Abstract
Climate change, represented by global warming, significantly affects the structure and function of alpine wetland ecosystems. Investigating the response strategies of alpine wetland plants to temperature changes is fundamental to understanding how alpine wetlands cope with global warming. This study, conducted at the typical alpine wetland Napahai, uses the latest predictions from the Intergovernmental Panel on Climate Change (IPCC) and employs open–top chamber warming experiments (OTCs) to study the responses of typical alpine wetland plants, Zizania caduciflor and Sparganium stoloniferum, to simulated warming. The results indicate that simulated warming significantly reduced the photosynthetic capacity of Z. caduciflor, and obviously decreased the biomass accumulation of both Z. caduciflor and S. stoloniferum (p < 0.05). The mean annual temperature (MAT) and annual maximum temperature (max) are the primary temperature factors affecting the photosynthetic and biomass parameters. Specifically, the net photosynthetic rate, stomatal conductance, transpiration rate, the aboveground, underground, and total biomasses, and the nitrogen contents of aboveground and underground buds of Z. caduciflor all showed significant negative correlations with MAT and max (p < 0.05). The parameters of S. stoloniferum mainly showed significant correlations with max, with its underground biomass, total biomass, and root nitrogen content all showing significant negative correlations with max, while its fibrous root carbon content and underground bud phosphorus content showed significant positive correlations with max (p < 0.05). The results are consistent with previous studies in high–altitude regions, indicating that warming reduces the photosynthetic capacity and biomass accumulation of alpine wetland plants, a trend that is widespread and will lead to a decline in the productivity of alpine wetlands and changes in vegetation composition. The study can provide a case for understanding the response strategies of alpine wetlands in the context of climate change.
1. Introduction
Climate change, with global warming as a prominent manifestation, is currently one of the most challenging environmental issues faced by humanity [1]. According to the Fifth Assessment Report on Climate Change by the Intergovernmental Panel on Climate Change (IPCC), atmospheric CO2 concentrations will reach 730–1000 ppm by 2100, with temperatures increasing by 1–3.7 °C in tandem [2]. Global warming significantly impacts the structure and function of wetland ecosystems, with the effects being particularly pronounced in high–altitude regions [3,4,5,6]. Highland wetlands in China account for 48.22% of the country’s total wetland area [7,8] and are an essential component of China’s “two screens and three belts” ecological security pattern [9,10,11]. The northwestern region of Yunnan, located on the southeastern edge of the Tibetan Plateau, possesses typical Asian subtropical Himalayan highland wetlands, with significant altitudinal differences and distinct environmental gradients [7,12]. Influenced by differential uplift due to neotectonic movements, fault subsidence, glacial erosion, and fluvial modification, this area has developed numerous small individual wetlands with high spatial heterogeneity and no interconnected waterways, forming unique closed and semi–closed wetland types. The wetland ecosystems in this region are extremely fragile [12]. The lakeshore zone is a primary component of the alpine wetland ecosystems in northwestern Yunnan, and plants, as the basis for the structure and function of the lakeshore zone, are highly sensitive to changes in climatic conditions [13,14]. Researching the ecological response strategies of lakeshore zone plants to climate warming in northwestern Yunnan is an important aspect for people to effectively understand the dynamics of alpine wetlands in northwestern Yunnan and to scientifically respond to climate change. It is also a hot topic of interest both domestically and internationally.
Plant functional traits can effectively modulate plant responses and adaptations to environmental changes, and are therefore often used to discuss patterns and mechanisms of plant environmental adaptation [15,16,17,18]. Research on plant adaptability emphasizes the quantitative determination of plant functional trait syndromes [19], one important aspect of which is to understand the trends, extents, and quantitative relationships of traits with environmental changes, and to elucidate the reasons for different traits working together to adapt to environmental changes [19]. Temperature is one of the most important environmental factors determining plant physiological functions, adaptive strategies, and distribution ranges [20]. Plants exhibit varying degrees of adaptation in their morphological structure and physiological functional traits in response to temperature changes [19,20]. Within the optimal temperature range for plants, the photosynthetic rate of most plants increases with rising temperatures, but as temperatures continue to rise beyond the optimal range, the photosynthetic rate declines [21,22]; environmental temperature affects plant growth, reproduction, and material accumulation by impacting photosynthetic functions [23,24]; high temperatures are a major abiotic factor limiting plant growth and production [25]; on a global scale, the annual mean temperature is significantly negatively correlated with plant nitrogen (N) and phosphorus (P) content [26]; and Roche et al. found in a study of 10 plant species across 8 sites in the southern Mediterranean that the annual mean minimum temperature is significantly negatively correlated with leaf biomass accumulation [27]. The significant correlation between plant functional traits and temperature fully reflects the response strategies of plants to temperature changes. However, these correlations have been less studied in alpine wetland plants, and the main temperature factors affecting plant functional traits and adaptability are not clear. There are also few reports on how climatic effects manifest in alpine wetland plant communities and their environmental responses [12].
Zizania latifolia and Sparganium stoloniferum are dominant emergent plants in the lakeshore zones of Northwest Yunnan, playing a significant role in the ecological structure and functions of these zones and being highly sensitive to environmental changes [28]. The presence of these two species is an important indicator of well–functioning wetland ecosystems in the wetlands of the area [29,30,31]. This study focuses on the ecological response of alpine wetland plants to climate change, selecting the typical alpine wetland Napahai in northwestern Yunnan as the research site, and targeting the large emergent plants Z. caduciflor and S. stoloniferum in the lakeshore zone. Based on the IPCC’s predictions for future atmospheric warming, an open–top warming control experiment is conducted using an artificial warming system. By observing the changes in the photosynthetic physiological parameters, biomass, and nutrient element content of Z. caduciflor and S. stoloniferum under different growth temperatures, the study explores the adaptive changes of plant functional traits to warming and the main temperature factors affecting plant functional traits. This research provides a case study for revealing the physiological adaptation strategies of alpine wetland plants to global warming.
2. Results
2.1. Differences in Functional Traits of Z. caduciflora and S. stoloniferum Under Three Different Growth Temperatures
Compared with the control group (CK), the net photosynthetic rate (Pn) and stomatal conductance (Gs) of Z. caduciflor under simulated warming treatment were significantly reduced, but there were no significant differences among the two parameters between the 2.0 ± 0.5 °C (ET–2) and 4.0 ± 0.5 °C (ET–4) warming treatments; the traspiration rate (Tr) of Z. caduciflor under the ET–2 simulated warming treatment was also significantly reduced, while the photosynthetic parameters of S. stoloniferum showed no significant differences among CK, ET–2, and ET–4 (Figure 1). Additionally, the photosynthetic parameters of S. stoloniferum were mostly numerically higher than those of Z. caduciflor overall (Figure 1).
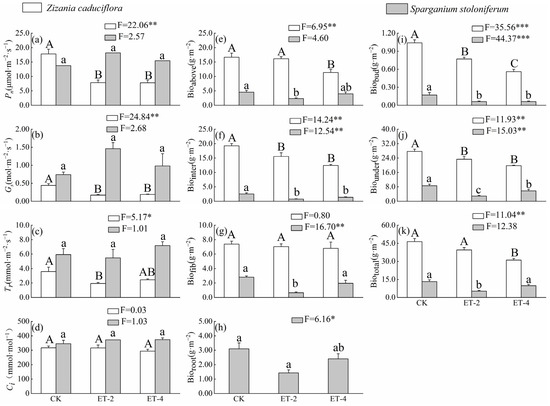
Figure 1.
Differences in photosynthetic traits and biomass of Z. caduciflor and S. stoloniferum among three different growth temperature groups. In this context, the underground part of the Z. caduciflor does not include the roots. Different lowercase letters indicate significant differences of plant traits in S. stoloniferum at the 0.05 level, while different uppercase letters indicate significant differences of plant traits in Z. caduciflor at the 0.05 level (p < 0.05). (a) Pn, net photosynthetic rate; (b) Gs, stomatal conductance; (c) Tr, transpiration rate; (d) Ci, intercellular CO2 concentration; (e) Bioabove, aboveground biomass; (f) Biointer, interval biomass; (g) Biofib, fibrous root biomass; (h) Bioroot, root biomass; (i) Biobud, bud biomass; (j) Biounder, underground biomass; (k) Biototal, total biomass. CK, ambient temperature (control group); ET–2, (2.0 ± 0.5) °C warming; ET–4, (4.0 ± 0.5) °C warming. * p < 0.05; ** p < 0.01; *** p < 0.001.
Simulated warming tends to reduce the biomass accumulation of both plants. Compared with CK, the aboveground biomass (Bioabove) and total biomass (Biototal) of Z. caduciflora showed no significant differences under the ET–2 treatment, but significantly decreased under the ET–4 treatment; the interval biomass (Biointer) and underground biomass (Biounder) significantly decreased under both the ET–2 and ET–4 treatments, but there were no significant differences between these two warming treatments (Figure 1). From the CK to the ET–2, and then to the ET–4, with the increase in temperature, the underground bud biomass (Biobud) of Z. caduciflor showed a significant decreasing trend, while the biomass changes in the fibrous roots were not significant (Figure 1).
The Bioabove, Biointer, and Biototal of S. stoloniferum showed no significant differences between the CK and ET–4 groups, but were significantly lower in the ET–2 group, indicating a trend of first decreasing and then increasing with the rise in temperature (Figure 1). Meanwhile, compared with CK, the Biobud and Biounder of this species were significantly reduced in both the ET–2 and ET–4 treatments. At the same time, the biomass of each part of Z. caduciflor was significantly higher than that of S. stoloniferum (Figure 1).
Compared with the CK group, the simulated warming had a smaller impact on the aboveground carbon content (Cabove) of both plant species, and the changes did not reach a significant level (p > 0.05; Figure 2). Among the belowground components, compared with the CK group, the fibrous root carbon content (Cfib) of Z. caduciflor significantly decreased under the ET–4 condition, while the underground bud carbon content (Cbud) was significantly higher under both warming conditions (p < 0.05; Figure 2). For S. stoloniferum, Cfib was significantly higher under the ET–2 condition, but returned to a level comparable to the CK group under the ET–4 condition, and there were no significant differences in the carbon content of other belowground components among the three groups (p > 0.05; Figure 2).
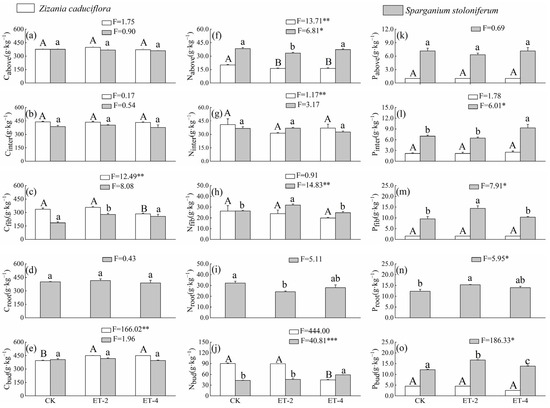
Figure 2.
Differences in elemental mass fractions of Z. caduciflora and S. stoloniferum among three different growth temperature groups. In this context, the underground part of the Z. caduciflor does not include the roots. Different lowercase letters indicate significant differences in plant traits in S.stoloniferum at the 0.05 level, while different uppercase letters indicate significant differences in plant traits in Z. caduciflor at the 0.05 level (p < 0.05). (a) Cabove, aboveground carbon comtent; (b) Cinter, interval carbon comtent; (c) Cfib, fibrous root carbon comtent; (d) Croot, root carbon comtent; (e) Cbud, bud carbon comtent; (f) Nabove, aboveground nitrogen comtent; (g) Ninter, interval nitrogen comtent; (h) Nfib, fibrous root nitrogen comtent; (i)Nroot, root nitrogen comtent; (j) Nbud, bud nitrogen comtent; (k) Pabove, aboveground phosphorus comtent; (l) Pinter, interval phosphorus comtent; (m) Pfib, fibrous root phosphorus comtent; (n) Proot, root phosphorus comtent; (o) Pbud, bud phosphorus comtent. CK, ambient temperature (control group); ET–2, (2.0 ± 0.5) °C warming; ET–4, (4.0 ± 0.5) °C warming. * p < 0.05; ** p < 0.01; *** p < 0.001.
Compared with the CK group, the aboveground nitrogen content (Nabove) of Z. caduciflor was significantly lower under the ET–2 and ET–4 treatments (p < 0.05), with no significant differences between the ET–2 and ET–4 treatments (Figure 2); the Nabove of S. stoloniferum significantly decreased under the ET–2 treatment (p < 0.05), and then returned to the original level under the ET–4 treatment (p > 0.05; Figure 2). Among the belowground components, compared with the CK group, the interval nitrogen content (Ninter) and fibrous root nitrogen content (Nfib) of Z. caduciflor showed no significant differences among the three groups (p > 0.05), while the underground bud nitrogen content (Nbud) significantly decreased under the ET–4 treatment (p < 0.05; Figure 2). For S. stoloniferum, Ninter showed no significant differences among the three groups (p > 0.05); Nfib significantly increased under the ET–2 treatment (p < 0.05), while the root nitrogen content (Nroot) significantly decreased under the ET–2 treatment (p < 0.05), and both returned to the CK level under the ET–4 treatment (p > 0.05); its Nfib showed no significant difference between the CK and ET–2 (p > 0.05) treatment, but significantly increased under the ET–4 treatment (p < 0.05; Figure 2).
Compared with the CK group, there were no significant differences in phosphorus content in various parts of Z. caduciflor among the three groups (p > 0.05). The aboveground phosphorus content (Pabove) of S. stoloniferum showed no significant differences among the three groups (p > 0.05), while the phosphorus content in each component of the belowground parts tended to increase under warming treatments (Figure 2). Specifically, the interval phosphorus content (Pinter) of S. stoloniferum significantly increased under the ET–4 condition; its fibrous root phosphorus content (Pfib) significantly increased under the ET–2 condition; and both the root phosphorus content (Proot) and the underground bud phosphorus content (Pbud) were significantly higher under both the ET–2 and ET–4 conditions (p < 0.05; Figure 2). Additionally, overall, the phosphorus content in each component of S. stoloniferum was higher than that in the corresponding components of Z. caduciflor (Figure 2).
2.2. The Relationship Between Z. caduciflora and S. stoloniferum Functional Traits and Environmental Factors
The stepwise regression models based on the functional traits of Z. caduciflor and S. stoloniferum and temperature factors indicate that the mean annual temperature (MAT) and the annual maximum temperature (max) are the main temperature factors affecting the trait variations in both species (Table 1 and Table 2). Specifically, the Bioabove, Cabove, Cfib, and Nbud of Z. caduciflora are mainly influenced by the MAT and max. The Pn, Gs, Tr, Cbud, and Nabove are primarily affected by the max. The Biointer, Biofib, Biobud, Biounder, and Biototal are mainly influenced by the MAT. However, its intercellular CO2 concentration (Ci), Biofib, Cinter, Ninter, Nfib, and phosphorus content in each component are less affected by temperature factors, with non–existent or non–significant stepwise regression models (Table 1).

Table 1.
Stepwise regression models based on the traits of Z. caduciflora and temperature factors.

Table 2.
Stepwise regression models based on the traits of S. stoloniferum and temperature factors.
For S. stoloniferum, most parameters such as the Pn and Gs, all biomass parameters of each component, the nitrogen content in each component, and the phosphorus content in each component are mainly influenced by the MAT and max. The Cfib is primarily affected by the max. The Tr, Ci, Bioabove, Cabove, Cinter, Croot, Cbud, Ninter, Nroot, and Pabove are less affected by temperature factors, with non–existent or non–significant models (Table 2).
The results of the redundancy analysis (RDA) based on the functional traits and temperature factors of Z. caduciflora show that the temperature factors account for a total cumulative explanation of 57.09% of the traits, with the first and second ordination axes explaining 42.93% and 14.16%, respectively (Figure 3a). The photosynthetic parameters, biomass parameters, and most elemental content parameters of Z. caduciflora are mainly distributed on the positive side of the first principal axis, while the Cbud and Pinter are mainly distributed on the negative side of the first principal axis (Figure 3a). The Cabove, Cfib Ninter, and Tr are mainly distributed on the second principal axis; the temperature factor parameters including the MAT, max, min, dat, nat, and sat are mainly distributed on the negative side of the first principal axis (Figure 3a). According to the distribution of traits and temperature factors, the photosynthetic parameters, biomass, and most elemental content parameters of Z. caduciflora are significantly influenced by temperature factors.
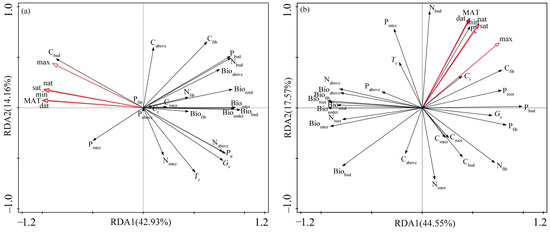
Figure 3.
Association between functional traits and temperature factors of two plants ((a) Z. caduciflora; (b) S. stoloniferum) based on RDA analysis. In this context, the underground part of the Z. caduciflor does not include the roots. Abbreviations for traits in the table are as shown in Table 5. MAT: mean annual temperature, max: annual maximum temperature, min: annual minimum temperature, sat: seasonal average temperature, dat: daytime accumulated temperature, nat: nighttime accumulated temperature.
The RDA results based on the functional traits and temperature factors of S. stoloniferum show that the temperature factors account for a total cumulative explanation of 62.12% of the traits, with the first two ordination axes explaining 44.55% and 17.57%, respectively (Figure 3b). The Pn, Gs, and Ci of S. stoloniferum are mainly distributed on the positive side of the first principal axis, while the biomass parameters of each part are mainly distributed on the negative side of the first principal axis, and the elemental content of each part is distributed on both the first and second principal axes (Figure 3b). Among the temperature factors, the max is mainly distributed on the positive side of the first principal axis, while the other temperature factors are mainly distributed on the positive side of the second principal axis (Figure 3b). According to the distribution of traits and temperature factors, the photosynthetic traits besides Tr and the biomass of S. stoloniferum are mainly related to the max, while its Tr and elemental content are more influenced by other temperature factors.
Combining the results of the stepwise regression model and RDA, the Ci, Biofib, Cinter, Ninter, Nfib, and phosphorus content in each component of Z. caduciflora is less affected by temperature factors, while other traits are mainly influenced by the MAT and max (Table 1, Figure 3a). According to the bivariate correlation analysis between the functional traits of Z. caduciflora and these two temperature factors (Table 3), among the photosynthetic parameters, the Pn and Gs have significant negative correlations with the MAT and max, and the Tr also has a significant negative correlation with the max (Table 3). Among the biomass parameters, the Biointer, Biobud, Biounder, and Biototal have significant negative correlations with the MAT and max, and the Bioabove also has a significant negative correlation with the MAT (Table 3). Among the elemental contents, the Cbud has significant positive correlations with the MAT and max; the Nabove shows significant negative correlations with the MAT and max; and the Nbud also has a significant negative correlation with the MAT (Table 3).

Table 3.
Pearson correlation between functional traits and temperature factors of Z. caduciflora and S. stoloniferum.
Table 3.
Pearson correlation between functional traits and temperature factors of Z. caduciflora and S. stoloniferum.
| Functional Traits | Mean Annual Temperature/MAT | Annual Maximum Temperature/Max | ||
|---|---|---|---|---|
| Z. caduciflora | S. stoloniferum | Z. caduciflora | S. stoloniferum | |
| net photosynthetic rate | −0.852 ** | 0.315 | −0.937 *** | 0.515 |
| stomatal conductance | −0.828 ** | 0.278 | −0.937 *** | 0.490 |
| transpiration rate | −0.586 | – | −0.743 * | – |
| intercellular CO2 concentration | – | – | – | – |
| aboveground biomass | −0.727 * | – | −0.523 | – |
| interval biomass | −0.908 *** | −0.638 | −0.832 ** | −0.826 ** |
| fibrous root biomass | – | −0.437 | – | −0.705 * |
| root biomass | – | −0.406 | – | −0.641 |
| bud biomass | −0.960 *** | −0.878 ** | −0.888 *** | −0.967 ** |
| underground biomass | −0.893 *** | −0.521 | −0.820 ** | −0.761 * |
| total biomass | −0.862 ** | −0.456 | −0.724 * | −0.710 * |
| aboveground carbon comtent | −0.043 | – | 0.184 | – |
| interval carbon comtent | – | – | – | – |
| fibrous root carbon comtent | −0.558 | 0.676 * | −0.259 | 0.821 ** |
| root carbon comtent | – | – | – | – |
| bud carbon comtent | 0.898 *** | – | 0.989 *** | – |
| aboveground nitrogen comtent | −0.822 ** | −0.229 | −0.904 *** | −0.509 |
| interval nitrogen comtent | – | −0.570 | – | −0.369 |
| fibrous root nitrogen comtent | – | −0.117 | – | 0.226 |
| root nitrogen comtent | – | −0.479 | – | −0.679 * |
| bud nitrogen comtent | −0.828 ** | 0.877 ** | −0.565 | 0.666 |
| aboveground phosphorus comtent | – | – | – | – |
| interval phosphorus comtent | – | – | – | – |
| fibrous root phosphorus comtent | – | 0.201 | – | 0.493 |
| root phosphorus comtent | – | – | – | – |
| bud phosphorus comtent | – | 0.444 | – | 0.740 * |
In this context, the underground part of the Z. caduciflor does not include the roots. Bold fonts indicate a significance level of less than 0.05. * p < 0.05; ** p < 0.01; *** p < 0.001.
The Tr, Ci, Bioabove, Cabove, Cinter, Croot, Cbud, Pabove, Pinter, and Proot of S. stoloniferum are less affected by temperature factors, while other traits are more significantly influenced by the max and relatively less influenced by the MAT (Table 2, Figure 3b). The photosynthetic parameters of S. stoloniferum do not show significant correlations with the MAT or max (Table 3). Among the biomass parameters, except for root biomass, other biomass parameters have significant negative correlations with the max, and Biobud also has a significant negative correlation with the MAT (Table 3). Among the elemental content parameters, Cfib has significant positive correlations with both the MAT and max; Nbud has a significant positive correlation with the MAT; and Pbud has a significant positive correlation with the max (Table 3).
3. Materials and Methods
3.1. Overview of the Study Area
Our study site is located in the Napahai Provincial Nature Reserve. Napahai Wetland (99°37′11″–99°40′20″ E, 27°48′56″–27°54′28″ N) is situated in Shangri–La City, Diqing Tibetan Autonomous Prefecture, Yunnan Province, in the middle section of the Hengduan Mountains, and is a seasonal lake wetland at low latitude and high altitude. Influenced by differential uplift due to neotectonic movements, fault subsidence, glacial erosion, and fluvial modification, the Hengduan Mountain area generally features small individual wetlands with high spatial heterogeneity and no interconnected waterways, forming unique closed and semi–closed wetland types, and Napahai Wetland also conforms to these characteristics. Due to its enclosed nature, small wetland area, and the characteristic of water source replenishment mainly relying on rainfall and snowmelt, the Napahai Wetland ecosystem is relatively fragile and highly sensitive to human activities and climate change.
The Shangri–La region, where Napahai Wetland is located, belongs to the western type of the cold temperate plateau monsoon climate zone. Influenced by the north–south arranged mountains and atmospheric circulation, the southerly and southwesterly winds prevail throughout the year. Due to its location in the southeastern extension of the Tibetan Plateau, it has distinct plateau climatic characteristics. The climatic features of Napahai include strong solar radiation, with an annual average of 2180 h of sunshine; a small annual temperature range, a large diurnal temperature range, long winters without summers, and short springs and autumns. The average annual temperature is 5.4 °C, the average temperature in the hottest month of July is 13.2 °C, and the average temperature in the coldest month of January is −3.7 °C, using a base temperature of 10 °C, and the accumulated temperature (or thermal time or Growing Degree Days, GDDs) reached 1392.8 °C. Napahai is situated in the transitional zone between rainy and less rainy areas, with an annual average precipitation of 620 mm, an annual frost period of about 125 days, and snow from September to May of the following year.
Napahai’s unique geographical environment has given rise to rich biodiversity, which is conducive to the differentiation of endemic species and the preservation of ancient species [32]. The wetland is rich in plant species, including two major categories: hydrophytes and emergent plants. Among them, submerged plants include Hydrailla verticillata and Myriophyllum spicatum; floating–leaf plants mainly consist of Potamogeton maackianus; and emergent plants include Scirpus validus, Zizania caduciflora, Sparganium stoloniferum, Hippuris vulgaris, and others.
3.2. Experimental Design
3.2.1. Construction of Artificial Simulated Warming Chambers
Open–top chambers (OTCs) were constructed in April 2014. Nine in situ research units with a bottom diameter of 2.4 m were established in an area similar to the Napahai wetland environment, with a 3 m interval between each research unit and connected with PVC pipes to ensure that the water depth in each research unit was consistent with the natural flooding depth in the field (approximately 18 cm). According to the IPCC’s assessment report for the end of the 21st century, the global near–surface atmospheric temperature is expected to rise by 0.3–1.7 °C under the RCP2.6 scenario and by 2.6–4.8 °C under the RCP8.5 scenario [2]. Based on this, we divided the research units into three groups, with three replicates per group (Figure 4). One group was set as the control group (CK), consistent with the surrounding environmental conditions, and the other two groups were set as experimental groups. Open–top simulated warming chambers were constructed on the ground with sunlight board materials, reaching a height of 2.4 m. By controlling the opening size of the chambers, different degrees of indoor temperature increase were achieved. One group of chambers had an opening size set to achieve an atmospheric warming of 2.0 ± 0.5 °C (ET–2), and the other group had an opening size set to achieve an atmospheric warming of 4.0 ± 0.5 °C (ET–4).
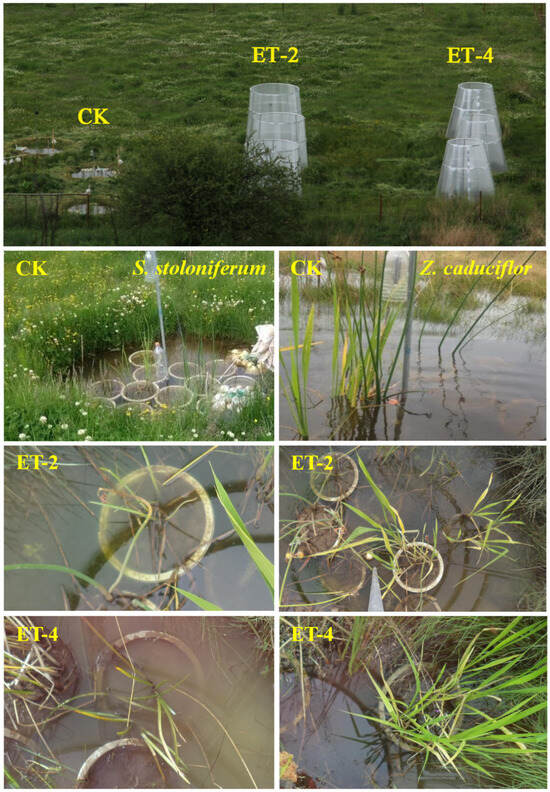
Figure 4.
Open–top chambers in Napahai wetland and the growth status of the two plant species in the chambers. CK, ambient temperature (control group); ET–2, (2.0 ± 0.5) °C warming; ET–4, (4.0 ± 0.5) °C warming.
In the center of each of the 9 differently treated research units, 1 m above the water surface, a TP–2200 temperature real–time recorder was placed. Each recorder automatically logged the atmospheric temperature of each research unit 24 times a day at a recording frequency of 1 time per hour. After the experiment concluded, the data from the temperature real–time recorders were exported, and the following data were organized and calculated for each group: the mean annual temperature (MAT, °C), annual maximum temperature (max, °C), annual minimum temperature (min, °C), seasonal average temperature (sat, °C), daytime accumulated temperature (dat, °C), and nighttime accumulated temperature (nat, °C). According to the analysis results of the full–year simulated warming in 2018, the average atmospheric temperature increase in the ET–2 and ET–4 groups was 2.17 °C and 3.76 °C, respectively (Table 4), indicating that the OTCs’ simulated warming system used in this study provided a stable warming effect.

Table 4.
Temperature variable values under different temperature conditions.
Table 4.
Temperature variable values under different temperature conditions.
| Process | Temperature Variable | |||||
|---|---|---|---|---|---|---|
| MAT | Max | Min | Sat | Dat | Nat | |
| CK | 8.83 | 37.69 | −22.38 | 15.12 | 2572.79 | 281.66 |
| ET–2 | 11.00 | 46.88 | −19.13 | 17.76 | 3319.56 | 470.34 |
| ET–4 | 12.59 | 47.50 | −16.75 | 19.09 | 3877.70 | 562.04 |
CK, ambient temperature (control group); ET–2, (2.0 ± 0.5) °C warming; ET–4, (4.0 ± 0.5) °C warming. MAT: mean annual temperature, max: annual maximum temperature, min: annual minimum temperature, sat: seasonal average temperature, dat: daytime accumulated temperature, nat: nighttime accumulated temperature.
3.2.2. Plant Transplantation
Based on preliminary surveys of dominant plants in the Napahai lakeshore zone, this study selected Z. caduciflor and S. stoloniferum as the subjects of research. In March–April 2018, healthy and similarly vigorous clones of these two plant species were excavated from the Napahai lakeshore zone. Soil from the Napahai lakeshore zone at a depth of 30 cm was collected as the cultivation substrate, and individual plants were transplanted into experimental pots with a diameter of 35 cm and a height of 40 cm, with an equal amount of soil in each pot. After a 15–day acclimation period under natural conditions, the transplanted plants were randomly divided into three groups (three pots per group) and then placed into different research units (Figure 4). To exclude edge effects and ensure uniform light conditions, the pots were randomly placed at the midpoint between the inner edge and the center of the growth chamber. The water depth in each research unit was based on the native waterlogged environment (20–30 cm) (Figure 4). Subsequent daily management of the plants was intensified to ensure the survival of the transplanted plants.
3.3. Measurement of Trait Parameters
3.3.1. Measurement of Photosynthetic Parameters
During the peak growth period of the plants in 2018 (July–August), from 9:00 to 12:00 on sunny mornings, the net photosynthetic rate, stomatal conductance, intercellular CO2 concentration, and transpiration rate of plants in different growth chambers were recorded in situ using the Li-6800XT portable photosynthesis system (LI-6800, LI-COR, 4647 Superior Street, Lincoln, NE 68504, USA). In each growth chamber, three mature plants were selected as subjects, and two healthy, fully expanded leaves per plant were chosen for measurement. During the measurement, the internal light intensity of the leaf chamber was set to 1500 μmol·m−2·s−1, the leaf temperature was maintained at 22–24 °C, the flow rate was set to 500 μmol·s−1, and the reference chamber CO2 concentration was set to 425 μmol·mol−1.
3.3.2. Determination of Biomass and Elemental Content
The entire plants of Z. caduciflor and S. stoloniferum, from which photosynthetic parameters had been measured, were carefully removed, rinsed with running water, marked, and brought back to the laboratory. In the laboratory, the aboveground and belowground parts were separated, dividing the entire plant into aboveground and belowground sections. The belowground part was further divided into interval, fibrous root, root, and underground buds; in this context, the underground part of the Z. caduciflor does not include the roots. After weighing the fresh weight of each part, they were individually placed into numbered kraft envelopes and dried in a 75 °C oven for at least 48 h until they reached a constant weight. This process yielded the biomass of each part, including the aboveground biomass, interval biomass, fibrous root biomass, root biomass, and underground bud biomass. The underground biomass was calculated as the sum of the latter four parts, and the total biomass was determined by adding the aboveground biomass to the underground biomass.
After weighing the biomass, the plant parts were ground separately using a plant grinder and mortar, and then sifted through a sieve with a mesh size of 0.25 mm. The sifted powder was packaged in labeled sealable bags and stored in a refrigerator at 4 °C for future use. Three milligrams of the ground plant samples was taken, treated with acid, dried, and wrapped in tin foil for analysis using the vario TOC select total organic carbon analyzer from Elementar, company is located in Hanau, a city near Frankfurt, Germany, to determine the total carbon mass fraction of each plant part, denoted as the total carbon content. Next, 0.2 g of the plant powder was digested using the concentrated sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) digestion method, after which the solution was made up to volume and filtered. The filtrate was then used to measure and calculate the total nitrogen and phosphorus mass fractions of the plants with an AA3 continuous flow analyzer, denoted as the total nitrogen content and total phosphorus content, respectively. The plant functional traits measured in this study and their abbreviations are shown in Table 5.

Table 5.
Measured plant functional traits and abbreviations.
Table 5.
Measured plant functional traits and abbreviations.
| Functional Traits | Abbreviations (Unit) | |
|---|---|---|
| Photosynthetic parameters | net photosynthetic rate | Pn (μmol·m−2·s−1) |
| stomatal conductance | Gs (mol·m−2·s−1) | |
| transpiration rate | Tr (μmol·mol−1) | |
| intercellular CO2 concentration | Ci (mmol·m−2·s−1) | |
| Biomass | aboveground biomass | Bioabove (g·m−2) |
| interval biomass | Biointer (g·m−2) | |
| fibrous root biomass | Biofib (g·m−2) | |
| root biomass | Bioroot (g·m−2) | |
| bud biomass | Biobud (g·m−2) | |
| underground biomass | Biounder (g·m−2) | |
| total biomass | Biototal (g·m−2) | |
| Elemental content | aboveground carbon comtent | Cabove (g·kg−1) |
| interval carbon comtent | Cinter (g·kg−1) | |
| fibrous root carbon comtent | Cfib (g·kg−1) | |
| root carbon comtent | Croot (g·kg−1) | |
| bud carbon comtent | Cbud (g·kg−1) | |
| aboveground nitrogen comtent | Nabove (g·kg−1) | |
| interval nitrogen comtent | Ninter (g·kg−1) | |
| fibrous root nitrogen comtent | Nfib (g·kg−1) | |
| root nitrogen comtent | Nroot (g·kg−1) | |
| bud nitrogen comtent | Nbud (g·kg−1) | |
| aboveground phosphorus comtent | Pabove (g·kg−1) | |
| interval phosphorus comtent | Pinter (g·kg−1) | |
| fibrous root phosphorus comtent | Pfib (g·kg−1) | |
| root phosphorus comtent | Proot (g·kg−1) | |
| bud phosphorus comtent | Pbud (g·kg−1) | |
3.4. Data Analysis
This study employed SPSS 25.0 software “www.ibm.com/products/spss-statistics (accessed on 10 August 2024)” to test the homogeneity of variance of the photosynthetic traits, biomass, and elemental mass fractions using Levene’s test. Then, to conduct a one–way ANOVA to test for differences in the functional traits between the three temperature treatment groups of Z. caduciflor and S. stoloniferum, a multiple comparisons were conducted by the least significant difference (LSD) method. The “Multiple–regression” package in the R 4.4.2 statistical analysis software “www.r-project.org (accessed on 17 August 2024)” was used to perform stepwise regression analysis on the functional traits of the two plants and the temperature factors, respectively, to select the main temperature factors affecting the traits of Z. caduciflor and S. stoloniferum. Redundancy analysis (RDA) was conducted using Canoco 5 software “www.canoco5.com (accessed on 28 August 2024)” to examine the relationships between the traits of Z. caduciflor and S. stoloniferum and the temperature factors. Pearson correlation analysis was performed using SPSS software to further detect the correlations between “traits and traits” and “traits and temperature factors” in the two plants. The significance levels for all data analyses in this study are defined as follows: p < 0.05 indicates significance, p < 0.01 indicates highly significant, and p < 0.001 indicates extremely significant. The figures in this study were created using Origin 2018 and Adobe Photoshop 2020 software.
4. Discussion
Z. caduciflora and S. stoloniferum are both typical emergent plants in the Napahai lakeshore zone. Simulated warming significantly reduced the net photosynthetic rate and stomatal conductance of Z. caduciflora (Figure 1), but these photosynthetic parameters of S. stoloniferum did not show significant changes (Figure 1). This indicates that Z. caduciflora is more sensitive to simulated warming, with a 2 °C increase already exceeding its optimal growth temperature, while S. stoloniferum can maintain a stable photosynthetic capacity under simulated warming conditions. Previous studies on aquatic plants such as Typha orientalis and Phragmites australis in high–altitude areas also showed that warming would decrease plant photosynthetic productivity [33,34], which is consistent with the trend observed in Z. caduciflora, suggesting that the decline in the photosynthetic productivity of large aquatic plants in plateau regions is a common phenomenon under the background of climate warming. When environmental temperatures exceed the optimal temperature for plant growth, the net photosynthetic rate will decline [35]. Even though an increase in temperature can enhance the actual photosynthetic rate of plants, the increase in photorespiration and plant respiration under high temperatures can also lead to a decrease in the net photosynthetic rate [36]. During the carbon fixation process, ribulose–1,5–bisphosphate carboxylase/oxygenase (Rubisco) can promote photosynthetic efficiency. The amount of active Rubisco is limited by the activity of Rubisco activase, which is inhibited by environmental conditions such as increased temperature, shading, or elevated CO2 concentrations [37,38]. In addition to this direct effect, warming can also affect the activity of Rubisco activase indirectly by reducing the rate of electron transport, redox potential, and the amount of nucleoside triphosphates, thereby decreasing photosynthetic efficiency and other processes related to photosynthesis [36,37]. Therefore, increasing the thermal stability of plants will be more conducive to maintaining higher net photosynthetic rates in plants [39].
Although the photosynthetic parameters of Z. caduciflora and S. stoloniferum respond differently to simulated warming, the biomass of both species shows a declining trend under simulated warming conditions, with decreases in the aboveground, belowground, and total biomass under warming conditions (Figure 1), indicating that high temperatures inhibit the biomass accumulation of both Z. caduciflora and S. stoloniferum. The aboveground part is the plant’s nutritional tissue, while the belowground part is related to the plant’s clonal reproduction; therefore, warming significantly reduces the nutritional growth and offspring clonal reproduction capabilities of both plants. Generally, an increase in temperature can extend the growing season by advancing the arrival of spring and delaying the onset of winter; however, for many plants, moderate warming can shorten the time required for plant development [40,41]. For instance, appropriate warming before plants reach maturity can shorten the leaf construction time, thereby shortening the plant development process [40,41]. This rapid development process in plants will reduce the total carbon fixation, which in turn decreases the energy investment in nutritional and reproductive structures [42]. Temperature increases beyond the species’ optimal temperature may affect the genetic potential of plants in terms of adaptation and biomass, thereby endangering the species’ survival capabilities [42]. Some studies have found that the regulatory capacity of the aboveground parts is stronger than that of the belowground parts; under resource–limited conditions, plants generally increase the proportion of underground biomass to ensure the reproductive capacity of offspring [33,43]. Compared to these plants, under the background of long–term climate warming, Z. caduciflora and S. stoloniferum may have reduced investment in offspring, leading to lower competitive ability, which is not conducive to the future development of the plants.
Consistent with the decline in photosynthetic capacity, the aboveground nitrogen content of Z. caduciflora and the nitrogen content of its underground buds were significantly reduced, and the aboveground nitrogen content and root nitrogen content of S. stoloniferum were also significantly decreased (Figure 2). Nitrogen is a structural element of photosynthetic proteins, especially the essential protein for photosynthesis–the Rubisco enzyme–and it is also the raw material for all protein metabolism and amino acid synthesis within plants. Global leaf economic spectra and wetland plant economic spectra have both demonstrated a significant positive correlation between the net photosynthetic rate and nitrogen and phosphorus content [44,45]. Higher photosynthetic rates, higher nitrogen and phosphorus content, and lower specific leaf weight are typical characteristics of resource–acquisitive plants, while lower net photosynthetic rates and nitrogen and phosphorus content, along with higher specific leaf weight, are typical characteristics of resource–conservative plants [26]. Wetland plants are generally clustered towards the “low investment–rapid return” end of resource acquisitive strategies [44]. The significant reduction in the net photosynthetic rate and nitrogen content of Z. caduciflora under warming conditions indicates that as temperatures rise, its resource utilization type is gradually shifting towards a resource–conservative strategy. Warming reduces the supply of nitrogen by decreasing the activity and quantity of enzymatic and other material activities, thereby reducing the net photosynthetic rate. Therefore, the deficiency of mineral nutrients under warming conditions is one of the reasons for the decline in the photosynthetic productivity of Z. caduciflora. S. stoloniferum’s response strategy differs from that of Z. caduciflora. In terms of the decrease in nitrogen content, the response strategies of Z. caduciflora and S. stoloniferum are consistent. However, regarding phosphorus, the phosphorus content of Z. caduciflora remains unchanged under all temperature conditions, while the phosphorus content of all underground parts of S. stoloniferum, including interval, fibrous roots, main roots, and underground buds, is significantly higher under warming conditions compared to the control group (Figure 2), indicating that simulated warming promotes the absorption of phosphorus in the underground parts of S. stoloniferum. Phosphorus is an important component of nucleic acids, lipid membranes, and the biological energy molecule ATP [46]. Under natural conditions, increasing plants’ intake of phosphorus can, to some extent, mitigate the impact of nitrogen deficiency on plant photosynthetic physiology and effectively ensure normal plant growth in the short term [46]. This may be one of the important reasons why S. stoloniferum can maintain stable photosynthetic rates under warming conditions.
The mean annual temperature (MAT) and the annual maximum temperature (max) are the main temperature factors affecting the functional traits of Z. caduciflora and S. stoloniferum (Table 3 and Table 4). The MAT reflects the average temperature level during the plant growth process and is directly related to the length of the growing season and the effective accumulated temperature during the growing period, while the max represents the extreme high temperatures in the plant growth environment [25,33,47]. These two temperature factors are mainly negatively correlated with the photosynthetic parameters, biomass of various parts, and nitrogen content in the aboveground parts and underground buds of Z. caduciflora and S. stoloniferum (Figure 3, Table 3), indicating that the increase in the MAT and max leads to a decrease in water vapor exchange capacity and nitrogen content in the aboveground parts and underground buds, resulting in insufficient photosynthetic raw materials and nutrient supply, a decline in photosynthetic productivity, and subsequently reduced biomass accumulation. Generally speaking, sustained high temperatures can reduce the photosynthetic efficiency of plants, shorten their life cycles, and lead to a decrease in productivity [48]. Under extreme high–temperature conditions, plants are further directly inhibited at the physiological level, and their metabolism is affected. Extreme high–temperature stress can impact the structure of chloroplasts and the thermal stability of photosynthetic system components, reducing the activity of Rubisco, the content of photosynthetic pigments, and the capacity for carbon fixation [49]. For plants, the survival of chloroplasts, which are essential for photosynthesis, has a certain temperature range. Excessively high temperatures can damage the thylakoid membrane, inhibit the activity of membrane–associated electron carriers and enzymes, and promote too rapid an electron transfer rate of chlorophyll a, leading to a decline or even interruption of the activity of heat–sensitive Photosystem II (PS II), causing irreversible tissue damage [50,51]. Plants possess a variety of mechanisms that help improve their heat tolerance, allowing them to endure high temperatures and prevent damage from excessively high leaf temperatures. These mechanisms include heat conduction, heat convection, and transpirational cooling [52,53]. Under water–limited conditions, plant species with low stomatal conductance due to isohydric behavior or inherently low transpiration capacity will open their stomata and increase stomatal conductance under high temperatures; however, under well–watered conditions, high temperatures will prompt plants to close their stomata, reducing transpiration rates and stomatal conductance [54]. The decrease in the transpiration rate and stomatal conductance reduces the capacity for gas exchange, leading to a decline in photosynthesis due to insufficient water and CO2 and, consequently, a reduction in biomass accumulation. There is, therefore, a balance between plant physiology and leaf energy [55]. For wetland plants, the limiting effect of water availability is relatively weak, so reducing tissue damage and ensuring the normal operation of the photosynthetic system under extreme high temperatures may be the main reason for the decrease in stomatal conductance and the transpiration rate in aquatic plants under high temperatures.
Nutrient supply, especially the supply of nitrogen and phosphorus, is an important aspect of photosynthesis in plants. Insufficient nutrients can directly lead to a lack of energy and material supply for the photosynthetic process, thereby reducing photosynthetic efficiency [56]. The relationship between the nitrogen content in the aboveground parts and underground buds of Z. caduciflora and S. stoloniferum and temperature factors is the strongest, while the relationship between carbon and phosphorus content and these two temperature factors is mostly insignificant. This indicates that under warming conditions, these two plants are more sensitive to nitrogen limitations and relatively less sensitive to carbon and phosphorus limitations. It also indicates that the aboveground parts and underground buds are key structures in determining the photosynthetic productivity of plants in response to warming. Studies on the absorption of nitrogen and phosphorus elements of short–eared rabbit grass on the Qinghai–Tibet Plateau under warming conditions also found that warming has a greater impact on nitrogen absorption and a less noticeable impact on phosphorus absorption [57]. The results of this study are in line with the conclusions of global pattern research on the terrestrial nitrogen and phosphorus limitation, which is that high–altitude and high–latitude ecosystems typically show a strong nitrogen limitation and have a lower demand for external phosphorus [58]. The aboveground parts, especially leaves, are the main structures for plants to carry out photosynthesis. According to the global plant leaf economic spectrum and wetland plant leaf economic spectrum, the nitrogen content in the aboveground parts is directly related to plant photosynthesis [26,44], and a decrease in its content directly leads to a decrease in the photosynthetic rate. Many studies have shown that climate change, especially temperature changes, can alter the content and combination ratios of elements within plants, thereby leading to changes in ecosystem functions [59,60,61]. However, the impact of climate change on plant elemental content is indirect [59]. For example, extreme high–temperature stress can lead to the production of free radicals within plants, affecting the recycling process of nitrogen, with incomplete nitrogen absorption being an important reason for the decline in plant nitrogen content [10]. The underground bud is the main structure for the clonal reproduction of aquatic plants. Under high temperatures, the decline in nitrogen accumulation and the reduction in underground bud biomass both indicate that the reproductive capacity of aquatic plants decreases under the influence of high temperatures. Therefore, the 2 °C and 4 °C warming in this study is both high–temperature stress for Z. caduciflora and S. stoloniferum. Against the backdrop of climate warming, the survival, reproduction, and development of Z. caduciflora and S. stoloniferum will face challenges, and the distribution pattern of plants and the stability of ecosystems will also be affected.
5. Conclusions
This study investigated the effects of warming on the photosynthetic traits, biomass, and elemental contents of two typical emergent plants (Z. caduciflora and S. stoloniferum) in the Napahai lakeshore zone through a simulated warming experiment. The results showed that simulated warming significantly reduced the net photosynthetic rate and stomatal conductance of Z. caduciflora, but had no significant impact on the photosynthetic parameters of S. stoloniferum. This indicates that Z. caduciflora is more sensitive to warming, while S. stoloniferum can maintain stable photosynthetic capacity under warming conditions. The biomass of both plants showed a declining trend under warming conditions, indicating that high temperatures inhibit biomass accumulation in both species. Warming not only shortened the growing season of the plants but also reduced their energy investment in vegetative growth and clonal reproduction. Additionally, warming led to a significant decrease in nitrogen content in both plants, particularly in the aboveground parts and underground buds of Z. caduciflora, which further limited its photosynthetic productivity. In contrast, S. stoloniferum alleviated the impact of nitrogen limitation by increasing phosphorus content in its underground parts, thus maintaining a stable photosynthetic rate. The mean annual temperature (MAT) and the annual maximum temperature (max) are the primary temperature factors influencing the traits of these two plants. The increase in the MAT and max led to a decrease in the plants’ water vapor exchange capacity and nitrogen content, thereby affecting photosynthetic productivity and biomass accumulation. Moreover, under high temperature stress, plants regulate their stomatal conductance and transpiration rates to maintain physiological balance, but this also restricts gas exchange capacity, further reducing photosynthetic efficiency.
Author Contributions
M.S. and Z.L. designed the research plan and organized the research work; T.W. and M.S. drafted the initial manuscript; T.W., Z.L. and X.Z. measured the functional traits; J.Y. and Y.Z. detected the water and sediment elements; Y.Z. and K.T. analyzed the data; M.S., J.Y. and K.T. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Yunnan Xingdian Youth Talent Project (XDYC-QNRC-2023-0218).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please contact the author at sm0510215@163.com.
Acknowledgments
The field experiment of this study was conducted in the Shangri–La Napahai International Important Wetland. We sincerely thank the wetland managers and the Yunnan Biodiversity Conservation Foundation Project for their support and assistance in this study.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Harrison, S. Plant community diversity will decline more than increase under climatic warming. Philos. Trans. R. Soc. B 2020, 375, 20190106. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Oxford, UK, 2014. [Google Scholar]
- Li, Y.; Yu, X.B.; Liu, Y.; Zhang, G.S.; Zhang, Q.J.; Duan, H.L. Response of wetland plant functional traits to hydrological processes: A review. Chin. J. Ecol. 2018, 37, 952–959. [Google Scholar]
- Yin, L.; Dai, E.; Zheng, D.; Wang, Y.; Ma, L.; Tong, M. What drives the vegetation dynamics in the Hengduan Mountain region, southwest China: Climate change or human activity? Ecol. Indic. 2020, 112, 106013. [Google Scholar] [CrossRef]
- Kuang, X.; Jiao, J.J. Review on climate change on the Tibetan Plateau during the last half century. J. Geophys. Res. Atmos. 2016, 121, 3979–4007. [Google Scholar] [CrossRef]
- Gopal, B. Future of wetlands in tropical and subtropical Asia, especially in the face of climate change. Aquat. Sci. 2013, 75, 39–61. [Google Scholar] [CrossRef]
- Mao, X.; Wei, X.; Engel, B.; Wei, X.; Zhang, Z.; Tao, Y.; Wang, W. Network–based perspective on water–air interface GHGs flux on a cascade surface–flow constructed wetland in Qinghai–Tibet Plateau, China. Ecol. Eng. 2020, 151, 105862. [Google Scholar] [CrossRef]
- Fu, W.C.; Tian, K.; Xiao, D.R.; Li, W.; Yue, H.T.; Zhao, X.J.; Yang, H. The ecological restoration effort of degraded estuarine wetland in Northwest Yunnan Plateau, China. Acta Ecol. Sin. 2014, 34, 2187–2194. [Google Scholar]
- Zhang, Y.; Yan, J.; Cheng, X.; He, X. Wetland changes and their relation to climate change in the Pumqu Basin, Tibetan Plateau. Int. J. Environ. Res. Public Health 2021, 18, 2682. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; Tian, K.; Xiao, D.; Yuan, X. Long–term responses of leaf litter decomposition to temperature, litter quality and litter mixing in plateau wetlands. Freshw. Biol. 2017, 62, 178–190. [Google Scholar] [CrossRef]
- Du, J.H.; Wang, Y.; Pan, Y.; Wang, J. Ecological risk assessment and ecological security pattern construction of county–level landscape in plateau valley: A case study in Yongping County, Dali Prefecture, Yunnan Province. Chin. J. Ecol. 2024, 43, 1509–1520. [Google Scholar]
- Xiao, D.R.; Zhang, C.; Tian, K.; Liu, G.; Yang, H.; An, S. Development of alpine wetland vegetation and its effect on carbon sequestration after dam construction: A case study of Lashihai in the northwestern Yunnan plateau in China. Aquat. Bot. 2015, 126, 16–24. [Google Scholar] [CrossRef]
- Walther, W.; Strauch, G.; Kersebaum, K.C.; Reinstorf, F.; Schäfer, W. About the knowledge in the field of nutrient metabolism in the drain zone and in groundwater and about their modelling. 2nd Part: The application of isotope tracer techniques and mathematical models for description of transport and turnover of plant nutrients in the water–saturated zone and the groundwater. Landnutz. Landentwickl. 2002, 43, 97–102. [Google Scholar]
- Erwin, J. Cacti: Studies on introducing a new group of ornamental plants. Acta Hortic. 2009, 813, 359–364. [Google Scholar] [CrossRef]
- Sun, M.; Feng, C.H.; Liu, Z.Y.; Tian, K. Evolutionary correlation of water–related traits between different structures of Dendrobium plants. Bot. Stud. 2020, 61, 16. [Google Scholar] [CrossRef]
- Chelli, S.; Marignani, M.; Barni, E.; Petraglia, A.; Puglielli, G.; Wellstein, C.; Acosta, A.; Bolpagni, R.; Bragazza, L.; Campetella, G.; et al. Plant–environment interactions through a functional traits perspective: A review of Italian studies. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2019, 153, 853–869. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, J.A.; Pineda, J.E.M.; Melgarejo, L.M.; Calderón, J.H.M. Functional traits of leaves and forest structure of neotropical mangroves under different salinity and nitrogen regimes. Flora 2018, 239, 52–61. [Google Scholar] [CrossRef]
- Bai, J.S.; Tang, H.R.; Lou, Y.J. Effects of water depth and nitrogen addition on functional traits of wetland plants: A review. Chin. J. Ecol. 2021, 40, 2987–2995. [Google Scholar]
- Soudzilovskaia, N.A.; Elumeeva, T.G.; Onipchenko, V.G.; Shidakov, I.I.; Salpagarova, F.S.; Khubiev, A.B. Functional traits predict relationship between plant abundance dynamic and long–term climate warming. Proc. Natl. Acad. Sci. USA 2013, 110, 18180–18184. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Bagley, J.; Rosenthal, D.M.; Ruiz-Vera, U.M.; Siebers, M.H.; Kumar, P.; Ort, D.R.; Bernacchi, C.J. The influence of photosynthetic acclimation to rising CO2 and warmer temperatures on leaf and canopy photosynthesis models. Glob. Biogeochem. Cycles 2015, 29, 194–206. [Google Scholar] [CrossRef]
- Zhang, C.C.; Han, G.; Guan, H.D.; Bao, C.H.; Zhang, X.P. Responses of optimal photosynthesis temperature to changes in ambient temperature for Cinnamomum camphora and Osmanthus fragrans. Chin. J. Ecol. 2014, 33, 2980–2987. [Google Scholar]
- Zhou, Y.Q.; Xu, Y.S.; He, L.X.; Zhang, Y.J.; Feng, Z.Z. Combined effects of elevated ozone concentration and waring on photosynthesis of rice leaves. Acta Ecol. Sin. 2025, 45, 1–12. [Google Scholar]
- Li, L.; Feng, S.D.; Wang, J.B.; Ni, H.W.; Fu, X.L.; Yang, L.Y.; Xu, M.H. Relationship between photosynthetic characteristics and leaf functional traits of 12 plant species of marshes in Sanjiang Plain. Wetl. Sci. 2010, 8, 225–232. [Google Scholar]
- Zhao, Y.; Sun, M.; Guo, H.; Feng, C.; Liu, Z.; Xu, J. Responses of leaf hydraulic traits of Schoenoplectus tabernaemontani to increasing temperature and CO2 concentrations. Bot. Stud. 2022, 63, 2. [Google Scholar] [CrossRef]
- Wright, I.J.; Groom, P.K.; Lamont, B.B.; Poot, P.; Prior, L.D.; Reich, P.B.; Schulze, E.-D.; Veneklaas, E.J.; Westoby, M. Leaf trait relationships in Australian plant species. Funct. Plant Biol. 2004, 31, 551–558. [Google Scholar] [CrossRef]
- Roche, P.; Díaz-Burlinson, N.; Gachet, S. Congruency analysis of species ranking based on leaf traits: Which traits are the more reliable? Plant Ecol. 2004, 174, 37–48. [Google Scholar] [CrossRef]
- Qian, Y.; Tian, K.; Xiao, D.; Yin, L.; Yu, D.; Yang, Y. Ecological restoration effect of closed and half–closed degraded wetlands in Northwest Yunnan Plateau, Southwest China. Yingyong Shengtai Xuebao 2012, 23, 1520–1526. [Google Scholar]
- Niu, M.; Guo, H.; Sun, M.; Xiao, D.R.; Liu, Z.Y.; Ai, J.; Zhao, P. Response of leaf morphological traits to environmental factors of an emergent aquatic plant Sparganium stoloniferum in northwest Yunnan. J. Southwest For. Univ. 2022, 42, 84–95. [Google Scholar]
- Wang, Z.B.; Tian, K.; Guan, D.X.; Li, H.; Zhang, Y.; Feng, C.; Sun, M. Responses of seed reproduction traits of a wetland dominant plant Schoenoplectus tabernaemontani to environmental changes in Hengduan Mountains. J. Northeast For. Univ. 2018, 46, 12–15. [Google Scholar]
- Liu, Z.Y.; Zhang, X.N.; Li, L.P.; Wang, H.; Zhang, Y.; Sun, M.; Xiao, D. Influence of simulated warming on light and CO2 utilization capacities of lakeside dominant plants in a typical plateau wetland in northwestern Yunnan. Acta Ecol. Sin. 2017, 37, 7821–7832. [Google Scholar]
- Xiao, D.R.; Tian, K.; Zhang, L.Q. Relationship between plant diversity and soil fertility in Napahai wetland of Northwestern Yunnan Plateau. Acta Ecol. Sin. 2008, 28, 3116–3124. [Google Scholar]
- Liu, Z.Y.; Zhao, Y.; Yu, H.Y.; Zhao, Y.; Guo, H.; Sun, M. Response of the functional traits of Schoenoplectus tabernaemontani to simulated warming in the Napahai wetland of northwestern Yunnan, China. Front. Ecol. Evol. 2024, 12, 1399584. [Google Scholar] [CrossRef]
- Yu, H.Y.; Sun, M.; Feng, C.H.; Xu, J.P.; Chen, H.Y.; Liu, Z.Y. Responses of leaf economic traits of Scirpus validus and Typha orientalis to simulated warming and CO2 concentration multiplication. Guihaia 2023, 43, 1588–1599. [Google Scholar]
- Kim, K.; Portis, A.R., Jr. Temperature dependence of photosynthesis in Arabidopsis plants with modifications in Rubisco activase and membrane fluidity. Plant Cell Physiol. 2005, 46, 522–530. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Robinson, S.P.; Portis, A.R., Jr. Adenosine triphosphate hydrolysis by purified Rubisco activase. Arch. Biochem. Biophys. 1989, 268, 93–99. [Google Scholar] [CrossRef]
- Kumar, A.; Li, C.; Portis, A.R. Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth. Res. 2009, 100, 143–153. [Google Scholar] [CrossRef]
- Ibañez, C.; Poeschl, Y.; Peterson, T.; Bellstädt, J.; Denk, K.; Gogol-Döring, A.; Marcel, Q.; Delker, C. Ambient temperature and genotype differentially affect developmental and phenotypic plasticity in Arabidopsis thaliana. BMC Plant Biol. 2017, 17, 114. [Google Scholar] [CrossRef]
- Frei, E.R.; Ghazoul, J.; Pluess, A.R. Plastic responses to elevated temperature in low and high elevation populations of three grassland species. PLoS ONE 2014, 9, e98677. [Google Scholar] [CrossRef]
- Lippmann, R.; Babben, S.; Menger, A.; Delker, C.; Quint, M. Development of wild and cultivated plants under global warming conditions. Curr. Biol. 2019, 29, R1326–R1338. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, M.; Wang, W.Y.; Li, Z.H.; Zhang, T.; Ma, L.; Bai, Y.F.; Wang, Y.L.; Shi, J.J.; Long, R.J.; et al. Variation characteristics of plant community diversity and above–ground biomass in alpine degraded slopes along altitude gradients in the headwaters region of three–river on Tibetan plateau. Acta Ecol. Sin. 2022, 42, 3640–3655. [Google Scholar]
- Pan, Y.; Cieraad, E.; Armstrong, J.; Armstrong, W.; Clarkson, B.R.; Colmer, T.D.; Pedersen, O.; Visser, E.J.W.; Voesenek, L.A.C.J.; van Bodegom, P.M. Global patterns of the leaf economics spectrum in wetlands. Nat. Commun. 2020, 11, 4519. [Google Scholar] [CrossRef]
- Gardner, R.C.; Finlayson, C. Global Wetland Outlook: State of the World’s Wetlands and Their Services to People; Ramsar Convention Secretariat: Gland, Switzerland, 2018. [Google Scholar]
- Sun, M.; Tian, K.; Yue, H.T.; Liu, Q.; Wang, Z.B. Correlated evolution between leaf nutrients and leaf biomass across epiphytic Dendrobium species. Ecol. Sci. 2019, 38, 1–8. [Google Scholar]
- Goraya, G.K.; Kaur, B.; Asthir, B.; Bala, S.; Kaur, G.; Farooq, M. Rapid injuries of high temperature in plants. J. Plant Biol. 2017, 60, 298–305. [Google Scholar] [CrossRef]
- Xalxo, R.; Yadu, B.; Chandra, J.; Chandrakar, V.; Keshavkant, S. Alteration in carbohydrate metabolism modulates thermotolerance of plant under heat stress. In Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; Wiley: New York, NY, USA, 2020; pp. 77–115. [Google Scholar]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Al Zeidi, M.; Siddique, K.H.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Schreiber, U.; Berry, J.A. Heat–induced changes of chlorophyll fluorescence in intact leaves correlated with damage of the photosynthetic apparatus. Planta 1977, 136, 233–238. [Google Scholar] [CrossRef]
- Bilger, H.W.; Schreiber, U.; Lange, O.L. Determination of leaf heat resistance: Comparative investigation of chlorophyll fluorescence changes and tissue necrosis methods. Oecologia 1984, 63, 256–262. [Google Scholar] [CrossRef]
- Schymanski, S.J.; Or, D.; Zwieniecki, M. Stomatal control and leaf thermal and hydraulic capacitances under rapid environmental fluctuations. PLoS ONE 2013, 8, e54231. [Google Scholar] [CrossRef]
- Hueve, K.; Bichele, I.; Rasulov, B.; Niinemets, Ü.L.O. When it is too hot for photosynthesis: Heat–induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 2011, 34, 113–126. [Google Scholar] [CrossRef]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought–induced mortality risk in vulnerable plant species. Glob. Change Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef] [PubMed]
- Gutschick, V.P. Leaf energy balance: Basics, and modeling from leaves to canopies. In Canopy Photosynthesis: From Basics to Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 23–58. [Google Scholar]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.H.; Cao, Z.; Li, X.G.; Scholten, T.; Kühn, P.; Wang, L.; Yu, R.P.; He, J.S. Soil phosphorus availability mediates the effects of nitrogen addition on community and species level phosphorus acquisition strategies in alpine grasslands. Sci. Total Environ. 2024, 906, 167630. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.L.; Zhang, S.H.; Wang, L.J.; Mao, Z.P.; Ma, X.Y. Numerical simulation of water purification effects of aquatic plant in artificial surface flow wetland. J. Hydraul. Eng. 2020, 51, 675–684. [Google Scholar]
- Tian, H.; Jin, H.T.; Sun, Q.F.; Liang, X.; Liu, Q.; Li, X. Ecolgical evaluation on Panjin wetland based on analytic hierarchy process. Geol. Resour. 2018, 27, 268–271. [Google Scholar]
- Manzoni, S.; Vico, G.; Katul, G.; Fay, P.A.; Polley, W.; Palmroth, S.; Porporato, A. Optimizing stomatal conductance for maximum carbon gain under water stress: A meta–analysis across plant functional types and climates. Funct. Ecol. 2011, 25, 456–467. [Google Scholar] [CrossRef]
- Güsewell, S.; Gessner, M.O. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009, 23, 211–219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).