Identification of a Papain-like Cysteine Protease Functioning as an Avirulence Factor in Striga–Cowpea Interactions
Abstract
1. Introduction
2. Results
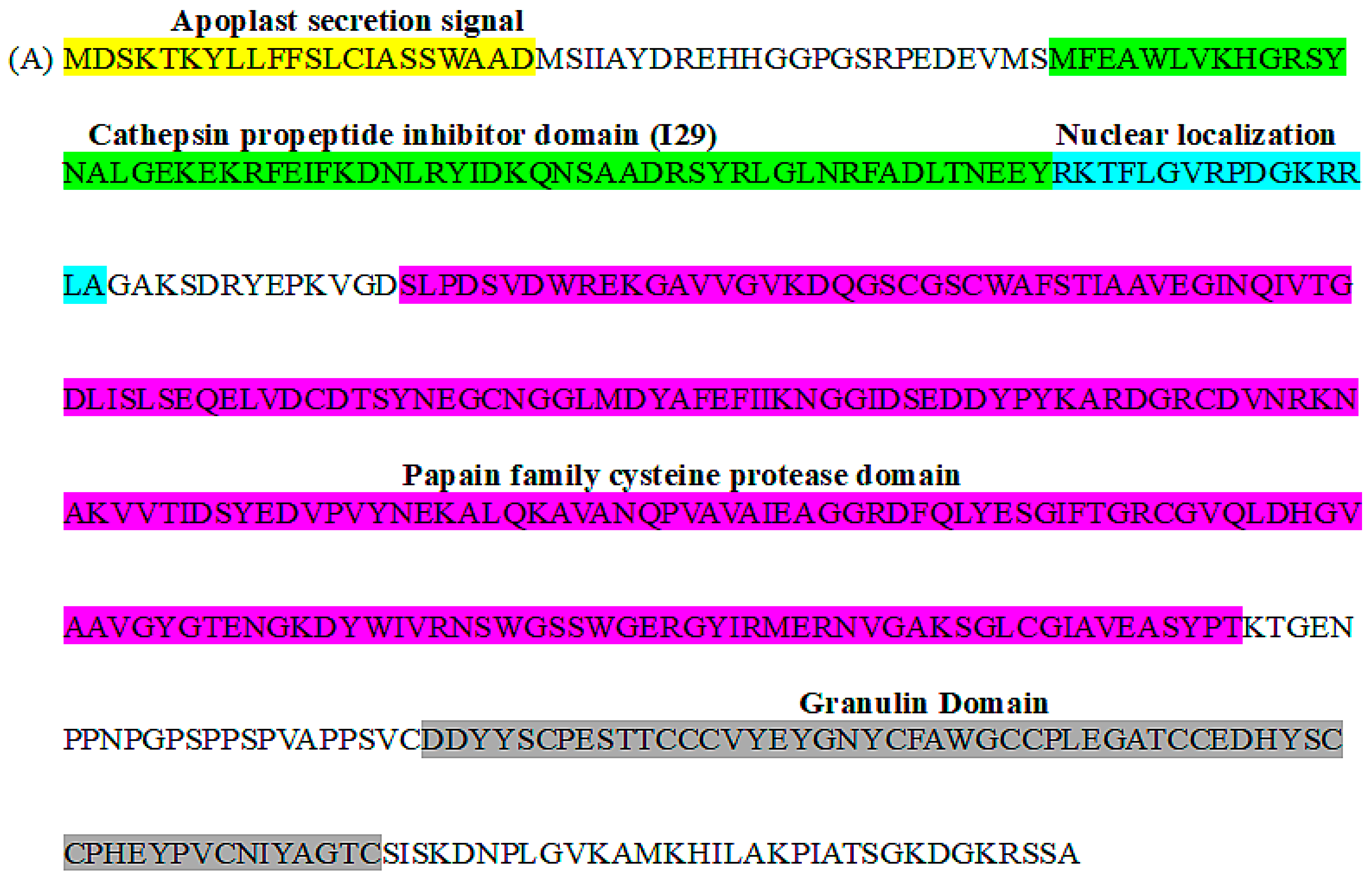
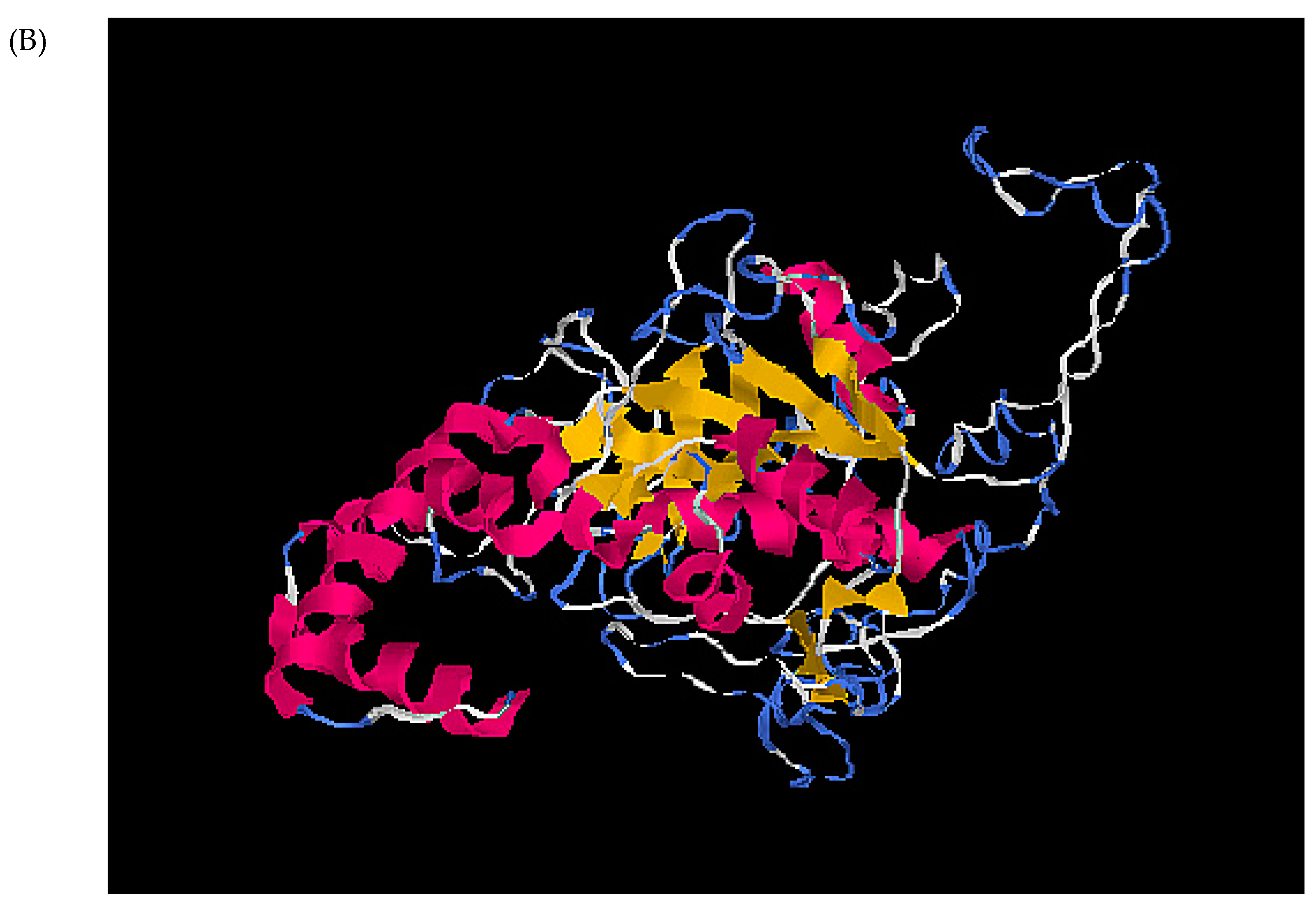
2.1. Characterization of a PLCP from Striga Haustoria
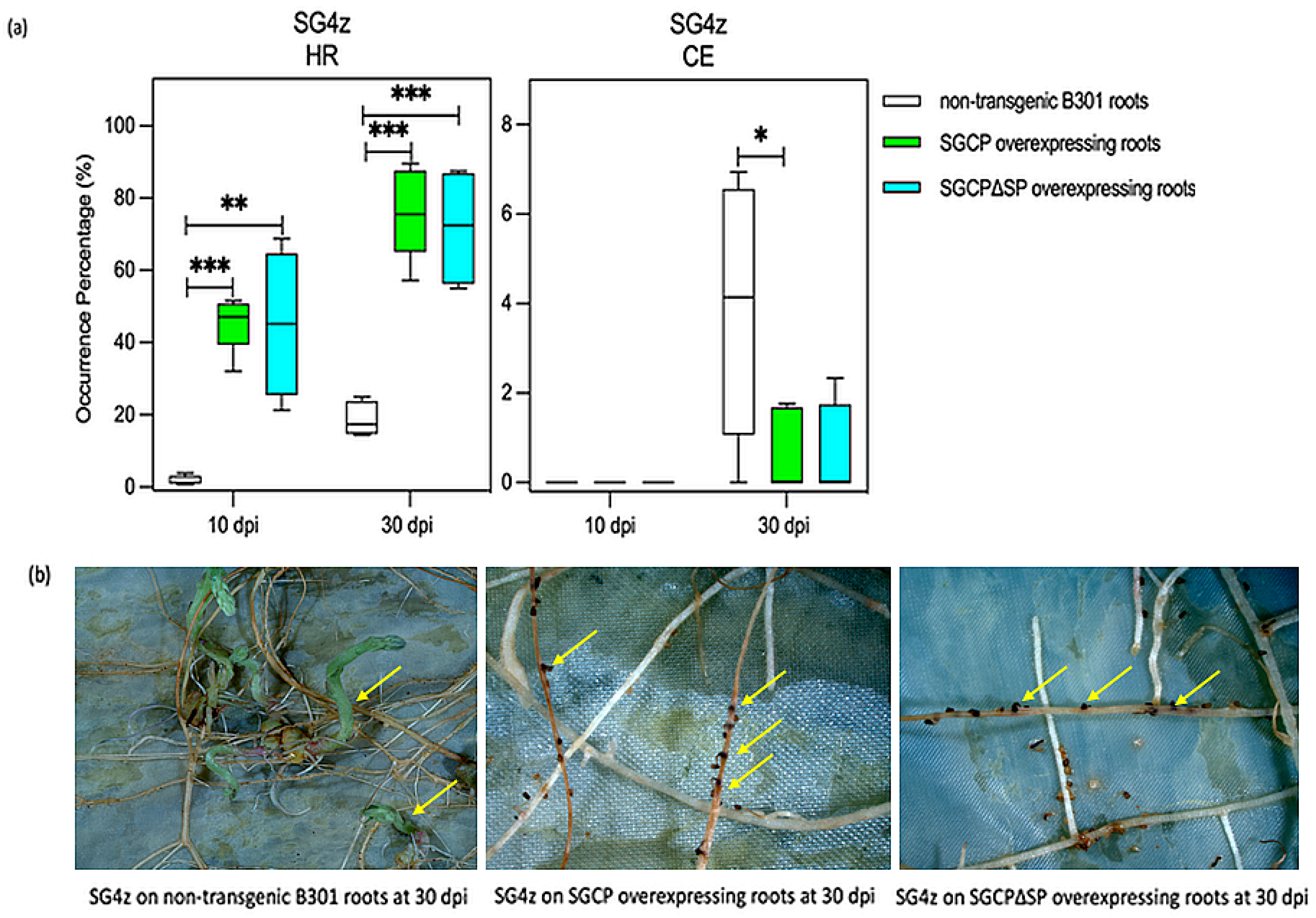
2.2. Expression of SGCP1 and SGCP∆SP in B301 Roots Enhances HR and Suppresses Parasite CE When Challenged by SG4z
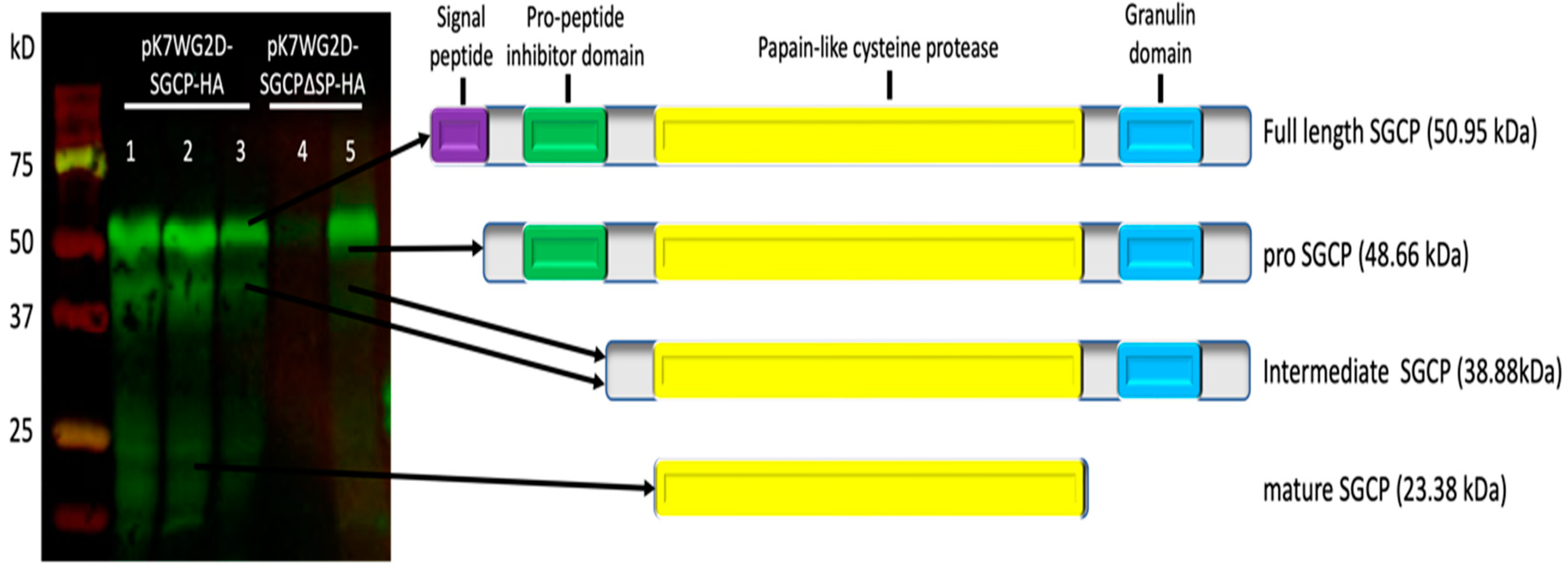
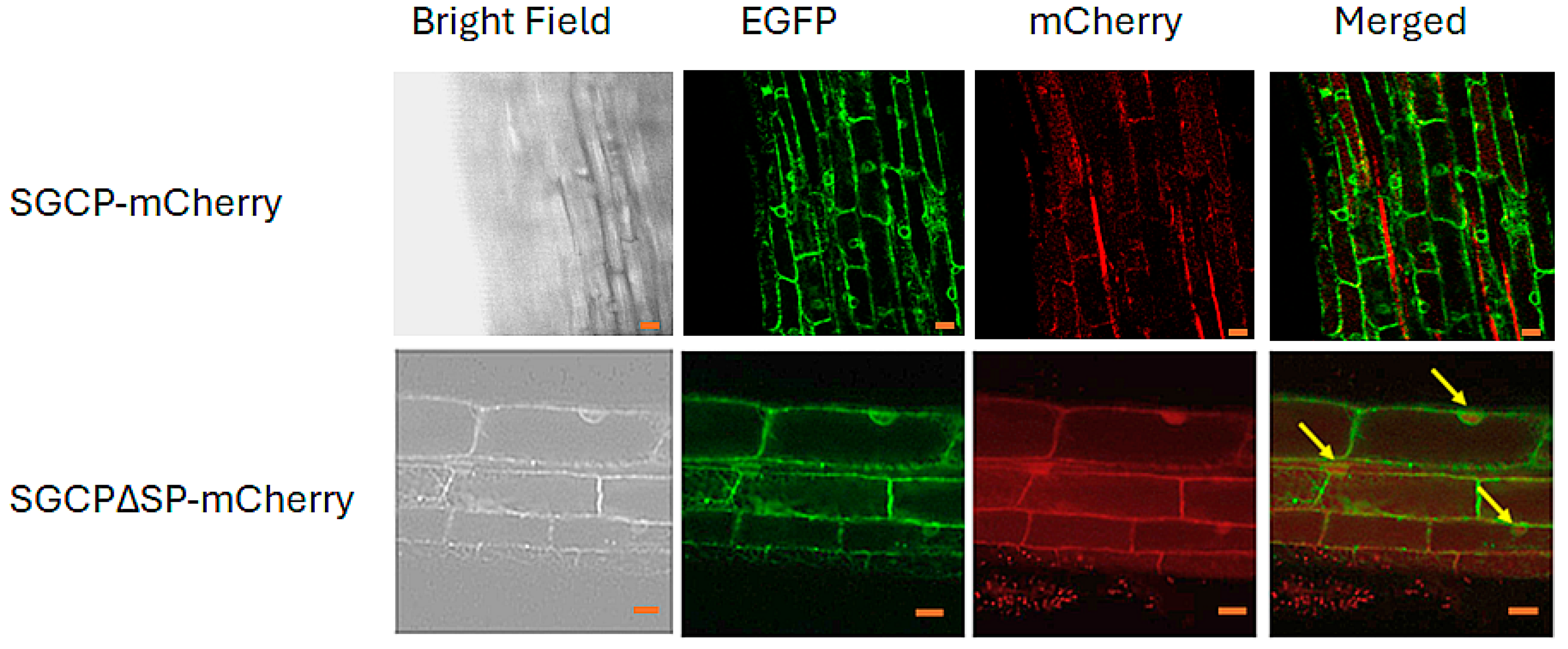
2.3. Molecular Characterization and Cellular Localization of Overexpressed SGCP1 in B301 Roots
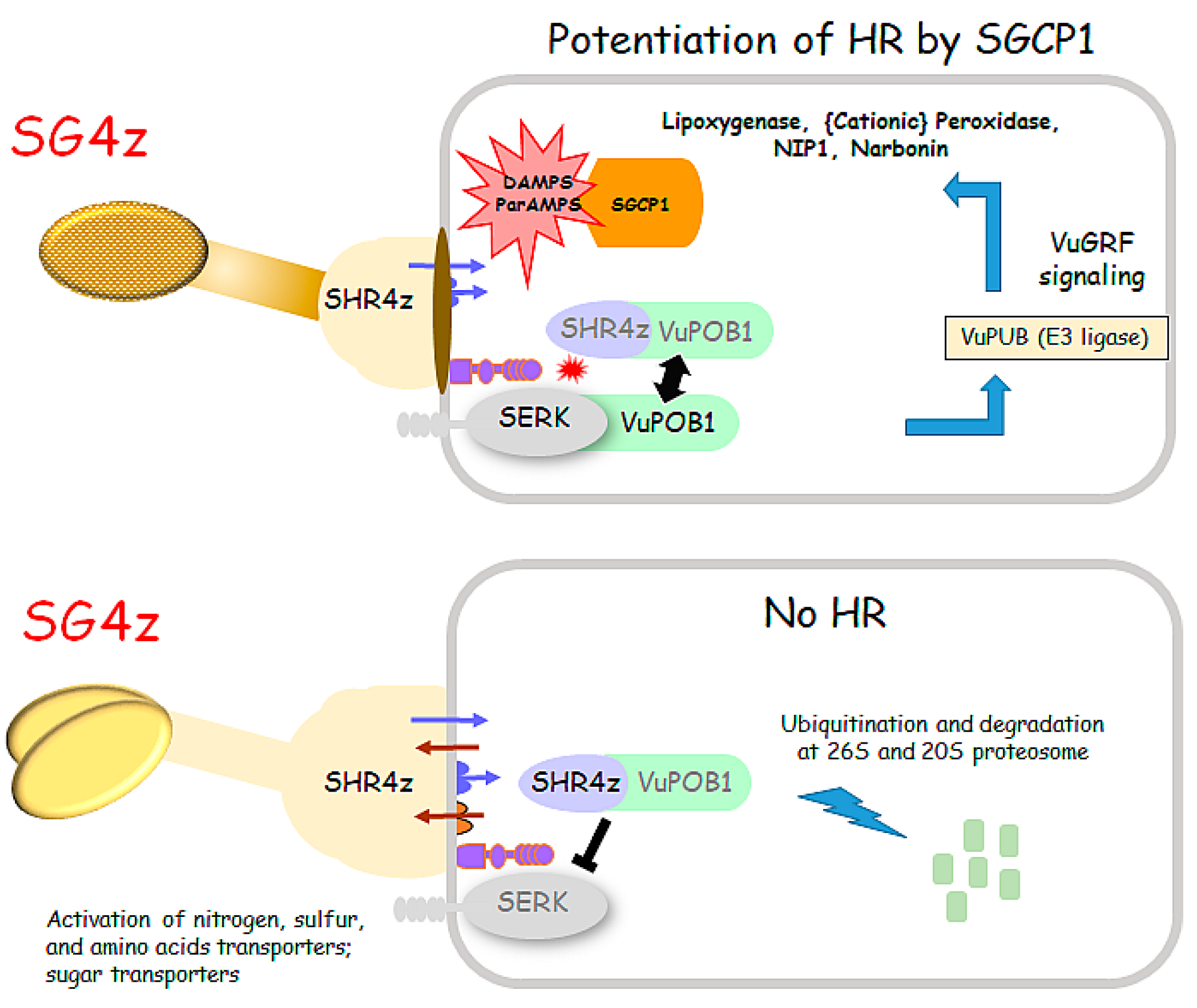
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Cloning of SGCP1 cDNA and Structural Analysis of SGCP1 Protein
4.3. Construction of Transgene Overexpression Plasmids and Generation of Ex Vitro Composite Plants
4.4. Striga–Host Root Interaction Assays
4.5. Immunoblot Analysis of SGCP Expression
4.6. Cellular Localization Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamil, M.; Kountche, B.A.; Al-Babili, S. Current progress in Striga management. Plant Physiol. 2021, 185, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Parker, C. The parasitic weeds of the Orobanchaceae. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Springer: Berlin/Heidelberg, Germany, 2013; pp. 313–344. [Google Scholar]
- Spallek, T.; Mutuku, M.; Shirasu, K. The genus Striga: A witch profile. Mol. Plant Pathol. 2013, 14, 861–869. [Google Scholar] [CrossRef]
- Barberi, P. Ecological weed management in sub-Saharan Africa: Prospects and implications on other agroecosystem services. Adv. Agron. 2019, 156, 219–264. [Google Scholar]
- Timko, M.P.; Singh, B.B. Chapter 10. Cowpea, a multifunctional legume. In Genomics of Tropical Crop Plants; Plant Genetics and Genomics: Crops and Models; Springer: New York, NY, USA, 2008; pp. 227–258. [Google Scholar]
- Botanga, C.J.; Timko, M.P. Phenetic relationships among different races of Striga gesnerioides (Willd.) Vatke from West Africa. Genome 2006, 49, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.A.; Moore, T.H.M.; Child, D.V.; Cardwell, K.F.; Singh, B.B.; Bailey, J.A. Virulence characteristics of a new race of the parasitic angiosperm, Striga gesnerioides, from southern Benin on cowpea (Vigna unguiculata). Euphytica 1994, 72, 183–188. [Google Scholar] [CrossRef]
- Ohlson, E.W.; Timko, M.P. Race structure of cowpea witchweed (Striga gesnerioides) in West Africa and its implications for Striga resistance breeding of cowpea. Weed Sci. 2020, 68, 125–133. [Google Scholar] [CrossRef]
- Lane, J.A.; Moore, T.H.M.; Child, D.V.; Cardwell, K.F. Characterization of virulence and geographic distribution of Striga gesnerioides on cowpea in West Africa. Plant Dis. 1996, 80, 299–301. [Google Scholar] [CrossRef]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The haustorium, a specialized invasive organ in parasitic plants. Annu. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef]
- Reiss, G.C.; Bailey, J.A. Striga gesnerioides parasitising cowpea: Development of infection structures and mechanisms of penetration. Ann. Bot. 1998, 81, 431–440. [Google Scholar] [CrossRef]
- Li, J.; Lis, K.E.; Timko, M.P. Molecular genetics of race-specific resistance of cowpea to Striga gesnerioides (Willd.). Pest Manag. Sci. 2009, 65, 520–527. [Google Scholar] [CrossRef]
- Li, J.; Timko, M.P. Gene-for-gene resistance in Striga-cowpea associations. Science 2009, 325, 1094. [Google Scholar] [CrossRef]
- Timko, M.P.; Huang, K.; Lis, K.E. Host resistance and parasite virulence in Striga–host plant interactions: A shifting balance of power. Weed Sci. 2012, 60, 307–315. [Google Scholar] [CrossRef]
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtel, M.J.; Westwood, J.H. Molecular dialog between parasitic plants and their hosts. RDAnnu. Rev. Phytopathol. 2019, 57, 279–299. [Google Scholar] [CrossRef]
- Huang, K.; Mellor, K.E.; Paul, S.N.; Lawson, M.J.; Mackey, A.J.; Timko, M.P. Global changes in gene expression during compatible and incompatible interactions of cowpea (Vigna unguiculata L.) with the root parasitic angiosperm Striga gesnerioides. BMC Genom. 2012, 13, 402. [Google Scholar] [CrossRef] [PubMed]
- Su, C. Uncovering the Molecular Mechanism Underlying the Virulence of Striga gernerioides. Ph.D. Thesis, University of Virginia, Biology-Graduate School of Arts and Sciences, Charlottesville, VA, USA, 2017. [Google Scholar]
- Su, C.; Liu, H.; Wafula, E.K.; Honaas, L.; de Pamphilis, C.W.; Timko, M.P. SHR4z, a novel decoy effector from the haustorium of the parasitic weed Striga gesnerioides, suppresses host plant immunity. New Phytol. 2020, 226, 891–908. [Google Scholar] [CrossRef]
- Qiu, S.; Bradley, J.M.; Zhang, P.; Chaudhuri, R.; Blaxter, M.; Butlin, R.K.; Scholes, J.D. Genome-enabled discovery of candidate virulence loci in Striga hermonthica, a devastating parasite of African cereal crops. New Phytol. 2022, 236, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.M.; Butlin, R.K.; Scholes, J.D. Comparative secretome analysis of Striga and Cuscuta species identifies candidate virulence factors for two evolutionarily independent parasitic plant lineages. BMC Plant Biol. 2024, 24, 251. [Google Scholar] [CrossRef]
- Davies, L.J.; Zhang, L.; Elling, A.A. The Arabidopsis thaliana papain-like cysteine protease RD21 interacts with a root-knot nematode effector protein. Nematology 2015, 17, 655–666. [Google Scholar] [CrossRef]
- Misas-Villamil, J.C.; van der Hoorn, R.A.; Doehlemann, G. Papain-like cysteine proteases as hubs in plant immunity. New Phytol. 2016, 212, 902–907. [Google Scholar] [CrossRef]
- Shindo, T.; Misas-Villamil, J.C.; Hörger, A.C.; Song, J.; van der Hoorn, R.A.L. A role in immunity for Arabidopsis cysteine protease RD21, the ortholog of the tomato immune protease C14. PLoS ONE 2012, 7, e29317. [Google Scholar] [CrossRef]
- Shindo, T.; van der Hoorn, R.A. Papain-like cysteine proteases: Key players at molecular battlefields employed by both plants and their invaders. Mol. Plant Pathol. 2008, 9, 119–125. [Google Scholar] [CrossRef] [PubMed]
- van der Hoorn, R.A.; Leeuwenburgh, M.A.; Bogyo, M.; Joosten, M.H.; Peck, S.C. Activity profiling of papain-like cysteine proteases in plants. Plant Physiol. 2004, 135, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, E.M.; Hein, I.; Van Der Hoorn, R.; Boevink, P.C.; Venter, E.; McLellan, H.; Kaffarnik, F.; Hrubikova, K.; Shaw, J.; Holeva, M.; et al. Involvement of cathepsin Bin the plant disease resistance hypersensitive response. Plant J. 2007, 52, 1–13. [Google Scholar] [CrossRef]
- Kaschani, F.; Shabab, M.; Bozkurt, T.; Shindo, T.; Schornack, S.; Gu, C.; Ilyas, M.; Win, J.; Kamoun, S.; Van Der Hoorn, R.A. An effector-targeted protease contributes to defense against Phytophthora infestans and is under diversifying selection in natural hosts. Plant Physiol. 2010, 154, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, P.; Mei, L.; Jiang, G.; Lv, Q.; Zhai, W.; Li, C. Knockout of a papain-like cysteine protease gene OCP enhances blast resistance in rice. Front. Plant Sci. 2022, 13, 1065253. [Google Scholar] [CrossRef]
- D’Silva, I.; Poirier, G.G.; Heath, M.C. Activation of cysteine proteases in cowpea plants during the hypersensitive response—A form of programmed cell death. Exp. Cell Res. 1998, 245, 389–399. [Google Scholar] [CrossRef]
- Bernoux, M.; Timmers, T.; Jauneau, A.; Brière, C.; de Wit, P.J.G.M.; Marco, Y.; Deslandes, L. RD19, an Arabidopsis cysteine protease required for RRS1-R–mediated resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 Effector. Plant Cell 2008, 20, 2252–2264. [Google Scholar] [CrossRef]
- Pogorelko, G.V.; Juvale, P.S.; Rutter, W.B.; Hütten, M.; Maier, T.R.; Hewezi, T.; Paulus, J.; van der Hoorn, R.A.; Grundler, F.M.; Siddique, S.; et al. Re-targeting of a plant defense protease by a cyst nematode effector. Plant J. 2019, 98, 1000–1014. [Google Scholar] [CrossRef]
- Pérez-López, E.; Hossain, M.M.; Wei, Y.; Todd, C.D.; Bonham-Smith, P.C. A clubroot pathogen effector targets cruciferous cysteine proteases to suppress plant immunity. Virulence 2021, 12, 2327–2340. [Google Scholar] [CrossRef]
- López-Solanilla, E.; Bronstein, P.A.; Schneider, A.R.; Collmer, A. HopPtoN is a Pseudomonas syringae Hrp (type III secretion system) cysteine protease effector that suppresses pathogen-induced necrosis associated with both compatible and incompatible plant interactions. Mol. Microbiol. 2004, 54, 353–365. [Google Scholar] [CrossRef]
- Bleischwitz, M.; Albert, M.; Fuchsbauer, H.L.; Kaldenhoff, R. Significance of cuscutain, a cysteine protease from Cuscuta reflexa, in host-parasite interactions. BMC Plant Biol. 2010, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Nabiabad, H.S.; Deljou, A. Host-synthesized cysteine protease-specific inhibitor disrupts Cuscuta campestris parasitism in tomato. Plant Biotechol. Rep. 2017, 11, 289–298. [Google Scholar] [CrossRef]
- Amini, M.; Nabiabad, H.S.; Deljou, A. The role of cuscutain-propeptide inhibitor in haustoria parasitism and enhanced resistance to dodder in transgenic alfalfa expressing this propeptide. Plant Biotechnol. Rep. 2018, 12, 165–173. [Google Scholar] [CrossRef]
- Axtell, M.J.; McNellis, T.W.; Mudgett, M.B.; Hsu, C.S.; Staskawicz, B.J. Mutational analysis of the Arabidopsis RPS2 disease resistance gene and the corresponding Pseudomonas syringae avrRpt2 avirulence gene. Mol. Plant-Microbe Interact. 2001, 14, 181–188. [Google Scholar] [CrossRef]
- Shao, F.; Merritt, P.M.; Bao, Z.; Innes, R.W.; Dixon, J.E. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 2002, 109, 575–588. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Yoshida, S.; Kim, S.; Wafula, E.K.; Tanskanen, J.; Kim, Y.-M.; Honaas, L.; Yang, Z.; Spallek, T.; Conn, C.E.; Ichihashi, Y.; et al. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr. Biol. 2019, 29, 3041–3052. [Google Scholar] [CrossRef]
- Corpet, F. Multiple sequence alignment with hierarchical clustering. Nucl Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef]
- Niño, M.; Kim, J.; Lee, H.J.; Abdula, S.E.; Nou, I.S.; Cho, Y.-G. Key roles of cysteine protease in different plant pathosystems. Plant Breed. Biotechnol. 2014, 2, 97–109. [Google Scholar] [CrossRef]
- Ozhelvaci, F.; Steczkiewicz, K. Identification and classification of papain-like cysteine proteinases. J. Biol. Chem. 2023, 299, 104801. [Google Scholar] [CrossRef]
- Russell, A.R.; Ashfield, T.; Innes, R.W. Pseudomonas syringae effector AvrPphB suppresses AvrB-induced activation of RPM1 but not AvrRpm1-induced activation. Mol. Plant-Microbe Interact. 2015, 28, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Hotson, A.; Mudgett, M.B. Cysteine proteases in phytopathogenic bacteria: Identification of plant targets and activation of innate immunity. Curr. Opin. Plant Biol. 2004, 7, 384–390. [Google Scholar] [CrossRef]
- Roden, J.; Eardley, L.; Hotson, A.; Cao, Y.; Mudgett, M.B. Characterization of the Xanthomonas AvrXv4 effector, a SUMO protease translocated into plant cells. Mol. Plant-Microbe Interact. 2004, 17, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, W.; Yang, J.; Xu, J.; Meng, Y.; Shan, W. Two Phytophthora parasitica cysteine protease genes, PpCys44 and PpCys45, trigger cell death in various Nicotiana spp. and act as virulence factors. Mol. Plant Pathol. 2020, 21, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Bhatia, D.; Mavi, G.S. Eighty years of gene-for-gene relationship and its applications in identification utilization of R genes. J. Genet. 2021, 100, 50. [Google Scholar] [CrossRef]
- Knepper, C.; Savory, E.A.; Day, B. The role of NDR1 in pathogen perception and plant defense signaling. Plant Signal. Behav. 2011, 6, 1114–1116. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signaling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef]
- Rodríguez-Ojeda, M.I.; Pineda-Martos, R.; Alonso, L.C.; Fernández-Escobar, J.; Fernández-Martínez, J.M.; Pérez-Vich, B.; Velasco, L. A dominant avirulence gene in Orobanche cumana triggers Or5 resistance in sunflower. Weed Res. 2013, 53, 322–327. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F.; Teofanova, D.R.; Zagorchev, L.I. Genomic and epigenomic mechanisms of the interaction between parasitic and host plants. Int. J. Mol. Sci. 2023, 24, 2647. [Google Scholar] [CrossRef] [PubMed]
- Saify Nabiabad, H.; Amini, M.; Kianersi, F. Ipomoea batatas: Papain propeptide inhibits cysteine protease in main plant parasites and enhances resistance of transgenic tomato to parasites. Physiol. Mol. Biol. Plants 2019, 25, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Tao, T. Standalone BLAST Setup for Windows, P.C. In BLAST® Help [Internet]; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK52637/ (accessed on 31 August 2020).
- Mellor, K.E.; Hoffman, A.M.; Timko, M.P. Use of ex vitro composite plants to study the interaction of cowpea (Vigna unguiculata L.) with the root parasitic angiosperm Striga gesnerioides. Plant Methods 2012, 8, 22. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Timko, M.P. Identification of a Papain-like Cysteine Protease Functioning as an Avirulence Factor in Striga–Cowpea Interactions. Plants 2025, 14, 1427. https://doi.org/10.3390/plants14101427
Zhang D, Timko MP. Identification of a Papain-like Cysteine Protease Functioning as an Avirulence Factor in Striga–Cowpea Interactions. Plants. 2025; 14(10):1427. https://doi.org/10.3390/plants14101427
Chicago/Turabian StyleZhang, Danhua, and Michael P. Timko. 2025. "Identification of a Papain-like Cysteine Protease Functioning as an Avirulence Factor in Striga–Cowpea Interactions" Plants 14, no. 10: 1427. https://doi.org/10.3390/plants14101427
APA StyleZhang, D., & Timko, M. P. (2025). Identification of a Papain-like Cysteine Protease Functioning as an Avirulence Factor in Striga–Cowpea Interactions. Plants, 14(10), 1427. https://doi.org/10.3390/plants14101427





