Response of Tree Seedlings to a Combined Treatment of Particulate Matter, Ground-Level Ozone, and Carbon Dioxide: Primary Effects
Abstract
1. Introduction
2. Results
2.1. Stem Growth Response to Pollutant Exposure
2.2. Biochemical Response to Pollutant Exposure
2.2.1. Pigment Content: Chlorophyll a and b, Carotenoids
2.2.2. Total Polyphenol Content
2.2.3. Total Flavonoid Content
2.2.4. Total Soluble Sugar Content
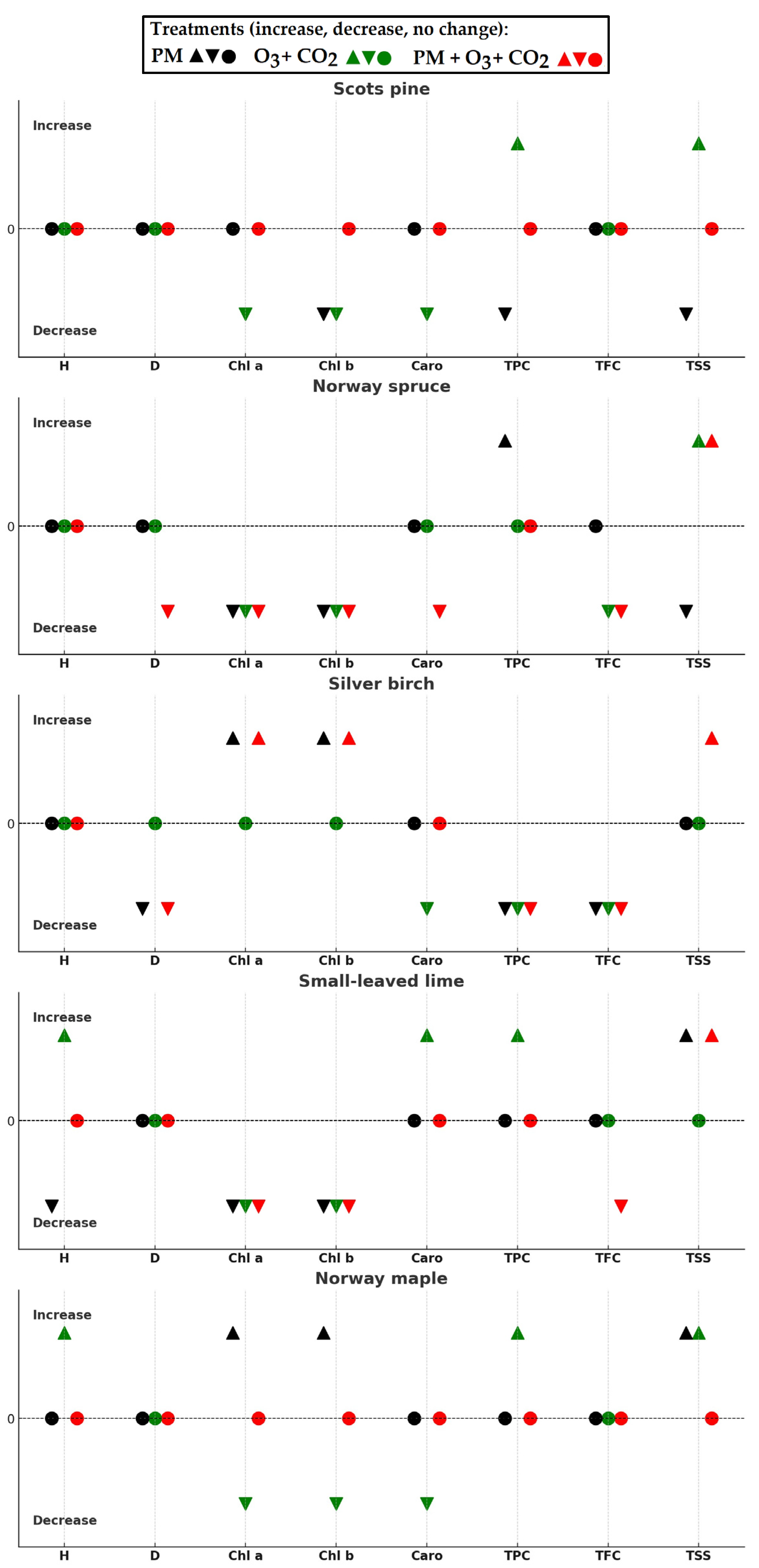
3. Discussion
3.1. Growth Response
3.2. Biochemical Response
4. Materials and Methods
4.1. Experiment Design: Planting Material, Growing Conditions, and Treatments
- Seedlings treated with particulate matter (PM) and exposed to O3 levels of 180 ppb in combination with CO2 levels of 650 ppm from 9 a.m. to 9 p.m. (PM + O3 + CO2);
- Seedlings without PM and exposed to an O3 level of 180 ppb in combination with a CO2 level of 650 ppm from 9 a.m. to 9 p.m. (O3 + CO2);
- Seedlings with PM and exposed to O3 levels below 40–45 ppb in combination with CO2 levels below 400 ppm (representing unchanged air conditions) (PM);
- Seedlings without PM were exposed to O3 levels below 40–45 ppb in combination with CO2 levels below 400 ppm, serving as the control group (Control).
4.2. Measurements
4.2.1. Seedling Stem Measurements
4.2.2. Biochemical Analyses
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, J.N.B.; Power, S.A.; Jarraud, N.; Agrawal, M.; Davies, C. The effects of air pollution on urban ecosystems and agriculture. Int. J. Sustain. Dev. World Ecol. 2011, 18, 226–235. [Google Scholar] [CrossRef]
- Bereitschaft, B.; Debbage, K. Urban form, air pollution, and CO2 emissions in large US metropolitan areas. Prof. Geogr. 2013, 65, 612–635. [Google Scholar] [CrossRef]
- United Nations. World Urbanization Prospects. 2017. Available online: https://www.unfpa.org/urbanization (accessed on 25 August 2024).
- Apte, J.S.; Brauer, M.; Cohen, A.J.; Ezzati, M.; Pope, C.A. Ambient PM2.5 reduces global and regional life expectancy. Environ. Sci. Technol. Lett. 2018, 5, 546–551. [Google Scholar] [CrossRef]
- Pataki, D.E.; Alberti, M.; Cadenasso, M.L.; Felson, A.J.; McDonnell, M.J.; Pincetl, S.; Pouyat, R.V.; Setälä, H.; Whitlow, T.H. The benefits and limits of urban tree planting for environmental and human health. Front. Ecol. Evol. 2021, 9, 603757. [Google Scholar] [CrossRef]
- Air Quality in Europe—2019 Report. EEA Report No 10/2019; Publications Office of the European Union: Luxembourg, 2019; Available online: https://www.miteco.gob.es/content/dam/miteco/es/calidad-y-evaluacion-ambiental/temas/atmosfera-y-calidad-del-aire/air-quality-in-europe_2019_tcm30-187944.pdf (accessed on 13 September 2024).
- Pope, C.A., III; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Cohen, A.J.; Anderson, H.R.; Ostro, B.; Pandey, K.D.; Krzyzanowski, M.; Künzli, N.; Gutschmidt, K.; Pope, A.; Romieu, I.; Samet, J.M.; et al. The global burden of disease due to outdoor air pollution. J. Toxicol. Environ. Health. Part A 2005, 68, 1301–1307. [Google Scholar] [CrossRef]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A., III; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef]
- Altimir, N.; Tuovinen, J.P.; Vesala, T.; Kulmala, M.; Hari, P. Measurements of ozone removal by Scots pine shoots: Calibration of a stomatal uptake model including the non-stomatal component. Atmos. Environ. 2004, 38, 2387–2398. [Google Scholar] [CrossRef]
- O’Driscoll, R.; Stettler, M.E.; Molden, N.; Oxley, T.; ApSimon, H.M. Real world CO2 and NOx emissions from 149 Euro 5 and 6 diesel, gasoline and hybrid passenger cars. Sci. Total Environ. 2018, 621, 282–290. [Google Scholar] [CrossRef]
- Yeung, L.Y.; Murray, L.T.; Martinerie, P.; Witrant, E.; Hu, H.; Banerjee, A.; Orsi, A.; Chappellaz, J. Isotopic constraint on the twentieth-century increase in tropospheric ozone. Nature 2019, 570, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Natasha; Dumat, C.; Niazi, N.K.; Xiong, T.T.; Farooq, A.B.U.; Khalid, S. Ecotoxicology of heavy metal (loid)-enriched particulate matter: Foliar accumulation by plants and health impacts. Rev. Environ. Contam. Toxicol. 2021, 253, 65–113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yin, D.; Yu, Y.; Kang, S.; Qin, D.; Dong, L. PM2.5 and O3 pollution during 2015–2019 over 367 Chinese cities: Spatiotemporal variations, meteorological and topographical impacts. Environ. Pollut. 2020, 264, 114694. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, E.; Kontunen-Soppela, S. Plants have different strategies to defend against air pollutants. Curr. Opin. Environ. Sci. Health 2021, 19, 100222. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Hoehn, R.E.; Lapoint, E. Carbon storage and sequestration by trees in urban and community areas of the United States. Environ. Pollut. 2013, 178, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Conte, A.; Alivernini, A.; Chianucci, F.; Grotti, M.; Zappitelli, I.; Petrella, F.; Corona, P. Testing removal of carbon dioxide, ozone, and atmospheric particles by urban parks in Italy. Environ. Sci. Technol. 2020, 54, 14910–14922. [Google Scholar] [CrossRef] [PubMed]
- Fares, S.; Matteucci, G.; Mugnozza, G.S.; Morani, A.; Calfapietra, C.; Salvatori, E.; Fusaro, L.; Manes, F.; Loreto, F. Testing of models of stomatal ozone fluxes with field measurements in a mixed Mediterranean forest. Atmos. Environ. 2013, 67, 242–251. [Google Scholar] [CrossRef]
- Lombardozzi, D.; Levis, S.; Bonan, G.; Hess, P.G.; Sparks, J.P. The influence of chronic ozone exposure on global carbon and water cycles. J. Clim. 2015, 28, 292–305. [Google Scholar] [CrossRef]
- Manes, F.; Incerti, G.; Salvatori, E.; Vitale, M.; Ricotta, C.; Costanza, R. Urban ecosystem services: Tree diversity and stability of tropospheric ozone removal. Ecol. Appl. 2012, 22, 349–360. [Google Scholar] [CrossRef]
- Sicard, P.; Agathokleous, E.; Araminiene, V.; Carrari, E.; Hoshika, Y.; De Marco, A.; Paoletti, E. Should we see urban trees as effective solutions to reduce increasing ozone levels in cities? Environ. Pollut. 2018, 243, 163–176. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Nowak, D.J.; Hirabayashi, S.; Doyle, M.; McGovern, M.; Pasher, J. Air pollution removal by urban forests in Canada and its effect on air quality and human health. Urban For. Urban Green. 2018, 29, 40–48. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Ash, R.M. Annual biomass loss and potential value of urban tree waste in the United States. Urban For. Urban Green. 2019, 46, 126469. [Google Scholar] [CrossRef]
- Currie, B.A.; Bass, B. Estimates of air pollution mitigation with green plants and green roofs using the UFORE model. Urban Ecosyst. 2008, 11, 409–422. [Google Scholar] [CrossRef]
- Sæbø, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427, 347–354. [Google Scholar] [CrossRef]
- Song, Y.; Maher, B.A.; Li, F.; Wang, X.; Sun, X.; Zhang, H. Particulate matter deposited on leaf of five evergreen species in Beijing, China: Source identification and size distribution. Atmos. Environ. 2015, 105, 53–60. [Google Scholar] [CrossRef]
- He, C.; Qiu, K.; Alahmad, A.; Pott, R. Particulate matter capturing capacity of roadside evergreen vegetation during the winter season. Urban For. Urban Green. 2020, 48, 126510. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, G. Air pollution tolerance, anticipated performance, and metal accumulation capacity of common plant species for green belt development. Environ. Sci. Pollut. Res. 2022, 29, 25507–25518. [Google Scholar] [CrossRef]
- Jim, C.Y.; Chen, W.Y. Assessing the ecosystem service of air pollutant removal by urban trees in Guangzhou (China). J. Environ. Manag. 2008, 88, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef] [PubMed]
- McPherson, E.G.; Simpson, J.R.; Peper, P.F.; Maco, S.E.; Xiao, Q. Municipal forest benefits and costs in five U.S. cities. J. For. 2005, 104, 411–416. [Google Scholar] [CrossRef]
- Ordóñez, C.; Duinker, P.N. Assessing the vulnerability of urban forests to climate change. Environ. Rev. 2014, 22, 311–321. [Google Scholar] [CrossRef]
- Locosselli, G.M.; de Camargo, E.P.; Moreira, T.C.L.; Todesco, E.; de Fátima Andrade, M.; de André, C.D.S.; de André, P.A.; Singer, J.M.; Ferreira, L.S.; Saldiva, P.H.N.; et al. The role of air pollution and climate on the growth of urban trees. Sci. Total Environ. 2019, 666, 652–661. [Google Scholar] [CrossRef]
- Baraldi, R.; Neri, L.; Costa, F.; Facini, O.; Rapparini, F.; Carriero, G. Ecophysiological and micromorphological characterization of green roof vegetation for urban mitigation. Urban For. Urban Green. 2019, 37, 24–32. [Google Scholar] [CrossRef]
- Singh, H.; Yadav, M.; Kumar, N.; Kumar, A.; Kumar, M. Assessing adaptation and mitigation potential of roadside trees under the influence of vehicular emissions: A case study of Grevillea robusta and Mangifera indica planted in an urban city of India. PLoS ONE 2020, 15, e0227380. [Google Scholar] [CrossRef]
- Singh, H. An integrated approach considering physiological- and biophysical-based indicators for assessing tolerance of roadside plantations of Alstonia scholaris towards urban roadside air pollution: An assessment of adaptation of plantations for mitigating roadside air pollution. Trees 2023, 37, 69–83. [Google Scholar] [CrossRef]
- Mulenga, C.; Clarke, C.; Meincken, M. Physiological and growth responses to pollutant-induced biochemical changes in plants: A review. Pollution 2020, 6, 827–848. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Dadkhah-Aghdash, H.; Rasouli, M.; Rasouli, K.; Salimi, A. Detection of urban trees sensitivity to air pollution using physiological and biochemical leaf traits in Tehran, Iran. Sci. Rep. 2022, 12, 15398. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Civerolo, K.L.; Rao, S.T.; Sistla, G.; Luley, C.J.; Crane, D.E. A modeling study of the impact of urban trees on ozone. Atmos. Environ. 2000, 34, 1601–1613. [Google Scholar] [CrossRef]
- Escobedo, F.J.; Nowak, D.J. Spatial heterogeneity and air pollution removal by an urban forest. Landsc. Urban Plan. 2009, 90, 102–110. [Google Scholar] [CrossRef]
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Urban woodlands: Their role in reducing the effects of particulate pollution. Environ. Pollut. 1998, 99, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Freer-Smith, P.H.; Beckett, K.P.; Taylor, G. Deposition velocities to Sorbus aria, Acer campestre, Populus deltoides trichocarpa ‘Beaupre’, Pinus nigra and Cupressocyparis leylandii for coarse, fine and ultra-fine particles in the urban environment. Environ. Pollut. 2005, 133, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Manes, F.; Marando, F.; Capotorti, G.; Blasi, C.; Salvatori, E.; Fusaro, L.; Ciancarella, L.; Mircea, M.; Marchetti, M.; Chirici, G.; et al. Regulating ecosystem services of forests in ten Italian metropolitan cities: Air quality improvement by PM10 and O3 removal. Ecol. Indic. 2016, 67, 425–440. [Google Scholar] [CrossRef]
- Popek, R.; Łukowski, A.; Bates, C.; Oleksyn, J. Accumulation of particulate matter, heavy metals, and polycyclic aromatic hydrocarbons on the leaves of Tilia cordata Mill. in five Polish cities with different levels of air pollution. Int. J. Phytoremediat. 2017, 19, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Dzierżanowski, K.; Popek, R.; Gawrońska, H.; Sæbø, A.; Gawroński, S.W. Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int. J. Phytoremediat. 2011, 13, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.R.; Nelson, T.A.; Coops, N.C.; Wulder, M.A. Biodiversity indicators show climate change will alter vegetation in parks and protected areas. Diversity 2013, 5, 352–373. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Kinose, Y. A review study on ozone phytotoxicity metrics for setting critical levels in Asia. Asian J. Atmos. Environ. (AJAE) 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Ashmore, M.R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005, 28, 949–964. [Google Scholar] [CrossRef]
- Karnosky, D.F.; Pregitzer, K.S.; Zak, D.R.; Kubiske, M.E.; Hendrey, G.R.; Weinstein, D.; Nosal, M.; Percy, K.E. Scaling ozone responses of forest trees to the ecosystem level in a changing climate. Plant Cell Environ. 2005, 28, 965–981. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Naidu, S.L.; Karnosky, D.F.; Long, S.P. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: A quantitative meta-analysis. Glob. Change Biol. 2009, 15, 396–424. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Duan, F.K.; He, K.B.; Qin, Y.; Tong, D.; Geng, G.N.; Liu, X.Y.; Li, H.; Yang, S.; Ye, S.Q.; et al. Air pollution characteristics and their relationship with emissions and meteorology in the Yangtze River Delta region during 2014–2016. J. Environ. Sci. 2019, 83, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Riondato, E.; Pilla, F.; Basu, A.S.; Basu, B. Investigating the effect of trees on urban quality in Dublin by combining air monitoring with i-Tree Eco model. Sustain. Cities Soc. 2020, 61, 102356. [Google Scholar] [CrossRef]
- Salmond, J.A.; Williams, D.E.; Laing, G.; Kingham, S.; Dirks, K.; Longley, I.; Henshaw, G.S. The influence of vegetation on the horizontal and vertical distribution of pollutants in a street canyon. Sci. Total Environ. 2013, 443, 287–298. [Google Scholar] [CrossRef]
- Abhijith, K.V.; Kumar, P.; Gallagher, J.; McNabola, A.; Baldauf, R.; Pilla, F.; Broderick, B.; Di Sabatino, S.; Pulvirenti, B. Air pollution abatement performances of green infrastructure in open road and built-up street canyon environments—A review. Atmos. Environ. 2017, 162, 71–86. [Google Scholar] [CrossRef]
- Rai, P.K. Impacts of particulate matter pollution on plants: Implications for environmental biomonitoring. Ecotoxicol. Environ. Saf. 2016, 129, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Kováts, N.; Hubai, K.; Diósi, D.; Sainnokhoi, T.A.; Hoffer, A.; Tóth, Á.; Teke, G. Sensitivity of typical European roadside plants to atmospheric particulate matter. Ecol. Indic. 2021, 124, 107428. [Google Scholar] [CrossRef]
- Meravi, N.; Prajapati, S.K. Temporal variation in chlorophyll fluorescence of different tree species. Biol. Rhythm Res. 2020, 51, 331–337. [Google Scholar] [CrossRef]
- Shabnam, N.; Oh, J.; Park, S.; Kim, H. Impact of particulate matter on primary leaves of Vigna radiata (L.) R. Wilczek. Ecotoxicol. Environ. Saf. 2021, 212, 111965. [Google Scholar] [CrossRef]
- Joshi, P.C.; Swami, A. Air pollution induced changes in the photosynthetic pigments of selected plant species. J. Environ. Biol. 2009, 30, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Kończak, B.; Cempa, M.; Deska, M. Assessment of the ability of roadside vegetation to remove particulate matter from the urban air. Environ. Pollut. 2021, 268, 115465. [Google Scholar] [CrossRef] [PubMed]
- Łukowski, A.; Popek, R.; Karolewski, P. Particulate matter on foliage of Betula pendula, Quercus robur, and Tilia cordata: Deposition and ecophysiology. Environ. Sci. Pollut. Res. 2020, 27, 10296–10307. [Google Scholar] [CrossRef]
- Oksanen, E. Birch as a model species for the acclimation and adaptation of northern forest ecosystem to changing environment. Front. For. Glob. Change 2021, 4, 682512. [Google Scholar] [CrossRef]
- Vapaavuori, E.; Holopainen, J.K.; Holopainen, T.; Julkunen-Tiitto, R.; Kaakinen, S.; Kasurinen, A.; Kontunen-Soppela, S.; Kostiainen, K.; Oksanen, E.; Peltonen, P.; et al. Rising atmospheric CO2 concentration partially masks the negative effects of elevated O3 in silver birch (Betula pendula Roth). AMBIO J. Hum. Environ. 2009, 38, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Araminienė, V.; Sicard, P.; Anav, A.; Agathokleous, E.; Stakėnas, V.; De Marco, A.; Varnagirytė-Kabašinskienė, I.; Paoletti, E.; Girgždienė, R. Trends and interrelationships of ground-level ozone metrics and forest health in Lithuania. Sci. Total Environ. 2019, 658, 1265–1277. [Google Scholar] [CrossRef]
- Jarraud, M.; Steiner, A. Summary for policymakers. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Malagoli, M.; Sut, S.; Kumar, G.; Dall’Acqua, S. Variations of elements, pigments, amino acids and secondary metabolites in Vitis vinifera (L.) cv Garganega after 501 biodynamic treatment. Chem. Biol. Technol. Agric. 2022, 9, 36. [Google Scholar] [CrossRef]
- Čėsnienė, I.; Miškelytė, D.; Novickij, V.; Mildažienė, V.; Sirgedaitė-Šėžienė, V. Seed treatment with electromagnetic field induces different effects on emergence, growth and profiles of biochemical compounds in seven half-sib families of silver birch. Plants 2023, 12, 3048. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and sensitive anthrone–sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef] [PubMed]
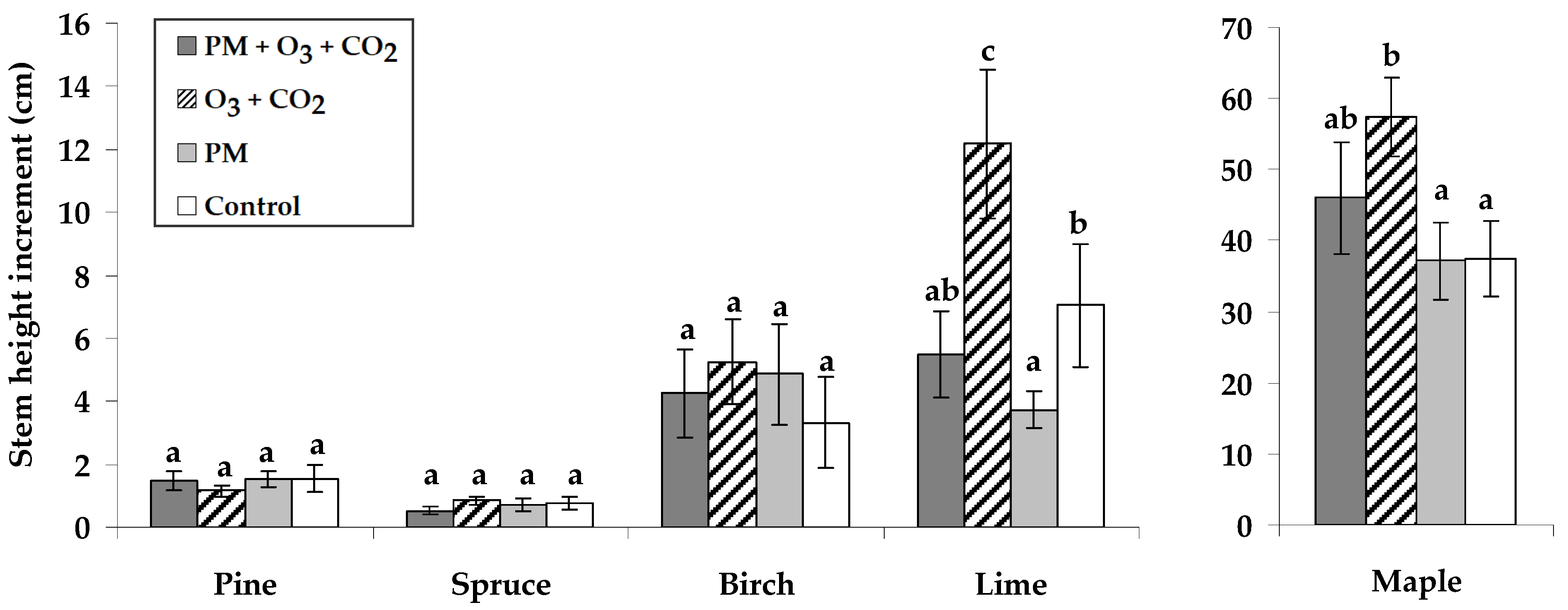
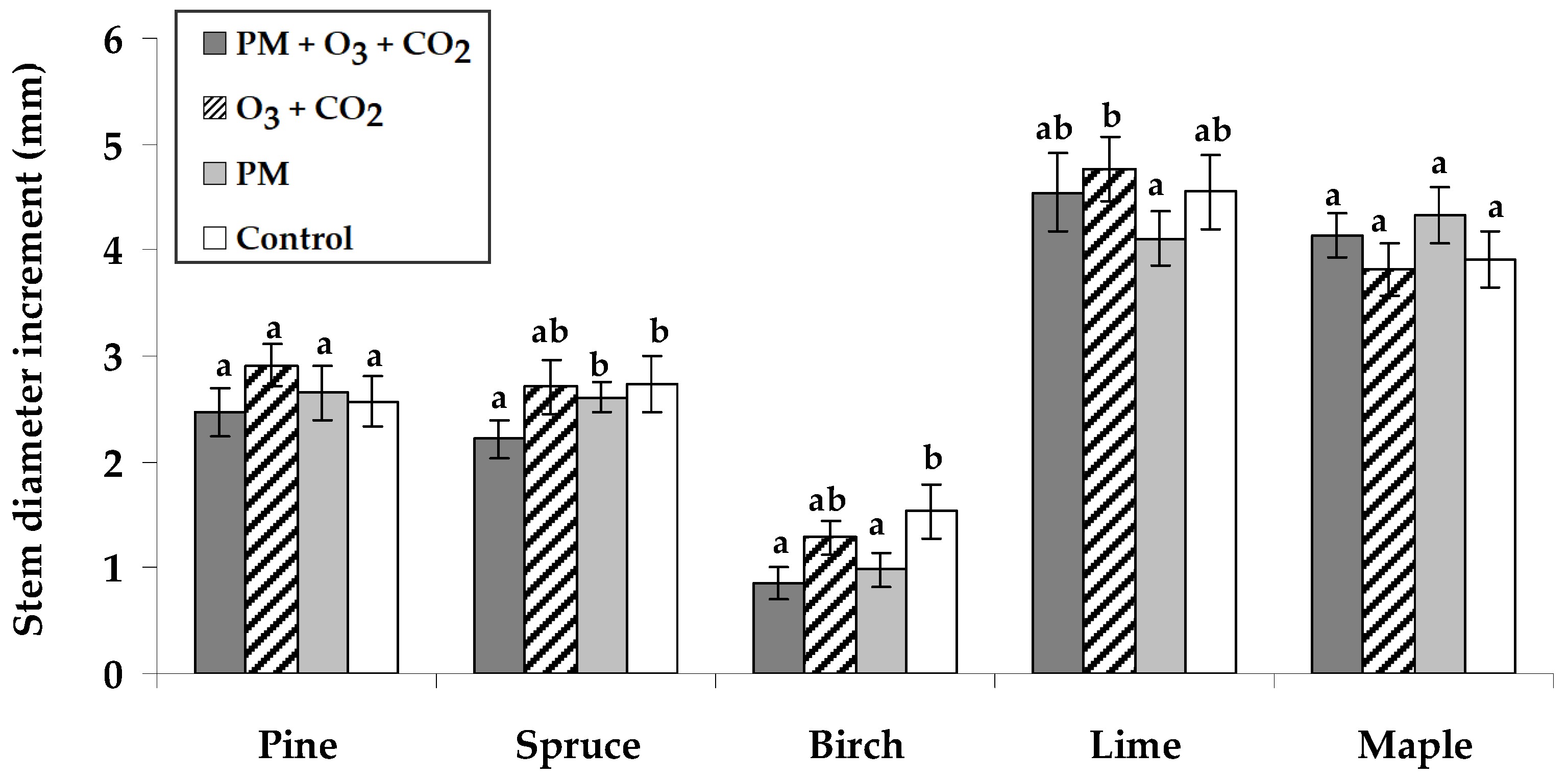


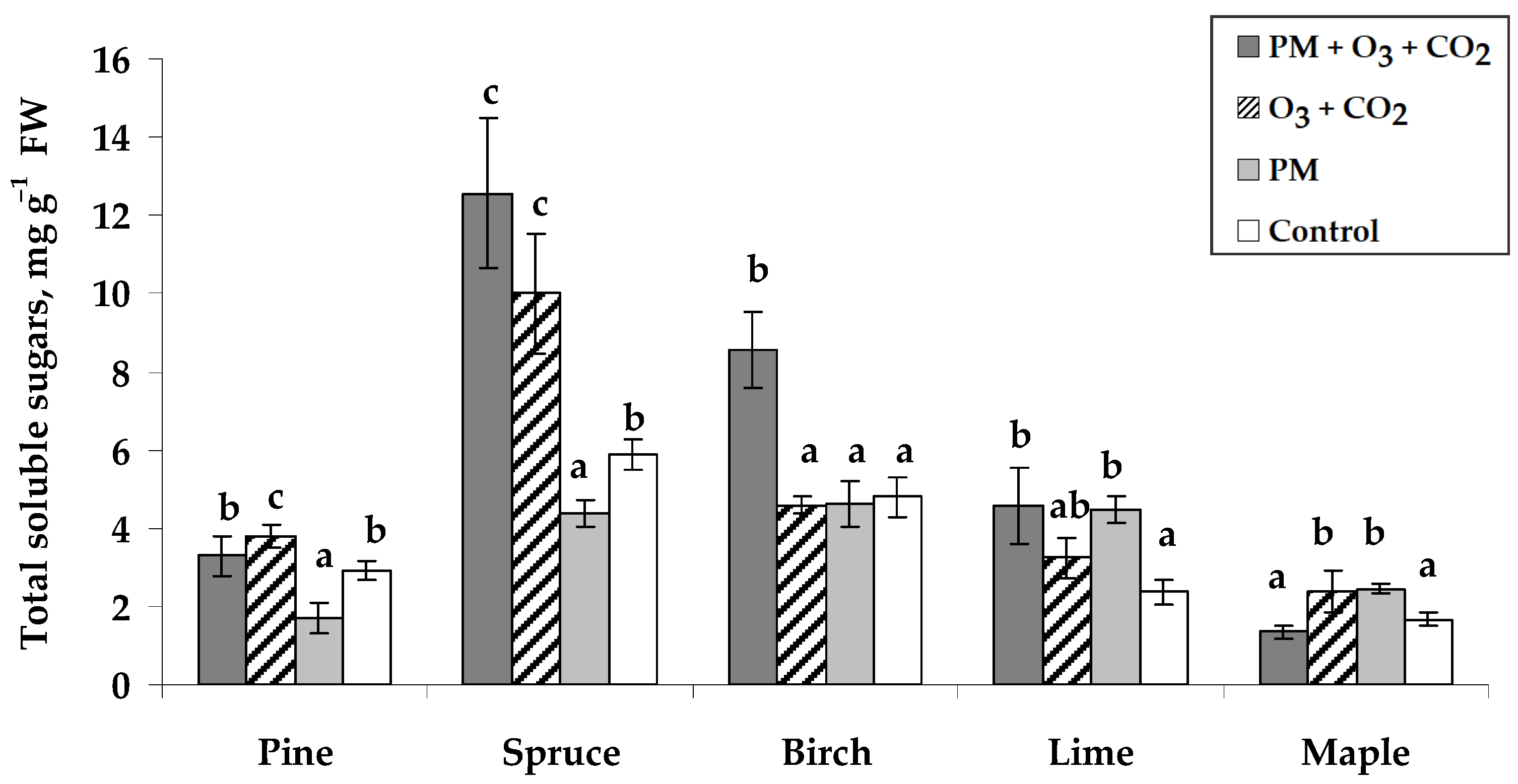
| Treatment * | Photosynthetic Pigment Content (µg g−1) ± SE | ||
|---|---|---|---|
| Chl a | Chl b | Carotenoid | |
| Scots pine seedlings | |||
| PM + O3 + CO2 | 343.8 ± 10.6 ab | 290.1 ± 7.8 b | 14.6 ± 0.2 b |
| O3 + CO2 | 325.1 ± 5.5 a | 262.2 ± 3.9 a | 13.9 ± 0.3 a |
| PM | 344.0 ± 23.8 ab | 268.1 ± 16.4 a | 14.4 ± 0.5 ab |
| Control | 366.3 ± 10.8 b | 283.6 ± 1.3 b | 14.8 ± 0.1 b |
| Norway spruce seedlings | |||
| PM + O3 + CO2 | 428.9 ± 5.5 a | 339.8 ± 7.1 a | 15.4 ± 0.6 a |
| O3 + CO2 | 459.6 ± 13.9 b | 349.2 ± 4.1 a | 17.3 ± 0.6 b |
| PM | 532.7 ± 5.4 c | 393.9 ± 4.1 b | 17.1 ± 0.2 b |
| Control | 615.9 ± 18.3 d | 427.8 ± 12.6 c | 17.3 ± 0.3 b |
| Silver birch seedlings | |||
| PM + O3 + CO2 | 385.4 ± 27.8 b | 271.7 ± 17.3 b | 20.0 ± 0.5 ab |
| O3 + CO2 | 308.0 ± 8.8 a | 226.8 ± 4.4 a | 19.6 ± 0.7 a |
| PM | 413.5 ± 36.4 b | 292.4 ± 25.3 b | 21.0 ± 0.6 ab |
| Control | 306.3 ± 14.8 a | 228.2 ± 7.3 a | 21.0 ± 0.2 b |
| Small-leaved lime seedlings | |||
| PM + O3 + CO2 | 411.9 ± 9.7 a | 296.7 ± 4.9 a | 28.6 ± 1.6 ab |
| O3 + CO2 | 595.5 ± 33.5 b | 398.2 ± 20.3 b | 29.7 ± 0.8 b |
| PM | 748.6 ± 36.0 c | 459.2 ± 20.0 c | 28.3 ± 0.7 ab |
| Control | 848.7 ± 24.8 d | 503.3 ± 10.6 d | 27.5 ± 1.1 a |
| Norway maple seedlings | |||
| PM + O3 + CO2 | 567.5 ± 26.5 b | 394.8 ± 27.0 b | 25.2 ± 1.9 ab |
| O3 + CO2 | 445.7 ± 26.4 a | 295.8 ± 17.9 a | 22.9 ± 1.0 a |
| PM | 815.3 ± 19.3 c | 520.2 ± 11.3 c | 24.2 ± 0.7 ab |
| Control | 619.6 ± 32.7 b | 421.6 ± 19.4 b | 25.1 ± 0.3 b |
| Parameter | Value | Analysis Method |
|---|---|---|
| pH | 12 | ISO 10390:2021 |
| Organic carbon (C, %) | 3.03 | LST EN 15936:2022 |
| Phosphorus (P, mg kg−1) | 14,352 | LST EN 13657:2003, LST EN ISO 6878:2004 |
| Potassium (K, mg kg−1) | 15,000 | LST EN 13657:2003, ISO 9964-3:1993 |
| Calcium (Ca, mg kg−1) | 237,250 | LST EN 13657:2003, LST EN ISO 7980:2000 |
| Magnesium (Mg, mg kg−1) | 30,083 | |
| Cadmium (Cd, mg kg−1) | 13.5 | LST EN 13657:2003, LST EN ISO 11885:2009 |
| Arsenic (As, mg kg−1) | 3.00 | |
| Nickel (Ni, mg kg−1) | 19.2 | |
| Lead (Pb, mg kg−1) | 98.0 | |
| Boron (B, mg kg−1) | 457 | |
| Vanadium (V, mg kg−1) | 9.3 | |
| Chromium (Cr, mg kg−1) | 58.8 | |
| Copper (Cu, mg kg−1) | 135 | |
| Zink (Zn, mg kg−1) | 2947 | |
| Mercury (Hg) | 0.143 | LST EN 13657:2003, LST EN ISO 12846:2012 |
| Benzo(a)pyrene | <0.5 | LST EN 17503:2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černiauskas, V.; Varnagirytė-Kabašinskienė, I.; Čėsnienė, I.; Armoška, E.; Araminienė, V. Response of Tree Seedlings to a Combined Treatment of Particulate Matter, Ground-Level Ozone, and Carbon Dioxide: Primary Effects. Plants 2025, 14, 6. https://doi.org/10.3390/plants14010006
Černiauskas V, Varnagirytė-Kabašinskienė I, Čėsnienė I, Armoška E, Araminienė V. Response of Tree Seedlings to a Combined Treatment of Particulate Matter, Ground-Level Ozone, and Carbon Dioxide: Primary Effects. Plants. 2025; 14(1):6. https://doi.org/10.3390/plants14010006
Chicago/Turabian StyleČerniauskas, Valentinas, Iveta Varnagirytė-Kabašinskienė, Ieva Čėsnienė, Emilis Armoška, and Valda Araminienė. 2025. "Response of Tree Seedlings to a Combined Treatment of Particulate Matter, Ground-Level Ozone, and Carbon Dioxide: Primary Effects" Plants 14, no. 1: 6. https://doi.org/10.3390/plants14010006
APA StyleČerniauskas, V., Varnagirytė-Kabašinskienė, I., Čėsnienė, I., Armoška, E., & Araminienė, V. (2025). Response of Tree Seedlings to a Combined Treatment of Particulate Matter, Ground-Level Ozone, and Carbon Dioxide: Primary Effects. Plants, 14(1), 6. https://doi.org/10.3390/plants14010006







