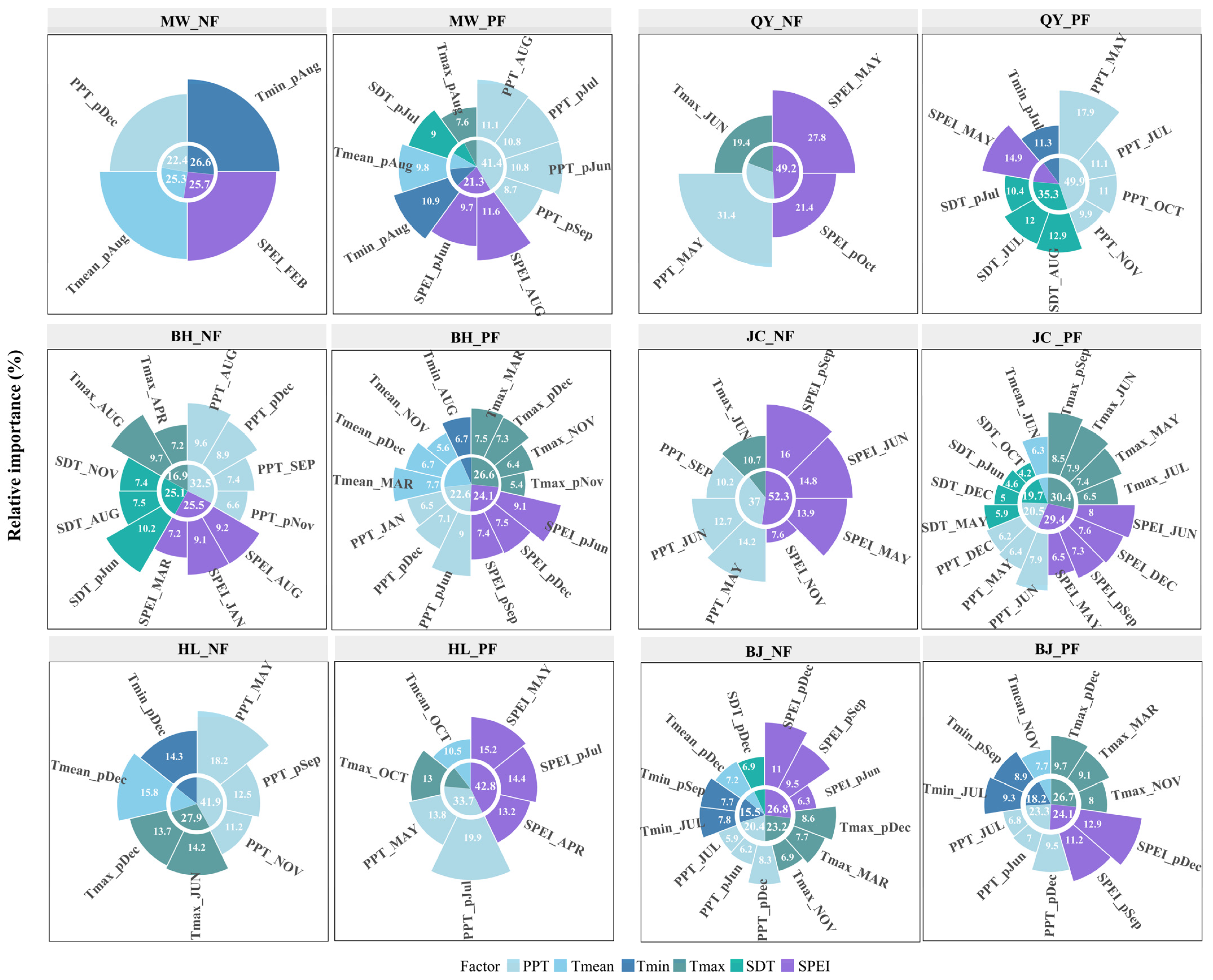
2.5. Relative Importance of Climatic Variables in NFs and PFs
3. Discussion
3.1. Relationships Between Radial Growth and Climatic Factors
3.2. Spatial Shifts in Climate–Growth Relationships
3.3. Relative Importance of Climatic Factors
4. Materials and Methods
4.1. Study Sites
4.2. Sample Processing and Chronology Development
4.3. Climate Variables
4.4. Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Roedenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Fang, J.; Houghton, R.A.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.Z.; Lewis, S.L.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Montagu, K.D.; Düttmer, K.; Barton, C.V.M.; Cowie, A.L. Developing general allometric relationships for regional estimates of carbon sequestration—An example using Eucalyptus pilularis from seven contrasting sites. For. Ecol. Manag. 2005, 204, 115–129. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Change Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Tei, S.; Kotani, A.; Sugimoto, A.; Shin, N. Geographical, climatological, and biological characteristics of tree radial growth response to autumn climate change. Front. For. Glob. Change 2021, 4, 687749. [Google Scholar] [CrossRef]
- Wang, H.; Duan, A.G.; Zhang, J.G. Research progress on the response of radial growth of tree species from different provenances to climate change. For. Res. 2023, 36, 198–204. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Springer: Berlin/Heidelberg, Germany, 1990. [Google Scholar]
- Hughes, M.K.; Swetnam, T.W.; Diaz, H.F. Dendroclimatology: Progress and Prospects; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Cherubini, P.; Battipaglia, G.; Innes, J.L. Tree vitality and forest health: Can tree-ring stable isotopes be used as indicators? Curr. For. Rep. 2021, 7, 69–80. [Google Scholar] [CrossRef]
- Conte, E.; Lombardi, F.; Battipaglia, G.; Palombo, C.; Altieri, S.; La Porta, N.; Marchetti, M.; Tognetti, R. Growth dynamics, climate sensitivity and water use efficiency in pure vs. mixed pine and beech stands in Trentino (Italy). For. Ecol. Manag. 2018, 409, 707–718. [Google Scholar] [CrossRef]
- Kukarskih, V.V.; Devi, N.M.; Moiseev, P.A.; Grigoriev, A.A.; Bubnov, M.O. Latitudinal and temporal shifts in the radial growth-climate response of Siberian larch in the Polar Urals. J. Mt. Sci. 2018, 15, 722–729. [Google Scholar] [CrossRef]
- Cao, X.G.; Hu, H.B.; Li, Y.J.; Dong, Z.P.; Lu, X.R.; Bai, M.W.; Zheng, Z.P.; Fang, K.Y. Differences in the ecological resilience of planted and natural Pinus massoniana and Cunninghamia lanceolata forests in response to drought in subtropical China. Chin. J. Appl. Ecol. 2021, 32, 3531–3538. [Google Scholar] [CrossRef]
- Jia, C.; Guo, M.M.; Wang, Q.; Cui, L.Z.; Guo, J.L. Response of the radial growth of Larix principis-rupprechtii plantations and natural forests to climate change. J. Cent. South Univ. For. Technol. 2022, 42, 120–128. [Google Scholar] [CrossRef]
- Grossiord, C. Having the right neighbors: How tree species diversity modulates drought impacts on forests. New Phytol. 2020, 228, 42–49. [Google Scholar] [CrossRef]
- Wei, J.S.; Li, Z.S.; Jiao, L.; Chen, W.L.; Wu, X.; Wang, X.C.; Wang, S. Climate effect on the radial growth of introduced and native tree species in the Yangjuangou catchment of the Loess Plateau. Acta Ecol. Sin. 2018, 38, 8040–8050. [Google Scholar] [CrossRef]
- Rodriguez-Vallejo, C.; Navarro-Cerrillo, R.M. Contrasting response to drought and climate of planted and natural Pinus pinaster Aiton forests in southern Spain. Forests 2019, 10, 603. [Google Scholar] [CrossRef]
- Bai, M.; Dong, Z.; Chen, D.; Zheng, H.; Zhou, F.; Cao, X.; Ou, T.; Fang, K. Different responses of the radial growth of the planted and natural forests to climate change in humid subtropical China. Geogr. Ann. Ser. A Phys. Geogr. 2020, 102, 235–246. [Google Scholar] [CrossRef]
- Cheng, Z.H.; Ding, K.Y.; Liu, Y.H. Relationship between arborous layer productivity and climatic factors in Pinus tabulaeformis natural forests and plantations in Beijing. J. Nanjing For. Univ. 2016, 40, 177–183. [Google Scholar] [CrossRef]
- Wang, H.; Ning, Y.; Liu, C.; Xu, P.; Zhang, W. Different radial growth responses to climate change of three dominant conifer species in temperate forest, northeastern China. Front. For. Glob. Change 2022, 4, 820800. [Google Scholar] [CrossRef]
- Podlaski, R. Variability in radial increment can predict an abrupt decrease in tree growth during forest decline: Tree-ring patterns of Abies alba Mill. in near-natural forests. For. Ecol. Manag. 2021, 479, 118579. [Google Scholar] [CrossRef]
- Li, L.; Li, L.G.; Chen, Z.; Zhou, Y.; Zhang, X.; Bai, X.; Chang, Y.; Xiao, J. Responses of Pinus sylvestris var. mongolica to gradient change of hydrothermal in plantations in Liaoning Province. Acta Ecol. Sin. 2015, 35, 4508–4517. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, X.; Zhang, Y.; Li, Z. Different responses of Korean pine (Pinus koraiensis) and Mongolia oak (Quercus mongolica) growth to recent climate warming in northeast China. Dendrochronologia 2017, 45, 113–122. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Linares, J.C.; Fajardo, A.; Colangelo, M.; Valeriano, C.; Sánchez-Salguero, R.; Sangüesa-Barreda, G.; Granda, E.; Gimeno, T.E. Differences in temperature sensitivity and drought recovery between natural stands and plantations of conifers are species-specific. Sci. Total Environ. 2021, 796, 148930. [Google Scholar] [CrossRef]
- Hua, F.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.; Wang, W.; McEvoy, C.; Peña-Arancibia, J.L.; et al. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef]
- Ni, Y.; Xiao, W.; Liu, J.; Jian, Z.; Li, M.; Xu, J.; Lei, L.; Zhu, J.; Li, Q.; Zeng, L.; et al. Radial growth-climate correlations of Pinus massoniana in natural and planted forest stands along a latitudinal gradient in subtropical central China. Agric. For. Meteorol. 2023, 334, 109422. [Google Scholar] [CrossRef]
- Cai, L.X.; Li, J.X.; Bai, X.P.; Jin, Y.T.; Chen, Z.J. Variations in the growth response of Pinus tabulaeformis to a warming climate at the northern limits of its natural range. Trees 2020, 34, 707–719. [Google Scholar] [CrossRef]
- Liang, E.; Dawadi, B.; Pederson, N.; Eckstein, D. Is the growth of birch at the upper timberline in the Himalayas limited by moisture or by temperature? Ecology 2014, 95, 2453–2465. [Google Scholar] [CrossRef]
- Su, J.R.; Xiao, S.C.; Peng, X.M.; Che, C.W.; Zhao, P. Regional differentiation of the response of radial growth of Pinus tabulaeformis to climate. Chin. Desert 2024, 44, 60–72. [Google Scholar] [CrossRef]
- Man, Z.; Zhang, J.; Liu, J.; Liu, L.; Yang, J.; Cao, Z. Process-based modeling of phenology and radial growth in Pinus tabuliformis in response to climate factors over a cold and semi-arid region. Plants 2024, 13, 980. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, Y.J.; Chen, F.H.; Yu, S.L.; Shang, H.M.; Zhang, T.W.; Zhang, R.B.; Qin, L. Reconstruction of spring temperature on the southern edge of the Gobi Desert, Asia, reveals recent climatic warming. Palaeogeogr. Palaeoclim. Palaeoecol. 2014, 409, 145–152. [Google Scholar] [CrossRef]
- Han, C.; Xiao, S.; Ding, A.; Teng, Z. Climate change recorded in tree rings of Picea crassifolia and Pinus tabulaeformis on the southern edge of the Tengger Desert. China Desert 2020, 40, 50–58. Available online: https://link.cnki.net/urlid/62.1070.p.20200114.2043.002 (accessed on 6 May 2022.).
- Du, D.; Jiao, L.; Chen, K.; Liu, X.; Qi, C.; Xue, R.; Wu, X. Response stability of radial growth of Chinese pine to climate change at different altitudes on the southern edge of the Tengger Desert. Glob. Ecol. Conserv. 2022, 35, e02091. [Google Scholar] [CrossRef]
- Yang, Y.R.; Zhang, M.S.; Zhang, L.N.; Lu, Q.Q.; Hong, Y.X.; Liu, X.H. Study on the response differences of radial growth of Pinus tabulaeformis to climate factors in the central and western Qinling Mountains. Acta Ecol. Sin. 2022, 42, 1474–1486. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, L.N.; Zeng, X.M.; Wang, K.Y.; Wang, Y.B.; Lu, Q.Q.; Liu, X.H. Different response of conifer and shrubs radial growth to climate in the middle Loess Plateau. Acta Ecol. Sin. 2020, 40, 5685–5697. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, Y.; Wang, M.; Kang, X.; Zhang, W.; Zhang, L.; Zhao, S. Responses to climate change in radial growth of Picea schrenkiana along elevations of the eastern Tianshan Mountains, northwest China. Dendrochronologia 2016, 40, 117–127. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, Y.; Gou, X.; Zhang, T.; Zou, C.; Ji, C.; Fan, Z.; Qin, L.; Shang, H.; Li, X. Intra-annual radial growth of Schrenk spruce (Picea schrenkiana Fisch. et Mey) and its response to climate on the northern slopes of the Tianshan Mountains. Dendrochronologia 2016, 40, 36–42. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Plavcová, L.; Anderegg, L.D.L.; Hacke, U.G.; Berry, J.A.; Field, C.B. Drought’s legacy: Multiyear hydraulic deterioration underlies widespread aspen forest die-off and portends increased future risk. Glob. Change Biol. 2013, 19, 1188–1196. [Google Scholar] [CrossRef]
- Deslauriers, A.; Beaulieu, M.; Balducci, L.; Giovannelli, A.; Gagnon, M.J.; Rossi, S. Impact of warming and drought on carbon balance related to wood formation in black spruce. Ann. Bot. 2014, 114, 335–345. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Anderegg, W.R.L.; Schwalm, C.R.; Biondi, F.; Camarero, J.J.; Koch, G.W.; Litvak, M.E.; Ogle, K.; Shaw, J.D.; Shevliakova, E.; et al. Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science 2015, 349, 528–532. [Google Scholar] [CrossRef]
- Gruber, A.; Pirkebner, D.; Florian, C.; Oberhuber, W. No evidence for depletion of carbohydrate pools in Scots pine (Pinus sylvestris L. ) under drought stress. Plant Biol. 2012, 14, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Xue, R.; Qi, C.; Chen, K.; Liu, X. Comparison of the responses of radial growth to climate change for two dominant coniferous tree species in the eastern Qilian Mountains, northwestern China. Int. J. Biometeorol. 2021, 65, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, X.; Li, M.; Zhang, J.; Cao, Y. Climate-growth pattern of Pinus tabulaeformis plantations and their resilience to drought events in the Loess Plateau. For. Ecol. Manag. 2021, 499, 119642. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Zhang, H.; Ren, S.Y.; Li, L.X.; Pan, N.; Yuan, Z.L.; Ye, Y.Z. Response of radial growth of different coniferous trees to climate in Baotianman Nature Reserve. Acta Ecol. Sin. 2016, 36, 5324–5332. [Google Scholar] [CrossRef]
- Peng, J.F.; Yang, A.R.; Tian, Q.H. Response of radial growth of Chinese pine (Pinus tabulaeformis) to climate factors in Wanxian Mountain of He’nan Province. Acta Ecol. Sin. 2011, 31, 5977–5983. [Google Scholar] [CrossRef]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.L.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef]
- Berdanier, A.B.; Clark, J.S. Multiyear drought-induced morbidity preceding tree death in Southeastern U.S. forests. Ecol. Appl. 2016, 26, 17–23. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Trugman, A.T.; Medvigy, D.; Anderegg, W.R.L.; Pacala, S.W. Differential declines in Alaskan boreal forest vitality related to climate and competition. Glob. Change Biol. 2018, 24, 1097–1107. [Google Scholar] [CrossRef]
- De Ridder, M.; Van den Bulcke, J.; Van Acker, J.; Beeckman, H. Tree-ring analysis of an African long-lived pioneer species as a tool for sustainable forest management. For. Ecol. Manag. 2013, 304, 417–426. [Google Scholar] [CrossRef]
- Barrette, M.; Thiffault, N.; Auger, I. Resilience of natural forests can jeopardize or enhance plantation productivity. For. Ecol. Manag. 2021, 482, 118872. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Zavala, M.A.; Bonet, F.J.; Zamora, R. Are pine plantations valid tools for restoring Mediterranean forests? An assessment along abiotic and biotic gradients. Ecol. Appl. 2009, 19, 2124–2141. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.C.; King, J.S.; Ward, E.; Oishi, A.C.; Palmroth, S.; Radecki, A.; Bell, D.M.; Miao, G.; Gavazzi, M.; Johnson, D.M.; et al. Conversion of natural forests to managed forest plantations decreases tree resistance to prolonged droughts. For. Ecol. Manag. 2015, 355, 58–71. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, S.; Wang, J.; Wei, X.; Schuler, J.; Sun, P.; Harper, R.; Zegre, N. Natural forests exhibit higher carbon sequestration and lower water consumption than planted forests in China. Glob. Change Biol. 2019, 25, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salguero, R.; Camarero, J.J.; Dobbertin, M.; Fernández-Cancio, Á.; Vilà-Cabrera, A.; Manzanedo, R.D.; Zavala, M.A.; Navarro-Cerrillo, R.M. Contrasting vulnerability and resilience to drought-induced decline of densely planted vs. natural rear-edge Pinus nigra forests. For. Ecol. Manag. 2013, 310, 956–967. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Griesbauer, H.P.; Green, D.S. Growth responses of three coexisting conifer species to climate across wide geographic and climate ranges in Yukon and British Columbia. For. Ecol. Manag. 2010, 259, 514–523. [Google Scholar] [CrossRef]
- Nichol, J.E.; Abbas, S. Evaluating Plantation Forest vs. Natural Forest Regeneration for Biodiversity Enhancement in Hong Kong. Forests 2021, 12, 593. [Google Scholar] [CrossRef]
- Levia, D.F.; Creed, I.F.; Hannah, D.M.; Nanko, K.; Boyer, E.W.; Carlyle-Moses, D.E.; van de Giesen, N.; Grasso, D.; Guswa, A.J.; Hudson, J.E.; et al. Homogenization of the terrestrial water cycle. Nat. Geosci. 2020, 13, 656–658. [Google Scholar] [CrossRef]
- DeWoody, J.A.; Harder, A.M.; Mathur, S.; Willoughby, J.R. The long-standing significance of genetic diversity in conservation. Mol. Ecol. 2021, 30, 4147–4154. [Google Scholar] [CrossRef]
- Shi, P.L.; Zhang, Y.X.; Hu, Z.Q.; Ma, K.; Wang, H.; Chai, T.Y. The response of soil bacterial communities to mining subsidence in the west China aeolian sand area. Appl. Soil Ecol. 2017, 121, 1–10. [Google Scholar] [CrossRef]
- Sheng, W.T. On the maintenance of long-term productivity of plantation in China. For. Res. 2018, 31, 1–14. [Google Scholar] [CrossRef]
- Schnabel, F.; Beugnon, R.; Yang, B.; Richter, R.; Eisenhauer, N.; Huang, Y.; Liu, X.; Wirth, C.; Cesarz, S.; Fichtner, A.; et al. Tree diversity increases forest temperature buffering via enhancing canopy density and structural diversity. Ecol. Lett. 2025, 28, e70096. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.E.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.G.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, F.-L.; Liu, W.-Z.; Flanagan, D.C. Spatial distribution and temporal trends of extreme temperature and precipitation events on the Loess Plateau of China during 2010, 1961–2007. Quat. Int. 2010; 226, 92–100. [Google Scholar] [CrossRef]
- Guo, Q.F.; Ren, H. Productivity as related to diversity and age in planted versus natural forests. Glob. Ecol. Biogeogr. 2014, 23, 1461–1471. [Google Scholar] [CrossRef]
- Zhang, G.; Hui, G.; Hu, Y.; Zhao, Z.; Guan, X.; von Gadow, K.; Zhang, G. Designing near-natural planting patterns for plantation forests in China. For. Ecosyst. 2019, 6, 28. [Google Scholar] [CrossRef]
- Liu, H.; Park Williams, A.; Allen, C.D.; Guo, D.; Wu, X.; Anenkhonov, O.A.; Liang, E.; Sandanov, D.V.; Yin, Y.; Qi, Z.; et al. Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Glob. Change Biol. 2013, 19, 2500–2510. [Google Scholar] [CrossRef]
- Fang, J.; Yu, G.; Liu, L.; Hu, S.; Chapin, F.S. Climate change, human impacts, and carbon sequestration in China. Proc. Natl. Acad. Sci. USA 2018, 115, 4015–4020. [Google Scholar] [CrossRef]
- Zhang, X.L.; Lv, P.C.; Xu, C.; Huang, X.R.; Rademacher, T. Dryness decreases average growth rate and increases drought sensitivity of Mongolia oak trees in North China. Agric. For. Meteorol. 2021, 308–309, 108611. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, D.; Du, J.; Yang, G.; Bai, M.; Zhou, F.; Zheng, Z.; Ruan, C.; Fang, K. A 241-year Cryptomeria fortune tree-ring chronology in humid subtropical China and its linkages with the pacific decadal oscillation. Atmosphere 2020, 11, 247. [Google Scholar] [CrossRef]
- Altmanová, N.; Fibich, P.; Doležal, J.; Bažant, V.; Černý, T.; Arco Molina, J.G.; Enoki, T.; Hara, T.; Hoshizaki, K.; Ida, H.; et al. Spatial heterogeneity of tree-growth responses to climate across temperate forests in Northeast Asia. Agric. For. Meteorol. 2025, 362, 110355. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Chhin, S.; Zhang, J. Effects of competition, age and climate on tree slenderness of Chinese fir plantations in southern China. For. Ecol. Manag. 2020, 458, 117815. [Google Scholar] [CrossRef]
- Rodriguez-Vallejo, C.; Navarro-Cerrillo, R.M.; Manzanedo, R.D.; Palacios Rodriguez, G.; Gazol, A.; Camarero, J.J. High resilience, but low viability, of pine plantations in the face of a shift towards a drier climate. For. Ecol. Manag. 2021, 479, 118537. [Google Scholar] [CrossRef]
- Mausolf, K.; Wilm, P.; Härdtle, W.; Jansen, K.; Schuldt, B.; Sturm, K.; von Oheimb, G.; Hertel, D.; Leuschner, C.; Fichtner, A. Higher drought sensitivity of radial growth of European beech in managed than in unmanaged forests. Sci. Total. Environ. 2018, 642, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Payn, T.; Carnus, J.-M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Neves Silva, L.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- Zhang, G.; Hui, G.; Zhao, Z.; Hu, Y.; Wang, H.; Liu, W.; Zang, R. Composition of basal area in natural forests based on the uniform angle index. Ecol. Inform. 2018, 45, 1–8. [Google Scholar] [CrossRef]
- Wan, P.; Zhang, G.; Wang, H.; Zhao, Z.; Hu, Y.; Zhang, G.; Hui, G.; Liu, W. Impacts of different forest management methods on the stand spatial structure of a natural Quercus aliena var. acuteserrata forest in Xiaolongshan, China. Ecol. Inform. 2019, 50, 86–94. [Google Scholar] [CrossRef]
- Babst, F.; Bouriaud, O.; Poulter, B.; Trouet, V.; Girardin, M.P.; Frank, D.C. Twentieth century redistribution in climatic drivers of global tree growth. Sci. Adv. 2019, 5, eaat4313. [Google Scholar] [CrossRef]
- Huang, X.; Huang, C.; Teng, M.; Zhou, Z.; Wang, P. Net primary productivity of Pinus massoniana dependence on climate, soil and forest characteristics. Forests 2020, 11, 404. [Google Scholar] [CrossRef]
- Su, H.; Axmacher, J.C.; Yang, B.; Sang, W. Differential radial growth response of three coexisting dominant tree species to local and large-scale climate variability in a subtropical evergreen broad-leaved forest of China. Ecol. Res. 2015, 30, 745–754. [Google Scholar] [CrossRef]
- Shen, B.B.; Song, S.F.; Zhang, L.J.; Wang, Z.Q.; Ren, C.; Li, Y.S. Characteristics of global temperature change from 1981 to 2019. Geogr. Res. 2021, 76, 2660–2672. [Google Scholar] [CrossRef]
- Zhang, F.; Quan, Q.; Ma, F.; Tian, D.; Hoover, D.L.; Zhou, Q.; Niu, S. When does extreme drought elicit extreme ecological responses? J. Ecol. 2019, 107, 2553–2563. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar] [CrossRef]


| Indicators | BH | MW | HL | JC | QY | BJ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NF | PF | NF | PF | NF | PF | NF | PF | NF | PF | NF | PF | |
| Time span | 1950–2022 | 1950–2022 | 1920–2022 | 1975–2022 | 1930–2022 | 1975–2022 | 1937–2022 | 1967–2022 | 1952–2022 | 1969–2022 | 1954–2024 | 1970–2022 |
| MD | 0.942 | 0.962 | 0.978 | 0.944 | 0.953 | 0.980 | 0.985 | 0.988 | 0.963 | 0.986 | 0.986 | 0.976 |
| MS | 0.166 | 0.170 | 0.096 | 0.163 | 0.132 | 0.141 | 0.118 | 0.167 | 0.188 | 0.148 | 0.241 | 0.178 |
| SD | 0.190 | 0.197 | 0.131 | 0.202 | 0.155 | 0.169 | 0.146 | 0.190 | 0.198 | 0.155 | 0.259 | 0.228 |
| AC1 | 0.587 | 0.417 | 0.558 | 0.457 | 0.439 | 0.506 | 0.498 | 0.328 | 0.302 | 0.201 | 0.186 | 0.544 |
| Rbt | 0.422 | 0.556 | 0.438 | 0.399 | 0.520 | 0.397 | 0.424 | 0.544 | 0.498 | 0.496 | 0.624 | 0.524 |
| SNR | 7.582 | 18.881 | 6.584 | 6.764 | 7.786 | 7.795 | 7.263 | 9.542 | 10.081 | 9.409 | 18.067 | 13.246 |
| EPS | 0.883 | 0.95 | 0.868 | 0.871 | 0.921 | 0.872 | 0.924 | 0.905 | 0.91 | 0.904 | 0.966 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhang, Y.; Han, D.; Fan, Y.; Liu, Y. Divergent Climate Sensitivity and Spatiotemporal Instability in Radial Growth of Natural and Planted Pinus tabulaeformis Forests Across a Latitudinal Gradient. Plants 2025, 14, 1441. https://doi.org/10.3390/plants14101441
Fan Y, Zhang Y, Han D, Fan Y, Liu Y. Divergent Climate Sensitivity and Spatiotemporal Instability in Radial Growth of Natural and Planted Pinus tabulaeformis Forests Across a Latitudinal Gradient. Plants. 2025; 14(10):1441. https://doi.org/10.3390/plants14101441
Chicago/Turabian StyleFan, Yue, Yujian Zhang, Dongqing Han, Yanbo Fan, and Yanhong Liu. 2025. "Divergent Climate Sensitivity and Spatiotemporal Instability in Radial Growth of Natural and Planted Pinus tabulaeformis Forests Across a Latitudinal Gradient" Plants 14, no. 10: 1441. https://doi.org/10.3390/plants14101441
APA StyleFan, Y., Zhang, Y., Han, D., Fan, Y., & Liu, Y. (2025). Divergent Climate Sensitivity and Spatiotemporal Instability in Radial Growth of Natural and Planted Pinus tabulaeformis Forests Across a Latitudinal Gradient. Plants, 14(10), 1441. https://doi.org/10.3390/plants14101441





