Functional Characterization of Grapevine VviMYC4 in Regulating Drought Tolerance by Mediating Flavonol Biosynthesis
Abstract
1. Introduction
2. Results
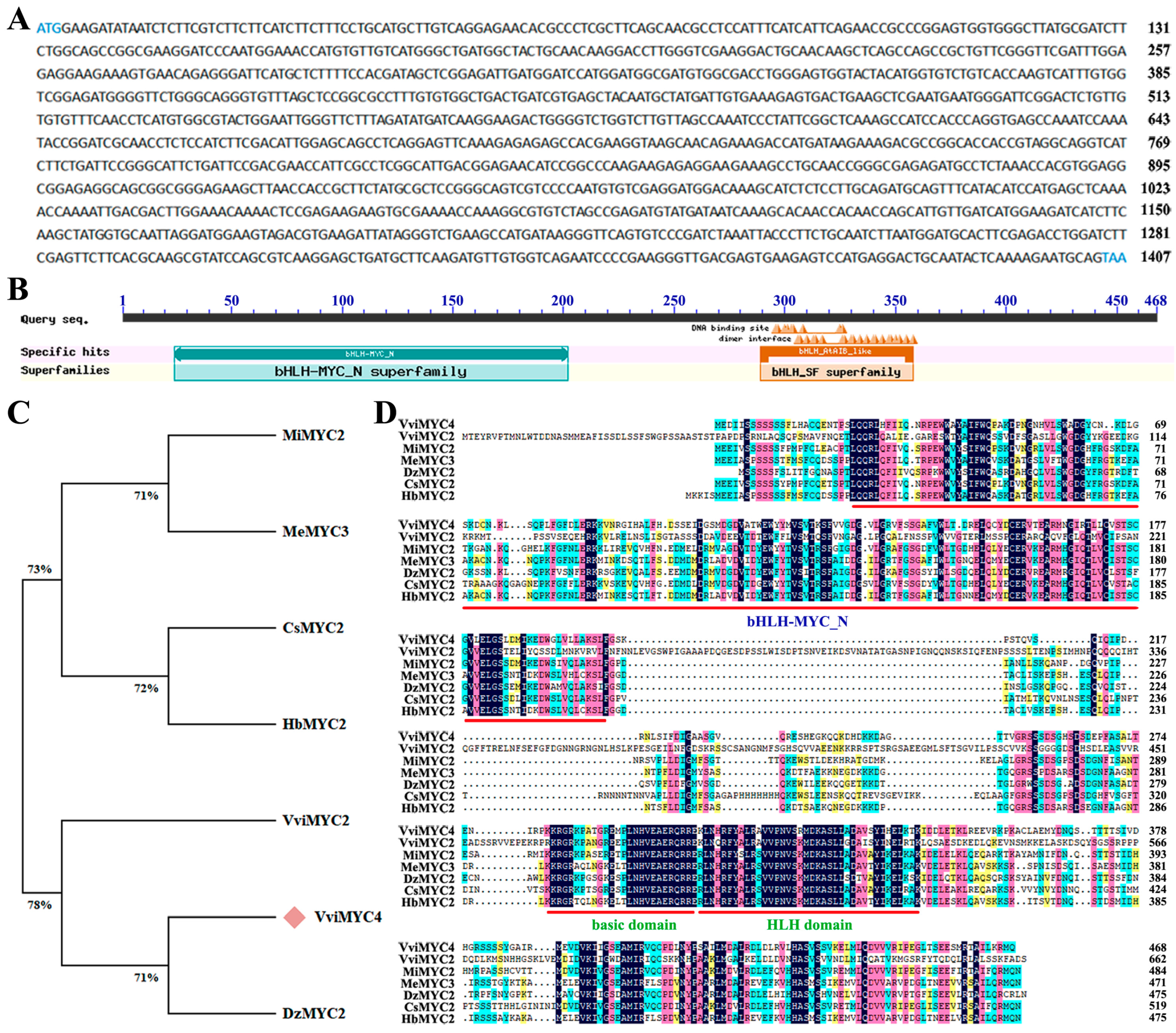
2.1. Molecular Cloning and Characterization of VviMYC4 in Grapevine
2.2. Expression Analysis of VviMYC4 in ‘Cabernet Sauvignon’ Grapevine Leaves Under Drought Stress
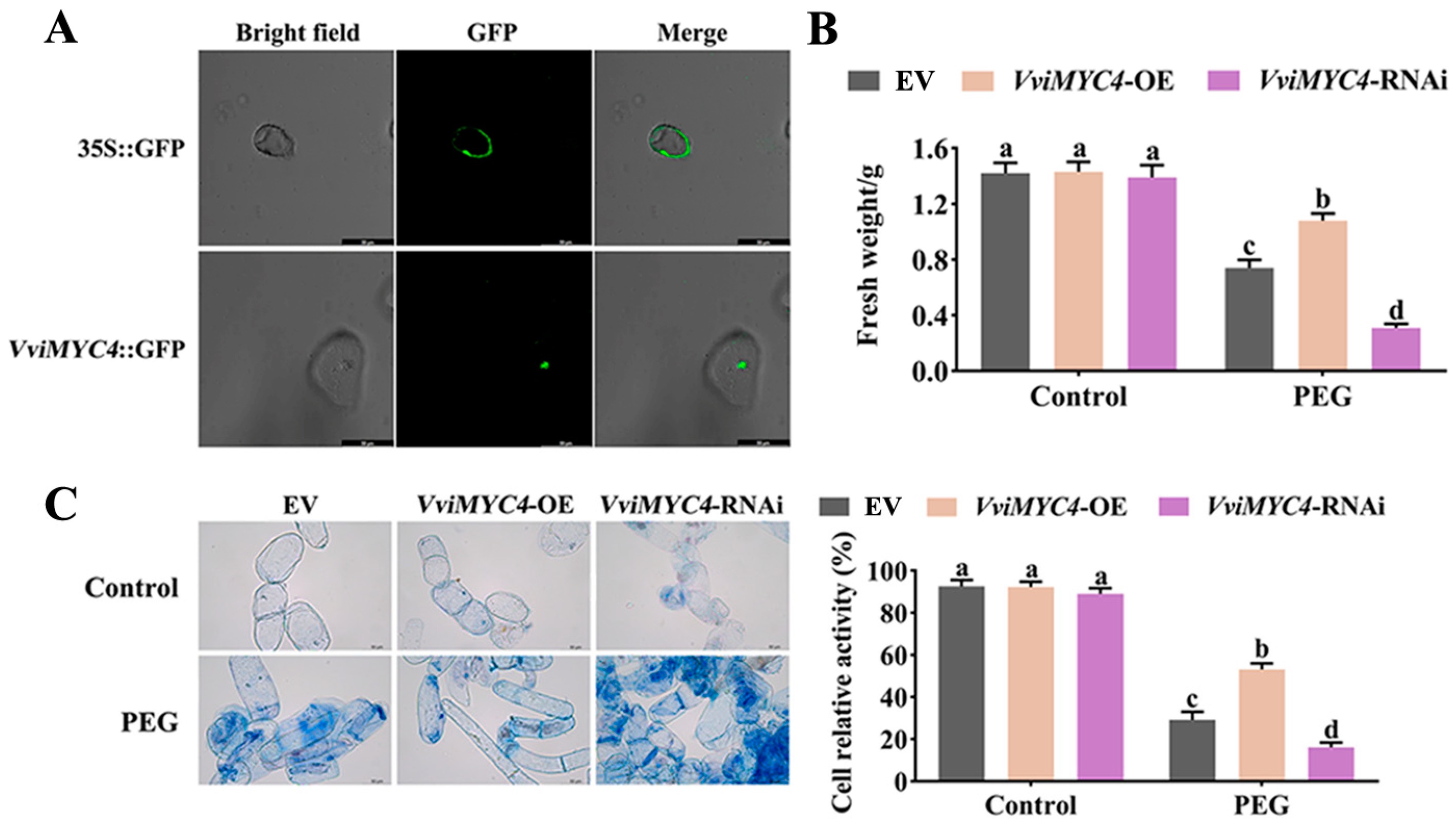
2.3. Functional Analysis of VviMYC4 in Enhancing Drought Tolerance of Transgenic Grape Suspension Cells
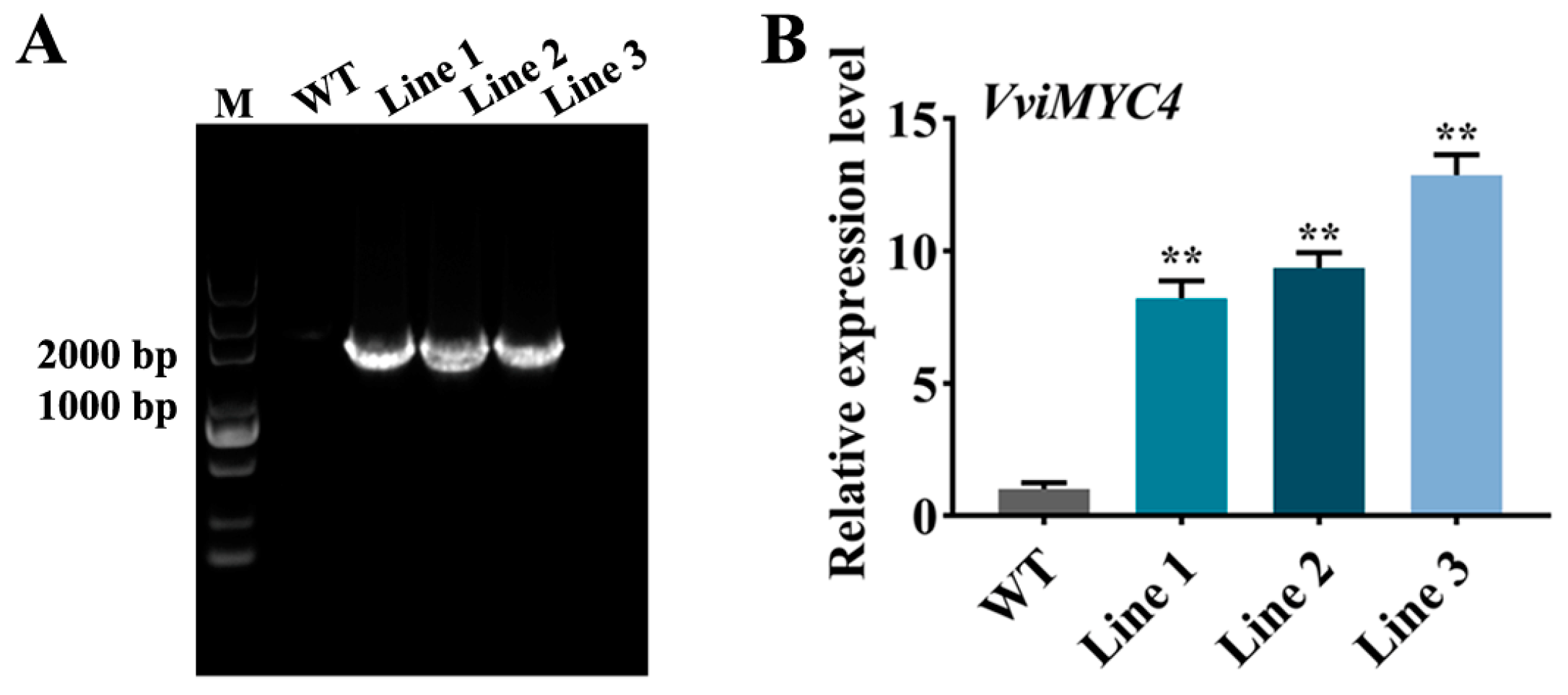
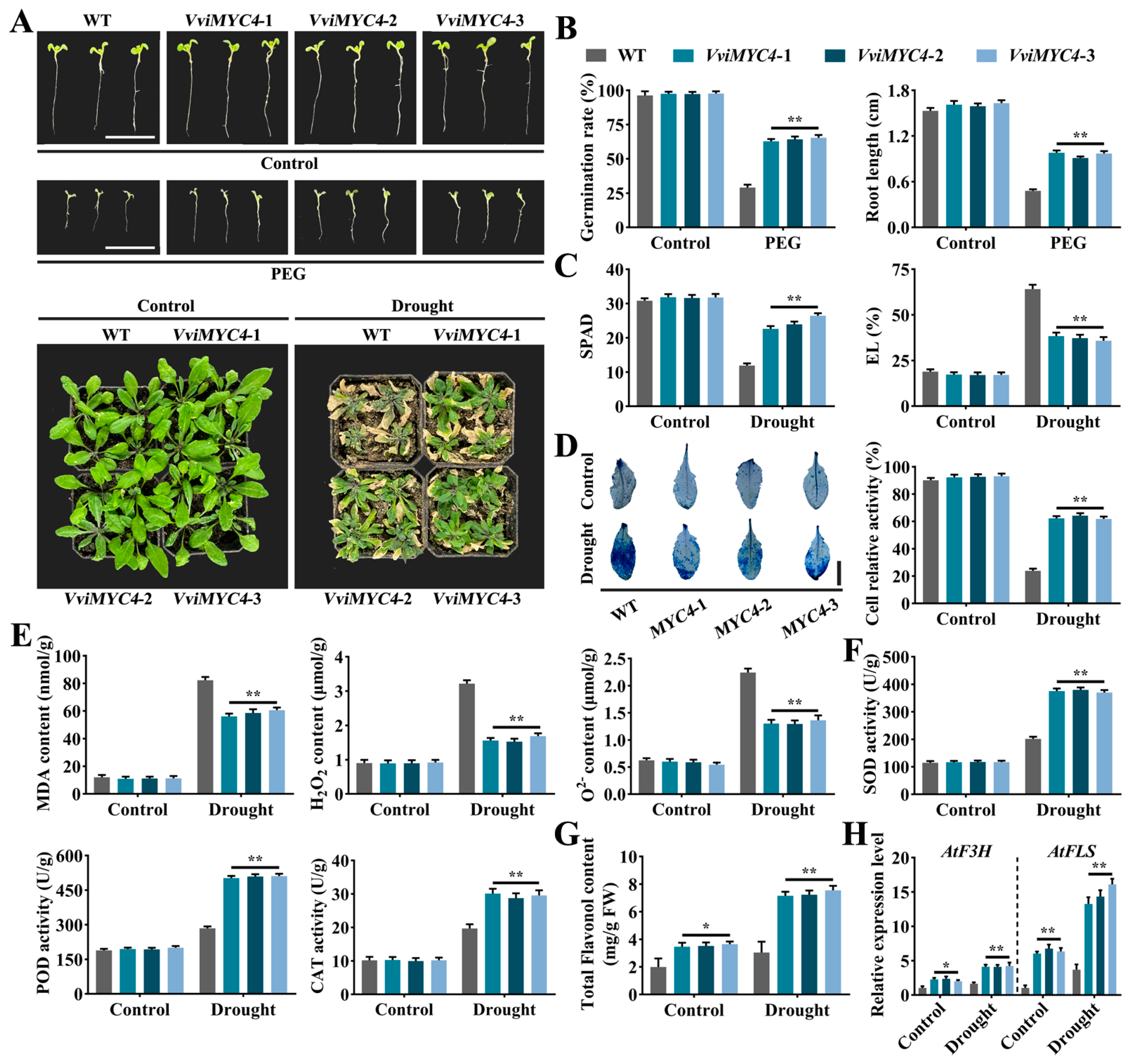
2.4. Functional Analysis of VviMYC4 in Mediating Flavonol Biosynthesis to Enhance Drought Tolerance in Transgenic A. thaliana
2.5. Functional Validation of VviMYC4 Gene Through Transient Overexpression in Tobacco Leaves
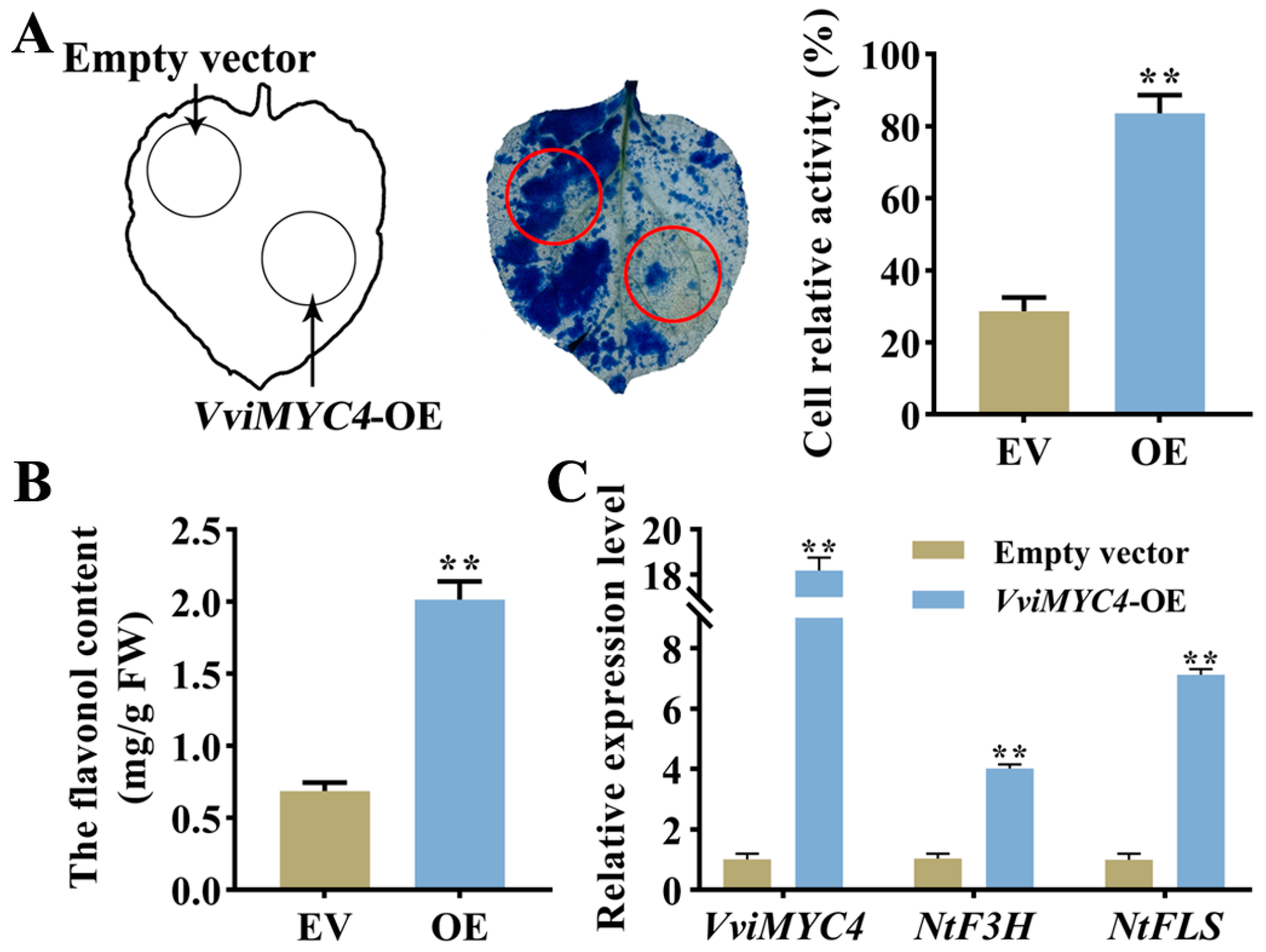
2.6. Functional Validation of VviMYC4 Gene Through Transient Overexpression in Grapevine Leaves
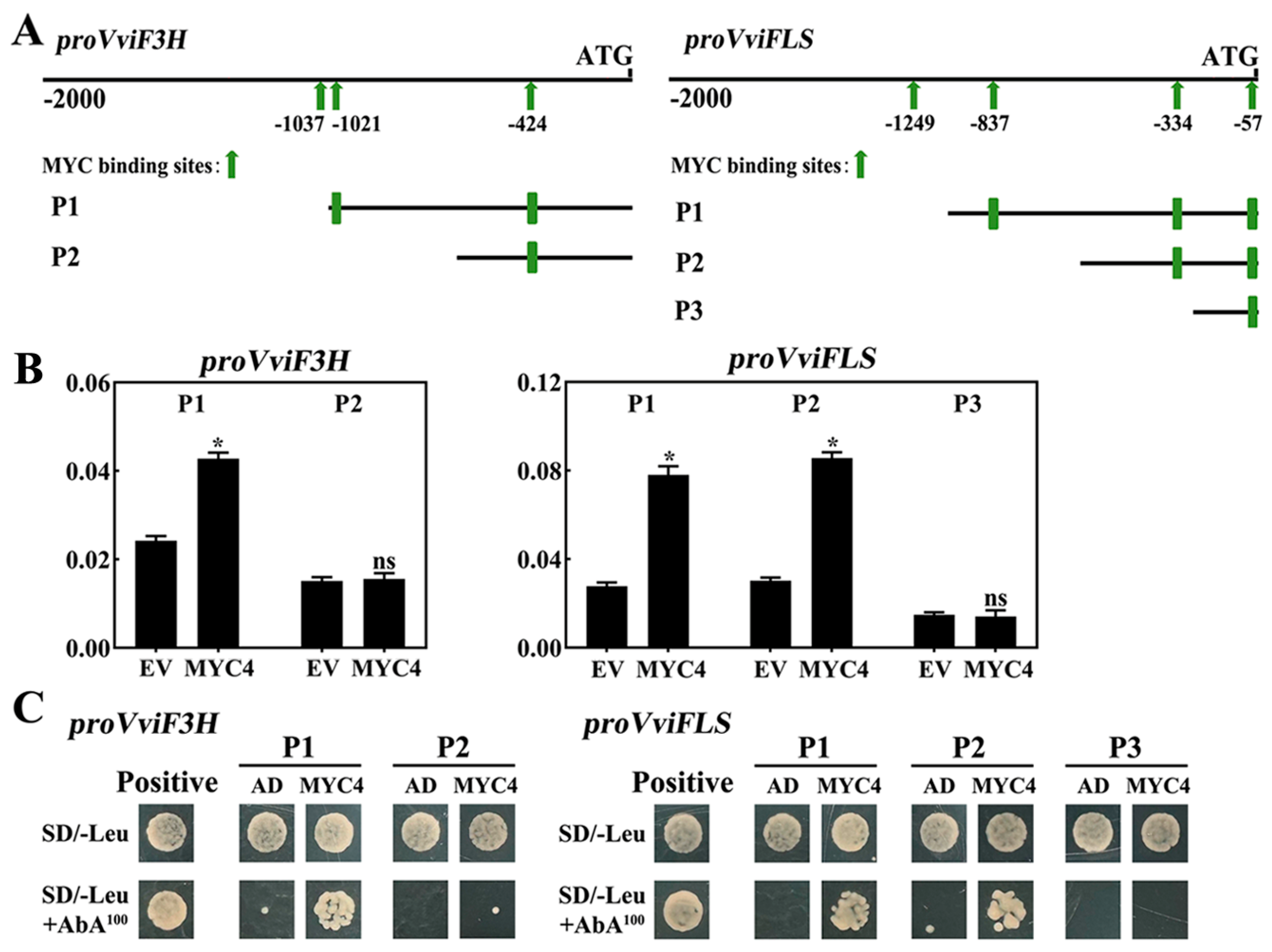
2.7. Analysis of the Targeted Regulatory Role of VviMYC4 in Relation to the Promoters of the VviF3H and VviFLS Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Gene Cloning and Sequence Analysis
4.3. Gene Expression Analysis
4.4. Evans Blue Staining
4.5. Vector Construction and Plant Transformation
4.6. Physiological and Biochemical Measurements
4.7. Cis-Acting Elements Analysis of Promoters
4.8. Dual-Luciferase (LUC) Reporter Assays
4.9. Yeast One-Hybrid (Y1H) Assays
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aanniz, T.; El Baaboua, A.; Aboulaghras, S.; Bouyahya, A.; Benali, T.; Balahbib, A.; El Omari, N.; Butnariu, M.; Muzammil, K.; Yadav, K.K.; et al. Impact of water stress to plant epigenetic mechanisms in stress and adaptation. Physiol. Plant. 2025, 177, e70058. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Xiong, L.Z. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.F.; Ge, Y.R.; Li, Y.F.; Li, R.L. Plants’ response to abiotic stress: Mechanisms and strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Costa, J.H.; Miranda, R.D.S. Molecular basis of crops and fruit plants in response to stress. Plants 2023, 12, 3813. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, S.H.; Yang, S.P.; Yang, Z.R.; Liu, S.X.; Wang, Y.J.; Gao, H.J.; Zhang, S.S.; Yang, X.H.; Jiang, C.F.; et al. Genome assembly and genetic dissection of a prominent drought-resistant maize germplasm. Nat. Genet. 2023, 55, 496–506. [Google Scholar] [CrossRef]
- Takahashi, F.; Kuromori, T.; Sato, H.; Shinozaki, K. Regulatory gene networks in drought stress responses and resistance in plants. Adv. Exp. Med. Biol. 2018, 1081, 189–214. [Google Scholar] [CrossRef]
- Debnath, T.; Dhar, D.G.; Dhar, P. Molecular switches in plant stress adaptation. Mol. Biol. Rep. 2023, 51, 20. [Google Scholar] [CrossRef]
- Wang, F.B.; Kong, W.L.; Won, G.; Fu, L.F.; Peng, R.; Li, Z.J.; Yao, Q.H. AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol. Genet. Genomics 2016, 291, 1545–1559. [Google Scholar] [CrossRef]
- Wu, J.T.; Lv, S.D.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.N.; Ma, F. Advances in the study of the function and mechanism of the action of flavonoids in plants under environmental stresses. Planta. 2023, 257, 108. [Google Scholar] [CrossRef]
- Li, B.Z.; Fan, R.N.; Guo, S.Y.; Wang, P.T.; Zhu, X.H.; Fan, Y.T.; Chen, Y.X.; He, K.Y.; Kumar, A.; Shi, J.P.; et al. The Arabidopsis MYB transcription factor, MYB111 modulates salt responses by regulating flavonoid biosynthesis. Environ. Exp. Bot. 2019, 166, 103807. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Waqas, M.; Park, J.R.; Asif, S.; Kim, N.; Lee, I.J.; Kim, K.M. Drought and UV radiation stress tolerance in rice is improved by overaccumulation of non-enzymatic antioxidant flavonoids. Antioxidants 2022, 11, 917. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Z.; Fan, R.N.; Sun, G.L.; Sun, T.; Fan, Y.T.; Bai, S.L.; Guo, S.Y.; Huang, S.Q.; Liu, J.; Zhang, H.; et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 2021, 461, 389–450. [Google Scholar] [CrossRef]
- Wang, F.B.; Zhu, H.; Chen, D.H.; Li, Z.J.; Peng, R.H.; Yao, Q.H. A grape bHLH transcription factor gene, VvbHLH1, increases the accumulation of flavonoids and enhances salt and drought tolerance in transgenic Arabidopsis thaliana. Plant Cell Tiss. Org. 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Yu, Z.M.; Dong, W.; Teixeira da Silva, J.A.; He, C.M.; Si, C.; Duan, J. Ectopic expression of DoFLS1 from Dendrobium officinale enhances flavonol accumulation and abiotic stress tolerance in Arabidopsis thaliana. Protoplasma 2021, 258, 803–815. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Zhu, C.Y.; Yao, X.Y.; Zheng, Z.; Tian, Z.N.; Cai, X. EkFLS overexpression promotes flavonoid accumulation and abiotic stress tolerance in plant. Physiol. Plant. 2021, 172, 1966–1982. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Y.; Feng, X.X.; Liu, L.B.; Xiong, L.J.; Wang, L.J. ALA-induced flavonols accumulation in guard cells is involved in scavenging H2O2 and inhibiting stomatal closure in Arabidopsis cotyledons. Front. Plant Sci. 2016, 7, 1713. [Google Scholar] [CrossRef]
- Cao, Y.L.; Mei, Y.Y.; Zhang, R.N.; Zhong, Z.L.; Yang, X.C.; Xu, C.J.; Chen, K.S.; Li, X. Transcriptional regulation of flavonol biosynthesis in plants. Hortic. Res. 2024, 11, uhae043. [Google Scholar] [CrossRef]
- Li, Z.W.; Huang, Y.S.; Shen, Z.W.; Wu, M.F.; Huang, M.J.; Hong, S.B.; Xu, L.; Zang, Y.X. Advances in functional studies of plant MYC transcription factors. Theor. Appl. Genet. 2024, 137, 195. [Google Scholar] [CrossRef]
- Qian, Y.C.; Zhang, T.Y.; Yu, Y.; Gou, L.P.; Yang, J.T.; Xu, J.; Pi, E. Regulatory mechanisms of bHLH transcription factors in plant adaptive responses to various abiotic stresses. Front. Plant Sci. 2021, 12, 677611. [Google Scholar] [CrossRef]
- Tan, Y.T.; Zhao, W.; Zhou, R.T.; Wang, B.B.; Liu, Y.F.; Ge, W.J.; Liang, J.J.; Zhao, Q.F.; Wen, P.F. VviMYB24 positively regulates flavonol biosynthesis in response to moderate drought stress in ‘Cabernet Sauvignon’ grape seedlings. Sci. Hortic. 2024, 338, 113769. [Google Scholar] [CrossRef]
- Zeng, G.H.; Gao, F.F.; Li, C.; Li, D.D.; Xi, Z.M. Characterization of 24-epibrassinolide-mediated modulation of the drought stress responses: Morphophysiology, antioxidant metabolism and hormones in grapevine (Vitis vinifera L.). Plant Physiol. Biochem. 2022, 184, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.L.; Wang, W.N.; Nan, Q.; Liu, J.W.; Ju, Y.L.; Fang, Y.L. VvNAC17, a grape NAC transcription factor, regulates plant response to drought-tolerance and anthocyanin synthesis. Plant Physiol. Biochem. 2025, 219, 109379. [Google Scholar] [CrossRef]
- Singh, A.K.; Pal, P.; Sahoo, U.K.; Sharma, L.; Pandey, B.; Prakash, A.; Sarangi, P.K.; Prus, P.; Pașcalău, R.; Imbrea, F. Enhancing crop resilience: The role of plant genetics, transcription factors, and next-generation sequencing in addressing salt stress. Int. J. Mol. Sci. 2024, 25, 12537. [Google Scholar] [CrossRef]
- Kozaki, A. INDETERMINATE DOMAIN transcription factors in crops: Plant architecture, disease resistance, stress response, flowering, and more. Int. J. Mol. Sci. 2024, 25, 10277. [Google Scholar] [CrossRef]
- Shomali, A.; Das, S.; Arif, N.; Sarraf, M.; Zahra, N.; Yadav, V.; Aliniaeifard, S.; Chauhan, D.K.; Hasanuzzaman, M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants 2022, 11, 3158. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; del Mar Rubio-Wilhelmi, M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Mori, T.; Saito, K. Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e29518. [Google Scholar] [CrossRef]
- Guo, J.R.; Sun, B.X.; He, H.R.; Zhang, Y.F.; Tian, H.Y.; Wang, B.S. Current understanding of bHLH transcription factors in plant abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 4921. [Google Scholar] [CrossRef]
- Hu, Y.F.; Zhao, H.Y.; Xue, L.Y.; Nie, N.; Zhang, H.; Zhao, N.; He, S.Z.; Liu, Q.C.; Gao, S.P.; Zhai, H. IbMYC2 contributes to salt and drought stress tolerance via modulating anthocyanin accumulation and ROS-scavenging system in sweet potato. Int. J. Mol. Sci. 2024, 25, 2096. [Google Scholar] [CrossRef]
- Su, W.B.; Xu, M.Y.; Radani, Y.; Yang, L.M. Technological development and application of plant genetic transformation. Int. J. Mol. Sci. 2023, 24, 10646. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.M.; Li, B.J.; Zhang, G.F.; Zhou, Y.J.; Gao, F. Jasmonate enhances cold acclimation in jojoba by promoting flavonol synthesis. Hortic. Res. 2024, 11, uhae125. [Google Scholar] [CrossRef]
- Xu, B.Q.; Wang, J.J.; Peng, Y.; Huang, H.; Sun, L.L.; Yang, R.; Suo, L.N.; Wang, S.H.; Zhao, W.C. SlMYC2 mediates stomatal movement in response to drought stress by repressing SlCHS1 expression. Front. Plant Sci. 2022, 13, 952758. [Google Scholar] [CrossRef] [PubMed]
- Canto, T. Transient expression systems in plants: Potentialities and constraints. Adv. Exp. Med. Biol. 2016, 896, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Gao, J.S.; Li, Y.Y.; Song, C.W.; Guo, Q.; Guo, L.L.; Hou, X.G. Functional characterization of PoEP1 in regulating the flowering stage of tree peony. Plants 2024, 13, 1642. [Google Scholar] [CrossRef]
- Ben-Amar, A.; Cobanov, P.; Buchholz, G.; Mliki, A.; Reustle, G. In planta agro-infiltration system for transient gene expression in grapevine (Vitis spp.). Acta Physiol. Plant. 2013, 35, 3147–3156. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Carvalho, S.D.; O’Grady, K.; Folta, K.M. Agroinfiltration of strawberry fruit-A powerful transient expression system for gene validation. Curr. Plant Biol. 2016, 6, 19–37. [Google Scholar] [CrossRef]
- Zeng, H.Q.; Xie, Y.W.; Liu, G.Y.; Wei, Y.X.; Hu, W.; Shi, H.T. Agrobacterium-mediated gene transient overexpression and Tobacco Rattle Virus (TRV)-based gene silencing in cassava. Int. J. Mol. Sci. 2019, 20, 3976. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, M.M.; Wang, S.Q.; Feng, S.; Wang, Y.; Wang, T.; Zhu, C.L.; Jiang, X.Y.; Wang, H.Y.; Wang, R.M.; et al. An insertion of transposon in DcNAP inverted its function in the ethylene pathway to delay petal senescence in carnation (Dianthus caryophyllus L.). Plant Biotechnol. J. 2023, 21, 2307–2321. [Google Scholar] [CrossRef]
- Liu, G.C.; Zhang, Z.; Tian, Y.; Yang, J.; Xu, X.F.; Liu, X. VvbZIP22 regulates quercetin synthesis to enhances cold resistance in grape. Plant Sci. 2025, 350, 112293. [Google Scholar] [CrossRef]
- Liu, Y.F.; Ma, K.X.; Qi, Y.W.; Lv, G.W.; Ren, X.L.; Liu, Z.D.; Ma, F.W. Transcriptional regulation of anthocyanin synthesis by MYB-bHLH-WDR complexes in kiwifruit (Actinidia chinensis). J. Agric. Food. Chem. 2021, 69, 3677–3691. [Google Scholar] [CrossRef]
- Xu, W.J.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Bulanov, A.N.; Andreeva, E.A.; Tsvetkova, N.V.; Zykin, P.A. Regulation of flavonoid biosynthesis by the MYB-bHLH-WDR (MBW) complex in plants and its specific features in cereals. Int. J. Mol. Sci. 2025, 26, 734. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Martínez, E.; Bovy, A.; Plazas, M.; Tikunov, Y.; Prohens, J.; Pereira-Dias, L. Genetics and breeding of phenolic content in tomato, eggplant and pepper fruits. Front. Plant Sci. 2023, 14, 1135237. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Zhang, R.N.; Xing, M.Y.; Ren, C.H.; Li, J.J.; Qian, J.F.; Mei, Y.Y.; Yang, X.C.; Sun, C.D.; Grierson, D.; et al. Synergistic actions of three MYB transcription factors underpins the high accumulation of myricetin in Morella rubra. Plant J. 2023, 115, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhao, J.Q.; Yuan, P.G.; Ding, S.Y.; Jiang, L.G.; Xi, Z.M. Hydrogen peroxide functioned as a redox signaling molecule in the putrescine-promoted drought tolerance in cabernet sauvignon. Sci. Hortic. 2024, 335, 113325. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.H.; Li, L.; Meng, H.J.; Yang, Y.; Dong, Z.B.; Wang, L.; Wu, G.L. Genome-wide identification and characterization of bHLH transcription factors related to anthocyanin biosynthesis in red walnut (Juglans regia L.). Front. Genet. 2021, 12, 632509. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, H.T.; Li, J.J.; He, Y. Overexpression of a novel E3 ubiquitin ligase gene from Coptis chinensis Franch enhances drought tolerance in transgenic tobacco. Z. Naturforsch. C. J. Biosci. 2020, 75, 417–424. [Google Scholar] [CrossRef]
- Liu, X.N.; Chen, H.Y.; Li, S.C.; Lecourieux, D.; Duan, W.; Fan, P.; Liang, Z.C.; Wang, L.J. Natural variations of HSFA2 enhance thermotolerance in grapevine. Hortic. Res. 2022, 10, uhac250. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Meng, G.H.; Zamin, I.; Wei, T.; Ma, D.D.; An, L.Z.; Yue, X.L. Genome-wide identification and functional analysis of the TIFY family genes in response to abiotic stresses and hormone treatments in Tartary Buckwheat (Fagopyrum tataricum). Int. J. Mol. Sci. 2023, 24, 10916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.H.; Fang, J.H.; Yin, W.C.; Yan, X.X.; Tu, M.X.; Liu, H.; Zhang, Z.D.; Li, Z.; Gao, M.; et al. VqWRKY56 interacts with VqbZIPC22 in grapevine to promote proanthocyanidin biosynthesis and increase resistance to powdery mildew. New Phytol. 2023, 237, 1856–1875. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Zhu, Q.G.; Deng, C.L.; Luo, Z.R.; Sun, N.J.; Grierson, D.; Yin, X.R.; Chen, K.S. Hypoxia-responsive ERFs involved in postdeastringency softening of persimmon fruit. Plant Biotechnol. J. 2017, 15, 1409–1419. [Google Scholar] [CrossRef]
- Wang, M.M.; Wu, Y.; Zhan, W.D.; Wang, H.; Chen, M.; Li, T.X.; Bai, T.H.; Jiao, J.; Song, C.H.; Song, S.W.; et al. The apple transcription factor MdZF-HD11 regulates fruit softening by promoting Mdβ-GAL18 expression. J. Exp. Bot. 2024, 75, 819–836. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Wang, W.; Tian, W.; Wang, B.; Zhao, Q.; Liang, J.; Zhao, W.; Wen, P. Functional Characterization of Grapevine VviMYC4 in Regulating Drought Tolerance by Mediating Flavonol Biosynthesis. Plants 2025, 14, 1409. https://doi.org/10.3390/plants14101409
Tan Y, Wang W, Tian W, Wang B, Zhao Q, Liang J, Zhao W, Wen P. Functional Characterization of Grapevine VviMYC4 in Regulating Drought Tolerance by Mediating Flavonol Biosynthesis. Plants. 2025; 14(10):1409. https://doi.org/10.3390/plants14101409
Chicago/Turabian StyleTan, Yiting, Wenjuan Wang, Wenbo Tian, Beibei Wang, Qifeng Zhao, Jinjun Liang, Wei Zhao, and Pengfei Wen. 2025. "Functional Characterization of Grapevine VviMYC4 in Regulating Drought Tolerance by Mediating Flavonol Biosynthesis" Plants 14, no. 10: 1409. https://doi.org/10.3390/plants14101409
APA StyleTan, Y., Wang, W., Tian, W., Wang, B., Zhao, Q., Liang, J., Zhao, W., & Wen, P. (2025). Functional Characterization of Grapevine VviMYC4 in Regulating Drought Tolerance by Mediating Flavonol Biosynthesis. Plants, 14(10), 1409. https://doi.org/10.3390/plants14101409






